Abstract
Explaining the mechanisms that produce the enormous diversity within and between tropical tree communities is a pressing challenge for plant community ecologists. Mechanistic hypotheses range from niche-based deterministic to dispersal-based stochastic models. Strong tests of these hypotheses require detailed information regarding the functional strategies of species. A few tropical studies to date have examined trait dispersion within individual forest plots using species trait means in order to ask whether coexisting species tend to be more or less functionally similar than expected given a null model. The present work takes an alternative approach by: (i) explicitly incorporating population-level trait variability; and (ii) quantifying the functional beta diversity in a series of 15 tropical forest plots arrayed along an elevational gradient. The results show a strong pattern of decay in community functional similarity with elevation. These observed patterns of functional beta diversity are shown to be highly non-random and support a deterministic model of tropical tree community assembly and turnover.
Keywords: community ecology, functional traits, leaf area, Puerto Rico, specific leaf area, wood density
1. Introduction
Tropical communities are renowned for their biodiversity. Not only is there an elevated level of species diversity within communities (i.e. alpha diversity) with respect to less diverse temperate communities, but species turnover between tropical communities (i.e. beta diversity) is also elevated. Perhaps, nowhere are patterns of tropical diversity more often documented than in tree communities (e.g. [1–5]), yet a substantial debate still exists on the extent to which these patterns are the result of deterministic or stochastic processes (e.g. [6–8]). Understanding the degree to which patterns of diversity are the result of deterministic or stochastic processes is particularly critical for those trying to conserve and predict the fate of tropical biodiversity.
The majority of research into patterns of tropical tree diversity and turnover has focused on one type of biodiversity: species diversity. The functional component of biodiversity in these communities has been less well quantified (but see [8–10]). This is unfortunate because documenting the functional strategies of species in communities may provide refined or novel mechanistic insights into their structure and diversity [11].
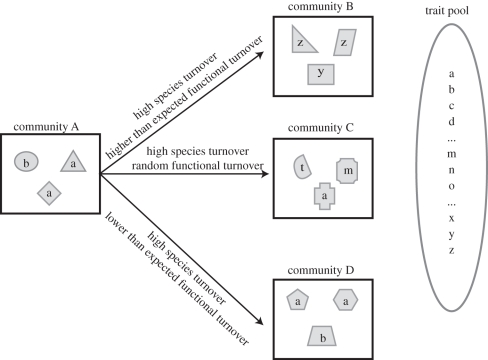
One principle concern for a community ecologist is that traditional analyses of species beta diversity are unable to determine whether or not the communities are functionally similar or dissimilar. In figure 1, we present one example of this where species may be dispersal limited causing complete species turnover between two communities, but the environment also plays a key role in determining the community assembly, thereby generating functionally analogous communities (figure 1). This would result in a decoupling of species and functional beta diversity where species beta diversity is high and functional beta diversity is low. We would expect this result to be particularly important when comparing assemblages between regions that share similar environments. Despite the potential insights one can gain from analysing the functional similarity of communities, the concept of ‘functional beta diversity’ is rather new and it still remains to be deeply explored (but see [12,13]). To date, the majority of the work on this topic has regarded the development of functional beta diversity metrics similar to those designed to analyse species beta diversity (e.g. [14]) or phylogenetic beta diversity [15,16]. To our knowledge, there have been no analyses of functional beta diversity of communities arrayed along broad environmental gradients and no analyses of diverse ecosystems such as tropical rainforests. Thus, tests using such datasets and multiple metrics are needed.
Figure 1.
A cartoon depicting the species and functional turnover between a set of four hypothetical communities. The shapes indicate species identity and the letters indicate the functional strategy of the species. The trait pool represents all of the functional strategies that could potentially colonize a community. Community A shares no species in common with any of the other communities and it therefore has the same level of species turnover from A to B, A to C and A to D. Community A and community B share no functional strategies. Further, the strategies present in community B are significantly more dissimilar from those in community A than expected if one were to randomly pull three strategies from trait pool. This would be expected if an underlying environmental gradient determines species turnover and community assembly. Community A and community C share no functional strategies, but the functional turnover is indistinguishable from a random pull of three functional strategies from the trait pool. This would be expected under a stochastic model of species turnover and community assembly. Community A and community D are functionally analogous and there is less functional turnover than expected given a random pull of three strategies from the trait pool. This would be expected where dispersal is limited but community assembly is deterministic with respect to the environment. The figure illustrates that three different ecological processes can be determined by examining the functional and species turnover between communities simultaneously, while analyses of species turnover alone could provide erroneous ecological inferences.
