Abstract
The retinal topography of three species of coleoid cephalopod (one cuttlefish, one squid and one octopus) was investigated to examine and compare the structure, density and organization of the photoreceptors. The aim was to determine if there were areas of increased cell density and/or cell specialization that might be related to lifestyle or phylogeny. The orientation of photoreceptors around the curved surface of the retina was also mapped to reveal how the overall arrangement of cell microvilli might enable the perception of polarized light stimuli. It was found that all species possessed an increase in photoreceptor density in a horizontal streak approximately placed at the position of a potential horizon in the habitat. The overall arrangement of photoreceptor microvillar arrangements followed lines of latitude and longitude in a global projection that has been rotated by 90°. This arrangement seems to map polarization sensitivities on the outside world in a vertical and horizontal grid. The potential significance of this and other retinal specializations is discussed in the context of phylogenetic and habitat differences between species.
Keywords: retinal topography, polarization sensitivity, coleoid cephalopod, e-vector, visual ecology
1. Introduction
(a). Retina topography
Photoreceptors and other cells in the retinae of many animals are arranged in a way that mirrors features of their specific visual field and facets of their environment. This is often achieved via specialized areas on the retina in which the photoreceptors may be more densely packed, elongated, shortened, banked or a combination of these anatomical specializations. Examples include: the fovea in humans and some teleosts—a point on the retina in which the photoreceptors are short and densely packed [1]; the horizontal streak in rabbits—a band of higher density ganglion cells across the horizontal equator of the eye [2]; the layers of photoreceptors in the banked retinae of some teleosts and the firefly squid—regions in the retina where there are layers of photoreceptors on top of each other, potentially allowing increased sensitivity and spectral tuning [3–5].
(b). Areas of specialization and ecological significance
The ecological significance of a specialized region on the retina often relates primarily to an increased level of resolving power, or increased sensitivity, for a part of the visual field that is for some reason significant for that species. Much in the same way as the spectral sensitivity of an animal's visual pigments are tuned to suit the spectral characteristics of their habitat, the photoreceptors are arranged and structured in accordance with the spatial and temporal characteristics of the visual field, and the requirements imposed by ethological and ecological factors [1]. An example of this correlation between the retinal features of many animals with their habitat, the ‘Terrain Theory’ [6], is the horizontal streak seen in many land and aquatic animals, such as rabbits, some fishes, crustaceans and cuttlefish [2,7–9]. This streak may allow the animal to visualize or ‘concentrate on’ the horizon without the need for eye movements required to perform the same task with a simple fovea or area centralis [7]. This may be of particular use for animals whose predators and prey are seen often laterally and monocularly [2,8]. The ‘horizon’, especially in the marine habitat, may be a local feature, rather than the far off edge of the globe as we think of it. In some cases, this ‘local horizon’, or main area of interest, may be on the substrate below the body position, or more related to the ocean surface for animals whose gaze is directed upwards to hunt prey out of the water, and therefore above the animal in question.
(c). Cephalopod eyes and retinae
The coleoid cephalopods have inhabited the oceans for well over 300 Myr [10], and currently occupy every type of marine habitat. Such substantial radiation has led to the evolution of remarkable visual systems. Coleoid eyes are structurally similar to those of marine vertebrates [8] in that they possess a camera-type eye with a spherical lens, a cornea and retractable pupil. Most commonly, the retina comprises a single type of photoreceptor cell. The outer segments possess two sets of stacked microvilli on opposite sides called rhabdomeres. The rhabdomeres on one cell sit orthogonally to the rhabdomeres on adjacent cells, forming a lattice-like square mosaic across the entire surface of the retina. In addition, dichroic photopigment molecules are positioned within these microvillar membranes. The absorption axes of their chromphores are roughly parallel to those of the microvilli, resulting in increased sensitivity to e-vectors of polarized light oriented parallel to the microvillar long axis [11–18]. Unlike vertebrates, irrespective of their habitat most cephalopod species are monochromatic, possessing a single visual pigment peaking in sensitivity between 470 and 500 nm [19]. Known exceptions to this are the firefly squid and relatives and their multiple spectral sensitivities seem specifically related to bioluminescent signalling [4]. It is, therefore, an attractive hypothesis to suggest that polarization vision may be more important and in some ways substituted for colour sense in these highly visual animals.
Coleoid eye morphology varies in terms of shape and size among species, while the orientation of the eye is often maintained constant relative to the horizontal plane (despite the position and the orientation of the body)—a characteristic probably related to maintaining a fixed orientation to the outside world and a feature of most visual systems in both invertebrates and vertebrates [20,21]. Here, we suggest that this eye stabilization also enables cephalopods to maintain a fixed e-vector sensitivity relative to the visual field. An external feature that is useful in determining this eye position relative to horizontal is the coleoid pupil, known to take on a variety of shapes such as: circles, horizontal slits, ‘u’ and ‘w’ shaped, ‘w’ with a ‘v’ on top and dorsally shifted horizontal slits.
These unusual features of eye design led to the hypothesis that there may be areas of specialization in the retina, which correlates with the pupil shape, possibly resulting in higher levels of acuity and/or sensitivity for parts of the visual field [8,22,23].
