Abstract
Although chemical communication is the most widespread form of communication, its evolution and diversity are not well understood. By integrating studies of a wide range of terrestrial plants and animals, we show that many chemicals are emitted, which can unintentionally provide information (cues) and, therefore, act as direct precursors for the evolution of intentional communication (signals). Depending on the content, design and the original function of the cue, there are predictable ways that selection can enhance the communicative function of chemicals. We review recent progress on how efficacy-based selection by receivers leads to distinct evolutionary trajectories of chemical communication. Because the original function of a cue may channel but also constrain the evolution of functional communication, we show that a broad perspective on multiple selective pressures acting upon chemicals provides important insights into the origin and dynamic evolution of chemical information transfer. Finally, we argue that integrating chemical ecology into communication theory may significantly enhance our understanding of the evolution, the design and the content of signals in general.
Keywords: chemical communication, signal, cue, volatiles, evolution of communication, pheromone
1. Introduction
Communication describes behaviours and traits that have evolved in order to influence other organisms and that are effective because the response of their receivers has also evolved [1–3]. As such, communication is frequently manipulative; a view that is consistent with the often pronounced conflict of interest between senders and receivers. Chemical communication is arguably the oldest and most widespread form of communication occurring widely among bacteria, fungi, plants and animals [4]. However, understanding the theoretical foundations of chemical communication—especially its origin and pronounced diversity—is challenging and has been rarely tackled.
Almost 60 years ago Tinbergen [5] proposed that communicative behaviour can evolve from functionally different behaviours through a process of ritualization where the communicative aspects are gradually emphasized so that they are more effective in eliciting a response in the receiver. This concept appears to be fundamentally correct and has been invoked for a number of different communication systems [6–8]. However, although the concept of ritualization does explain how communication can evolve, it does not explain why communication systems can be so diverse. Chemical communication is particularly diverse. For example, the chemical class of emitted terpenoids comprises over 40 000 substances, many of which have various protective, defensive and attractive functions [9]. If chemicals are involved in species/mate recognition, speciation and character displacement may play a predominant role in their diversification [10,11]. However, speciation mechanisms cannot alone account for the ubiquity and high abundance of chemical signals. Here, we present other important, but so far underappreciated evolutionary trajectories responsible for the remarkable diversity of chemical communication. Most organisms emit a multitude of chemicals into the environment, such as the complex blends found on the cuticles of insects, the multi-faceted bouquets of flowers, or the elaborate marking compounds of mammals. The emission of chemicals can occur owing to the volatility of produced metabolic by-products or because organisms have to eliminate waste products or protect themselves against enemies and abiotic stress [12–15]. It is thus not surprising that only a few of the emitted substances have a communicative function. For example, only nine out of greater than 60 compounds in the floral scent of Datura wrightii are behaviourally active [16]. Similarly, out of over 40 cuticular hydrocarbons, pentacosane alone induces policing behaviour in the social ant Aphaenogaster cockerellii [17]. Since various substances are emitted for non-communicative purposes, these chemicals provide multiple starting points for the evolution of communication.
One starting point for the evolution of communication is that chemicals released for non-communicative purposes yield inadvertent information to receivers. Inadvertent information can be defined as covariance between chemical stimulus and phenotypic quality of the sender. This applies to mammalian excretions, spider silk, plants' defensive compounds or the waxy layer of the cuticle of insects. The chemicals may merely indicate the presence or location of an individual, but because they are frequently very plastic and respond to many extrinsic (e.g. temperature and nutrition) and intrinsic (e.g. hormones and age) factors, they may inform about sex, health, reproductive state or dominance status. That such information is originally often only an environmental or physiological by-product, can be exemplified by the cuticular hydrocarbons used by red harvester ants Pogonomyrmex barbatus to identify individual task specialization [18]. The different cuticular patterns of foragers and nest-maintenance workers do not result from actively signalling individual specialization but from differences in the time spent outside the nest [19]. The warm, dry conditions that foragers experience outside the nest trigger a change in cuticular chemistry as an adaptation to reduce water loss.
Compounds that supply information without being selected for this function are cues (see figure 1 and electronic supplementary material, S1 for the distinction of cues and signals). If receivers have the ability to detect these cues, they can use the inadvertent information in their decision making. If their reaction to the cue is selectively neutral to the sender, no evolution towards increased effectiveness of communication is expected (e.g. [26,27]). Because incidental transmission of chemical information presents the simplest and arguably the most widespread form of information transfer it should serve as a null hypothesis against which to compare more complex, evolved communication systems.
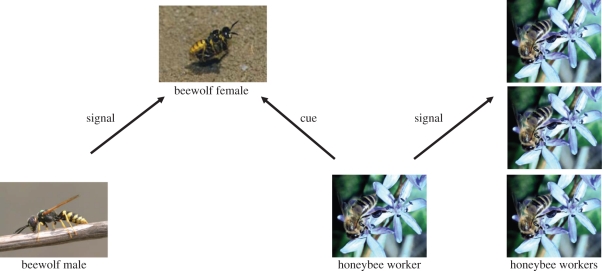
Figure 1.
Intentional (signal) and unintentional (cue) information transfer in the European beewolf Philanthus triangulum and the honeybee A. mellifera. Defining a substance as a cue or signal depends on the selective pressures under consideration. (Z)-11-Eicosen-1-ol functions as signal or cue according to the multiple selection pressures acting upon it. As a major compound of an alarm pheromone in the sting apparatus of honeybees, it is selected as an intraspecific signal to lure conspecifics to the site of emission for concerted defence behaviour. It also prolongs the activity of the more volatile isopentyl acetate, a further compound of the alarm pheromone [20,21]. Emitting (Z)-11-eiconsen-1-ol from a reservoir appears thus to be adaptive for honeybee workers. It is thus a signal that is selected because it increases the fitness of its sender. At the same time, traces of this alcohol spread over the cuticle of honeybee workers causing a permanent smell. Female beewolves exploit this smell as a cue to identify their prey [22,23]. Remarkably, the use of a chemical cue during foraging impinges on the epigamic communication system among beewolf mates. Beewolf males apparently exploit the sensory biases that females exhibit to locate their prey by producing a pheromone to attract females that mainly consist of (Z)-11-eicosen-1-ol [24]. The males produce this compound in a large postpharyngial gland and scent mark small territories on a lek, which are defended against conspecific males [25]. Thus, sensory exploitation of cues used in a different context can trigger the evolution of intraspecific sexual communication to facilitate mate location. Photographs reproduced with permission from Martin Kaltenpoth (male beewolf), Gudrun Herzner (female beewolf) and Claudia Gack (honeybee).
If the reaction of the receiver to the cue is beneficial to the sender, evolution towards signalling, that is increased effectiveness of communication is expected [28]. This is a process that we term ‘chemical ritualization’. While it is long known that cues can evolve into signals, there are two previously neglected aspects of signal evolution. First, the various functions of chemicals represent a cornerstone for understanding under which circumstances signalling evolves. Second, distinct selective pressures can turn a cue into a signal that leads to distinct evolutionary trajectories of communication. Here, we focus on these two cornerstones and show how they affect signal evolution. We explain chemical ritualization among terrestrial plants and insects, and also show how it applies to the excretions of mammals (for species in aquatic environments, e.g. review of [29]). We aim to describe different evolutionary trajectories of refinement, which generate a particular dynamic evolution of communication. We start with describing evolutionary trajectories in the senders before turning to evolution in the sensory system of receivers.