When comparing communities along an environmental gradient it is probable that species beta diversity and functional beta diversity will be, on average, positively related. In such instances, the question becomes whether the functional turnover between communities is higher or lower than that expected given the species turnover. For example, a higher than expected functional turnover between communities given the species turnover could occur if the species in the two communities are more functionally divergent than expected given the species pool. This would indicate that the species turnover along the gradient is not simply owing to dispersal limitation and that the underlying environmental gradient plays a key role in influencing species turnover and community assembly. Thus given the importance of quantifying the functional strategies of species within and among communities, ecologists are increasingly promoting a functional trait-based approach to community ecology [11].
Plant community ecologists are increasingly integrating functional ecology into their research programmes by quantifying functional traits on the individuals and species in their study systems (e.g. [17–21]). Functional traits are morphological and physiological traits that are indicative of the ecological strategies of species. An advantage of functional traits is that they allow for a rapid and standardized inventory of several major axes of plant functional differentiation across all species in diverse communities [22–24]. Indeed this advantage has led to the pioneering work into the relative influence of deterministic and stochastic processes on patterns of species coexistence in tropical tree communities [8].
Despite the promise of a functional trait-based paradigm in community ecology there are several outstanding challenges. First, the appropriateness of the functional traits used in community ecology investigations is routinely questioned and determining which are the ‘right’ traits to use is important. In recent years, there has been a great deal of focus on several plant functional traits that have been proposed to be important indicators of plant ecological strategies [22–24]. These ‘soft’ functional traits are proxies of actual physiological processes and they are relatively easy to measure compared with ‘hard’ functional traits that are often more closely linked to the physiological processes of interest, but much more difficult to quantify across diverse communities [25]. While the debate of which are the best traits or the right traits for community ecology investigations will continue, an initial step is to determine when and where those often used soft functional traits can provide novel insights into the mechanisms determining community assembly and diversity.
A second challenge has been that of intraspecific functional trait variability [20,26]. Many functional trait investigations of communities have assigned species-level trait means to all individuals or populations (e.g. [8,10]). In some cases, these mean values are calculated from a few individuals from the study system, and in others these values come from global databases where the measured individuals may be from a very different geographical region (e.g. [9,19]). Given that the traits of interest are known to vary with respect to the local-scale environment, assigning species-level means may introduce several biases and reduce the power of functional trait-based community ecology. Perhaps nowhere will this be more important than when examining the functional similarity of communities (i.e. functional beta diversity) arrayed along a substantial environmental gradient. In particular, large changes in population-level trait values owing to physiological responses to an environmental could promote a further decoupling of species and functional beta diversity, whereby functional beta diversity would increase at a faster pace than species beta diversity. A result such as this would provide further evidence that deterministic factors play a large role in determining the functional composition and assembly of communities. Thus, when feasible, quantifying trends in population-level trait means along environmental gradients are a critical next step in functional trait-based community ecology.
The present study provides, to our knowledge, the first analysis of functional beta diversity along a substantial environmental gradient. Specifically, here we quantify several important functional traits representing plant ecological strategies in a series of 15 permanent forest inventory plots arrayed along 700 m elevational gradient in Puerto Rico. Given the potential for substantial intraspecific variation in trait values for species with large elevational ranges, all analyses in the present work use population-level trait means in lieu of species-level trait means. The trait data were used to quantify the functional beta diversity of the forest plots along the gradient in order to address the fundamental question of whether tropical tree community turnover is stochastic or deterministic and to ask the following sub-questions: (i) is there a distance decay in functional similarity between the forest plots similar to the distance decay in species similarity? and (ii) is the functional turnover between forest plots faster or slower than expected given the observed patterns of species turnover? We propose that deterministic species turnover and directional species functional responses to the environmental gradient should lead to higher than expected functional turnover given the species turnover. If the functional turnover is random with respect to species turnover or lower than expected, this would indicate a larger role for stochastic processes influencing the species turnover and community assembly in the study system.