(d). Retinal topography and pupil shape
The purpose of an adjustable pupil is to alter the amount of light entering the eye, depending on ambient light levels [24], so as to avoid saturating the photoreceptors with light (high light conditions) or increase their chance of detecting light (in low light conditions). It also can help an animal focus on a particular area or item of interest, such as a prey item on the horizon, within the visual field by increasing sensitivity or resolution [8,25] and eliminating distracting input. Possession of a complementary, horizontal area on the retina with increased visual acuity, by way of increased photoreceptor density, and sensitivity through increased photoreceptor length or banking of the retina, might be expected. Before this study, however, this has only been discussed qualitatively for some coleoid species including Octopus vulgaris and Sepia officinalis [8,25].
Thus, this study investigates whether coleoids possess retinal specializations that are correlated with their environment and secondarily their pupil shape, and discuss what purposes this may serve in relation to survival in their specific habitat.
(e). Photoreceptor arrangement
The ability of coleoid cephalopods to detect and respond to polarized light is a function of the photoreceptor morphology, and photopigment alignment within the photoreceptor microvilli [15,18,26,27]. The main question we pose here is, do coleoids maintain a constant orientation of rhabdomeric microvilli parallel to the expected orientations of e-vectors entering the eye from anywhere within the visual field? Can they do this without either making postural changes, or confounding the signals by detecting multiple different e-vectors simultaneously, resulting in cancellation or fluctuations of the polarized signal? Shashar et al. [16] investigated the retinae of three other coleoids and found that, although the microvilli are arranged orthogonally to one another, their axes are not always fitted exactly to horizontal and vertical axes, but fluctuate by up to 20° across the retina. Thus, how should the orthogonally aligned microvilli be arranged across the curved surface of the retina in order to avoid, or at least minimize, such complications?
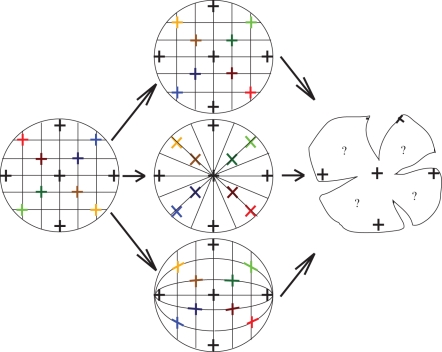
There are several theoretical possibilities for the arrangement of a square, lattice array of orthogonal microvilli across the curved retinal surface. They include: square, radial and along lines of latitude and longitude with lines in this instance corresponding to the axes along which the horizontal and vertical axes of the photoreceptors (and therefore their e-vector sensitivities) are aligned (figure 1).
Figure 1.
Three possible photoreceptor arrangements. The left picture represents a non-standard visual field that has been gridded with horizontal and vertical lines to represent horizontal and vertical e-vectors, the middle column shows three possible placements of individual orthogonal photoreceptors, (rhabdoms—the crosses) which correspond to the crosses in the visual field on the left viewed two-dimensionally (as if looking at a picture of a globe of the world), and the right column represents a single hypothetical wholemounted retina. Note that this projection is not to scale, but a simple representation of how horizontally and vertically oriented components in the visual field may translate onto the coleoid retina. The top row shows a simple square arrangement of photoreceptors, where the horizontal and vertical axes of the microvilli are aligned on horizontal and vertical axes across the retinal surface relative to the outside world. The middle row shows a radial arrangement of the photoreceptors, whose horizontal and vertical microvillar axes are arranged along lines that radiate out from the centre of the retina to the peripheral retina. The bottom row shows an arrangement of photoreceptors whose horizontal and vertical axes are aligned along lines of latitude and longitude rotated 90°.
We hypothesize here an arrangement that should allow horizontal and vertical e-vectors from the external polarization field to be detected by a corresponding set of orthogonal microvilli oriented horizontally and vertically, and then test this through photoreceptor orientation mapping. Maintaining a fixed eye position with habitat may optimize sensitivity or discriminability to the most ecologically important e-vectors. This is especially important to understand given that coleoids are highly flexible animals (especially the octopus) but typically seem to keep their eyes in a relatively fixed position relative to the environment, despite body movement and contortion during locomotion (figure 2).
Figure 2.
Coleoid cephalopods tend to keep their eyeball in a stable position relative to the environment no matter the pose or means of locomotion. This is especially obvious in octopus. Maintaining this stable state could ensure constant, maximum sensitivity or discriminability to horizontal and vertical e-vectors at all times.
2. Material and methods
One representative of each coleoid group was selected for this study. Cuttlefish, Sepia plangon, the mourning cuttlefish; squid, Sepioteuthis lessoniana, the bigfin reef squid; octopus, Octopus cyanea, the day octopus. All specimens were caught on North Stradbroke Island, Queensland, using seine nets or hand-capture on SCUBA. Specimens were kept at Moreton Bay Research Station, Dunwich, North Stradbroke Island, Queensland, in individual flow through tanks until required for analysis.
(a). Wholemount preparation
Following anaesthesia and decapitation, eye balls were removed and either fixed in a solution of 4–10% formalin and sea water for later processing, or processed immediately using the following modified wholemount techniques:
A small incision was made just dorsal to the pupil to maintain the orientation of the eye during preparation for analysis. The front corneal and scleral surface of the eye ball was removed, leaving just the eye cup containing the retina. The retina was removed carefully and relaxation slits were made to allow it to be opened out flat. Particular attention was paid to the location of the dorsal slit to allow reconstruction of the photoreceptor arrangement later in the process.
