Abstract
Haplodiploid species display extraordinary sex ratios. However, a differential investment in male and female offspring might also be achieved by a differential provisioning of eggs, as observed in birds and lizards. We investigated this hypothesis in the haplodiploid spider mite Tetranychus urticae, which displays highly female-biased sex ratios. We show that egg size significantly determines not only larval size, juvenile survival and adult size, but also fertilization probability, as in marine invertebrates with external fertilization, so that female (fertilized) eggs are significantly larger than male (unfertilized) eggs. Moreover, females with on average larger eggs before fertilization produce a more female-biased sex ratio afterwards. Egg size thus mediates sex-specific egg provisioning, sex and offspring sex ratio. Finally, sex-specific egg provisioning has another major consequence: male eggs produced by mated mothers are smaller than male eggs produced by virgins, and this size difference persists in adults. Virgin females might thus have a (male) fitness advantage over mated females.
Keywords: gamete size, fertilization probability, sex ratio, offspring fitness, sexual dimorphism, haplodiploid species
1. Introduction
The allocation of resources to male versus female offspring is an important reproductive decision for the parents and provides one of the best grounds to study the way in which natural selection acts [1–4]. Whenever daughters and sons provide different fitness returns, parents are selected to bias their investment towards the most rewarding sex [5]. Theory predicts a number of situations in which parents are expected to adjust their sex allocation, and there is a large body of empirical evidence supporting these predictions [4,6].
The most obvious way to adjust sex allocation is to produce a biased sex ratio in the progeny [1,3,7]. This aspect of sex allocation has been extensively studied in a wide range of organisms including invertebrates and vertebrates [3,8,9]. The most striking patterns are found in haplodiploid arthropods, in which the sex determination mechanism (males are haploid and females are diploid) may allow females to precisely control the sex of their offspring [2,4,10].
However, increasing the relative number of eggs of the preferred sex is not the only way by which parents can adjust sex allocation. An alternative, non exclusive, strategy may be to alter the quality of these eggs, e.g. by providing them with different amounts of resources [11]. For organisms without parental care, parental investment is restricted to prenatal provisioning of eggs, which represents the complete energy supply of the offspring until they start feeding on their own [12]. The quantity of resources that parents invest in their eggs can thus dramatically affect offspring fitness [13,14]. One of the most widely used predictors of such investment is egg size [12]. This approach assumes that offspring resulting from larger eggs have higher fitness [15–17], which has been supported by empirical studies across a wide variety of taxa [13,18]. Furthermore, several studies have shown adaptive plasticity of egg size in response to environmental changes in food or density conditions [19–21]. However, very few studies have investigated the adjustment of egg size depending on the sex of the embryo. Such sex-biased parental strategies have been demonstrated in birds [22–28] and recently in a lizard [29], but so far only a single study has addressed this question in haplodiploid arthropods [30], although they are key models in the study of sex allocation.
Sex allocation in haplodiploids might thus be more finely tuned than previously thought, and the bias towards the preferred sex might be higher (if eggs of the more frequent sex are the largest) or lower (if eggs of the more frequent sex are the smallest) than usually described. We investigated this hypothesis using the spider mite Tetranychus urticae as a model system. This species is particularly suited for such study, as it exhibits extremely female-biased sex ratios (female : male ratio varies from 2 : 1 to 9 : 1), the main explanatory factor put forward being local mate competition (LMC; [1,31]). In this species, males develop from unfertilized eggs, whereas females develop from fertilized eggs, so that females might control the offspring sex ratio by controlling the fertilization process [3]. Indeed, females were found able to adjust their sex ratio in response to mating delays [32] and the amount of LMC [33]. In the present study, we first assessed the consequences of egg size variation for offspring fitness. We then compared the size of eggs bearing female or male embryos, and studied the mechanisms behind the difference we observed. Finally, we studied the consequences of differential sex allocation through egg size for male offspring of virgin females (which produce only male eggs) and mated females (which produce both male and female eggs). Although our findings raise very interesting evolutionary questions and might allow a test of various evolutionary hypotheses, in this paper, we focus on egg size as a possible proximate mechanism leading to biased sex allocation.
2. Material and methods
Spider mites (T. urticae) were reared in large numbers (more than 10 000) on cucumber plants (variety: Ventura provided by Rijkzwaan France), under controlled conditions (25°C). Our mite culture was established in September 2007 from approximately 5000 individuals sampled from a laboratory population of the University of Amsterdam, which was originally created in 1994 from individuals collected in a cucumber greenhouse in Pijnacker, The Netherlands.
In our population, developmental time at 25°C takes about 13 days. Females can live for 30–40 days and lay 2–10 eggs per day on the host plant used (cucumber; [34]) throughout their life. Virgin individuals can easily be isolated prior to mating because females and males can be distinguished when they are in their final quiescent stage of development.
All experiments were performed in a growth chamber at 25°C, under continuous light.
(a). Consequences of egg size variation for offspring fitness
To test whether egg size was an appropriate predictor of energy content in T. urticae, and whether the hypothesis that ‘bigger is better’ applies to this species, we investigated the consequences of egg size for the following offspring fitness components: hatching probability, juvenile survival, developmental time, larval size and adult size. These traits were observed in the progeny of either mated or virgin mothers.
(i). Juvenile fitness traits (experiment 1)
Adult females (n = 120) were randomly sampled from our population and allowed to lay eggs for 24 h on cucumber leaves placed on wet cotton (30 females per 5 cm2 leaf cutlets). A total of 350 eggs were evenly laid across the leaf cutlets. Larvae hatching from these eggs were then individually put on 1 cm2 leaf cutlets to complete their development. Before reaching sexual maturity, males were discarded, whereas 80 females were haphazardly chosen from those who emerged. These females were either left alone on their leaf, thus remaining virgin throughout the experiment (n = 37), or individually exposed to two males for 48 h to ensure mating (n = 39). The 78 males used in the second treatment had been previously isolated from our base population during 72 h, to guarantee sperm replenishment.
Subsequently, virgin and mated females were placed on a 1 cm2 leaf cutlet (one female per leaf cutlet) and allowed to oviposit during 24 h. The resulting eggs (n = 275 and 130 eggs for mated and virgin females, respectively) were collected and measured, and the females were transferred to a new leaf cutlet for another 24 h period, to increase the number of eggs (n = 325 and 249 eggs for mated and virgin females, respectively). We have (yet) no proximate explanation as to why virgins produced fewer eggs than mated females. To measure egg size, each egg was individually placed on a glass slide under a binocular microscope (lens 2×, 40×), and photographed using AVT SmartView software. For each egg-laying period, all pictures were taken on the same day. The pictures were subsequently analysed with Optimas 6.5 software, which automatically calculates the projected area covered by each egg. From this area (s), we calculated the total egg surface (S) assuming a spherical shape, hence S = 4s. All the results shown here are similar whether we considered the surface or the volume of eggs.