2. Material and methods
(a). Study area and forest inventory plots
The present study took place in the Luquillo Experimental Forest (LEF) located in eastern Puerto Rico (18°19.6′ N, 65°49.4′ W). The LEF is approximately 11 330 ha in area with elevations ranging from 100 to 1075 m above sea level (a.s.l.) [27]. At 100 m a.s.l. the temperature averages 24.5°C during the warmest month of September and annual rainfall totals 2300 mm on average. At 1075 m a.s.l. the temperature averages 20.0°C during September and the annual rainfall totals 3600 mm on average. The elevational gradient spans from premontane rainforest to cloud forest. Permanent forest inventory plots were established from 2001 to 2002 along the Sonadora River watershed within the LEF [28]. The forest plots were arrayed every 50 m in elevation from 300 to 1000 m a.s.l. The sites selected for each forest plot had little to no previous human disturbance and did not include ravines [28]. Each forest plot is 0.1 ha in area (50 × 20 m) with all free-standing woody stems 1 cm in diameter at breast height (130 cm above the ground) mapped, tagged and identified. As the flora of the LEF is well-known, over 99 per cent of the stems could be identified to species and voucher specimens were deposited in the El Verde Field Station Herbarium. There are a total of 6562 stems and 102 species found in the 15 forest plots. In each plot, we calculated the species alpha and beta diversity. The species beta diversity was calculated using a Bray–Curtis Distance in the R package ‘Vegan’.
(b). Functional traits and dendrogram
A total of six traits were chosen for this study to quantitatively represent several axes of plant functional strategy [24]. Specific leaf area (SLA), leaf nitrogen content (%N) and leaf phosphorus content (%P) were used to represent the well-documented leaf economics spectrum [29]. Wood-specific gravity was used to represent a wood economics spectrum [30]. Leaf area was used to represent a trade-off between area deployed for light capture and leaf temperatures [31], and maximum height was used to represent the adult light niche of the species [32].
Each trait was measured on multiple individuals in each forest inventory plot following the protocols in Cornelissen et al. [33]. When possible, 15 individuals were sampled for leaf and height trait measurements and 10 individuals were used for wood-specific gravity measurements. A recent power analysis of leaf functional traits in a tropical forest community has suggested that a similar sampling intensity is necessary for reliable estimations of population mean trait values [26]. Sampling was fewer than 15 and 10 individuals respectively, only when that many individuals could not be located at the given elevation.
For each trait, a distance matrix was constructed to represent the trait similarity between all populations using population-level mean trait values for each species. This distance matrix and UPGMA clustering were used to construct a dendrogram for each trait [34]. This dendrogram represented the trait similarity between populations. Two of the functional dissimilarity metrics shown below, Dnn and Dpw, can also be implemented without using trait dendrograms, whereas the other, Fsor, cannot. Thus, for consistency we have chosen to use the dendrograms for each metric. Further studies are needed to address the degree to which using dendrograms versus raw trait distances influence functional beta diversity metrics. As this study was also interested in a composite measure of functional similarity between species, we used a principle coordinates analysis (PCA). To perform the PCA, we first logged the trait data and converted it to standard normal deviates to identify the major axes of multivariate functional similarity. The first three PCA axes explained over 96 per cent of the variation (see the electronic supplementary material, table S1) and the Euclidean distance between populations in this three-dimensional space and UPGMA clustering were used to construct a composite functional dendrogram representing the overall functional similarity between populations.
(c). Functional beta diversity
The present study implemented three metrics of functional beta diversity. The concept and measurement of functional beta diversity are still in their infancy and further work will be needed to compare and contrast the relative benefits of various metrics. Here, we have chosen to use three potential metrics of functional similarity between communities as a starting point without making any particular judgement with respect to their relative merits. The first metric used we term Functional Sorensen's Index, Fsor. This metric is presence–absence weighted and defined as:
where 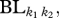 is the total dendrogram branch length common to all species in communities k1 and k2, and
is the total dendrogram branch length common to all species in communities k1 and k2, and 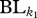 and
and 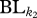 are the total dendrogram branch length common to the species within communities k1 and k2, respectively. This metric is a functional trait analogue of the phylogenetic metric, PhyloSor, introduced by Bryant et al. [35]. It is also analogous to a traditional Sorensen's Index. Thus, the Fsor metric provides an overall indicator of the shared function between two communities.
are the total dendrogram branch length common to the species within communities k1 and k2, respectively. This metric is a functional trait analogue of the phylogenetic metric, PhyloSor, introduced by Bryant et al. [35]. It is also analogous to a traditional Sorensen's Index. Thus, the Fsor metric provides an overall indicator of the shared function between two communities.