Retinae were placed into a solution of 4 per cent formalin in sea water for up to 15 min to preserve the tissue, and then rinsed in 425 mOsm phosphate buffer solution (PBS). Retinae were then placed into a solution of either 3 (if freshly fixed) or 6 per cent (if previously fixed) hydrogen peroxide with 425 mOsm PBS, set to a pH of approximately 11.94 using 0.1 M KOH. This protocol was adapted from Kröger & Wagner [28] who used this method on fish to bleach retinal pigment (e.g. the retinal pigment epithelium) and allow unobstructed visualization of photoreceptors. Retinae were left to bleach for between 1 and 3 days, depending on the length of time they had been in fixative for. Bleaching was also conducted to remove the screening pigment from the photoreceptors, which can make it difficult to count individual cells.
One bleached retina from each species was then placed onto a slide inside a small well made using thin strips of tape built up no higher than the thickness of the retina. A drop of glycerol was placed onto the retina, which was then covered with a coverslip. The coverslip was sealed with nail polish and left to dry. Mounted retinae were placed onto a flatbed scanner with a standard ruler aligned next to the glass slide. Images were imported into Adobe Photoshop and a 1 mm scale bar was made using the ruler, and placed under the retina. The retina and scale bar were then printed onto an A4 paper. Using a light box, the outline of the retina and the scale bar was traced onto a sheet of 1 mm A4 graph paper. This was to obtain a larger outline of the retina onto which the density counts could be recorded and followed the protocols established by Hughes et al. (in [29]), but particular care was taken again to ensure that the retina maintained a fixed orientation after removal.
(b). Counting cell densities
Photoreceptor cells within wholemounts were counted under a light microscope (Zeiss Axioscop—HBO 50) rotated 180° so that the image seen through the eyepiece was the correct way up. A point on the retina was chosen as the reference, and its coordinates were recorded on a graph paper onto which an outline of the retina was drawn. At 100× magnification, cells within a 10 × 10 minigrid were counted (an area of 0.01 mm2 at 100×). These were counted at 0.5 mm intervals across the entire surface of the retina. Areas containing similar numbers of cells (within 5000) were joined with contour lines and colour coded to produce a density topographic map.
(c). Modelling photoreceptor arrangement
A large, white, plastic hemisphere (1 m diameter) mounted into a goniometric frame, allowing rotation along both the horizontal and vertical axes, was used to replicate a retinal cup onto which horizontal and vertical ‘e-vectors’ were projected through a circular pupil (15 cm diameter) suspended in the middle of the open entrance of the hemisphere—there was no object representing a ‘lens’ in this model, as refraction of incoming e-vectors was not considered to effect their e-vector angle. A laser producing a small bar was placed outside the hemisphere, aimed in through the pupil, and its location on the hemisphere traced with a black marker (this was done when the laser bar was orientated horizontally and vertically to create crosses representing the orthogonal pairs (rhabdoms) of microvilli on the photoreceptors). The laser was projected into the hemisphere from approximately 15° intervals both horizontally and vertically outside the hemisphere, until it was covered in crosses, revealing the arrangement of orthogonal microvilli (as denoted in the middle column of figure 1) that would be aligned parallel to the incoming horizontal and vertical e-vectors.
Having determined this theoretical arrangement, a small rubber ball was cut in half to produce another hemisphere that was to represent a retinal cup. The arrangement in the large hemisphere was drawn onto the inside surface of the ball. Several cuts were then made from the rim of the rubber ball hemisphere in towards the middle to enable it to be opened out flat—the same method used to open out a real retina for wholemounting. Thus, it was possible to see how this proposed arrangement on the model should look on a wholemounted retina. This was compared with the real wholemounted retinae of the species used in this study to see if there was correspondence. Photos of different regions of the wholemounted retinae were taken using an RTO Colour camera (SPOT Diagnostic Instruments Inc) using SPOT software and a Zeiss Axioscop light microscope, and compared with the corresponding part of the wholemount model, taking into account the individual shape of the wholemounted retina. The model could be adjusted to resemble the shape of each retina using corresponding sets of cuts to open out the model. Photos could then be compared with the model.
3. Results
(a). Photoreceptor density
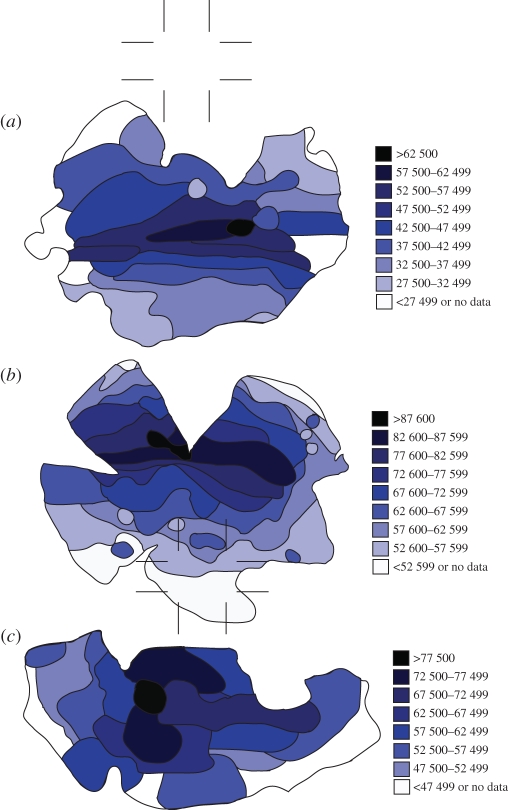
Each species was found to possess an area on the retina containing an increased level of photoreceptor density in the form of a streak in the cuttlefish and octopus and a weak streak and a centralized area in the squid (figure 3). The visual streak in O. cyanea appeared slightly dorsally shifted while that of S. plangon was positioned along the equator of the eye. Octopus cyanea possessed the highest photoreceptor density at close to 90 000 cells mm−1. Table 1 contains a list of the species included in this study, detailing the shape of their pupil, the type and the location of that specialization on the retina and the typical habitat that species occupies.