After being photographed, eggs were individually placed on a 1 cm2 cucumber leaf cutlets to complete their development, and surveyed every 2 days until adulthood. Thus, each egg was characterized by its size, hatching date, age at maturity and sex. As visual discrimination between males and females is not possible at the egg stage, the sex of the resulting offspring was recorded once the adults emerged. The sex of the larvae that died before adulthood was thus unknown. Moreover, as sex could only be determined in survivors, the sex ratios shown here correspond to secondary sex ratios.
Egg-laying period (clutch 1 or 2) did not influence egg size (F1,978 = 0, p = 0.99). Hence, these data were pooled throughout the rest of the analyses. Eggs and larvae that died accidentally were excluded from the analysis. We combined hatching probability with survival from hatched egg to adulthood (similar results were obtained when studying the two traits separately). Using Proc GLIMMIX (SAS 9.2, 2000) with the dist = binary option, we performed a mixed logistic regression to model the probability of an egg to survive to adulthood as a function of its size and of its mother's mating status (MMS, i.e. virgin or mated), with mother identity nested within MMS as a random factor.
Using Proc GLIMMIX (SAS 9.2, 2000) with the dist = normal option, we then performed a mixed linear regression to model the development time from egg to adulthood as a function of egg size, MMS and sex nested within MMS as fixed factors, and mother identity nested within MMS as a random factor.
(ii). Larval size (experiment 2)
As larvae in experiment 1 had not been measured, experiment 2 was performed to determine the consequences of egg size for larval size.
Larval sex cannot be visually determined, and male and female have very different sizes at adulthood; hence, we studied the relationship between egg size and larval size using larvae exclusively produced by virgin mothers, which were thus necessarily male. To this purpose, 25 virgin females of the same age were isolated and allowed to lay eggs for 24 h on a 1 cm2 cucumber leaf cutlet. Each egg was collected and measured as described for experiment 1, then placed back on leaf cutlets and observed twice per day until hatching. As soon as they hatched, larvae were killed with chloroform and measured. The larvae which were measured (n = 46) had not eaten yet, thus their development was only through the resources contained in the eggs. Each larva was individually placed on a glass slide under a binocular microscope and photographed in the same way as eggs. The outline of the body was traced manually using Optimas 6.5 software, which then automatically calculated the body area. Contrary to the size of eggs, which was expressed as a surface, the size of the offspring is expressed as a projected area.
Using Proc GLIMMIX (SAS 9.2, 2000) with the dist = normal option, we performed a mixed linear regression to model the body size of larva as a function of egg size, with mother identity as a random factor.
(iii). Adult male size
Data for this analysis were directly obtained from experiment 1, in which 30 adult males from mated mothers and 50 adult males from virgin mothers had been haphazardly chosen to be measured at the end of the experiment, in the same manner as larvae were measured. For these males, both egg size and adult size are thus known.
Using Proc GLIMMIX (SAS 9.2, 2000) with the dist = normal option, we performed a mixed linear regression to model the body size of adult male as a function of its egg size and of its MMS (virgin or mated), with mother identity nested within MMS as a random factor.
(iv). Adult female size (experiment 3)
As adult females in experiment 1 were not measured, experiment 3 was performed to determine the consequences of egg size for female adult size. Forty mated females were allowed to lay eggs for 24 h, producing a total of 261 eggs. These eggs were individually measured and isolated on cucumber leaflets to complete development. Thirteen days later, those female offspring that had reached maturity were measured in the same manner as larvae (n = 42 offspring, from 22 different mothers).
Using Proc GLIMMIX (SAS 9.2, 2000) with the dist = normal option, we performed a mixed linear regression to model body size of an adult female as a function of its egg size, with mother identity as a random factor.
(b). Egg size and sex allocation
(i). Comparative size of male and female eggs
To quantify maternal investment in male and female offspring, we compared the size of fertilized (female) and unfertilized (male) eggs produced by mated mothers, using eggs of experiment 1. Using Proc GLM (SAS 9.2, 2000), we first ran a mixed ANOVA on egg size, with sex as a fixed factor and mother identity as a random factor. In this way, we obtained an LSmeans estimate of mean egg size per sex and its standard deviation. We then performed a mixed logistic regression to model the probability of an egg being male or female as a function of its size. We used Proc GLIMMIX (SAS 9.2, 2000) with the dist = binary option with egg size as a fixed factor and mother identity as a random factor.
(ii). Proximate mechanisms of asymmetric allocation between male and female eggs
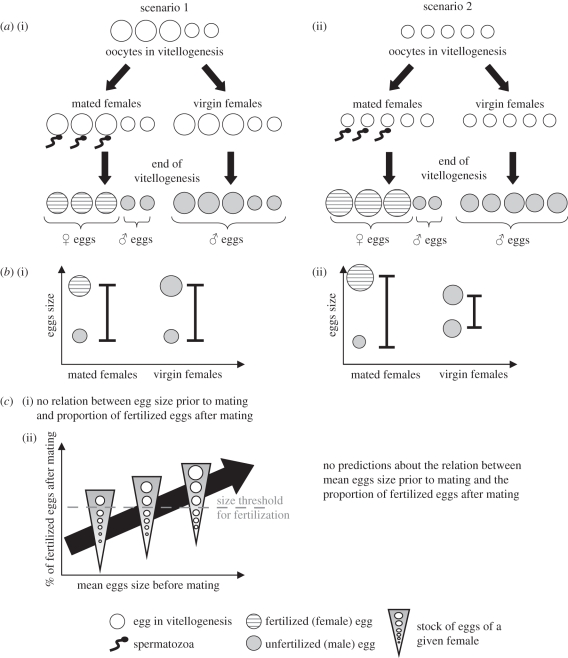
Comparison of size distribution of eggs laid by mated and virgin mothers. An asymmetric allocation between male and female eggs could occur either prior to fertilization, if egg size per se determines the probability of an egg being fertilized (scenario 1, figure 1a), or after fertilization, if eggs draw more (or less) resources once fertilized, which is possible since eggs are fertilized before the end of vitellogenesis ([35]; scenario 2, figure 1a). Under scenario 1, the range of egg size should be similar in mated mothers (which produce both male and female eggs) and virgin mothers (which produce only male eggs), whereas under scenario 2, size variation should be greater in mated than in virgin mothers (figure 1b). To discriminate between the two scenarios, we compared the size distribution of eggs produced by mated and virgin mothers, using eggs of experiment 1 (600 eggs from mated mothers and 379 eggs from virgin mothers).
