Abstract
The offspring of brood parasitic birds benefit from hatching earlier than host young. A proposed but little-known strategy to achieve this is ‘internal incubation’, by retaining the egg in the oviduct for an additional 24 h. To test this, we quantified the stage of embryo development at laying in four brood parasitic birds (European cuckoo, Cuculus canorus; African cuckoo, Cuculus gularis; greater honeyguide, Indicator indicator; and the cuckoo finch, Anomalospiza imberbis). For the two cuckoos and the honeyguide, all of which lay at 48 h intervals, embryos were at a relatively advanced stage at laying; but for the cuckoo finch (laying interval: 24 h) embryo stage was similar to all other passerines laying at 24 h intervals. The stage of embryo development in the two cuckoos and honeyguide was similar to that of a non-parasitic species that lay at an interval of 44–46 h, but also to the eggs of the zebra finch Taeniopygia guttata incubated artificially at body temperature immediately after laying, for a further 24 h. Comparison with the zebra finch shows that internal incubation in the two cuckoos and honeyguide advances hatching by 31 h, a figure consistent with the difference between the expected and the observed duration of incubation in the European cuckoo predicted from egg mass. Rather than being a specific adaptation to brood parasitism, internal incubation is a direct consequence of a protracted interval between ovulation (and fertilization) and laying, but because it results in early hatching may have predisposed certain species to become brood parasitic.
Keywords: brood parasite, embryo development, eggs, honeyguide, cuckoo
1. Introduction
For many brood parasitic birds, early hatching relative to their hosts' eggs is advantageous because it gives their offspring a competitive advantage over host young. Early hatching facilitates the ejection or killing of host eggs or young as in the cuckoos Cuculus spp., or honeyguides Indicator spp., respectively [1–3]. In cases where parasite and host young are reared together, early hatching gives parasitic offspring a competitive advantage in terms of food acquisition [2,3].
Incubation periods, reflecting rates of embryo development, can be modified by several different mechanisms including parental incubation behaviour, egg size and content such as the relative amount of yolk and deposition of growth-promoting maternal steroids in the yolk, and by embryonic communication [4,5], and in some brood parasites early hatching may be facilitated by disruption of host incubation behaviour [6]. One of the least-studied mechanisms of reducing the incubation period is egg retention and internal incubation, evinced by the fact that at laying the cuckoo embryo is advanced relative to those of its hosts ([7–10]; see also [11]).
Montagu [7: xv] was the first to suggest—apparently on the basis of dissections—that egg retention by the female cuckoo could account for the fact that the European cuckoo chick Cuculus canorus often hatched before the hosts' eggs: ‘the consequence of this retention would be a dilation of the embryo by the internal heat of the body, and the foetus advanced towards perfection in proportion to the time the egg remained in that state’. Egg collectors also commented on the fact that embryo development in cuckoo eggs was advanced relative to that of their hosts' eggs in the same nest (e.g. [12]). Liversidge [9] reported that the ovum of an egg from a Jacobin cuckoo Clamator jacobinus examined within 2 h of laying, exhibited a stage of embryo development equivalent to 17–20 h of true post-laying incubation compared with the domestic fowl Gallus domesticus. Perrins [10] similarly examined a single unincubated egg of a European cuckoo, which ‘had clearly started to develop, there being a circular area of some four millimetres in diameter which had differentiated from the yolk’, although he was unable to estimate how many hours of incubation the development represented.
Although it seems intuitively obvious that internal incubation might be an adaptation to brood parasitism, the evidence is clearly extremely limited. The aim of the present study is to compare the stage of embryo development (and hence the extent of internal incubation) at laying of several brood parasitic species, together with some host species and a range of other non-parasitic birds to estimate the incubation advantage of internal incubation and to assess whether internal incubation is an adaptation to brood parasitism.