Figure 3.
The retinal topography of each of the three species in this study: (a) S. plangon and (b) O. cyanea show a prominent, horizontally orientated band of increased photoreceptor density across the horizontal equator of the retina; (c) S. lessoniana shows a more centrally positioned area of increased photoreceptor density. Densities peak at over 87 600 cells mm−2 in O. cyanea. In each case, there were areas of retina (mostly in the periphery) where no cell counts could be taken owing to damaged tissue; however, the tissues approaching these areas give an indication of most probable density.
Table 1.
The specimens included in this study, their retinal specialization type, location of specialization on the retina and typical habitat types.
| species | pupil shape | type of specialization | position of specialization | typical habitat |
|---|---|---|---|---|
| S. plangon | ‘w’ | horizontal band | horizontal equator | seagrass, mudflats, rocky reef, benthic |
| O. cyanea | horizontal band | horizontal band | horizontal equator | coral reef, rocky reef |
| S. lessoniana | ‘u’ | centralized area | centre | seagrass, rocky reef midwater |
(b). Photoreceptor arrangement
Using the large hemisphere model and laser, the axes of the orthogonal pairs of photoreceptors were found to be approximately aligned along lines of latitude and longitude that have been rotated 90° in all species (figure 4).
Figure 4.
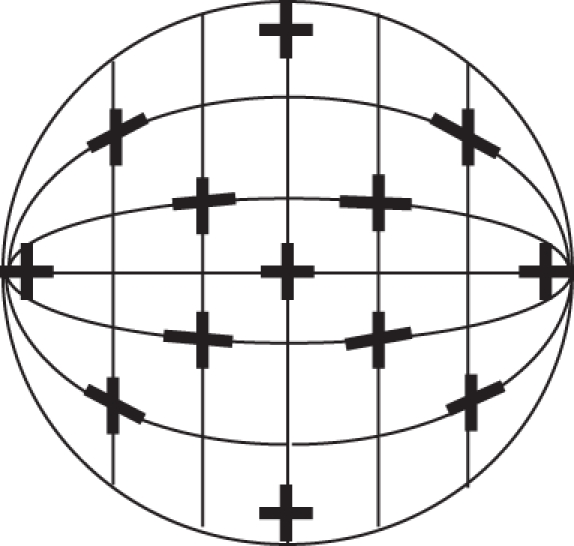
Representation of the hemisphere model eye cup (viewed from the front). The axes of the orthogonally paired rhabdomeres (represented by black crosses at approximately 30° intervals) are aligned along lines of latitude and longitude that have been rotated 90°, as determined by the hemisphere model.
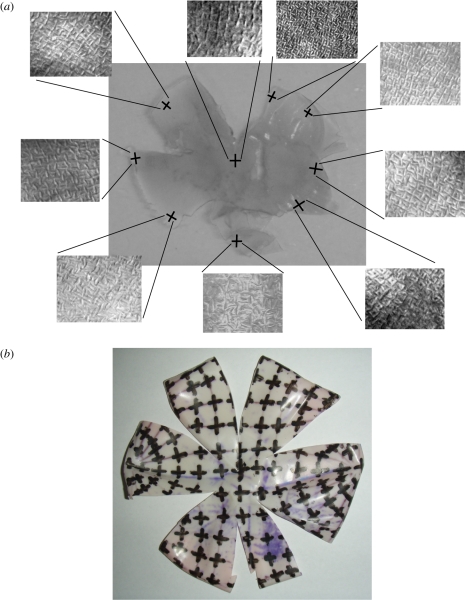
Using the small rubber ball hemisphere model, which had the large hemisphere model arrangement translated onto its inside surface, it was found that the suggested arrangement matched that observed in the wholemounted retinae of all three species quite closely. Figure 5 shows the local orientation of orthogonally arranged cells from different areas of the wholemounted retina of O. cyanea compared with the local orientation of crosses in the same areas on the rubber ball model.
Figure 5.
The wholemounted retina of O. cyanea. (a) Pictures of photoreceptors were taken from around the wholemount to show local orientation of orthogonal cells. (b) These orientations were compared with the rubber ball model containing the hypothesized arrangement proposed in this study.
4. Discussion
This visual ecological study is the first illustration of comparative retinal topography among the three coleoid cephalopod groups. It examines potential correlations between retinal specializations, habitat and pupil shape. All three species are found in the coastal waters of South Queensland and inhabit similar basic environments and climates. They do, however, maintain quite different attitudes to the substrate, as well as modes of predation, and it is interesting to examine and compare the visual adaptations between these distantly related members of the same sub-class.
