Abstract
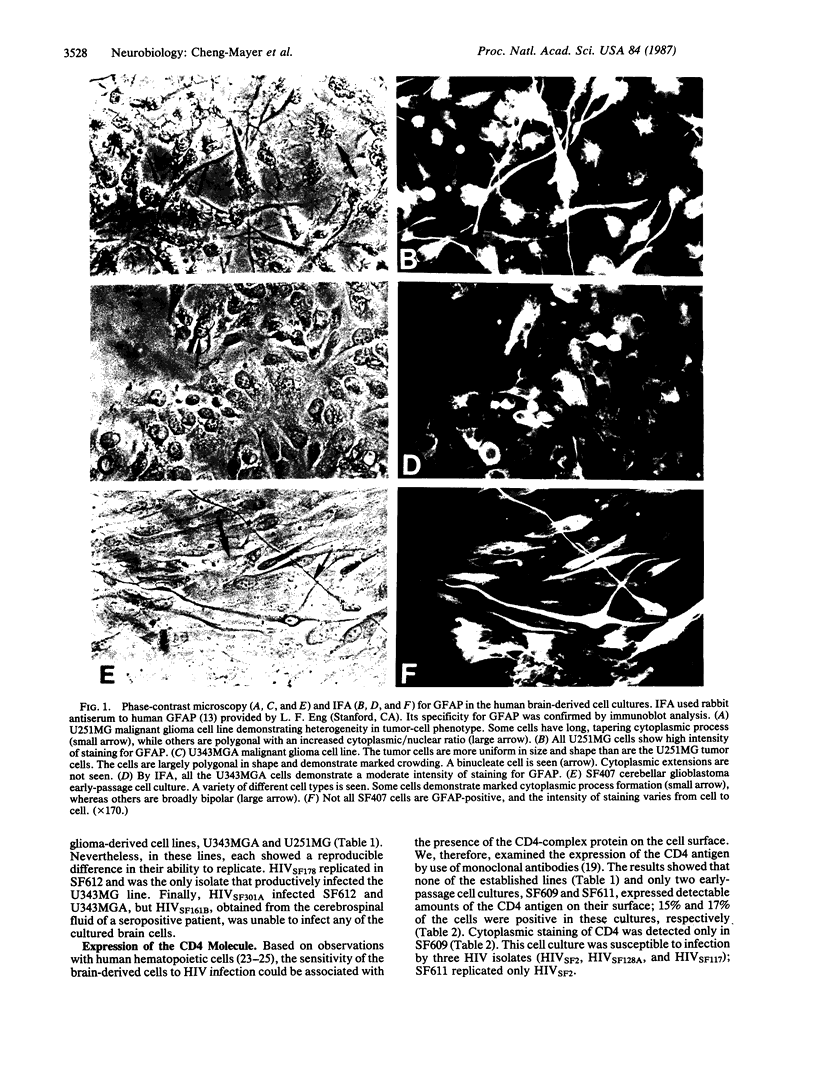
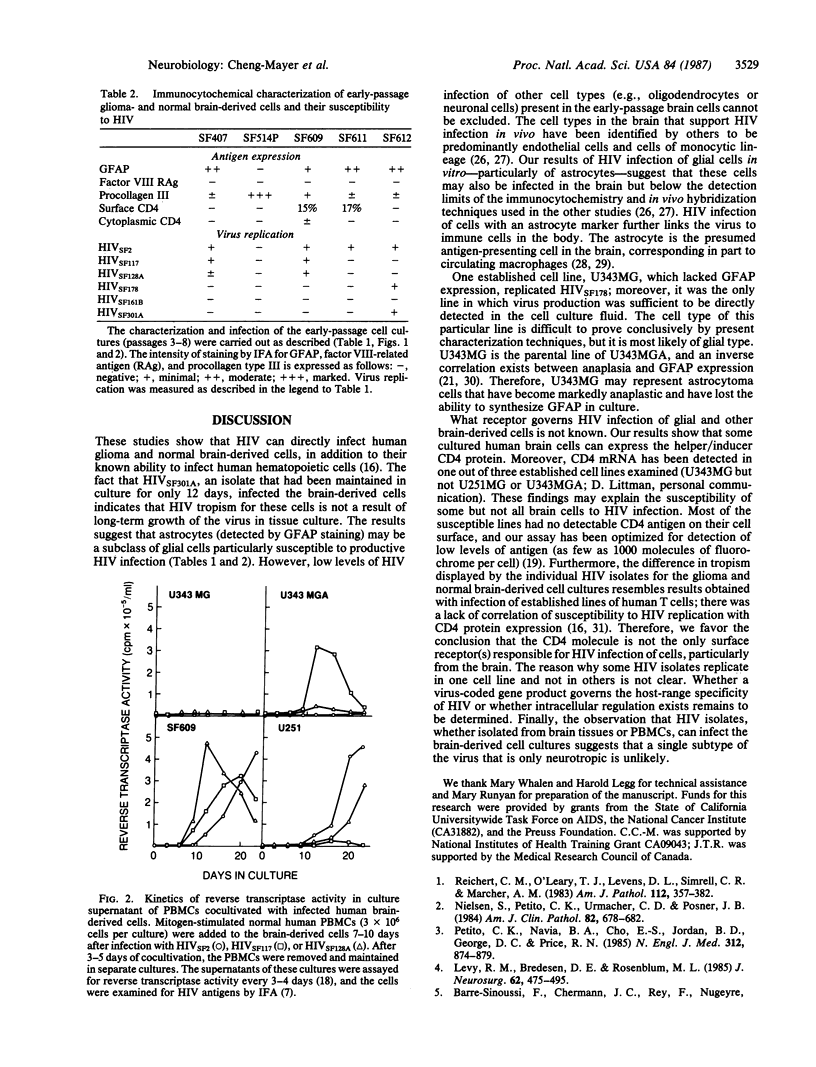
Six isolates of the human immunodeficiency virus (HIV) showed differences in their ability to productively infect glioma-derived cell lines and early-passage human brain cell cultures. Susceptibility to HIV infection correlated well with the expression of the astrocyte marker glial fibrillary acidic protein. The CD4 molecule was expressed on some, but not all, of the brain-derived cells; however, no correlation was observed between CD4 protein expression and susceptibility to virus infection. The results show that HIV can productively infect human brain cells, particularly those of glial origin, and suggest that these cell types in the brain can harbor the virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Nilsson K., Westermark B., Pontén J., Sundström C., Larsson E., Bergh J., Påhlman S., Busch C., Collins V. P. Formation and growth of multicellular spheroids of human origin. Int J Cancer. 1983 May 15;31(5):523–533. doi: 10.1002/ijc.2910310502. [DOI] [PubMed] [Google Scholar]
- Coffin J., Haase A., Levy J. A., Montagnier L., Oroszlan S., Teich N., Temin H., Toyoshima K., Varmus H., Vogt P. Human immunodeficiency viruses. Science. 1986 May 9;232(4751):697–697. doi: 10.1126/science.3008335. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Deck J. H., Eng L. F., Bigbee J., Woodcock S. M. The role of glial fibrillary acidic protein in the diagnosis of central nervous system tumors. Acta Neuropathol. 1978 Jun 30;42(3):183–190. doi: 10.1007/BF00690355. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Schooley R. T., Kaplan J. C., Allan J. D., Groopman J. E., Resnick L., Felsenstein D., Andrews C. A., Hirsch M. S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Jacque C. M., Kujas M., Poreau A., Raoul M., Collier P., Racadot J., Baumann N. GFA and S 100 protein levels as an index for malignancy in human gliomas and neurinomas. J Natl Cancer Inst. 1979 Mar;62(3):479–483. doi: 10.1093/jnci/62.3.479. [DOI] [PubMed] [Google Scholar]