Figure 1.
Hypothetical mechanisms of differential allocation into male and female eggs (in the case where female eggs are bigger than male eggs). Scenario 1: (a) (i) differences in egg size exist prior to fertilization, and egg size per se determines the probability of an egg being fertilized (with a higher probability for larger eggs). (b) (i) Under this scenario, the range variation of egg size should be the same between mated and virgin mothers. (c) This scenario can be divided into two sub-scenarios: (i) there is no size threshold for fertilization, and the larger eggs of each female are preferentially fertilized. In this case, no relation is expected between mean egg size prior to mating and the proportion of fertilized eggs after mating. (ii) There is a size threshold for fertilization. In this case, the proportion of eggs above the threshold is expected to increase with mean egg size. Scenario 2: (a) (ii) the differential allocation is due to a differential provisioning of male and female eggs following fertilization, with fertilized eggs receiving more resources. (b) (ii) Under this scenario, the range variation of egg size should be larger among eggs from mated mothers, compared with eggs from virgin mothers. Indeed, if fertilized eggs draw more resources, fewer resources are available for unfertilized ones, leading to very large female eggs and very small male ones.
Using the complete dataset, we compared the size of eggs laid by virgins and mated mothers independently of offspring sex using a two-way analysis of variance, with MMS as a fixed factor and mother identity nested within MMS as a random factor. We also compared the size of the smallest and of the largest egg laid by each individual female, depending on whether she was mated or not, to test the hypothesis that egg size and its range of variation were determined prior to fertilization.
Sex ratio as a function of mean egg size prior to mating (experiment 4). We further asked whether fertilization occurred for a fixed proportion of eggs per female, or for eggs whose size exceeded (or was below) a certain threshold value. Under the first hypothesis, the sex ratio produced by a female should not depend on her mean egg size prior to mating, whereas it should under the second scenario (figure 1c). To discriminate between these two hypotheses, we measured the size of eggs prior to mating and the offspring sex ratio after mating for 56 females (experiment 4). These females were first sampled from our population as quiescent sub-adults (virgins), and isolated on a 1 cm2 cucumber leaf cutlet (one female per leaf). At sexual maturity, they were transferred to a new leaf cutlet and allowed to lay eggs for 24 h. Four haphazardly chosen eggs per female were then measured as described above. Subsequently, each female was put with two virgin males for 24 h to ensure mating, then isolated and allowed to lay eggs for 3 days. The sex ratio of the resulting offspring (10–28 per female) was then recorded. All females produced both sons and daughters.
We performed a logistic regression to model the offspring sex ratio of mated females as a function of mean egg size of each female prior to mating. We used Proc LOGISTIC (SAS 9.2, 2000) with mean egg size prior to mating as the independent (fixed) factor and the number of daughters/total number of offspring per female as the response variable.
(iii). Comparative size of male offspring produced by mated or virgin mothers
Data for this analysis were obtained from experiment 1, in which male offspring from mated and virgin mothers were measured, at both egg and adult stages. Using only male eggs, we ran a mixed ANOVA (Proc GLM, SAS 9.2, 2000) on egg size, with MMS as a fixed factor and mother nested within MMS as a random factor. We did the same analysis on adult male size.
3. Results
(a). Consequences of egg size variation for offspring fitness traits
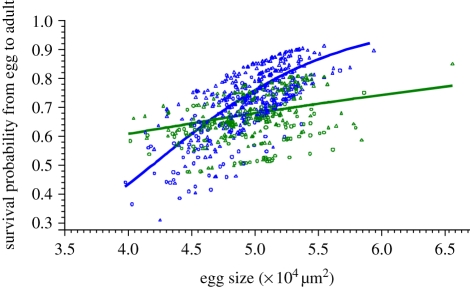
Of the 730 eggs recovered following measurement, 515 survived to adulthood (322 from mated mothers and 193 from virgins; electronic supplementary material, table S1). Egg size, MMS and the interaction between egg size and MMS all significantly or marginally significantly influenced the probability of egg surviving to adulthood, with larger eggs and eggs from mated mothers being more likely to survive than smaller eggs and eggs from virgins (egg size: F1,653 = 9.52, p = 0.0021; MMS: F1,73 = 3.41, p = 0.069; egg size * MMS: F1,653 = 3.85, p = 0.050; figure 2). We thus analysed the effect of egg size separately for mated and virgin mothers. We found that the effect of egg size on egg probability of reaching adulthood was highly significant in mated females (F1,402 = 12.79, p = 0.0004), but not significant in virgin females (F1,251 = 0.73, p = 0.39).
Figure 2.
Mixed logistic regression of the probability for eggs to reach adulthood on egg size. Line, mean predicted value; individual symbols, best linear unbiased predictors, which depends on the random factor mother nested within MMS (triangle, alive at adulthood; circle, dead before adulthood). Colours indicate the MMS (blue, mated; green, virgin).
Using the full model, developmental time from egg to adulthood was not significantly influenced by egg size (F1,361 = 1.02, p = 0.31), whereas it was significantly influenced by the MMS (F1,72 = 4.83, p = 0.031), by sex within MMS (F1,361 = 4.66, p = 0.031) and by the interaction between egg size and MMS (F1,361 = 5.26, p = 0.022). On average, female eggs reached adulthood in 14.32 days (standard error, s.e. = 0.21); males from mated mothers took 13.31 days (s.e. = 0.43); and males from virgins took 13.28 days (s.e. = 0.23; LSmeans statement, Proc GLIMMIX).
We thus considered the effect of egg size separately for males and females (N = 219 each, with mother identity nested within MMS as random). For males, developmental time was not significantly affected by either egg size, or MMS, or their interaction (egg size: F1,156 = 0.19, p = 0.66; MMS: F1,59 = 0.41, p = 0.52; egg size * MMS: F1,156 = 0.30, p = 0.58). We obtained the same type of results when sequentially deleting factors. Conversely for females, developmental time was significantly affected by egg size (F1,40 = 6.23, p = 0.017), with larger eggs taking more time to develop. However, the fit was very poor, and a linear regression performed on mean values per mother led to a non-significant relationship (with F1,39 = 1.28, p = 0.26, R2 = 0.032).
In offspring from virgins studied in experiment 2, larval size was significantly influenced by egg size, with larger eggs producing larger larvae (figure 3a; F1,20 = 17.25, p = 0.0005).