(a). Retinal specialization and pupil shape
The cuttlefish and octopus species in this study each possess pupils elongated horizontally across the eye. Their retinae also possess a band of higher photoreceptor density across the horizontal equator of the retina, much like a rabbit [2] and it is tempting to speculate that these two eye features are correlated in these cases. The squid possesses a u-shaped pupil and a more circular, centrally positioned area of higher photoreceptor density, again perhaps reflecting the pupil aperture shape. All three species are capable of dilating their pupils to such a degree that there is little iris remaining visible and this occurs during periods of low light (figure 6). Cuttlefish also show local pupil dilations, that is only the front or back of the pupil may dilate, when the animal fixates or becomes interested in specific objects such as prey or approaching divers from different directions (personal observation, 2006–2010). It is in fact not well known if cephalopod optical elements, including the pupil shape, in any way result in the retinal specializations seen. Alternatively, odd pupil shapes are also known to be effective for camouflage in a number of animals as eyes are a strong and salient feature in any interaction [23]. With the cephalopods' astonishing camouflage capability [27,30], their strange pupil shape may also aid in disguising the eyes of these lurking predators. Interestingly, some cuttlefish can also pinch their pupil into two smaller mini-pupils (one anterior and one posterior), and would particularly benefit from this specialization as they typically swim backwards away from predators, and hunt prey items from the front [8]. An interesting study by Malmström & Kröger [23] suggested that differently shaped pupils in vertebrates may be adaptations for reducing chromatic aberration, using multi-focal lenses during periods where the pupil is contracted, such as in high light conditions. A contracted circular pupil prevents much of the ambient light passing through the peripheral zones of the lens, which in some animals may result in loss of focused images for certain wavelengths. A contracted elongated pupil slit would, however, still allow light to pass through the periphery of the lens and provide the ability to obtain focused images even in high light conditions. However, given that most coleoids are indeed monochromatic, the effects of chromatic aberration at the lens would be negligible [31,32]. Thus, the purpose of the elongated pupils seems more related to improving focal range and maintaining high levels of resolution for important parts of the visual field (in conjunction with a retinal specialization), than with dealing with the effects of lens aberration.
Figure 6.
Top three rows are schematic diagrams of the eye from each coleoid group included in this study, the bottom row shows a photograph of the light adapted eyes of: left—O. cyanea, middle—S. plangon, right—S. lessoniana. Of the schematic pictures, the middle row shows the shape of the pupil in normal light adapted conditions (dark line) and the general shape of the retinal specialization for each group (thin line). Top row shows the contracted pupil in bright light conditions, bottom row shows the dilated pupil in low light conditions.
(b). Photoreceptor density, habitat and habits
Horizontal streaks are typically found in animals that may fixate the horizon or local horizon, looking for objects of interest, such as predators and prey, without having to necessarily rely on conspicuous eye movements, such as in some fish, reptiles and amphibians (a list of such animals are included in [7]). This may be especially useful for animals that need to maintain a certain level of crypsis during predatory behaviour or predator avoidance [2]. It also places the area of highest acuity on the part of the environment that may contain critical information, such as an approaching animal breaking the horizon line [33]. Some pelagic species of fish are known to possess less pronounced visual streaks, while those closely associated with the surface may show upward directed streaks, presumably matched to detecting prey on the surface of the water [7,34]. The squid in our study S. lessoniana is not specifically surface oriented and its lack of, or having at most a weak, horizontal streak may reflect life in mid water with no specific horizontal feature, and a need to detect potential predators and prey from all directions.
The octopus O. cyanea spends almost all its life in contact with the substrate, while the cuttlefish, S. plangon, primarily hovers just over the sea bed. The horizontally weighted visual sampling of these two species appears matched to a flat benthic topography. This is consistent with the findings in some teleost fish [7] in that animals whose primary visual field is occupied by the sand–water interface, or air–land interface for terrestrial animals, typically possess a horizontal streak-like specialization providing a greater ability to focus on features in the plane of the horizon. Muntz [8] concluded that such horizontal streaks in octopus and cuttlefish are appropriate for these benthic species, as their vision is indeed adapted for best resolution near the horizon, because their prey are typically detected and hunted from the front or side, and because they are capable of swimming sideways and backwards on the sea floor, as well as forward.
The position of the visual streak in the octopus is slightly dorsally shifted compared with the cuttlefish, perhaps reflecting the slightly elevated attitude of the cuttlefish in the water column where they may be more concerned with more distant objects. This may be expected for the relatively fast locomotion of the cuttlefish, while octopus is generally a slow mover over the bottom.
(c). Photoreceptor arrangement and e-vector projection
Before determining the actual orientation of orthogonal microvilli within the rhabdoms of each species, we hypothesized that any cephalopod may benefit from examining polarized light in the environment around it, mainly along horizontal and vertical directions. For much of the day and for most conditions, scattering from particles in the water column provides a predominantly horizontally polarized light field [35–38]. The result is that any animal possessing a vertical polarizing analyser, looking into this strongly (20–40%) horizontally polarized illumination, will be able to detect some objects more effectively against the resultant ‘dark field’ background provided by the vertical analysis of horizontally polarized light. While Johnsen et al. [39] provide precautionary evidence, it has previously been hypothesized that transparent objects such as potential zooplankton prey, may be more easily detected in this way [40,41]. The purpose of the second e-vector detector matched to, and thus analysing, the horizontal spacelight is not known, but may allow opponent processing. Any such neural analysis of stimuli is critically dependant on the way in which the rhabdoms sensitive to horizontally or vertically polarized light are connected, and the resultant interneuronal and indeed brain processing. Unfortunately, in the cephalopods, we know very little about such processes [18].