2. From cues to signals—distinct evolutionary trajectories of chemical information transfer
The different functions of non-communicative compounds can lead to a variety of evolutionary trajectories of signal evolution. Functional shifts (exaptations) can include a major switch from unavoidable metabolic by-products to attractants [14,30], minor modification from defence to alarm or a strong functional change from a defensive agent to an attractive substance as has been suggested for floral scents [31,32]. For example, resin excreted by blossom glands in the flowers of Dalechampia vines is collected by pollinating bees for use in nest construction [33]. This resin reward system appears to have originated as a taxonomically widespread defence system in plants, which exude terpenoid resins in response to wounding [9]. Foliar and floral resins are chemically similar; both deter feeding and leaf-cutting. Thus, the most parsimonious explanation is that the resin excretion was secondarily adopted as a reward, when resin-collecting bees began visiting flowers incidentally to steal resins [33].
Exaptations of defensive substances also occur in insects where they have been co-opted to function as sex, aggregation, alarm or trail pheromones (e.g. [34–37]). For example, various eusocial Hymenoptera emit alarm pheromones, sex pheromones and components of trail pheromones from the venom gland, which primarily functions as reservoir for defensive substances [34,38]. This fact illustrates that even chemicals with an originally similar function can undergo different forms of exaptations leading to distinct communicative functions.
The evolutionary route that will be adopted depends on the type of receiver and on the information it can extract. In social insects, for examples, defensive substances are released to repel intruders from a colony. This function is a pre-adaptation towards the evolution of alarm pheromones because nest-mates perceiving the defensive compounds may use them as reliable information about danger. This kind of information is not inherent to floral defensive cues because plants and pollinators rarely share common predators. However, pollinators perceiving defensive floral compounds can use them for localizing the flower. Obviously, defensive substances are not the only starting point for the evolution of communication. Anti-desiccation agents can also be exapted to function as sex pheromones or trail pheromones [39,40]. Functional shifts can even occur across different development stages of the same individual, with larvae emitting certain chemicals for other purposes than adults. Volatile compounds released by grubs of the scarab beetle, Cyclocephala lurida, are retained by females (but not males) as sex pheromones [7].
Another form of exaptation is the amplification of an already existing internal communication system. For example, plants release volatiles as private messages from damaged parts to inform distant parts of the same plants about the imminent risk of attack [41]. The adaptive significance is that the gaseous information transfer is quicker than a systemic response by the plant. This private information system presumably represents the precursor to communication between different plants as well as between plants and the natural enemies of herbivorous insects because the former can use them to locate their prey [42,43]. Given that plants benefit from attracting the enemies of herbivores, it is not astonishing that a wide range of plants release induced chemicals to attract predators of attacking herbivores, thereby potentially reducing the overall number of herbivores [44].
In sum, different forms of exaptations are possible depending on what kind of information the receiver extracts from the emitted chemicals and on what the signaller does to improve the probability that the receiver responds to the signal. The second step is far from trivial and in the following, we will show that there are different ways of signal amplification depending upon the information content, design and the original function of the cue (see also figure 2).
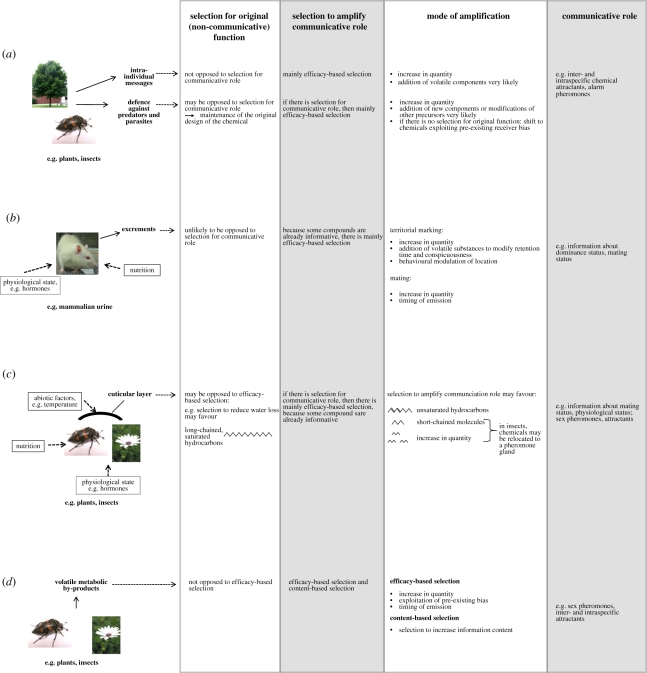
Figure 2.
Precursor models of the evolution of chemical communication. The model is simplified and does not include all, but only the main evolutionary trajectories. (a) Intraplant messages and defense compounds as precursors for attractants or alarm pheromones. (b) Components of excrements as precursors for communication of dominance or mating status. (c) Chemicals of the cuticle act as precursors for the communication of mating status or physiological status or as precursors for sex pheromones or interspecific attractants. (d) Volatile metabolic by-products are precursors for sex pheromones and interspecific attractants. Photographs by SS and Tak Cheung.
(a). Increasing signal quantity
A simple way to enhance the efficacy of chemical communication is to increase the quantity of an emitted cue. In plants, there is good evidence for the underlying assumption that increasing scent production is fitness-relevant. Augmenting odour extracts in the damask violet (Hesperis matronalis) leads to a higher visitation rate of insects [45]. Furthermore, floral scent emission rates in damask violet during the night are positively associated with seed production; probably because more pollinators visit flowers with high scent production. Yet, we should not expect selection to always increase the quantity of emitted chemicals. Riffell et al. [16] showed that the floral blend of Datura wrightii elicited equivalent foraging behaviour in their pollinator over a 1000-fold range of concentration demonstrating that changes in quantity will not attract more pollinators. Comparing these studies elucidates an inherent difficulty in studying the evolution of chemical communication, which is that the behavioural response of receivers can often not be generalized. Differences in the sensory and also the perceptual abilities of receivers may thus partly explain the pronounced diversity of chemical communication systems. Many animals have independently evolved glands enabling them to produce, store and release higher quantities of their pheromones. These glands probably represent a convergent evolutionary response to selection for an increase in the quantity of emitted chemical [38]. However, it has to be noted that not all glands containing pheromones have evolved primarily for communicative functions (e.g. [46]).
An increase in signal quantity can also be informative to others, if the emission of the chemical is predictably linked to the quality of the sender [47]. The moth Utetheisa ornatrix acquires protection by sequestering pyrolizidine alkaloids from host plants. These compounds are toxic to many predators protecting larvae and adults [48]. In this species, female mate choice depends entirely on the quantity of males' courtship pheromone, which is directly derived from the pyrolizidine alkaloids [49]. By choosing males with a higher pheromone titre females gain direct (alkaloids received with the spermatophore) as well as indirect genetic benefits (genes for large size; [50]).
Although enhancing the efficacy of chemical information transfer by an increase in a chemical's quantity can make individuals of some species more vulnerable to eavesdropping (e.g. [51]), we still predict that it is a widespread phenomenon and not restricted to a specific class of chemicals. An important reason why increases in the quantity of emitted volatiles are common is that this mode of amplification does not conflict with other, non-communicative functions of the cue.