Figure 3.
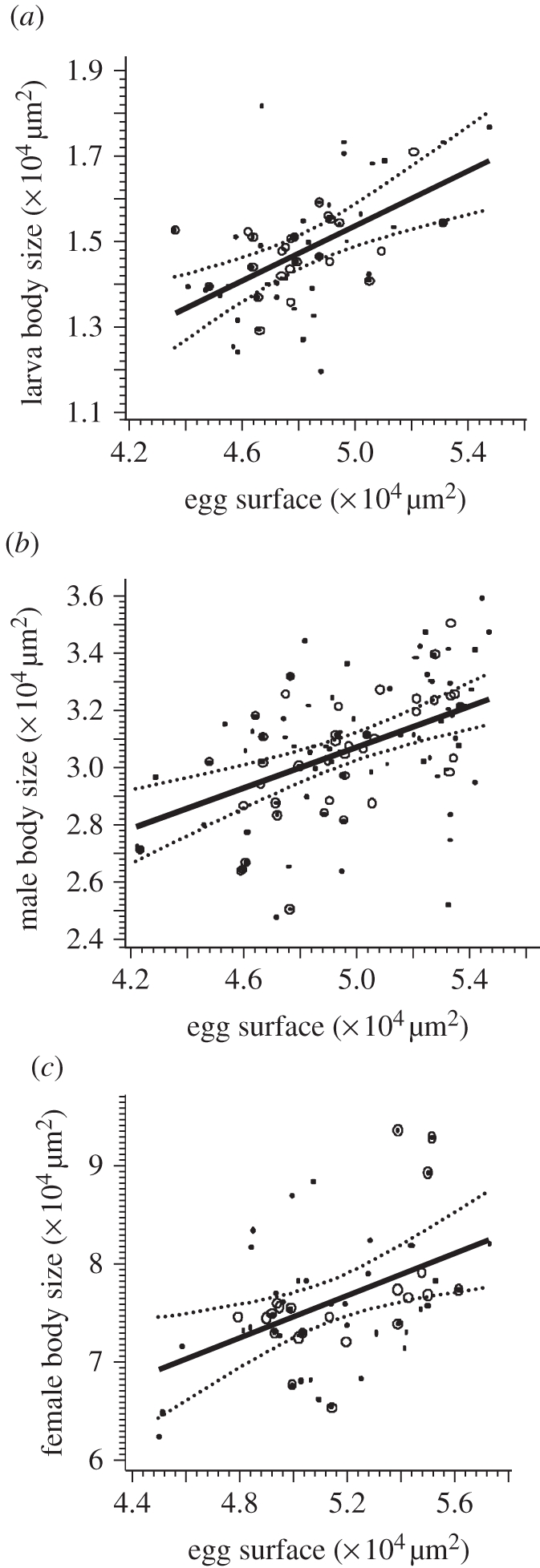
Mixed linear regression of body size on egg size. (a) Larvae (y = −0.038 + 0.3141x; p = 0.0005). (b) Adult male offspring (y = 1.2796 + 0.3569x; p < 0.0001). (c) Adult female offspring (y = 2.0894 + 1.0725x; p = 0.0073). Dotted lines, lower and upper 95% prediction limits for linear predictor; solid lines, linear predictor; circles, observed mean values per female; dots, observed individual data.
From experiment 1, the regression of male adult size on male egg size was also significant, with larger adults emerging from larger eggs (figure 3b; F1,37 = 5.86, p = 0.021 for the full model; F1,38 = 21.90, p < 0.0001 for the final model). In the full model, there was no significant effect of either MMS or the interaction between egg size and MMS on male adult size (F1,39 = 1.53, p = 0.22 and F1,37 = 1.79, p = 0.19, respectively).
From experiment 3, the regression of female adult size on female egg size was highly significant, with larger adults emerging from larger eggs (figure 3c; F1,19 = 9.01, p = 0.0073).
(b). Egg size and sex allocation
(i). Comparative egg size of male and female eggs
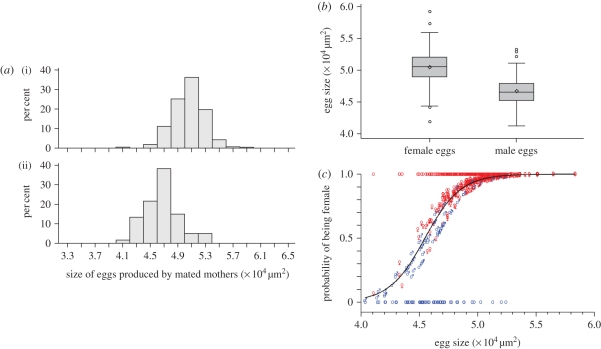
From the 600 eggs measured in mated mothers, 516 were recovered following measurement and 321 attained adulthood (261 females and 60 males; electronic supplementary material, table S1). Female eggs were significantly larger than male eggs (figures 4 and 5a,b, LSmeans female: 5.04 × 104 µm2, 95% CI 5.01–5.07 × 104 µm2; male: 4.66 × 104 µm2, 95% CI 4.60–4.72 × 104 µm2, F1,281 = 125.3, p < 0.0001). Egg size significantly influenced the probability of an egg to be female, with larger eggs more likely to be female than smaller ones (figure 5c; F1,281 = 55.60, p < 0.0001). In particular, the probability for an egg larger than 5.04 × 104 µm2 (the average size of female eggs) to be female exceeded 93 per cent, whereas it was below 54 per cent for eggs smaller than 4.66 × 104 µm2 (the average size of male eggs).
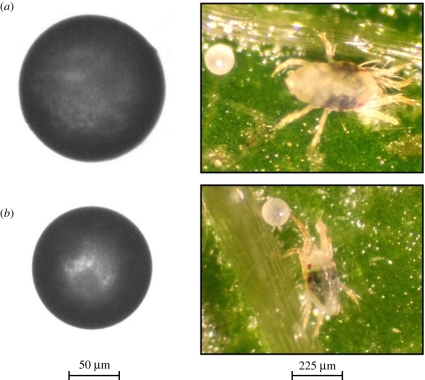
Figure 4.
Eggs and adults of T. urticae, representing the extremes of the egg size distribution in experiment 1. (a) Fertilized egg and adult female; (b) unfertilized egg and adult male.
Figure 5.