2. Methods
Eggs of the following brood parasitic species were collected (under licence) soon after oviposition or before any incubation by the host during 2008 and 2009: (i) European cuckoo (n = 8), a non-passerine that lays at 48 h intervals and where the chick evicts host eggs or young [3]; (ii) African cuckoo, Cuculus gularis (n = 3), non-passerine, laying at 48 h intervals (all cuckoos lay at 48 h intervals: [3,13,14]) and whose chick evicts host eggs or young [15]; (iii) greater honeyguide Indicator indicator (n = 3), a non-passerine, with a 48 h laying interval [1,16] and whose chick kills host eggs or young by piercing with modified egg-tooth [1]; (iv) cuckoo finch, Anomalospiza imberbis (n = 2), a passerine that lays at 24 h intervals ([2]; C. N. Spottiswoode 2009, personal observation) and whose chick is reared together with host chicks, although host chicks rarely survive [17]. For all species, sample sizes were relatively small because finding unincubated eggs is difficult since brood parasites are unpredictable in where they lay.
For comparison, we obtained unincubated eggs of the following species (some of which were hosts to particular brood parasites) by regular nest inspections, from both wild and captive birds.
— Zebra finch, a passerine with a 24 h laying interval, altricial development and whose embryo development has been studied in detail (N. Hemmings 2010, unpublished data). For this species we measured: (i) the stage of embryo development at laying; (ii) after 24 h of artificial incubation at 40°C; and (iii) at 24 h intervals throughout incubation at the normal incubation temperature of 36°C (in an artificial incubator). The birds used were captive, domesticated, wild-type zebra finches maintained at the University of Sheffield [18,19].
— Feral pigeon, Columba livia, a non-passerine with altricial development and a laying interval of 44–46 h, but whose ovum is known to spend 40 h in the oviduct [20,21]. These were free-flying domesticated feral pigeons.
— Little bee-eater, Merops pusillus, a non-passerine with altricial development and a laying interval of 48 h (C. N. Spottiswoode 2009, personal observation). This species is a host of the greater honeyguide.
— Several wild (altricial) passerines (table 1), all of which lay at 24 h intervals (collected under licence); we also compared their stage of embryo development with several non-passerine, precocial species whose stage of development of laying was previously reported by Sellier et al. [22].
Table 1.
Mean ± s.d. stage of embryo development at laying (from Hamburger & Hamilton [25]) for four brood parasites and a range of other species, including host species. (The little bee-eater is a host species for the greater honeyguide; the prinia is a host of the cuckoo finch and the reed warbler is a host of the European cuckoo.)
| species | mean stage of development | s.d. | n | source |
|---|---|---|---|---|
| 0 h incubation—brood parasites | ||||
| African cuckoo (C. gularis)a | 4.33 | 0.58 | 3 | this study |
| European cuckoo (C. canorus)a | 4.59 | 0.50 | 8 | this study |
| greater honeyguide (I. indicator)a | 4.33 | 0.58 | 3 | this study |
| cuckoo finch (A. imberbis) | 1.50 | 0.80 | 2 | this study |
| 0 h incubation—other species | ||||
| guinea fowl (N. meleagris)b | <1d | — | 563 | [22] |
| domestic turkey (M. gallopavo)b | <1d | — | 80 | [22] |
| Japanese quail (Coturnix japonica)b | <1d | — | 142 | [22] |
| domestic fowl (G. domesticus)b | 1.00 | — | — | [25] |
| domestic goose (Anser anser domesticus)b | <1d | — | 251 | [22] |
| muscovy duck (Cairina moschata)b | <1d | — | 524 | [22] |
| mallard duck (Anas platyrhynchos)b | <1d | — | 285 | [22] |
| pekin duck (Anas platyrhynchos domestica)b | <1d | — | 492 | [22] |
| feral pigeon (Columba livia)a,c | 3.75 | 0.52 | 6 | this study |
| little bee-eater (Merops pusillus)a | 1.13 | 0.25 | 4 | this study |
| wryneck (Jynx torquilla) | 1.0 | 0 | 2 | this study |
| tawny-flanked prinia (Prinia subflava) | 1.92 | 0.54 | 6 | this study |
| reed warbler (Acrocephalus scirpaceus) | 1.20 | 0.45 | 5 | this study |
| redpoll (Carduelis flammea) | 1.00 | — | 1 | this study |
| zebra finch (T. guttata) | 1.00 | 0 | 5 | this study |
| 24 h incubation at 40°C | ||||
| zebra finch (T. guttata) | 4.88 | 0.79 | 8 | this study |
aSpecies laying at 48 h intervals, all others lay at 24 h intervals.
bDomestic, precocial species.
cComparison between feral pigeon and European cuckoo (p = 0.043), African cuckoo (p = 0.34) and honeyguide (p = 0.34) (Mann–Whitney U-tests), but note that the pigeon has 40 h of internal incubation, where the other species probably have 42 h (see text).
dMean stage is earlier than Stage 1 as described in Hamburger & Hamilton [25].