Two orthogonal polarization-sensitive photoreceptors may also increase the visibility of objects through a different mechanism: by the subtraction of scattered ‘veiling light’ between the object and observer [42,43]. Contrast increase through removing intervening scatter between the observer and object, while maintaining image quality, is a suggested function of two orthogonally arranged e-vector-sensitive photoreceptor populations [43,44]. This works because, as we have already seen, light scattered from particles in water is partially polarized horizontally for much of the time [35,45]. For such a two-channel system to function well, the e-vector sensitivities should be arranged to provide a maximum difference between channels, that is, one horizontal and one vertical. Our proposed arrangement of photoreceptors, aligned along lines of latitude and longitude rotated 90°, should theoretically provide the ideal alignment of rhabdoms relative to such a horizontally polarized external environment. Known fluctuations of this horizontal field that occur towards the beginning and end of the day or as the Sun seasonally departs from being mainly overhead [26,45], could be compensated for small rotational eye movements and all cephalopods are known to be capable of such eye movement [46].
When our hypothesized model was compared with actual wholemounted retinae belonging to each species used in this study, using the laser projection and cut open rubber ball approach, a close match was found with the observed arrangement of the photoreceptor array of each species. Thus, we can show that the horizontal and vertical axes of the orthogonally arranged microvilli in the retinae of these three species of coleoid cephalopod, are aligned along lines of latitude and longitude rotated 90°, and thus maintain constant orientation to horizontal and vertical e-vectors entering the eye from anywhere within the visual field, as long as the position of the eye is maintained horizontal with respect to the horizon.
Cephalopod species from the cuttlefish, to the squid and a lesser extent to the octopus, are known to reflect polarized light and the suspected function of these markings is communication [47,48]. It is also possible that the polarization-sensitive visual system in cephalopods is used to interpret these signals, the functions of which remain largely unknown. It is also interesting to note that these polarization reflections could, in principle, be detected by any properly constructed arrangement of polarization sensitivities [49].
(d). Evolution and phylogeny
The three species used in this study represent three orders of the coleoid class of cephalopods—Octopoda (octopus), Teuthida (squid) and Sepiida (cuttlefish). All three species inhabit environments that overlap both spatially and temporally [50], but vary with the amount each is ‘wedded’ to the substrate. The octopus and cuttlefish may have independently evolved a retinal streak to sample efficiently from such a world, or share a common ancestor with such a visual system, but this remains unknown. The more pelagic squid is more closely related to cuttlefish than octopus (see phylogenetic tree—[51]) and has a weak retinal streak and more of a central area of heightened photoreceptor density. We suggest it probably that these species may have evolved their respective visual features as a result of environmental pressures, rather than phylogenetic processes. Our future studies will examine more closely related species, for instance, a number of species of octopus, but with variable habitat (benthic versus pelagic) to learn more about how retinal design responds to the evolutionary pressures associated with different visual worlds.
Acknowledgements
All experiments were performed in accordance with the following permits: the University of Queensland Animal Ethics Committee, permit number SBS/738/08/ARC; Moreton Bay Marine Parks Regulation Permit, permit number QS2008/CVL625; Queensland Government Department of Primary Industries and Fisheries, permit number 55604.
Many thanks to Prof. Shaun Collin and Dr Susan Theiss for their technical and methodological advice. This research was funded by the Research Scholarships Office at the University of Queensland, the Australian Research Council, Asian Office of Aerospace Research and Development and Air Force Office of Scientific Research.
Footnotes
One contribution of 20 to a Theme Issue ‘New directions in biological research on polarized light’.
References
- 1.Wagner J. 1990. Retinal structure of fishes. In The visual system of fish (eds Douglas R., Djamgoz M.), pp. 109–157 London, UK: Chapman and Hall Ltd Publishers [Google Scholar]
- 2.Hughes A. 1971. Topographic relationships between the anatomy and physiology of the rabbit visual system. Documenta ophth 30, 33–159 10.1007/BF00142518 (doi:10.1007/BF00142518) [DOI] [PubMed] [Google Scholar]