(a) Size distribution of (i) female (n = 261, mean = 5.051, s.d. = 0.233) and (ii) male (n = 60, mean = 4.674, s.d. = 0.253) eggs produced by mated mothers. The statistical analysis was performed taking into account a random mother effect nested within egg sex. (b) Box plot for the size of female and male eggs produced by mated mothers. (c) Mixed logistic regression of individual egg sex on egg size, performed on eggs produced by mated females. Dark line, mean predicted value; individual symbols (♂ and ♀), best linear unbiased predictors (which depend on the random factor mother). Circles which take the values ‘0’ or ‘1’ indicate the observed data. Colours indicate the sex (blue, male; red, females).
(ii). Mechanisms underlying the asymmetric allocation between male and female eggs
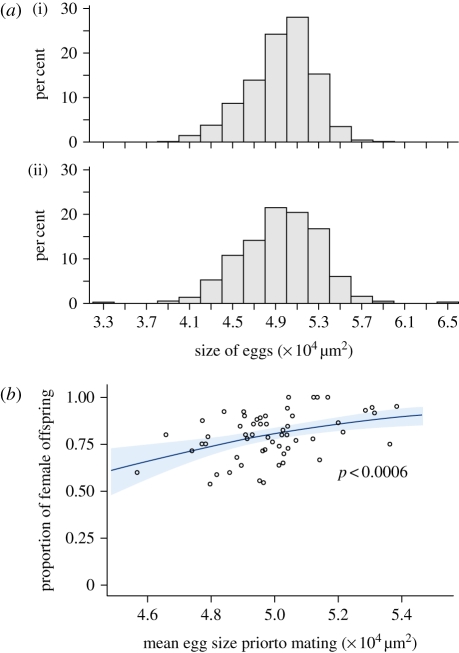
Comparison of size distribution of eggs laid by mated and virgin mothers. The size distributions of eggs were similar between mated and virgin mothers (figure 6a), as were the minimum, maximum and mean egg size per female (electronic supplementary material, figure S1, p = 0.19, 0.97 and 0.97, respectively). These results indicate that differences in egg size occur prior to mating, suggesting that females have a stock of eggs of different sizes among which the largest will be fertilized (figure 1, scenario 1).
Figure 6.
(a) Size distribution of all eggs laid by (i) mated mothers (n = 600, mean = 4.946, s.d. = 0.301) and (ii) virgin mothers (n = 379, mean = 4.946, s.d. = 0.365). (b) Logistic regression of sex-ratio (proportion of female offspring produced after mating) on mean egg size prior to mating. Grey area represents 95% confidence limits.
Sex ratio as a function of mean egg size prior to mating. Mothers with larger eggs when virgin produced a significantly larger proportion of fertilized (female) eggs once mated (figure 6b; χ2 = 11.9, p < 0.0006), suggesting the existence of a threshold size for egg fertilization. However, most of the variation remained unexplained (R2 = 0.17), suggesting that sex-ratio determination was partly independent of female mean egg size before mating.
(iii). Comparative size of male offspring of mated and virgin mothers
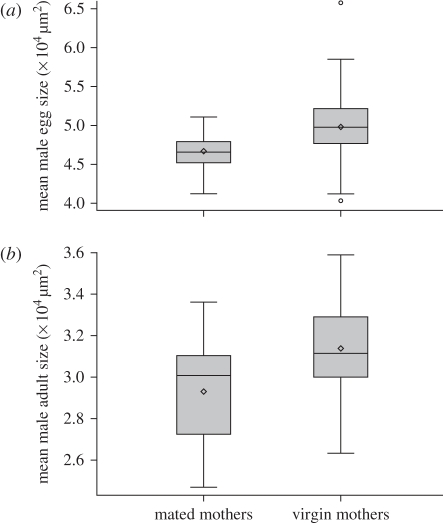
On average, male egg size of mated mothers was significantly smaller than that of virgins (figure 7a, LSmeans male eggs from mated mothers: 4.67 × 104 µm2, 95% CI 4.57–4.75 × 104 µm2; from virgins: 4.96 × 104 µm2, 95% CI 4.92–5.01 × 104 µm2; F1,109.48 = 20.34, p < 0.0001). This difference persisted in adults, since adult males produced by mated mothers were smaller than those produced by virgins (figure 7b, LSmeans projected area of males from mated mothers: 2.93 × 104 µm, 95% CI 2.85–3.01 × 104 µm2; from virgins: 3.11 × 104 µm2, 95% CI 3.04–3.17 × 104 µm2; F1,55.2 = 8.44, p = 0.0053).
Figure 7.
(a) Box plot for size of male eggs produced by virgins and by mated females (F = 20.34, p < 0.0001). (b) Box plot for size of adult males produced by virgins and by mated females (F = 8.44, p = 0.0053).
4. Discussion
(a). Variation of egg size affects offspring fitness in T. urticae
The relationship between egg size and offspring fitness has been extensively studied in animals [13,36], although never in mites. Larger eggs are generally more likely to hatch and produce fast-developing juveniles with higher survivorship than smaller eggs, suggesting that egg size is a good predictor of energy content in most cases. In T. urticae, however, egg size did not significantly affect developmental time in males, whereas it even increased it in females (as in some invertebrates [37]). However, as expected, larger eggs were more likely to reach adulthood than smaller eggs. Interestingly, this latter effect was more pronounced in the progeny of mated mothers than in that of virgin mothers. Mated females, unlike virgins, produce both female and male offspring. In T. urticae, adult females are larger than adult males [38], so the differential susceptibility of survival to egg size could result from female larvae requiring more resources than male larvae to develop successfully. Such sex-specific resource requirement has been demonstrated in birds [39,40]. Furthermore, independent of egg size, survival of eggs from mated mothers was slightly greater than from virgin mothers (figure 2). This difference suggests that female larvae survived better than male larvae. Thus, egg size in T. urticae has short-term fitness consequences for the offspring, by affecting juvenile survival. Furthermore, as usually found in arthropods [13], larger eggs of T. urticae also produced larger larvae and larger adults in both male and female offspring.
These results have two major implications. First, they strongly suggest that egg size differences in T. urticae are not due to secondary differences in water content or shell thickness, but rather to differences in nutrient content. Second, through its effects on adult size, egg size probably affects adult fitness. Indeed, adult body size can have important consequences for fitness, and particularly for reproductive success. The common trend among arthropods is that large females can lay larger or more eggs than smaller ones [41]. A larger body size might also be generally advantageous for males, mainly because of sexual selection [41]. In T. urticae, competition between males can be very intense, because males are sexually long-lived and individual females, for which only the first mating is effective, are available for reproduction only for a short period [38]. Adult males thus guard quiescent females prior to their final moult to enhance their chance of being the first male to mate, and have to resist attempted interference from other males [38,42]. Larger males have a better chance of winning the competition than smaller males [43]. Furthermore, larger males guard more selectively (i.e. spend less time guarding) and are better able to resist attempted interference in mating than smaller ones [42]. By determining body size, egg size thus probably influences the future reproductive success of the offspring.