(b). Adding signal components
We have argued that inadvertent information provides a starting point for communication to evolve. Often, the substances indicating qualities will be inconspicuous. For example, non-volatile chemicals are often more closely associated with quality aspects of a sender (e.g. sex, age and fertility; e.g. [52–54]) than chemicals with low molecular weight, but they are less conspicuous to receivers. We, therefore, propose that in this situation selection for more effective communication will primarily result in adding signal components to the informative but inconspicuous substances. This is because major modifications of the design of the reliable compounds to make them easier to perceive will probably reduce their reliability. As such, the concomitant emission of conspicuous substances or adding signal components of other modalities can solve the inherent trade-off between the communicative and informative aspect of communication.
The easiest way to enhance the efficacy of chemical information transfer without modifying the molecules is adding behavioural components and modulating the timing or location of emission so that it coincides with the presence of the receiver [55]. Modifying the location is often found in the context of dominance and territorial marking. Wolves, for example, leave some of their faeces in exposed sites. These faeces are characterized by higher sex hormone levels than other faeces indicating that they serve to advertise dominance status [56]. Similarly, mice engage in counter-marking or leave behind a line of chemical flags that indicate the boundaries of their territory [57]. Females show behavioural adaptations urinating most during the period of oestrous to advertise their reproductive status, and some species even engage in elaborate ‘olfactory displays’ during which they spray scents at potential partners [58]. In most cases, the excrements are cues, but the behavioural modulations where and when the excretions are released constitute signals helping to draw receivers' attention to the cues.
A prediction derived from our hypothesis on adding signal components is that organisms emit blends of signals/cues, which comprise volatile components that attract attention and less volatile components that indicate quality. Urine is again a good example. Quality aspects are often indicated by hormones, non-volatile proteins or peptides present in the urine, whereas volatiles, such as methylthiomethanethiol in mice, enhance the probability that the urine of the male will be detected and investigated by females [52,59]. Hence, quality-indicating chemicals are thus often cues, whereas the volatiles that amplify their conspicuousness are frequently signals. This conclusion is not trivial given that the selection on communication is traditionally assumed to enhance the information content, whereas we propose that selection is more likely to enhance the detectability of information transfer.
Another important corollary is that receivers can exert diversifying selection leading to the evolution of diverse volatile substances within chemical blends. This diversifying selection can be seen in burying beetles, which produce on the one hand, long-chained cuticular hydrocarbons that are used by conspecifics to identify sex and reproductive state [54,60], and on the other hand an ester with lower molecular weight and consequently higher volatility to attract mates from afar [61].
The maintenance of the original cue and the concomitant emission of conspicuous substances might not only be favoured when there is an inherent trade-off between the communicative and the informative aspect of communication, but also when there is an inherent trade-off between the communicative and the original non-communicative function of a cue. For example, formic acid is used by some ant species in direct defence against enemies but acts at the same time as an alarm pheromone [34]. Modification towards improved communication would presumably result in a decrease in the effectiveness of formic acid as a defence. Instead of modifying the molecule, ants have added signal components to enhance or prolong their efficiency as alarm substance [62].
In sum, selection upon communication can favour the maintenance of the original design of the cue, but enhances its detectability by adding chemical signal components or behavioural components. This evolutionary trajectory explains at least partly some of the observed diversity of chemical substances involved in communication.
(c). Modifying the design of the cue
A different starting point for communication to evolve occurs, if receivers select cues primarily to locate the sender without any further informative aspect. If a sender benefits from attracting a receiver, efficacy-based selection can lead to a modification of the original cue to a very conspicuous signal, irrespective of its content. In other words, selection on communication can lead to a shift of the original blend towards the most conspicuous molecules or towards the most effective combination of chemicals. Such signals are expected to possess low molecular weights and other chemical and physical properties that improve dispersion in atmospheric plumes. We would also expect that they are very conspicuous against background noise that will be influenced by wind, humidity and the surrounding vegetation [4].
A slightly different trajectory occurs if cues evolve into signals so that they match pre-existing biases of the sensory system of desired recipients [63]. Examples of sensory exploitation in flowers include the mimicry of carrion, fungi or faecal odours in Araceae, Aristolociaceae and Apocynaceae to exploit female insects as pollinators that oviposit in decaying matter [64]. Similarly, Ophrys orchids attract their pollinators by mimicking the sex pheromones of female Hymenoptera [65]. Interestingly, Ophrys exaltata are imperfect mimics that emit chemicals whose relative proportions differ from the pheromone of females. Imperfect mimicry does not reduce the attractiveness of the flowers. On the contrary, males prefer flower bouquets to the sexual pheromone of local females, presumably because O. exaltata exploits pre-existing sensory biases of their pollinators [66,67]. We predict that perceptual exploitation is more commonly found in chemical communication than hitherto known, particularly in species that rely on chemical signals to attract mates or mutualists from a long distance.
Reconstructing the evolution of olfactory conspicuous signals can be challenging because it is difficult to ascertain if the starting point was a cue which became ritualized during evolution or if it was employed in sensory exploitation. Schiestl [32] proposed that the chemical similarity of floral and insect aromatics is explicable by plants exploiting pre-existing biases of their pollinators that have been formed in the context of their intraspecific communication. However, this hypothesis should be tested against the alternative one that the protective functions of chemicals provide a common starting point for the evolution of communication in both plants and insects, since a number of aromatics are known for their antibactericidal, antifungal and nematocidal properties (e.g. [68–70]). This hypothesis is also supported by the behavioural active substances in Ophrys that mimic the sex pheromone of their pollinators. These substances are cuticular hydrocarbons that primarily function as a protective barrier against desiccation and micro-organisms; both are selection factors that were probably important in the evolution of plants and insects. Hence, we suggest that the starting point for communication was very likely a blend of protective chemicals (and as such a cue), which was shaped in a co-evolutionary process to meet pre-existing receiver properties.
3. Multi-functionality of chemicals and its implication for the evolution of communication
We have argued that chemicals released for non-communicative functions can be the precursor for the evolution of more complex communication. Yet, selection for non-communicative functions can constrain and even oppose selection for communication. Flowers, for example, have to protect themselves against nectar thieves and florivores, but also have to attract visitors at the same time. Faced by these opposing selection factors, the native tobacco Nicotiana attenuata produces nicotine as a repellent and benzyl acetone as an attractant, and the synergistic effect of both substances is required to maximize fitness [71]. The evolution of complex blends thus constitutes an adaptive response to accommodate the opposing selective pressure of communication on one hand and those of protection against biotic and abiotic factors on the other hand.