- Kikukawa R., Koyanagi Y., Harada S., Kobayashi N., Hatanaka M., Yamamoto N. Differential susceptibility to the acquired immunodeficiency syndrome retrovirus in cloned cells of human leukemic T-cell line Molt-4. J Virol. 1986 Mar;57(3):1159–1162. doi: 10.1128/jvi.57.3.1159-1162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Rowe W. P. Lack of requirement of murine leukemia virus for early steps in infection of mouse embryo cells by murine sarcoma virus. Virology. 1971 Sep;45(3):844–847. doi: 10.1016/0042-6822(71)90211-x. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., Hollander H., Mills J., Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985 Sep 14;2(8455):586–588. [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., McHugh T., Casavant C., Stites D., Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985 Dec;147(2):441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Levy R. M., Bredesen D. E., Rosenblum M. L. Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): experience at UCSF and review of the literature. J Neurosurg. 1985 Apr;62(4):475–495. doi: 10.3171/jns.1985.62.4.0475. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Dörries R., ter Meulen V. Viral particles induce Ia antigen expression on astrocytes. Nature. 1986 Apr 10;320(6062):543–546. doi: 10.1038/320543a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Nielsen S. L., Petito C. K., Urmacher C. D., Posner J. B. Subacute encephalitis in acquired immune deficiency syndrome: a postmortem study. Am J Clin Pathol. 1984 Dec;82(6):678–682. doi: 10.1093/ajcp/82.6.678. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Glazer A. N., Stryer L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol. 1982 Jun;93(3):981–986. doi: 10.1083/jcb.93.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito C. K., Navia B. A., Cho E. S., Jordan B. D., George D. C., Price R. W. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985 Apr 4;312(14):874–879. doi: 10.1056/NEJM198504043121402. [DOI] [PubMed] [Google Scholar]
- Reichert C. M., O'Leary T. J., Levens D. L., Simrell C. R., Macher A. M. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983 Sep;112(3):357–382. [PMC free article] [PubMed] [Google Scholar]
- Rutka J. T., Giblin J., Dougherty D. V., McCulloch J. R., DeArmond S. J., Rosenblum M. L. An ultrastructural and immunocytochemical analysis of leptomeningeal and meningioma cultures. J Neuropathol Exp Neurol. 1986 May;45(3):285–303. doi: 10.1097/00005072-198605000-00012. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]