To estimate the amount of time saved by internal incubation, we compared the stage of embryo development in the European cuckoo and other brood parasites laying at 48 h intervals, with the stage of development in the zebra finch (which lays at 24 h intervals) after an additional 24 h of incubation (in a commercial incubator) at 40°C, immediately after laying. To achieve this we video-recorded egg-laying (N. Hemmings & T. R. Birkhead 2010, unpublished data) and within 10 min of oviposition placed eggs in a commercial incubator (Brinsea Octagon 20) at 40°C, which is the normal body temperature of both the zebra finch [23] and cuculiformes [24] for 24 h, for comparison with the brood parasite eggs. We performed similar artificial incubation on pheasant, Phasianus colchicus eggs.
We opened the eggs and placed the ovum in 5 per cent formalin solution, fixing them for later analysis, when we carefully removed and examined the blastoderm using a Leica binocular dissecting microscope. The stage of embryo development was assessed using the scheme devised by Hamburger & Hamilton [25], originally for the domestic fowl, but developed and used by us for captive, domesticated zebra finches, Taeniopygia guttata and other species (N. Hemmings 2010, unpublished data), which varies between 1 (pre-streak: blastoderm prior to development of primitive streak) and 46 (newly hatched chick). Means are expressed as ±s.d.
3. Results
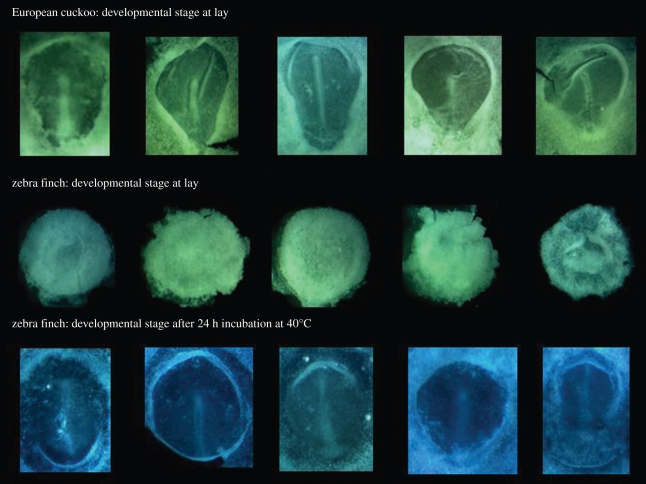
The stage of embryo development at laying and prior to any incubation, for the European cuckoo (4.59 ± 0.50; figure 1), African cuckoo (4.33 ± 0.58) and greater honeyguide (4.33 ± 0.58) was, as predicted, consistent with a 48 h period of internal incubation. Moreover, the stage of embryo development in the two cuckoos and the honeyguide was similar to zebra finch eggs artificially incubated at body temperature for an additional 24 h (4.88 ± 0.79, n = 8) (table 1). The stage of embryo development in the cuckoos and honeyguide eggs was also similar to the feral pigeon, which has a 44–46 h interval between its two eggs (table 1; see figure 1). Interestingly, in the little bee-eater, the mean embryo stage at laying (1.13 ± 0.25, n = 4) strongly suggests that despite a 48 h laying interval ([2]; C. Spottiswoode 2009, personal observation), females probably ovulate around 24 h before laying. By comparison, all other species laying at 24 h intervals had an embryo stage of around 1, or <1 in the case of non-passerines with precocial development (table 1).
Figure 1.
Blastodisc of eggs of European cuckoo at laying (top line); zebra finch at laying (middle line) and zebra finch after 24 h of artificial incubation at body temperature (40°C), showing that the stage of embryo development is similar to that of the European cuckoo at laying.
In the cuckoo finch, the stage of embryo development at laying (1.5 ± 0.71, n = 2) is consistent with its 24 h laying interval. This is similar to that of their hosts, and to that of all other passerines that lay at 24 h intervals (table 1). The eggs of the cuckoo finch typically hatch either one day before (six cases) or on the same day (one case) as their hosts (C. N. Spottiswoode 2009, personal observation). Their head start in hatching comes from the fact that the cuckoo finch (usually) removes all the host eggs when she lays her own [17], giving her egg an automatic one day head start on any subsequently laid eggs.