Protection against abiotic factors is a particularly widespread constraint on the evolution of communication through the cuticle. Volatiles with a low molecular weight have a wider detection range than those with high molecular weight and—all else being equal—are more likely to evolve into signals. However, water loss through the cuticular layer of insects is apparently negatively correlated with carbon-chain length, but positively with hydrocarbon unsaturation and methyl branching [12]. Hence, protection against desiccation and also against micro-organisms can impose constraints on the likelihood that chain length and the number of unsaturated and methyl-branched hydrocarbons change towards more efficient communication. In plants, the relative importance of distinct selection pressures is visible by comparing the cuticular patterns across plant organs. In the early spider orchid Ophrys sphegodes, leaves and flowers share similar cuticular chemicals, but in different proportions. Ophrys flowers attract pollinators by their scent, whereas the cuticle of leaves is presumably selected mainly to prevent water loss. The higher proportion of the more volatile unsaturated hydrocarbons in flowers compared with leaves may pinpoint that these function in pollinator attraction [72]. Yet, the evaluation of the relationship between abiotic constraints and communicational function of a chemical or chemical blend is still an under-represented research area and we suggest it would be very fruitful for future studies to conduct selection experiments to reveal the relative contribution of each of the functions.
In nature, a combination of distinct selection pressures on chemical blends can result in a spatially and temporarily variable selection mosaic that can, for example, depend on local temperatures [73,74]. The scenario of a selection mosaic is supported by studies documenting geographical variability in scent patterns [75]. Local environmental adaptation of chemical compounds involved in mate recognition may also result incidentally in divergence in mating preferences among populations and therefore contribute to a rapid speciation rate [76]. Therefore, an important research avenue will be to unravel the factors responsible for population-specific patterns in chemical blends. Owing to the multiple functions of chemical blends and the probable spatial variation in the selective pressures associated with them, we propose that they represent an ideal study system to investigate the geographical mosaic of coevolution [77] where populations differ in their evolutionary response to intraspecific or interspecific interactions.
4. The dynamic evolution of chemical communication and receiver aspects
Apart from selection for species specificity in substances that are used in species recognition (see review of [11]), we have identified three additional causes for the pronounced diversity of chemical communication. First, waste-products and chemical compounds that serve non-communicative functions provide distinct, multiple origins for the evolution of intentional communication. Second, chemical communication can diversify from each starting point depending on the respective receiver and the information the receiver extracts from the chemicals. Third, several distinct trajectories of efficacy-based selection are possible that either increases or modifies an original component or add new components to it.
Reconstructing the evolution of chemical communication is thus a difficult task, even more so because few chemical compounds have been analysed in a phylogenetic context [78–80]. Phylogenetic analyses have revealed that sibling species can use strikingly different pheromones that can be produced by distinct enzymes of a given pathway and therefore require only simple genetic changes [11]. Reconstruction of the phylogeny should be applied to the full spectrum of chemicals emitted by a species and not only to pheromones that trigger a behavioural response. If the entire blend emitted by an organism is taken into consideration, a more complete picture emerges that traces how by-products of more ancestral species can evolve into signals in derived species. Because extrinsic factors like nutrition can have further effects on chemical blends [81], the relevance of ecological factors can thus be compared throughout phylogenetic history. We predict that expanding phylogenetic analyses to account for behaviourally inactive substances will greatly improve our picture on the evolutionary trajectory of chemical signals in general.
Another crucial factor to improve our understanding of the evolution of chemical communication is the incorporation and expansion of our current knowledge of olfactory systems. Recent progress on olfactory systems already shed light on two of the main questions associated with the evolution from cues to signals: (i) have organism the ability to detect novel cues and if so, (ii) are there certain cues that are more likely to be detected than others and therefore more likely to provide the starting point for signal evolution?
Several olfactory receptor neurons respond to small differences in chain length, functional group, double bond position or even stereoisomers [82]. If odorant receptors (OR) are so finely tuned, how should organisms be able to detect novel cues and use their information, the prerequisite for signal evolution via chemical ritualization?
First, it has to be recognized that an animal's olfactory system mediates a wide range of behaviours, such as feeding, mating with suitable partners, escaping predators or interacting with kin. Therefore, it is not astonishing that insects and vertebrates are equipped with a repertoire of ORs. Most vertebrate species possess between 600 and 1300 OR genes [83]. The insect OR repertoire is smaller, consisting of 62 OR genes in Drosphila melanogaster [84], 79 in Anopheles gambiae [85], and 162 in Apis mellifera [86]. In addition, not all ORs are narrowly tuned. In mammals, the so-called ‘generalists’ can be found, which have a broad receptive range (see [83]), but also in insects, studies have demonstrated that most receptors are sensitive to more than one substance [87–89]. Overall, we can say that insects as well as mammals are sensitive to a broad range of chemicals of different structures and different classes [87,90] and although the overall number of chemical substances that can be detected is unknown, rough estimates suggest that rodents can detect several thousands or even tens of thousands of odorant molecules [83].
Second, the precursor model that we suggest entails that even if the current OR repertoire of an organism is not adequate to detect a specific chemical cue, evolution will not directly lead to the loss of that chemical in the sender, if the chemicals fulfil other functions. These functions increase the longevity of a substance in evolutionary times and thereby increase the likelihood that mutations occur in a population of receivers, which enable individuals to detect the previously imperceptible cue. Recent studies provide us with an idea how receptor repertoires may expand and evolve (see [91] for references). A prevalent mechanism appears to be tandem gene duplication with a subsequent functional divergence of the duplicated receptors, which can create novel pheromone preferences in receivers [92].
A comparative study of the OR repertoire, and how the chemical world is reflected by it, suggests that one-sided evolution can operate on the olfactory system leading to an OR repertoire that corresponds to the ecology of a species [90]. Fruitflies and mosquitoes respond both to aromatics and esters, but the percentage of odorant–receptor combinations that generate strong responses is higher for aromatics in A. gambiae and higher for esters in D. melanogaster. Whereas some aromatics are major components in human emanations, A. gambiae's hosts, esters are often generated by fermentation processes and may therefore be used by D. melanogaster to find its food source. Likewise, phytophagous insects, but not Drosphila, have a large variety of ORs to detect and discriminate among terpenoids—a major chemical class emitted by plants [82,90].
Overall, olfactory systems appear to be evolutionarily flexible enough to adjust to important environmental chemical cues, therefore providing a first step towards signal evolution. The OR repertoire might be either already capable of detecting a newly evolved chemical, leading to a subsequent refinement of the receptor or increased investment to detect the specific cue or the olfactory system might be sufficiently plastic for a novel suitable receptor to emerge over time. Although we cannot easily predict which novel cue might be detected and subsequently modified into a signal, the likelihood for a chemical of a certain structure or class will depend strongly on the current OR repertoire of a species and how it has been shaped by ecological factors. In addition, as the response profiles of many OR are broader at high ligand concentrations [83], the probability may increase with the emission rate of a chemical.
5. Evolution of chemical communication versus other signalling channels
We have highlighted in our review that chemical information can emerge as an incidental by-product, feasibly long before any suitable receptors exist. This scenario differs from recent concepts about the evolution of acoustic communication [2]. The release of calls, songs and other forms of sounds often has no other functions than communication itself (although they might be produced as by-product of breathing or movements). Fittingly, recent evolutionary concepts of acoustic signalling emphasize the role of sensory exploitation, pre-existing receiver bias and receiver psychology [2], and the authors suggest a paradigm shift in communication theory. They advocate abandoning information-based approaches in favour of manipulation-based approaches. While this shift is reasonable, it fails partly to capture the evolution of olfactory communication. Although the evolutionary step from chemical cue to signal is indeed first and foremost a matter of how cues are enhanced to better influencing others, the starting point can be—as we have highlighted—chemical information. We therefore emphasize that inadvertent information is important in the evolution of chemical communication. Given that pleiotropic effects are also apparent in pigments such as anthocyanins and melanins [93,94], inadvertent information is probably also more widespread than currently acknowledged in the evolution of visual signals.