The next two metrics we employed use abundance information. First, we employed an abundance weighted nearest functional neighbour dissimilarity, Dnn, between two communities, k1 and k2. This metric was originally published by Ricotta & Burrascano [14] and is defined as:
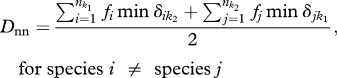 |
where 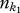 is the number of species in community k1, fi the relative abundance of species i in community k1, and min
is the number of species in community k1, fi the relative abundance of species i in community k1, and min 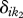 the dendrogram branch lengths between species i in community k1 and its nearest functional neighbour in community k2 that is not the same species. Thus if two communities share functionally analogous or nearly analogous species, the average nearest neighbour distances will be much lower than two communities that have very dissimilar community trait distributions.
the dendrogram branch lengths between species i in community k1 and its nearest functional neighbour in community k2 that is not the same species. Thus if two communities share functionally analogous or nearly analogous species, the average nearest neighbour distances will be much lower than two communities that have very dissimilar community trait distributions.
The third metric was an abundance weighted pairwise dissimilarity, Dpw, between communities k1 and k2 modified from the Dnn metric described above. This metric is defined as:
where 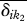 is the mean pairwise distance on the dendrogram between all species in community k1 and all species in community k2 excluding conspecific species. The Dpw is somewhat different than most beta diversity metrics because it compares all pairwise distances between species in the two communities. Thus we may not expect it to correlate with patterns of species beta diversity as well as the previous two metrics.
is the mean pairwise distance on the dendrogram between all species in community k1 and all species in community k2 excluding conspecific species. The Dpw is somewhat different than most beta diversity metrics because it compares all pairwise distances between species in the two communities. Thus we may not expect it to correlate with patterns of species beta diversity as well as the previous two metrics.
(d). Null model and standardized effect sizes
As discussed above, it is reasonable to expect species and functional beta diversity to be correlated. While we were interested in documenting patterns of functional beta diversity in the study system, we were also interested in determining whether the functional beta diversity was higher or lower than expected given the species beta diversity. In order to accomplish this we performed null modelling analyses. A null distribution of functional beta diversity values was generated for each trait by randomizing the names of the populations across the tips of the trait dendrograms 9999 times. During each iteration, the functional beta diversity was calculated across the entire elevational gradient. These values were used to generate the null distribution. Therefore, the randomization procedure only randomized the functional similarity of populations while maintaining the observed species occupancy rates, community species richness levels, community abundance distributions and species beta diversity. Further, as the null model maintains the observed distributional patterns of species and their populations, the observed dispersal limitation of species is conserved in the null model.
A standardized effect size (SES; [36]) was calculated for functional beta diversity using the mean and standard deviation of the null distribution as follows:
where Xobs is the observed dissimilarity value (i.e. Dpw) between two communities, 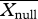 the mean of the null distribution and s.d.(Xnull) the standard deviation of the null distribution. Values greater than 1.96 indicate a higher than expected functional dissimilarity between the communities and values below −1.96 indicate a lower than expected functional dissimilarity between the two communities. Non-random SES values could be generated by functionally non-random replacement of species along the gradient and/or population-level trait means shifting directionally along the gradient.
the mean of the null distribution and s.d.(Xnull) the standard deviation of the null distribution. Values greater than 1.96 indicate a higher than expected functional dissimilarity between the communities and values below −1.96 indicate a lower than expected functional dissimilarity between the two communities. Non-random SES values could be generated by functionally non-random replacement of species along the gradient and/or population-level trait means shifting directionally along the gradient.
3. Results
(a). Species alpha and beta diversity
There was a general decline in species alpha diversity with increasing elevation (see the electronic supplementary material, figure S1) although the pattern may have become unimodal if lower elevation forests could have been censused. The species alpha diversity in the plots ranged from 18 species at 950 m a.s.l. to a maximum of 52 species at 300 m a.s.l. The species beta diversity was also calculated and the results show a general increase in community dissimilarity with increasing elevation using a Bray–Curtis Distance (see the electronic supplementary material, figures S1 and S2).