4. Discussion
Our results are consistent with the hypothesis that internal incubation in the European cuckoo, African cuckoo and the greater honeyguide contributes to their shorter duration of incubation.
Relative to naturally incubated host eggs, the additional 24 h of internal incubation in the cuckoos and honeyguides provides a hatching advantage of at least 24 h because internal incubation occurs at body temperature (approx. 40°C; [24]), whereas external incubation occurs at approximately 36°C [26]. The full hatching advantage can be calculated as follows, making two assumptions: (i) that the interval between ovulation and oviposition in the two cuckoos and the honeyguide is around 48 h (see below), and (ii) that the rate of development of the cuckoo embryo during the first few days after fertilization is similar to that of the zebra finch. In the zebra finch, the period of internal incubation, i.e. the interval between ovulation (and fertilization) and oviposition, is 24 h. We also know that a zebra finch embryo requires 55 h of external incubation to reach an embryo stage of 4.6 (N. Hemmings 2009, personal observation). To reach this stage, the zebra finch embryo has therefore had 24 h at 40°C (internal incubation) plus 55 h at 36°C (external incubation); 79 h in total. At laying, the eggs of the two cuckoos and honeyguide are at embryo stage 4.3–4.6, following a period of about 48 h internal incubation at 40°C. Subtracting the 48 h of internal incubation in the cuckoos and honeyguide from 79 h, gives a 31 h advantage. The validity of using the zebra finch for this comparison is illustrated by the fact that incubating zebra finch eggs for 24 h at 40°C results in an embryo stage of 4.88, which is not significantly different from that of the European cuckoo, African cuckoo or greater honeyguide (Wilcoxon rank-sum tests, p > 0.05). The estimated saving of 31 h is consistent with the difference between the expected and the observed duration of incubation in the European cuckoo predicted from egg mass (see below).
Liversidge [9] suggested a shorter saving of 17–20 h in the Jacobin cuckoo (which also lays at intervals of about 48 h), but his comparison was made against the domestic fowl, which (because its chicks are precocial) has a more rapid rate of embryo development than the zebra finch, and whose embryo after 17–20 h of natural incubation is indeed at stage 4–5 [25].
Our conclusions are based on two assumptions. The first is that a 48 h laying interval in the two cuckoos and the honeyguide is equivalent to a 48 h period between ovulation (and fertilization) and oviposition, and hence a 48 h period of internal incubation. The only species with a greater than 24 h laying interval for which the timing of ovulation is accurately known is the domestic pigeon. In this species, the two eggs are typically laid about 44–46 h apart and dissection at different times after oviposition of the first egg shows that the second ovum is ovulated and fertilized about 5 h after oviposition, and hence spends around 40 h in the oviduct [20,21]. Our data on the embryo development in the pigeon at laying (stage 3.75; table 1) is consistent with this. However, our data for the little bee-eater on the stage of embryo development at laying show that a 48 h laying interval can also be associated with a 24 h interval between ovulation and oviposition. In other words, a 48 h laying interval—as occurs in the two cuckoos and the honeyguide—does not automatically result in a 48 h a period of internal incubation.
However, there are two reasons for assuming that in the two cuckoos and the greater honeyguide, a laying interval of 48 h does indeed result in a period of approximately 48 h of internal incubation. First, the stage of embryo development at laying in these species (4.3–4.6) is similar to that in a zebra finch (4.88) incubated for an additional 24 h at body temperature (40°C). Second, although the stage of embryo development at laying in the cuckoos and honeyguide could, with very rapid embryo growth, arise without an extra 24 h of internal incubation, this seems unlikely because a high rate of very early embryo development would imply that development during the remainder of the incubation period would be similarly high and result in early hatching. There is no evidence for this, at least in the European cuckoo for which we have detailed information. Indeed, the observed duration of incubation in the European cuckoo is 11.6 days ± 0.29 s.d. (n = 9) [27], which is 1.2 days or 28.8 h (i.e. about 10%) shorter than that predicted from egg mass (3.4 g, [3,28]), a difference that is almost exactly accounted for by the time saving generated by internal incubation, described below. It therefore seems likely that the relatively advanced stage of embryo development in the two cuckoos and honeyguide is a result of an additional period of internal incubation.