(b). Egg size and sex allocation
Eggs bearing female embryos were larger than those bearing male embryos. As it was found that egg size probably reflects energy content, sex allocation in T. urticae is thus biased towards females not only via the sex ratio, but also via the amount of resources provided to the eggs. It should be noted that in our study, the sex of the offspring was only recorded at adulthood. Thus, if egg size affected juvenile mortality differently in male and female offspring, e.g. with females tending to die more often when starting life as small eggs, the difference of size observed between male and female eggs might simply result from this differential mortality. We would then expect surviving eggs from mated mothers to be on average larger than surviving eggs from virgin mothers, since only the former produce female eggs. Instead, we found that the mean egg size of mated females and of virgins were exactly equal, whether we considered all eggs (mean egg size = 4.94 × 104 µm2 for each) or only eggs that survived until adulthood (mean egg size = 4.98 × 104 µm2 for each). The size difference observed between male and female eggs is thus more probably due to a sex-specific egg provisioning, rather than to a differential mortality between the two sexes. This differential investment in eggs of each sex has been observed in birds, such as the American kestrel or the Bengalese finch, with male eggs larger than female eggs, probably to compensate for the greater vulnerability of males to sibling competition [22–28]. However, egg sexual dimorphism has never been investigated so far in haplodiploids, except in the predatory mite Phytoseiulus persimilis, where female eggs were also found to be larger than male eggs [30].
To investigate the proximate mechanisms underlying the asymmetric allocation between male and female eggs, we tested whether this differential allocation occurred prior or after fertilization. If fertilization induced an increase in egg provisioning, mated mothers should have a larger distribution of egg size than virgin mothers, with female (i.e. fertilized) eggs being very large. We did not observe such a pattern. Instead, we found that egg size distribution was similar for mated and virgin mothers, suggesting that the differential allocation occurred prior to fertilization. Moreover, we found that the mean size of eggs produced by virgin mothers predicted the proportion of female offspring produced after mating. These results suggest that large eggs have a higher probability of being fertilized than smaller eggs. Thus, it is not fertilization that determined egg size, but rather egg size that determined fertilization probability. Such phenomenon is already known in marine invertebrates [44]. An alternative, less likely hypothesis would be that mothers are able to control their relative allocation into male and female eggs. In many animals, spatial or temporal segregation of eggs or embryos of different sex enables sex-specific parental resource allocation [25]. In T. urticae, the ventral area of the ovary contains fewer sperm than the dorsal area [35,38], suggesting that oocytes present in the ventral area are less likely to be fertilized. Hence, a spatial segregation of small and large oocytes in the ovary might allow mothers to adjust their relative investment in male and female eggs. Note that the mechanism that we have envisaged in T. urticae, namely that larger eggs are more likely to be fertilized, cannot be at work in the predatory mite P. persimilis, in which female eggs were also found to be larger than male eggs [30]: P. persimilis is a pseudoarrenotokous species, i.e. all eggs are fertilized, but in males the paternal genome is aborted. Moreover, it should be noted that we observed secondary sex ratios, which could be more female biased than primary sex ratio since egg size influenced egg survival, so that the final sex ratio could be achieved not through decreased probability of fertilization of small eggs, but through increased mortality of these eggs or of larvae emerging from them. However, even though the proximate mechanisms underlying the sex-specific provisioning of eggs in mites remain unclear, our study strongly suggests that the size difference between male and female eggs in T. urticae occurs prior to fertilization.
Egg size in T. urticae determines the probability of an egg being fertilized, thus mediating differential egg provisioning to male and female offspring, individual sex and progeny sex ratio: sex allocation in this haplodiploid species is more finely tuned than previously thought. This asymmetric sex allocation has an unexpected consequence: male offspring of mated mothers are smaller than male offspring of virgins, leading to the potential evolution of split sex ratios [45–47]. The next step will be to document the relative reproductive success of these males in various conditions.
The results presented in this paper can be interpreted in a variety of ways in the context of sex allocation theory. We have shown that, in spider mites, adult size is correlated to egg size. If it is the case that larger size benefits female offspring more than male offspring (something for which we have yet very limited evidence), our finding that female eggs are larger than male eggs could illustrate the prediction that whenever one sex, here female, benefits more than the other one from good conditions, offspring of this sex should be given more resources than offspring of the other sex [5]. This is similar to what has been shown in solitary parasitoids, whereby female eggs are laid in larger hosts and male eggs in smaller hosts [48]. Theory also predicts that biased sex allocation could result from heterogeneity in female condition. Still assuming that female offspring benefit more than male offspring from increased allocation, females in good conditions should produce a higher proportion of females compared with females in bad conditions [5]. In the present study, we found that females producing larger eggs prior to mating produced a higher proportion of females once mated, compared with females producing smaller eggs. If mean egg size prior to mating reflects female condition, then our study could illustrate condition-dependent optimal sex allocation. However, trade-offs between egg size and fecundity in females would strongly affect the hypothesis that large eggs reflect female condition. Finally, in the context of superparasitism and LMC, it has been theoretically and empirically shown that females with a lower fecundity should produce more males [49]. This is because when a female arrives in a patch where a majority of female offspring have been laid and this female has only a few eggs to lay, it is advantageous to lay male eggs, so as to fertilize all the females. If however this female has many eggs to lay, it is no longer advantageous to lay too many male eggs, because those will compete to access few females. Strongly female-biased sex ratios in spider mites are assumed to result from LMC [30–31]. Our finding that females with smaller eggs produce relatively more males could illustrate such kin-selection argument, if egg size reflects the quality of females and is correlated to their overall fecundity. This latter hypothesis, as well as the assumption that females benefit more than males from a larger size, remains to be tested. Alternatively, egg size and sex ratio might be the result of an arm race between males (selected to fertilize all offspring) and females (selected to produce at least some males). Hence, the findings presented in this paper raise fascinating questions concerning the evolutionary forces driving egg size and sex allocation in haplodiploids.
Acknowledgements
This work benefited from helpful discussions with Denis Bourguet, Martijn Egas, Michael Hochberg, Arne Janssen, Mark Kirkpatrick, Yannis Michalakis, Maria Navajas, Ana Rivero, Maurice Sabelis, Thomas Tully and Jacques Van Alphen. Two anonymous reviewers made helpful comments on the submitted version. The study was supported by a PhD grant from CNRS to E.M., a CNRS post-doc grant to H.D-T.K. and B.F., an ANR post-doc grant to S.M. and to A.F., a PESSOA travel grant and a Project for International Scientific Cooperation (PICS CNRS—Universidade de Lisboa) to I.O. and S.M. This is publication ISEM-2010–092 from The Institut des Sciences de l'Evolution, Montpellier.