- 3.Denton E. J., Locket N. A. 1989. Possible wavelength discrimination by multibank retinae in deep-sea fishes. J. Mar. Biol. Assoc. UK 69, 409–435 10.1017/S0025315400029507 (doi:10.1017/S0025315400029507) [DOI] [Google Scholar]
- 4.Michinomae M., Masuda H., Seidou M., Kito Y. 1994. Structural basis for wavelength discrimination in the banked retina of the firefly squid Watasenia scintillans. J. Exp. Biol. 193, 1–12 [DOI] [PubMed] [Google Scholar]
- 5.Pumphrey R. J. 1948. The theory of the fovea. J. Exp. Biol. 25, 299–312 [Google Scholar]
- 6.Hughes A. 1977. The topography of vision in mammals of contrasting lifestyle: comparative optics and retinal organisation. In Handbook of sensory physiology, vol. 7/5 (ed. Crescitelli F.), pp. 613–756 Berlin, Germany: Springer [Google Scholar]
- 7.Collin S. P., Pettigrew J. D. 1988. Retinal topography in reef teleosts: II. Some species with prominent horizontal streaks and high-density areae. Brain Behav. Evol. 31, 283–295 10.1159/000116595 (doi:10.1159/000116595) [DOI] [PubMed] [Google Scholar]
- 8.Muntz W. 1999. Visual systems, behaviour and environment in cephalopods. In Adaptive mechanisms in the ecology of vision (ed. Archer, et al.), pp. 467–484 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 9.Zeil J., Hemmi J. M. 2006. The visual ecology of fiddler crabs. J. Comp. Physiol. A 192, 1–25 10.1007/s00359-005-0048-7 (doi:10.1007/s00359-005-0048-7) [DOI] [PubMed] [Google Scholar]
- 10.Boyle P., Rodhouse P. 2005. Origin and evolution. In Cephalopods—ecology and fisheries, pp. 36–49 Oxford, UK: Blackwell Publishing [Google Scholar]
- 11.Moody M., Parriss J. 1960. Discrimination of polarized light by Octopus. Nature 186, 839–840 10.1038/186839a0 (doi:10.1038/186839a0) [DOI] [PubMed] [Google Scholar]
- 12.Moody M., Parriss J. 1961. The discrimination of polarized light by Octopus: a behavioural and morphological study. Z. Vergl. Physiol. 44, 268–291 10.1007/BF00298356 (doi:10.1007/BF00298356) [DOI] [Google Scholar]
- 13.Nilsson D.-E., Warrant E. 1999. Visual discrimination: seeing the third quality of light. Curr. Biol. 9, R535–R537 10.1016/S0960-9822(99)80330-3 (doi:10.1016/S0960-9822(99)80330-3) [DOI] [PubMed] [Google Scholar]
- 14.Saibil H. R., Langmack K. A., Venien-Bryan C., Wilkinson J. R. 1995. Squid Rhodopsin. In Cephalopod neurobiology: neuroscience studies in squid, octopous and cuttlefish (eds Joan Abbott N. J., Williamson R., Maddock L.), pp. 479–489 Oxford, UK: Oxford University Press [Google Scholar]
- 15.Saidel W., Lettvin J., MacNicol E. 1983. Processing of polarized light by squid photoreceptors. Nature 304, 534–536 10.1038/304534a0 (doi:10.1038/304534a0) [DOI] [PubMed] [Google Scholar]
- 16.Shashar N., Milbury C., Hanlon R. 2001. Polarization vision in cephalopods: neuroanatomical and behavioural features that illustrate aspects of form and function. Mar. Freshw. Behav. Phy. 35, 57–68 10.1080/10236240290025617 (doi:10.1080/10236240290025617) [DOI] [Google Scholar]
- 17.Young J. Z. 1960. The visual system of octopus. Nature 186, 836–839 10.1038/186836a0 (doi:10.1038/186836a0) [DOI] [PubMed] [Google Scholar]
- 18.Young J. 1962. The retina of cephalopods and its degeneration after optic nerve section. Phil. Trans. R. Soc. Lond. B 245, 19–58 10.1098/rstb.1962.0005 (doi:10.1098/rstb.1962.0005) [DOI] [Google Scholar]
- 19.Muntz W., Johnson M. 1978. Rhodopsins of oceanic decapods. Vis. Res. 18, 601–602 10.1016/0042-6989(78)90210-9 (doi:10.1016/0042-6989(78)90210-9) [DOI] [PubMed] [Google Scholar]
- 20.Carpenter R. 1993. Distribution of quick-phase intervals in optokinetic nystagmus. Ophthalmic Res. 25, 91–93 10.1159/000267270 (doi:10.1159/000267270) [DOI] [PubMed] [Google Scholar]
- 21.Wehner R. 1987. ‘Matched filters’—neural models of the external world. J. Comp. Physiol. A 161, 511–531 10.1007/BF00603659 (doi:10.1007/BF00603659)3316619 [DOI] [Google Scholar]
- 22.Land M. F. 2006. Visual optics: the shapes of pupils. Curr. Biol. 16, 167–168 10.1016/j.cub.2006.02.046 (doi:10.1016/j.cub.2006.02.046) [DOI] [PubMed] [Google Scholar]
- 23.Malmström T., Kröger R. H. H. 2006. Pupil shapes and lens optics in the eyes of terrestrial vertebrates. J. Exp. Biol. 209, 18–25 10.1242/jeb.01959 (doi:10.1242/jeb.01959) [DOI] [PubMed] [Google Scholar]
- 24.Douglas R. H., Williamson R., Wagner J. 2005. The papillary response of cephalopods. J. Exp. Biol. 208, 261–265 10.1242/jeb.01395 (doi:10.1242/jeb.01395) [DOI] [PubMed] [Google Scholar]
- 25.Muntz W. 1977. Pupillary responses of cephalopods. Symp. Zool. Soc. Lond. 38, 277–285 [Google Scholar]
- 26.Cronin T., Shashar N. 2001. The linearly polarized light field in clear, tropical marine waters: spatial and temporal variation of light intensity, degree of polarization and e-vector angle. J. Exp. Biol. 204, 2461–2467 [DOI] [PubMed] [Google Scholar]
- 27.Hanlon R., Messenger J. 1996. Cephalopod behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 28.Kröger R. H. H., Wagner J. 1998. A fluorescent double stain for visualization of neural tissue by confocal laser scanning microscopy. J Neurosci. Methods 84, 87–92 10.1016/S0165-0270(98)00093-4 (doi:10.1016/S0165-0270(98)00093-4) [DOI] [PubMed] [Google Scholar]