With a few exceptions (e.g. [7,29]), most previous brood-parasite researchers do not appear to have recognized that the eggs of all bird species show some embryo development (albeit microscopic) at laying. This is because development typically starts within 5–7 h of fertilization [20,22,30,31]. A comparison of six, domestic precocial bird species (domestic fowl, turkey Meleagris gallopavo, duck Anas platyrhynchos, goose Anser domesticus, guinea fowl Numida meleagris and Japanese quail Coturnix c. japonica), all of which lay at 24 h intervals, showed that the onset of development was very similar—about 5–7 h after ovulation and fertilization—across these different species ([22]; see also Romanoff [20] for feral pigeon). Assuming that the onset of development starts at a similar time after ovulation in the zebra finch, the two cuckoos and the greater honeyguide, the actual duration of internal incubation is more likely to be: (i) 18 h (rather than 24 h cf. above) in the zebra finch (and other passerines), and (ii) 42 h in the two cuckoos and greater honeyguide.
Internal incubation in cuckoos is clearly not an adaptation to brood parasitism. Rather, it is an automatic consequence of the 48 h interval between ovulation and oviposition and the fact that embryo development starts soon after fertilization. Moreover, as several authors have noted (e.g. [3]), even the cuckoos' habit of laying at 48 h intervals is unlikely to be an adaptation to brood parasitism, since non-parasitic cuckoos also lay at intervals of more than 24 h [13,14] and have relatively short incubation periods [13,14,32], indicating that laying intervals of more than 24 h may be the ancestral state in cuckoos. However, a 48 h laying may have predisposed cuckoos to brood parasitism, not least because of the advantage it confers in terms of hatching in advance of the host eggs [3,33,34]. As far as we are aware, there have been no experiments to establish whether early hatching in cuckoo chicks is adaptive. Common sense suggests that it is, by facilitating the ejection of host eggs and young, and allowing the cuckoo chick to monopolize the food supply brought by the parents. The only information available is observational and shows that in those rare cases, where cuckoos lay after their great warbler Acrocephalus arundinaceus hosts had started to incubate (n = 9), none of the cuckoo eggs hatched, compared with about 80 per cent for cuckoo eggs laid before the hosts' incubation started (C. Moskát 2009, unpublished data).
Interestingly, greater honeyguides lay at 48 h intervals [16,35], yet their closest relatives, the toucans, barbets and woodpeckers [36], typically lay at intervals of 24 h [35]. The longer laying interval in honeyguides may therefore be an adaptation to brood parasitism.
The cuckoo finch may be constrained to lay at 24 h intervals since very few passerines lay at intervals greater than 24 h (Tullett, in [37]; some Gerygone spp. seem to be the only examples of passerines laying at intervals of 48 h [38])—and accordingly achieves its hatching advantage by removing all the host eggs when it lays its own, so that any host eggs present at hatching are laid subsequent to the cuckoo finch's and therefore hatch after it.
The European cuckoo is the best-studied brood parasite. Of the various ways that early hatching can be achieved, it is mediated in this species partly through: (i) having a small egg for its body size [3], because across birds in general, the duration of incubation period is positively correlated with egg size [28]; and (ii) internal incubation which results in a 31 h hatching advantage when compared with host eggs. There is no evidence in the European cuckoo that the maternal hormones in the yolk are higher than that of its host the great reed warbler, although the total amount of yolk in the cuckoo egg is relatively large and may account for the chick's vigour on hatching [39]. However, in the parasitic shiny cowbird Molothrus bonariensis, a relatively small yolk contributes to a reduced incubation period [40], so it seems unlikely that yolk size in the European cuckoo facilitates early hatching. It is not known whether embryonic communication occurs between cuckoo and host eggs, that might advance hatching in the cuckoo [5].
In conclusion, we present evidence that internal incubation, resulting from an interval of approximately 42 h between ovulation (and fertilization) and oviposition in two species of cuckoo and the greater honeyguide, facilitates early hatching. This is only one of several ways that early hatching can be achieved. The cuckoo finch, which lays at 24 h intervals, shows no evidence of internal incubation any different from any other passerine.
Acknowledgements
Natural England and the Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management provided licences.