Chemical communication differs from communication in other sensory modes also in a number of other important ways. Chemical compounds are—like pigments—discrete. Unlike pigments they do not combine to form a continuous, physical trait such as reflectance that is later combined by the neuronal system of the receiver into colour categories. From the limited evidence available [95], it seems as though the discrete chemical compounds that make up blends are perceived separately according to chain length and functional group (e.g. alcohols, ketones, alkanes and aldehydes). Given the extraordinary quantity and variability among odour receptors, there is a higher probability than in other sensory modes that compounds evolve that match the sensitivities of the intended receivers but not those of unintended receivers such as predators (private communication channels). Likewise, the discrete nature of chemical blends coupled with the multiple selective pressures acting upon them leads to the evolution of new signal components that reflect an adaptive balance between communication and non-communicative functions. As such efficacy-based selection is particularly likely to result in the evolution of new signals components leading to the extraordinary diversity that characterizes chemical communication.
6. Conclusions
We propose a dynamic framework on the evolution of chemical communication that places more emphasis than has traditionally been done on the origin of communication. More specifically, we emphasize that organisms, from plants to mammals, release waste-products and other chemicals that incidentally carry information. We have argued that such products provide multiple starting points for the evolution of chemical communication thereby explaining the prevalence of this mode of communication and its diversity. Cues are often precursors of signals that evolve via ‘chemical ritualization’. Since information is often present before there is any communicative intent, chemical signal evolution is predominantly driven by efficacy-based selection. Importantly, we can predict how cues are refined to enhance the efficacy of communication. Apart from understanding the distinct forms of amplification in the evolution of signals, accounting for the multi-functionality of chemical blends and the multiple selective pressures acting on them will help to unravel the constraints acting on the evolution of chemical communication.
Acknowledgements
We thank Scott Sakaluk, Tristam Wyatt and three anonymous referees for helpful comments on the manuscript. S.S. was supported by a Feodor Lynen Fellowship provided by the Alexander von Humboldt Foundation; T.S. and H.M.S. were supported by DFG grants (Schm 2645/1-1, Scha 1008/5-1, respectively).
References
- 1.Dawkins R., Krebs J. R. 1978. Animal signals: information and manipulation. In Behavioural ecology: an evolutionary approach. (eds Krebs J. R., Davies N. B.), pp. 282–309 Oxford, UK: Blackwell Scientific [Google Scholar]
- 2.Rendall D., Owren M. J., Ryan M. J. 2009. What do animal signals mean? Anim. Behav. 78, 233–240 10.1016/j.anbehav.2009.06.007 (doi:10.1016/j.anbehav.2009.06.007) [DOI] [Google Scholar]
- 3.Scott-Phillips T. C. 2010. Animal communication: insights from linguistic pragmatics. Anim. Behav. 79, E1–E4 10.1016/j.anbehav.2009.10.013 (doi:10.1016/j.anbehav.2009.10.013) [DOI] [Google Scholar]
- 4.Wyatt T. 2003. Pheromones and animal behaviour: communication by smell and taste. Cambridge, UK: University Press [Google Scholar]
- 5.Tinbergen N. 1952. ‘Derived’ activities; their causation, biological significance, origin, and emancipation during evolution. Q. Rev. Biol. 27, 1–32 10.1086/398642 (doi:10.1086/398642) [DOI] [PubMed] [Google Scholar]
- 6.Bradbury J., Vehrencamp S. 1998. Principles of animal communication. Sunderland, MA: Sinauer Associates [Google Scholar]
- 7.Haynes K. F., Potter D. A. 1995. Sexual response of a male scarab beetle to larvae suggests a novel evolutionary origin for a pheromone. Am. Entomol. 41, 169–175 [Google Scholar]
- 8.Otte D. 1974. Effects and functions in the evolution of signaling systems. Annu. Rev. Ecol. Syst. 5, 385–417 10.1146/annurev.es.05.110174.002125 (doi:10.1146/annurev.es.05.110174.002125) [DOI] [Google Scholar]
- 9.Theis N., Lerdau M. 2003. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 164, S93–S102 10.1086/374190 (doi:10.1086/374190) [DOI] [Google Scholar]
- 10.Smadja C., Butlin R. K. 2009. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102, 77–97 10.1038/hdy.2008.55 (doi:10.1038/hdy.2008.55) [DOI] [PubMed] [Google Scholar]
- 11.Symonds M. R. E., Elgar M. A. 2008. The evolution of pheromone diversity. Trends Ecol. Evol. 23, 220–228 10.1016/j.tree.2007.11.009 (doi:10.1016/j.tree.2007.11.009) [DOI] [PubMed] [Google Scholar]
- 12.Gibbs A. G. 1998. Water-proofing properties of cuticular lipids. Am. Zool. 38, 471–482 [Google Scholar]
- 13.Herzner G., Strohm E. 2007. Fighting fungi with physics: food wrapping by a solitary wasp prevents water condensation. Curr. Biol. 17, R46–R47 10.1016/j.cub.2006.11.060 (doi:10.1016/j.cub.2006.11.060) [DOI] [PubMed] [Google Scholar]
- 14.Rosenstiel T. N., Ebbets A. L., Khatri W. C., Fall R., Monson R. K. 2004. Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biol. 6, 12–21 10.1055/s-2003-44722 (doi:10.1055/s-2003-44722) [DOI] [PubMed] [Google Scholar]
- 15.Rostas M., Blassmann K. 2009. Insects had it first: surfactants as a defence against predators. Proc. R. Soc. B 276, 633–638 10.1098/rspb.2008.1281 (doi:10.1098/rspb.2008.1281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riffell J. A., Lei H., Christensen T. A., Hildebrand J. G. 2009. Characterization and coding of behaviorally significant odor mixtures. Curr. Biol. 19, 335–340 10.1016/j.cub.2009.01.041 (doi:10.1016/j.cub.2009.01.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith A. A., Hölldober B., Liebig J. 2009. Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social Insect. Curr. Biol. 19, 78–81 10.1016/j.cub.2008.11.059 (doi:10.1016/j.cub.2008.11.059) [DOI] [PubMed] [Google Scholar]
- 18.Greene M. J., Gordon D. M. 2003. Social insects — cuticular hydrocarbons inform task decisions. Nature 423, 32–32 10.1038/423032a (doi:10.1038/423032a) [DOI] [PubMed] [Google Scholar]