- 29.Stone J. 1981. The whole mount handbook. Sydney, Australia: Maitland Publications Pty Ltd [Google Scholar]
- 30.Messenger J. 2001. Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 76, 473–528 [DOI] [PubMed] [Google Scholar]
- 31.Kröger R. H. H., Gislén A. 2004. Compensation for longitudinal chromatic aberration in the eye of the firefly squid Watasenia scintillans.. Vis. Res. 44, 2129–2134 10.1016/j.visres.2004.04.004 (doi:10.1016/j.visres.2004.04.004) [DOI] [PubMed] [Google Scholar]
- 32.Land M. F. 1981. Optics vision in invertebrates. In Handbook of sensory physiology, vol. V11/6B (ed. Autrum H.), pp. 471–592 Berlin, Germany: Springer [Google Scholar]
- 33.Layne J., Land M. F., Zeil J. 1997. Fiddler crabs use the visual horizon to distinguish predators from conspecifics: a review of the evidence. J. Mar. Biol. Assoc. UK 77, 43–54 10.1017/S0025315400033774 (doi:10.1017/S0025315400033774) [DOI] [Google Scholar]
- 34.Collin S. P., Shand J. 2003. Retinal sampling and the visual field in fishes. In Sensory processing in aquatic environments (eds Collin S. P., Marshall J.), pp. 139–169 Berlin, Germany: Springer [Google Scholar]
- 35.Cronin T. W., Marshall J. 2011. Patterns and properties of polarized light in air and water. Phil. Trans. R. Soc. B 366, 619–626 10.1098/rstb.2010.0201 (doi:10.1098/rstb.2010.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerner A., Sabbah S., Erlick C., Shashar N. 2011. Navigation by light polarization in clear and turbid waters. Phil. Trans. R. Soc. B 366, 671–679 10.1098/rstb.2010.0189 (doi:10.1098/rstb.2010.0189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shashar N., Sabbah S., Cronin T. 2004. Transmission of linearly polarized light in seawater: implications for polarization signalling. J. Exp. Biol. 207, 3619–3628 10.1242/jeb.01187 (doi:10.1242/jeb.01187) [DOI] [PubMed] [Google Scholar]
- 38.Wehner R. 2001. Polarization vision—a uniform sensory capacity? J. Exp. Biol. 204, 2589–2596 [DOI] [PubMed] [Google Scholar]
- 39.Johnsen S., Marshall N. J., Widder E. A. 2011. Polarization sensitivity as a contrast enhancer in pelagic predators: lessons from in situ polarization imaging of transparent zooplankton. Phil. Trans. R. Soc. B 366, 655–670 10.1098/rstb.2010.0193 (doi:10.1098/rstb.2010.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shashar N., Hagen R., Boal J., Hanlon R. 2000. Cuttlefish use polarization sensitivity in predation on silvery fish. Vis. Res. 40, 71–75 10.1016/S0042-6989(99)00158-3 (doi:10.1016/S0042-6989(99)00158-3) [DOI] [PubMed] [Google Scholar]
- 41.Shashar N., Hanlon R., Petz A. 1998. Polarization vision helps detect transparent prey. Nature 393, 222–223 10.1038/30380 (doi:10.1038/30380)9607759 [DOI] [Google Scholar]
- 42.Lythgoe J. N. 1979. The ecology of vision. Oxford, UK: Oxford University Press [Google Scholar]
- 43.Schechner Y., Karpel N. 2005. Recovery of underwater visibility and structure by polarization analysis. IEEE J. Oceanic Eng 30, 570–587 10.1109/JOE.2005.850871 (doi:10.1109/JOE.2005.850871) [DOI] [Google Scholar]
- 44.Schechner Y. Y. 2011. Inversion by P4: polarization-picture post-processing. Phil. Trans. R. Soc. B 366, 638–648 10.1098/rstb.2010.0205 (doi:10.1098/rstb.2010.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivanoff A., Waterman T. 1958. Factors, mainly depth and wavelength, affecting the degree of underwater light polarization. J. Mar. Res. 16, 283–307 [Google Scholar]
- 46.Budelmann B. U., Young J. Z. 1993. The oculomotor system of decapods cephalopods: eye muscles, eye muscles nerves, and the oculomotor neurons in the central nervous system. Phil. Trans. R. Soc. Lond. B 340, 93–125 10.1098/rstb.1993.0051 (doi:10.1098/rstb.1993.0051) [DOI] [PubMed] [Google Scholar]
- 47.Cronin T., Shashar N., Caldwell R., Marshall J., Cheroske G., Chiao T. 2003. Polarization vision and its role in biological signaling. Integr. Comp. Biol. 43, 549–558 10.1093/icb/43.4.549 (doi:10.1093/icb/43.4.549) [DOI] [PubMed] [Google Scholar]
- 48.Shashar N., Rutledge P., Cronin T. 1996. Polarization vision in cuttlefish—a concealed communication channel? J. Exp. Biol. 199, 2077–2084 [DOI] [PubMed] [Google Scholar]
- 49.Chiou T.-H., Mäthger L. M., Hanlon R. T., Cronin T. 2007. Spectral and spatial properties of polarized light reflections from the arms of squids (Loligo pealeii) and cuttlefish (Sepia officinalis). J. Exp. Biol. 210, 3624–3635 10.1242/jeb.006932 (doi:10.1242/jeb.006932) [DOI] [PubMed] [Google Scholar]
- 50.Norman M., Debelius H. 2000. Cephalopods: a world guide. Hackenheim, Germany: Conch Books [Google Scholar]
- 51.Jereb P., Roper C. F. E. 2005. Cephalopods of the World. An annotated and illustrated catalogue of cephalopod species known to date. Volume 1: chambered nautiluses and sepioids, vol. 1 Rome, Italy: FAO Species Catalogue for Fishery Purposes no. 4. Food and Agriculture Organisation of the United Nations [Google Scholar]