References
- 1.Hamilton W. D. 1967. Extraordinary sex ratios. Science 156, 477–488 10.1126/science.156.3774.477 (doi:10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 2.Charnov E. L. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Hardy I. C. W. 2002. Sex ratios: concepts and research methods. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.West S. A. 2009. Sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 5.Trivers R. L., Willard D. E. 1973. Natural selection of parental ability to vary sex-ratio of offspring. Science 179, 90–92 10.1126/science.179.4068.90 (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 6.Werren J. H., Beukeboom L. W. 1998. Sex determination, sex ratios and genetic conflicts. Annu. Rev. Ecol. Syst 29, 233–261 10.1146/annurev.ecolsys.29.1.233 (doi:10.1146/annurev.ecolsys.29.1.233) [DOI] [Google Scholar]
- 7.Clutton-Brock T. H., Albon S. D., Guiness F. E. 1984. Maternal dominance, breeding success and birth sex ratios in red deer. Nature 308, 358–360 10.1038/308358a0 (doi:10.1038/308358a0) [DOI] [Google Scholar]
- 8.Frank S. A. 1990. Sex allocation theory for birds and mammals. Annu. Rev. Ecol. Syst. 21, 13–55 10.1146/annurev.es.21.110190.000305 (doi:10.1146/annurev.es.21.110190.000305) [DOI] [Google Scholar]
- 9.Komdeur J. 1998. Long-term fitness benefits of egg sex modification by the Seychelles warbler. Ecol. Lett. 1, 56–62 10.1046/j.1461-0248.1998.00009.x (doi:10.1046/j.1461-0248.1998.00009.x) [DOI] [Google Scholar]
- 10.Ueno T. 1999. Host-size-dependent sex ratio in a parasitoid wasp. Res. Popul. Ecol. 41, 47–57 10.1007/PL00011982 (doi:10.1007/PL00011982) [DOI] [Google Scholar]
- 11.Berthouly A., Helfenstein F., Tanner M., Richner H. 2008. Sex-related effects of maternal egg investment on offspring in relation to carotenoid availability in the great tit. J. Anim. Ecol. 77, 74–82 10.1111/j.1365-2656.2007.01309.x (doi:10.1111/j.1365-2656.2007.01309.x) [DOI] [PubMed] [Google Scholar]
- 12.Clutton-Brock T. H. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Fox C. W., Czesak M. E. 2000. Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 45, 341–369 10.1146/annurev.ento.45.1.341 (doi:10.1146/annurev.ento.45.1.341) [DOI] [PubMed] [Google Scholar]
- 14.Fischer K., Zwaan B. J., Brakefield P. M. 2002. How does egg size relate to body size in butterflies? Oecologia 131, 375–379 10.1007/s00442-002-0913-9 (doi:10.1007/s00442-002-0913-9) [DOI] [PubMed] [Google Scholar]
- 15.Smith C. C., Fretwell S. D. 1974. The optimal balance between size and number of offspring. Am. Nat. 108, 499–506 10.1086/282929 (doi:10.1086/282929) [DOI] [Google Scholar]
- 16.Parker G. A., Begon M. 1986. Optimal egg size and clutch size: effects of environment and maternal phenotype. Am. Nat. 128, 573–592 10.1086/284589 (doi:10.1086/284589) [DOI] [Google Scholar]
- 17.McGinley M. A., Temme D. H., Geber M. A. 1987. Parental investment in offspring in variable environments: theoretical and empirical considerations. Am. Nat. 130, 370–398 10.1086/284716 (doi:10.1086/284716) [DOI] [Google Scholar]
- 18.Allen R. M., Buckley Y. M., Marshall D. J. 2008. Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am. Nat. 171, 225–237 10.1086/524952 (doi:10.1086/524952) [DOI] [PubMed] [Google Scholar]
- 19.Fox C. W., Thakar M. S., Mousseau T. A. 1997. Egg size plasticity in a seed beetle: an adaptive maternal effect? Am. Nat. 149, 149–163 10.1086/285983 (doi:10.1086/285983) [DOI] [PubMed] [Google Scholar]
- 20.Tully T., Ferrière R. 2008. Reproductive flexibility: genetic variation, genetic costs and long-term evolution in a Collembola. PLoS ONE 39 See http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0003207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stillwell R. C., Blanckenhorn W. U., Teder T., Davidowitz G., Fox C. W. 2010. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: from physiology to evolution. Ann. Rev. Entomol. 55, 227–245 10.1146/annurev-ento-112408-085500 (doi:10.1146/annurev-ento-112408-085500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson D. J., Reeve J., Bird D. M. 1997. Sexually dimorphic eggs, nestling growth and sibling competition in American kestrels Falco sparverius. Funct. Ecol. 11, 331–335 10.1046/j.1365-2435.1997.00091.x (doi:10.1046/j.1365-2435.1997.00091.x) [DOI] [Google Scholar]
- 23.Cordero P. J., Griffith S. C., Aparicio J. M., Parkin D. T. 2000. Sexual dimorphism in house sparrow eggs. Behav. Ecol. Sociobiol. 48, 353–357 10.1007/s002650000252 (doi:10.1007/s002650000252) [DOI] [Google Scholar]
- 24.Cordero P. J., Vinuela J., Aparicio J. M., Veiga J. P. 2001. Seasonal variation in sex-ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. J. Evol. Biol. 14, 829–834 10.1046/j.1420-9101.2001.00320.x (doi:10.1046/j.1420-9101.2001.00320.x) [DOI] [Google Scholar]
- 25.Blanco G., Martinez-Padilla J., Serrano D., Davila J. A., Vinuela J. 2003. Mass provisioning to different-sex eggs within the laying sequence: consequences for adjustment of reproductive effort in a sexually dimorphic bird. J. Anim. Ecol. 72, 831–838 10.1046/j.1365-2656.2003.00753.x (doi:10.1046/j.1365-2656.2003.00753.x) [DOI] [Google Scholar]
- 26.Badyaev A. V., Oh K. P., Mui R. 2006. Evolution of sex-biased maternal effects in birds: II. Contrasting sex-specific oocyte clustering in native and recently established populations. J. Evol. Biol. 19, 909–921 10.1111/j.1420-9101.2005.01041.x (doi:10.1111/j.1420-9101.2005.01041.x) [DOI] [PubMed] [Google Scholar]