- 19.Wagner D., Tissot M., Gordon D. 2001. Task-related environment alters the cuticular hydrocarbon composition of harvester ants. J. Chem. Ecol. 27, 1805–1819 10.1023/A:1010408725464 (doi:10.1023/A:1010408725464) [DOI] [PubMed] [Google Scholar]
- 20.Free J., Williams I., Pickett J., Ferguson A., Martin A. 1982. Attractiveness of (Z)-11-Eicosen-1-ol to foraging honeybees. J. Apic. Res. 21, 151–156 [Google Scholar]
- 21.Pickett J., Williams I., Martin A. 1982. (Z)-11-Eicosen-1-ol, an important new pheromonal component from the sting of the honey bee, Apis mellifera L. (Hymenoptera, Apidae). J. Chem. Ecol. 8, 163–175 10.1007/BF00984013 (doi:10.1007/BF00984013) [DOI] [PubMed] [Google Scholar]
- 22.Herzner G., Schmitt T., Linsenmair K. E., Strohm E. 2005. Prey recognition by females of the European beewolf and its potential for a sensory trap. Anim. Behav. 70, 1411–1418 10.1016/j.anbehav.2005.03.032 (doi:10.1016/j.anbehav.2005.03.032) [DOI] [Google Scholar]
- 23.Schmitt T., Herzner G., Weckerle B., Schreier P., Strohm E. 2007. Volatiles of foraging honeybees Apis mellifera (Hymenoptera: Apidae) and their potential role as semiochemicals. Apidologie 38, 164–170 10.1051/apido:2006067 (doi:10.1051/apido:2006067) [DOI] [Google Scholar]
- 24.Schmitt T., Strohm E., Herzner G., Bicchi C., Krammer G., Heckel F., Schreier P. 2003. (S)-2,3-dihydrofarnesoic acid, a new component in cephalic glands of male European beewolves Philanthus triangulum. J. Chem. Ecol. 29, 2469–2479 10.1023/A:1026305901049 (doi:10.1023/A:1026305901049) [DOI] [PubMed] [Google Scholar]
- 25.Kroiss J., Schmitt T., Schreier P., Strohm E., Herzner G. 2006. A selfish function of a ‘social’ gland? A postpharyngeal gland functions as a sex pheromone reservoir in males of the solitary wasp Philanthus triangulum. J. Chem. Ecol. 32, 2763–2776 10.1007/s10886-006-9198-5 (doi:10.1007/s10886-006-9198-5) [DOI] [PubMed] [Google Scholar]
- 26.Saleh N., Scott A. G., Bryning G. P., Chittka L. 2007. Distinguishing signals and cues: bumblebees use general footprints to generate adaptive behaviour at flowers and nest. Arthropod Plant Interact. 1, 119–127 10.1007/s11829-007-9011-6 (doi:10.1007/s11829-007-9011-6) [DOI] [Google Scholar]
- 27.Wilms J., Eltz T. 2008. Foraging scent marks of bumblebees: footprint cues rather than pheromone signals. Naturwissenschaften 95, 149–153 10.1007/s00114-007-0298-z (doi:10.1007/s00114-007-0298-z) [DOI] [PubMed] [Google Scholar]
- 28.Wyatt T. D. 2010. Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J. Comp. Physiol. A 196, 685–700 10.1007/s00359-010-0564-y (doi:10.1007/s00359-010-0564-y) [DOI] [PubMed] [Google Scholar]
- 29.Hay M. E. 2009. Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Annu. Rev. Mar. Sci. 1, 193–212 10.1146/annurev.marine.010908.163708 (doi:10.1146/annurev.marine.010908.163708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penuelas J., Llusia J. 2004. Plant VOC emissions: making use of the unavoidable. Trends Ecol. Evol. 19, 402–404 10.1016/j.tree.2004.06.002 (doi:10.1016/j.tree.2004.06.002) [DOI] [PubMed] [Google Scholar]
- 31.Raguso R. A. 2009. Floral scent in a whole-plant context: moving beyond pollinator attraction. Funct. Ecol. 23, 837–840 10.1111/j.1365-2435.2009.01643.x (doi:10.1111/j.1365-2435.2009.01643.x) [DOI] [Google Scholar]
- 32.Schiestl F. P. 2010. The evolution of floral scent and insect chemical communication. Ecol. Lett. 13, 643–656 10.1111/j.1461-0248.2010.01451.x (doi:10.1111/j.1461-0248.2010.01451.x) [DOI] [PubMed] [Google Scholar]
- 33.Armbruster W. S., Howard J. J., Clausen T. P., Debevec E. M., Loquvam J. C., Matsuki M., Cerendolo B., Andel F. 1997. Do biochemical exaptations link evolution of plant defense and pollination systems? Historical hypotheses and experimental tests with Dalechampia vines. Am. Nat. 149, 461–484 10.1086/286000 (doi:10.1086/286000) [DOI] [Google Scholar]
- 34.Blum M. S. 1996. Semiochemical parsimony in the Arthropoda. Annu. Rev. Entomol. 41, 353–374 10.1146/annurev.en.41.010196.002033 (doi:10.1146/annurev.en.41.010196.002033) [DOI] [PubMed] [Google Scholar]
- 35.Geiselhardt S., Schmitt T., Peschke K. 2009. Chemical composition and pheromonal function of the defensive secretions in the subtribe Stizopina (Coleptera, Tenebrionidae, Opatrini). Chemoecology 19, 1–6 10.1007/s00049-008-0001-7 (doi:10.1007/s00049-008-0001-7) [DOI] [Google Scholar]
- 36.Löfstedt C., Kozlov M. 1997. A phylogenetic analysis of pheromone communication in primitive moths. In Insect pheromone research: new directions (eds Cardé R. T., Minks A. K.). New York, NY: Chapman & Hall [Google Scholar]
- 37.Ruther J., Reinecke A., Tolasch T., Hilker M. 2001. Make love not war: a common arthropod defence compound as sex pheromone in the forest cockchafer Melolontha hippocastani. Oecologia 128, 44–47 10.1007/s004420100634 (doi:10.1007/s004420100634) [DOI] [PubMed] [Google Scholar]
- 38.Greenfield M. D. 2002. Signalers and receivers—mechanisms and evolution of arthropod communication. New York, NY: Oxford University Press [Google Scholar]
- 39.Howard R. W., Blomquist G. J. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393 10.1146/annurev.ento.50.071803.130359 (doi:10.1146/annurev.ento.50.071803.130359) [DOI] [PubMed] [Google Scholar]
- 40.Steinmetz I., Schmolz E., Ruther J. 2003. Cuticular lipids as trail pheromone in a social wasp. Proc. R. Soc. Lond. B 270, 385–391 10.1098/rspb.2002.2256 (doi:10.1098/rspb.2002.2256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heil M., Silva Bueno J. C. 2007. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl Acad. Sci. USA 104, 5467–5472 10.1073/pnas.0610266104 (doi:10.1073/pnas.0610266104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Moraes C. M., Lewis W. J., Pare P. W., Alborn H. T., Tumlinson J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393, 570–573 10.1038/31219 (doi:10.1038/31219) [DOI] [Google Scholar]
- 43.Turlings T. C. J., Tumlinson J. H., Lewis W. J. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250, 1251–1253 10.1126/science.250.4985.1251 (doi:10.1126/science.250.4985.1251) [DOI] [PubMed] [Google Scholar]
- 44.Kessler A., Baldwin I. T. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144 10.1126/science.291.5511.2141 (doi:10.1126/science.291.5511.2141) [DOI] [PubMed] [Google Scholar]