We thank L. Hamusikili, K. Moto, C. Moya and others for finding nests in Zambia, Phil Young, Lynsey Gregory and Dirk Tolmitt for technical support and the Zambian Wildlife Authority for research permits, and those people in Zambia who provided hospitality, in particular the Bruce-Miller family. Thanks also to two anonymous referees for their suggestions. C.N.S. was funded by a Royal Society Dorothy Hodgkin Fellowship and N.H. was funded by a Natural Environment Research Council (NERC) studentship. M.B. was funded by the INCORE: FP6-2005-NEST-Path, no. 043318, Integrating Cooperation Research across Europe.
References
- 1.Friedmann H. 1955. The honey-guides. US National Museum (Bulletin 208) [Google Scholar]
- 2.Payne R. B. 1977. The ecology of brood parasitism in birds. Ann. Rev. Ecol. Syst. 8, 1–28 10.1146/annurev.es.08.110177.000245 (doi:10.1146/annurev.es.08.110177.000245) [DOI] [Google Scholar]
- 3.Davies N. B. 2000. Cuckoos, cowbirds and other cheats. London, UK: Poyser [Google Scholar]
- 4.Martin T. E., Schwabl H. 2007. Variation in maternal effects and embryonic development rates among passerine species. Phil. Trans. R. Soc. B 363, 1663–1674 10.1098/rstb.2007.0009 (doi:10.1098/rstb.2007.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown C. R. 1984. Laying eggs in a neighbor's nest: benefit and cost of colonial nesting in swallows. Science 224, 518–519 10.1126/science.224.4648.518 (doi:10.1126/science.224.4648.518) [DOI] [PubMed] [Google Scholar]
- 6.McMaster D. G., Sealy S. G. 1998. Short incubation periods of brown-headed cowbird: how do cowbird eggs hatch before yellow warbler eggs? Condor 100, 102–111 10.2307/1369901 (doi:10.2307/1369901) [DOI] [Google Scholar]
- 7.Montagu G. 1802. Ornithological dictionary. London, UK: White [Google Scholar]
- 8.Jenson R. A. C., Vernon C. J. 1970. On the biology of the didric cuckoo in southern Africa. Ostrich 41, 237–246 [Google Scholar]
- 9.Liversidge R. 1961. Pre-incubation development of Clamator jacobinus. Ibis 103, 624 [Google Scholar]
- 10.Perrins C. M. 1967. The short apparent incubation period of the cuckoo. Brit. Birds 60, 51–52 [Google Scholar]
- 11.Schulze-Hagen K., Stokke B., Birkhead T. R. 2009. Reproductive biology of the European cuckoo Cuculus canorus: early insights, persistent errors and the acquisition of knowledge. J. Ornithol. 150, 1–16 10.1007/s10336-008-0340-8 (doi:10.1007/s10336-008-0340-8) [DOI] [Google Scholar]
- 12.Claudon M. 1955. Nouvelles observations sur Cuculus c. canorus Linné en Alsace. ĹOiseaux 25, 44–49 [Google Scholar]
- 13.Hughes J. M. 1999. Yellow-billed cuckoo (Coccyzus americanis). In The birds of North America (eds Poole A., Gill F.), p. 418 Philadelphia, PA: Cornell Laboratory of Ornithology and American Ornithologists' Union. Retrieved from: http://bna.birds.cornell.edu/bna/species/ [Google Scholar]
- 14.Hughes J. M. 1999. Black-billed cuckoo (Coccyzus erythrothalmus). In The birds of North America (eds Poole A., Gill F.), p. 587 Philadelphia, PA: Cornell Laboratory of Ornithology and American Ornithologists' Union. Retrieved from: http://bna.birds.cornell.edu/bna/species/ [Google Scholar]
- 15.Tarboton W. 2001. A guide to the nests and eggs of southern African birds. Cape Town, South Africa: Struik [Google Scholar]
- 16.Payne R. B. 1992. Clutch size, laying periodicity and behavior in the honeyguides Indicator indicator and I. minor. Proc. 7 Pan-African Ornithol. Congress (ed. Bennun L.), pp. 537–547 Nairobi, Kenya: Watts [Google Scholar]
- 17.Vernon C. J. 1964. The breeding of the cuckoo weaver Anomalospiza imberbis in southern Rhodesia. Ostrich 35, 260–263 [Google Scholar]