- 27.Soma M., Saito D. S., Hasegawa T., Okanoya K. 2007. Sex-specific maternal effect on egg mass, laying order, and sibling competition in the Bengalese finch Lonchura striata var. domestica. Behav. Ecol. Sociobiol. 61, 1695–1705 10.1007/s00265-007-0400-8 (doi:10.1007/s00265-007-0400-8) [DOI] [Google Scholar]
- 28.Saino N., Romano M., Caprioli M., Ambrosini R., Rubolini D., Fasola M. 2010. Sex allocation in yellow-legged gulls (Larus michahellis) depends on nutritional constraints on production of large last eggs. Proc. R. Soc. B 277, 1203–1208 10.1098/rspb.2009.2012 (doi:10.1098/rspb.2009.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radder R. S., Pike D. A., Quinn A. E., Shine R. 2009. Offspring sex in a lizard depends on egg size. Curr. Biol. 19, 1102–1105 10.1016/j.cub.2009.05.027 (doi:10.1016/j.cub.2009.05.027) [DOI] [PubMed] [Google Scholar]
- 30.Nagelkerke C. J., Sabelis M. W. 1998. Precise control of sex allocation in pseudo-arrhenotokous phytoseiid mites. J. Evol. Biol. 11, 649–684 10.1007/s000360050112 (doi:10.1007/s000360050112) [DOI] [Google Scholar]
- 31.Roeder C., Harmsen R., Mouldey S. 1996. The effects of relatedness on progeny sex ratio in spider mites. J. Evol. Biol. 9, 143–151 10.1046/j.1420-9101.1996.9020143.x (doi:10.1046/j.1420-9101.1996.9020143.x) [DOI] [Google Scholar]
- 32.Krainacker D. A., Carey J. R. 1990. Effect of age at first mating on primary sex-ratio in the two-spotted spider mite. Exp. Appl. Acarol. 9, 169–175 10.1007/BF01193426 (doi:10.1007/BF01193426) [DOI] [Google Scholar]
- 33.Roeder C. 1992. Sex ratio response of the two-spotted spider mite Tetranychus urticae Koch to changes in density under local mate competition. Can. J. Zool. 70, 1965–1967 10.1139/z92-266 (doi:10.1139/z92-266) [DOI] [Google Scholar]
- 34.Magalhães S., Blanchet E., Egas M., Olivieri I. 2009. Are adaptation costs necessary to build up a local adaptation pattern? BMC Evol. Biol. 9, 182. 10.1186/1471-2148-9-182 (doi:10.1186/1471-2148-9-182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helle W. 1967. Fertilization in 2-spotted spider mite Tetranychus urticae: Acari. Entomol. Exp. Appl. 10, 103–110 [Google Scholar]
- 36.Azevedo R. B. R., French V., Partridge L. 2001. Life-history consequences of eggs size in Drosophila melanogaster. Am. Nat. 150, 250–282 [DOI] [PubMed] [Google Scholar]
- 37.Levitan D. R. 2000. Optimal egg size in marine invertebrates: theory and phylogenetic analysis of the critical relationship between egg size and development time in echinoids. Am. Nat. 156, 175–192 10.1086/303376 (doi:10.1086/303376) [DOI] [PubMed] [Google Scholar]
- 38.Helle W., Sabelis M. W. 1986. Spider mites: their biology, natural enemies and control, vol. 1B Amsterdam, The Netherlands: Elsevier Science Publishing Company [Google Scholar]
- 39.Sheldon B. C., Merilä J., Lindgren G., Ellegren H. 1998. Gender and environmental sensitivity in nestling collared flycatchers. Ecology 79, 1939–1948 10.1890/0012-9658(1998)079[1939:GAESIN]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[1939:GAESIN]2.0.CO;2) [DOI] [Google Scholar]
- 40.von Engelhardt N., Carere C., Dijkstra C., Groothuis T. G. G. 2006. Sex-specific effects of yolk testosterone on survival, begging and growth of zebra finches. Proc. R. Soc. B 273, 65–70 10.1098/rspb.2005.3274 (doi:10.1098/rspb.2005.3274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savalli U. M., Fox C. W. 1998. Sexual selection and the fitness consequences of male body size in the seed beetle Stator limbatus. Anim. Behav. 55, 473–483 10.1006/anbe.1997.0622 (doi:10.1006/anbe.1997.0622) [DOI] [PubMed] [Google Scholar]
- 42.Enders M. M. 1993. The effect of male size and operational sex ratio on male mating success in the common spider mite, Tetranychus urticae Koch Acari: Tetranychidae. Anim. Behav. 46, 835–846 10.1006/anbe.1993.1269 (doi:10.1006/anbe.1993.1269) [DOI] [Google Scholar]
- 43.Potter D. A., Wrensh D. L., Johnston D. E. 1976. Guarding, aggressive behaviour and mating success in male twospotted spider mites. Ann. Entomol. Soc. Am. 69, 707–711 [Google Scholar]
- 44.Levitan D. R. 2006. The relationship between egg size and fertilization success in broadcast-spawning marine invertebrates. Int. Comp. Biol. 46, 298–311 10.1093/icb/icj025 (doi:10.1093/icb/icj025) [DOI] [PubMed] [Google Scholar]
- 45.Godfray H. C. J. 1990. The causes and consequences of constrained sex allocation in haplodiploid animals. J. Evol. Biol. 3, 3–17 10.1046/j.1420-9101.1990.3010003.x (doi:10.1046/j.1420-9101.1990.3010003.x) [DOI] [Google Scholar]
- 46.Krantz B. D., Schwarz M. P., Giles L. C., Crespi B. J. 2001. Split sex ratios and virginity in a gall-inducing thrips. J. Evol. Biol. 13, 700–706 [Google Scholar]
- 47.Helms K. R., Reuter M., Keller L. 2005. Sex-ratio conflict between queens and workers in eusocial Hymenoptera: mechanisms, costs and the evolution of split colony sex ratios. Evolution 59, 2626–2638 [PubMed] [Google Scholar]
- 48.Charnov E. L., Losdenhartogh R. L., Jones W. T., Vandenassem J. 1981. Sex ratio evolution in a variable environment. Nature 289, 27–33 10.1038/289027a0 (doi:10.1038/289027a0) [DOI] [PubMed] [Google Scholar]
- 49.Werren J. H. 1980. Sex-ratio adaptations to local mate competition in a parasitic wasp. Science 208, 1157–1159 10.1126/science.208.4448.1157 (doi:10.1126/science.208.4448.1157) [DOI] [PubMed] [Google Scholar]