- 45.Majetic C. J., Raguso R. A., Ashman T. L. 2009. The sweet smell of success: floral scent affects pollinator attraction and seed fitness in Hesperis matronalis. Funct. Ecol. 23, 480–487 10.1111/j.1365-2435.2008.01517.x (doi:10.1111/j.1365-2435.2008.01517.x) [DOI] [Google Scholar]
- 46.Herzner G., Schmitt T., Peschke K., Hilpert A., Strohm E. 2007. Food wrapping with the postpharyngeal gland secretion by females of the European beewolf Philanthus triangulum. J. Chem. Ecol. 33, 849–859 10.1007/s10886-007-9263-8 (doi:10.1007/s10886-007-9263-8) [DOI] [PubMed] [Google Scholar]
- 47.Ruther J., Matschke M., Garbe L. A., Steiner S. 2009. Quantity matters: male sex pheromone signals mate quality in the parasitic wasp Nasonia vitripennis. Proc. R. Soc. B 276, 3303–3310 10.1098/rspb.2009.0738 (doi:10.1098/rspb.2009.0738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisner T., Meinwald J. 2003. Alkaloid-derived pheromone and sexual selection in Lepidoptera. In Pheromone biochemistry (eds Prestwich G. D., Blomquist G. J.), pp. 251–269 Orlando, FL: Academic [Google Scholar]
- 49.Iyengar V. K., Rossini C., Eisner T. 2001. Precopulatory assessment of male quality in an arctiid moth (Utetheisa ornatrix): hydroxydanaidal is the only criterion of choice. Behav. Ecol. Sociobiol. 49, 283–288 10.1007/s002650000292 (doi:10.1007/s002650000292) [DOI] [Google Scholar]
- 50.Iyengar V. K., Eisner T. 1999. Female choice increases offspring fitness in an arctiid moth (Utetheisa ornatrix). Proc. Natl Acad. Sci. USA 96, 15 013–15 016 10.1073/pnas.96.26.15013 (doi:10.1073/pnas.96.26.15013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halitschke R., Stenberg J. A., Kessler D., Kessler A., Baldwin I. T. 2008. Shared signals—‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 11, 24–34 [DOI] [PubMed] [Google Scholar]
- 52.Brennan P. A., Zufall F. 2006. Pheromonal communication in vertebrates. Nature 444, 308–315 10.1038/nature05404 (doi:10.1038/nature05404) [DOI] [PubMed] [Google Scholar]
- 53.Holman L., Jorgensen C. G., Nielsen J., d'Ettorre P. 2010. Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. B. 277, 3793–3800 10.1098/rspb.2010.0984 (doi:10.1098/rspb.2010.0984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steiger S., Peschke K., Francke W., Müller J. K. 2007. The smell of parents: breeding status influences cuticular hydrocarbon pattern in the burying beetle Nicrophorus vespilloides. Proc. R. Soc. B 274, 2211–2220 10.1098/rspb.2007.0656 (doi:10.1098/rspb.2007.0656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Appelt C. W., Sorensen P. W. 2007. Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim. Behav. 74, 1329–1338 10.1016/j.anbehav.2007.02.032 (doi:10.1016/j.anbehav.2007.02.032) [DOI] [Google Scholar]
- 56.Barja I., Silvan G., Illera J. C. 2008. Relationships between sex and stress hormone levels in feces and marking behavior in a wild population of Iberian wolves (Canis lupus signatus). J. Chem. Ecol. 34, 697–701 10.1007/s10886-008-9460-0 (doi:10.1007/s10886-008-9460-0) [DOI] [PubMed] [Google Scholar]
- 57.Rich T. J., Hurst J. L. 1999. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Anim. Behav. 58, 1027–1037 10.1006/anbe.1999.1217 (doi:10.1006/anbe.1999.1217) [DOI] [PubMed] [Google Scholar]
- 58.Johnston R. E. 1983. Chemical signals and reproductive behavior. In Pheromones and reproduction in mammals (ed. Vandenbergh J. G.). New York, NY: Academic Press [Google Scholar]
- 59.Lin D. Y., Zhang S. Z., Block E., Katz L. C. 2005. Encoding social signals in the mouse main olfactory bulb. Nature 434, 470–477 10.1038/nature03414 (doi:10.1038/nature03414) [DOI] [PubMed] [Google Scholar]
- 60.Steiger S., Whitlow S., Peschke K., Müller J. K. 2009. Surface chemicals inform about sex and breeding status in the biparental burying beetle Nicrophorus vespilloides. Ethology 115, 178–185 10.1111/j.1439-0310.2008.01600.x (doi:10.1111/j.1439-0310.2008.01600.x) [DOI] [Google Scholar]
- 61.Haberer W., Schmitt T., Peschke K., Schreier P., Müller J. K. 2008. Ethyl 4-methyl heptanoate: a male-produced pheromone of Nicrophorus vespilloides. J. Chem. Ecol. 34, 94–98 10.1007/s10886-007-9406-y (doi:10.1007/s10886-007-9406-y) [DOI] [PubMed] [Google Scholar]
- 62.Löfqvist J. 1976. Formic acid and saturated hydrocarbons as alarm pheromones for the ant Formica rufa. J. Insect Physiol. 22, 1331–1346 10.1016/0022-1910(76)90155-4 (doi:10.1016/0022-1910(76)90155-4) [DOI] [Google Scholar]
- 63.Endler J. A., Basolo A. L. 1998. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 13, 415–420 10.1016/S0169-5347(98)01471-2 (doi:10.1016/S0169-5347(98)01471-2) [DOI] [PubMed] [Google Scholar]
- 64.Jürgens A., Dötterl S., Meve U. 2006. The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae). New Phytol. 172, 452–468 10.1111/j.1469-8137.2006.01845.x (doi:10.1111/j.1469-8137.2006.01845.x) [DOI] [PubMed] [Google Scholar]
- 65.Vereecken N. J., Schiestl F. P. 2008. The evolution of imperfect floral mimicry. Proc. Natl Acad. Sci. USA 105, 7484–7488 10.1073/pnas.0800194105 (doi:10.1073/pnas.0800194105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raguso R. A. 2008. Wake up and smell the roses: the ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 39, 549–569 10.1146/annurev.ecolsys.38.091206.095601 (doi:10.1146/annurev.ecolsys.38.091206.095601) [DOI] [Google Scholar]
- 67.Schaefer H. M., Ruxton G. D. 2009. Deception in plants: mimicry or perceptual exploitation? Trends Ecol. Evol. 24, 676–685 10.1016/j.tree.2009.06.006 (doi:10.1016/j.tree.2009.06.006) [DOI] [PubMed] [Google Scholar]
- 68.Kai M., Effmert U., Berg G., Piechulla B. 2007. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 187, 351–360 10.1007/s00203-006-0199-0 (doi:10.1007/s00203-006-0199-0) [DOI] [PubMed] [Google Scholar]
- 69.Kim J., Seo S. M., Lee S. G., Shin S. C., Park I. K. 2008. Nematicidal activity of plant essential oils and components from coriander (Coriandrum sativum), oriental sweetgum (Liquidambar orientalis), and valerian (Valeriana wallichii) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 56, 7316–7320 10.1021/jf800780f (doi:10.1021/jf800780f) [DOI] [PubMed] [Google Scholar]