- 18.Birkhead T. R., Pellatt E. J., Matthews I. M., Roddis N. J., Hunter F. M., McPhie F., Castillo-Juarez H. 2006. Genic capture and the genetic basis of sexually selected traits in the zebra finch. Evolution 60, 2389–2398 [PubMed] [Google Scholar]
- 19.Slate J., Hale M.C., Birkhead T.R. 2007. Simple sequence repeats in zebra finch (Taeniopygia guttata) expressed sequence tags: a new resource for evolutionary genetic studies of passerlines. BMC Genomics 8, 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanoff A. L. 1960. The avian embryo: structural and functional development. New York, NY: Macmillan [Google Scholar]
- 21.Levi W. M. 1945. The pigeon. Columbia, SC: Racing Pigeon [Google Scholar]
- 22.Sellier N., Brillard J.-P., Dupuy V., Bakst M. R. 2006. Comparative staging of embryo development in chicken, turkey, duck, goose, guinea fowl, and Japanese quail assessed from five hours after fertilization through seventy-two hours of incubation. J. Appl. Poultry Res. 15, 219–228 [Google Scholar]
- 23.Zann R. A. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press [Google Scholar]
- 24.Prinzinger R., Pressmar A., Schleucher E. 1991. Body temperature in birds. Comp. Biochem. Physiol. 99A, 499–506 [Google Scholar]
- 25.Hamburger V., Hamilton H. L. 1951. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 10.1002/jmor.1050880104 (doi:10.1002/jmor.1050880104) [DOI] [PubMed] [Google Scholar]
- 26.Drent R. 1975. Incubation. In Avian biology, vol. V (eds Farner D. S., King J. R.), pp. 333–420 New York, NY: Academic Press [Google Scholar]
- 27.Wyllie I. 1981. The cuckoo. London, UK: Batsford [Google Scholar]
- 28.Rahn H., Ar A. 1974. The avian egg: incubation time and water loss. Condor 76, 147–152 10.2307/1366724 (doi:10.2307/1366724) [DOI] [Google Scholar]
- 29.Hoffman E. C. 1929. Cowbirds-decoys-incubation period. Bull. N.E. Bird-Banding Assoc. 5, 118–119 [Google Scholar]
- 30.Eyal-Giladi H., Kochav S. 1976. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. Dev. Biol. 49, 321–337 [DOI] [PubMed] [Google Scholar]
- 31.Perry M. M. 1987. Nuclear events from fertilisation to the early cleavage stages in the domestic fowl (Gallus domesticus). J. Anat. 150, 99–109 [PMC free article] [PubMed] [Google Scholar]
- 32.Davies N. B., Brooke M. de L. 1988. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284 10.1016/S0003-3472(88)80269-0 (doi:10.1016/S0003-3472(88)80269-0) [DOI] [Google Scholar]
- 33.Hamilton W. J., Orians G. H. 1965. Evolution of brood parasitism in altricial birds. Condor 67, 361–382 10.2307/1365631 (doi:10.2307/1365631) [DOI] [Google Scholar]
- 34.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen [Google Scholar]
- 35.Short L. L., Horne J. F. M. 2001. Toucans, barbets and honeyguides. Oxford, UK: Oxford University Press [Google Scholar]
- 36.Hackett S. J., et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 10.1126/science.1157704 (doi:10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 37.Campbell B., Lack E. (eds) 1985. A dictionary of birds. Waterhouse, UK: T & A.D. Poyser [Google Scholar]
- 38.Del Hoyo J., Elliot A., Sargatel J., Christie D.A. 2007. Handbook of birds of the world, vol. 12 Madrid, Spain: Lynx [Google Scholar]
- 39.Hargitai R., Moskát C., Bán M., Gil D., López-Rull I., Solymos E. 2010. Eggshell characteristics and yolk composition in the common cuckoo Cuculus canorus: are they adapted to brood parasitism? J. Avian Biol. 41, 177–185 10.1111/j.1600-048X.2009.04818.x (doi:10.1111/j.1600-048X.2009.04818.x) [DOI] [Google Scholar]
- 40.Kattan G. H. 1995. Mechanisms of short incubation period in brood-parasitic cowbirds. Auk 112, 335–342 [Google Scholar]