- 70.Lucchini J. J., Corre J., Cremieux A. 1990. Antibacterial activity of phenolic-compounds and aromatic alcohols. Res. Microbiol. 141, 499–510 10.1016/0923-2508(90)90075-2 (doi:10.1016/0923-2508(90)90075-2) [DOI] [PubMed] [Google Scholar]
- 71.Kessler D., Gase K., Baldwin I. T. 2008. Field experiments with transformed plants reveal the sense of floral scents. Science 321, 1200–1202 10.1126/science.1160072 (doi:10.1126/science.1160072) [DOI] [PubMed] [Google Scholar]
- 72.Schiestl F. P., Ayasse M., Paulus H. F., Lofstedt C., Hansson B. S., Ibarra F., Francke W. 2000. Sex pheromone mimicry in the early spider orchid (Ophrys sphegodes): patterns of hydrocarbons as the key mechanism for pollination by sexual deception. J. Comp. Physiol. A 186, 567–574 10.1007/s003590000112 (doi:10.1007/s003590000112) [DOI] [PubMed] [Google Scholar]
- 73.Rouault J., Capy P., Jallon J. M. 2000. Variations of male cuticular hydrocarbons with geoclimatic variables: an adaptative mechanism in Drosophila melanogaster? Genetica 110, 117–130 10.1023/A:1017987220814 (doi:10.1023/A:1017987220814) [DOI] [PubMed] [Google Scholar]
- 74.Rouault J. D., Marican C., Wicker-Thomas C., Jallon J. M. 2004. Relations between cuticular hydrocarbon (HC) polymorphism, resistance against desiccation and breeding temperature; a model for HC evolution in D. melanogaster and D. simulans. Genetica 120, 195–212 10.1023/B:GENE.0000017641.75820.49 (doi:10.1023/B:GENE.0000017641.75820.49) [DOI] [PubMed] [Google Scholar]
- 75.Tregenza T., Buckley S. H., Pritchard V. L., Butlin R. K. 2000. Inter- and intrapopulation effects of sex and age on epicuticular composition of meadow grasshopper, Chorthippus parallelus. J. Chem. Ecol. 26, 257–278 10.1023/A:1005457931869 (doi:10.1023/A:1005457931869) [DOI] [Google Scholar]
- 76.Mullen S. P., Mendelson T. C., Schal C., Shaw K. L. 2007. Rapid evolution of cuticular hydrocarbons in a species radiation of acoustically diverse Hawaiian crickets (Gryllidae: Trigonidiinae: Laupala). Evolution 61, 223–231 10.1111/j.1558-5646.2007.00019.x (doi:10.1111/j.1558-5646.2007.00019.x) [DOI] [PubMed] [Google Scholar]
- 77.Thompson J. N. 2005. Coevolution: the geographic mosaic of coevolutionary arms races. Curr. Biol. 15, R992–R994 10.1016/j.cub.2005.11.046 (doi:10.1016/j.cub.2005.11.046) [DOI] [PubMed] [Google Scholar]
- 78.Symonds M. R. E., Elgar M. A. 2004. The mode of pheromone evolution: evidence from bark beetles. Proc. R. Soc. Lond. B 271, 839–846 10.1098/rspb.2003.2647 (doi:10.1098/rspb.2003.2647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Symonds M. R. E., Moussalli A., Elgar M. A. 2009. The evolution of sex pheromones in an ecologically diverse genus of flies. Biol. J. Linnean Soc. 97, 594–603 10.1111/j.1095-8312.2009.01245.x (doi:10.1111/j.1095-8312.2009.01245.x) [DOI] [Google Scholar]
- 80.Symonds M. R. E., Wertheim B. 2005. The mode of evolution of aggregation pheromones in Drosophila species. J. Evol. Biol. 18, 1253–1263 10.1111/j.1420-9101.2005.00971.x (doi:10.1111/j.1420-9101.2005.00971.x) [DOI] [PubMed] [Google Scholar]
- 81.Liang D., Silverman J. 2000. ‘You are what you eat’: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87, 412–416 10.1007/s001140050752 (doi:10.1007/s001140050752) [DOI] [PubMed] [Google Scholar]
- 82.De Bruyne M., Baker T. C. 2008. Odor detection in insects: volatile codes. J. Chem. Ecol. 34, 882–897 10.1007/s10886-008-9485-4 (doi:10.1007/s10886-008-9485-4) [DOI] [PubMed] [Google Scholar]
- 83.Kaupp U. B. 2010. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat. Rev. Neurosci. 11, 188–200 [DOI] [PubMed] [Google Scholar]
- 84.Robertson H. M., Warr C. G., Carlson J. R. 2003. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100, 14 537–14 542 10.1073/pnas.2335847100 (doi:10.1073/pnas.2335847100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hill C. A., et al. 2002. G protein coupled receptors in Anopheles gambiae. Science 298, 176–178 10.1126/science.1076196 (doi:10.1126/science.1076196) [DOI] [PubMed] [Google Scholar]
- 86.Robertson H. M., Wanner K. W. 2006. The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 16, 1395–1403 10.1101/gr.5057506 (doi:10.1101/gr.5057506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hallem E. A., Carlson J. R. 2006. Coding of odors by a receptor repertoire. Cell 125, 143–160 10.1016/j.cell.2006.01.050 (doi:10.1016/j.cell.2006.01.050) [DOI] [PubMed] [Google Scholar]
- 88.Miura N., Nakagawa T., Touhara K., Ishikawa Y. 2010. Broadly and narrowly tuned odorant receptors are involved in female sex pheromone reception in Ostrinia moths. Insect Biochem. Mol. Biol. 40, 64–73 [DOI] [PubMed] [Google Scholar]
- 89.Wanner K. W., Nichols A. S., Allen J. E., Bunger P. L., Garczynski S. F., Linn C. E., Robertson H. M., Luetje C. W. 2010. Sex pheromone receptor specificity in the European corn borer moth, Ostrinia nubilalis. PLoS ONE 5, e8685. 10.1371/journal.pone.0008685 (doi:10.1371/journal.pone.0008685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carey A. F., Wang G. R., Su C. Y., Zwiebel L. J., Carlson J. R. 2010. Odorant reception in the malaria mosquito. Anopheles gambiae. Nature 464, 66–77 10.1038/nature08834 (doi:10.1038/nature08834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramdya P., Benton R. 2010. Evolving olfactory systems on the fly. Trends Genet. 26, 307–316 10.1016/j.tig.2010.04.004 (doi:10.1016/j.tig.2010.04.004) [DOI] [PubMed] [Google Scholar]
- 92.Heckel D. G. 2010. Smells like a new species: gene duplication at the periphery. Proc. Natl Acad. Sci. USA 107, 9481–9482 10.1073/pnas.1004511107 (doi:10.1073/pnas.1004511107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ducrest A. L., Keller L., Roulin A. 2008. Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol. Evol. 23, 502–510 10.1016/j.tree.2008.06.001 (doi:10.1016/j.tree.2008.06.001) [DOI] [PubMed] [Google Scholar]
- 94.Schaefer H. M., Rolshausen G. 2006. Plants on red alert: do insects pay attention? Bioessays 28, 65–71 10.1002/bies.20340 (doi:10.1002/bies.20340) [DOI] [PubMed] [Google Scholar]
- 95.Chittka L., Brockmann A. 2005. Perception space—the final frontier. PLoS Biol. 3, e137. 10.1371/journal.pbio.0030137 (doi:10.1371/journal.pbio.0030137) [DOI] [PMC free article] [PubMed] [Google Scholar]