(b). Functional beta diversity and standardized effect sizes
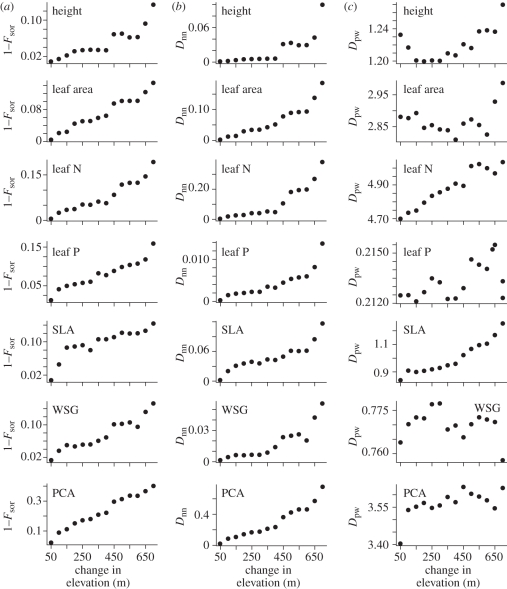
The functional beta diversity of the forest plots was determined using three different metrics. In the main text, we only present the functional dissimilarity between the plot at the lowest elevation and the other 14 forest plots along the gradient, but all pairwise comparisons between plots are presented in the electronic supplementary material. The results show a general decay in functional similarity with elevation across all traits using both the presence–absence weighted Fsor metric and the abundance weighted nearest neighbour metric, Dnn (figure 2 and electronic supplementary material, figure S3). The results were not as consistent using the abundance weighted pairwise metric, Dpw. Specifically, dissimilarity increased with elevation for maximum height, leaf area, leaf nitrogen content, leaf phosphorus content and PCA, but the wood-specific gravity results were unimodal (figure 2 and electronic supplementary material, figure S3).
Figure 2.
The beta functional diversity along the elevational gradient in the Luquillo Experimental Forest presented as the dissimilarity between the plot at the lowest elevation (300 m a.s.l.) and the other 14 forest plots spaced every 50 m in elevation. (a) Calculates beta functional diversity using the presence–absence weighted Fsor metric, (b,c) calculate beta functional diversity using the abundance weighted nearest neighbour metric, Dnn and the pairwise metric, Dpw, respectively.
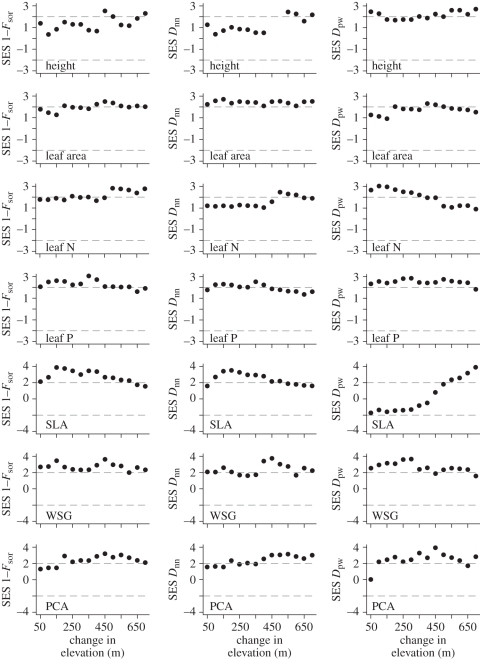
In an additional analysis we compared the trait dissimilarity values between plots with the Bray–Curtis Distance between plots. The Bray–Curtis Distance was positively correlated with the Fsor and Dnn trait dissimilarity metrics (see the electronic supplementary material, figure S4). The Dpw metric on the other hand was generally not correlated with the Bray–Curtis Distance (electronic supplementary material, figure S4). Given the strong relationship between some of the functional beta diversity metrics and species beta diversity and our desire to know whether the functional beta diversity was higher or lower than that expected given the species beta diversity, we conducted null model analyses. The SESs for the Fsor, Dnn and Dpw, metrics were generally positive across all traits and consistent across elevation with effect sizes often greater than 1.96 (figure 3). The constancy in the positive values greater than 1.96 is indicative of higher than expected functional trait dissimilarity or turnover with elevation. A notable exception to this general pattern is the SLA results for the Dpw metric where there was lower than expected turnover initially along the gradient followed by a larger than expected turnover when comparing the lowest plot to the high elevation plots (figure 3).
Figure 3.
The beta functional dispersion along the elevational gradient in the Luquilllo Experimental Forest. The data are presented using the standardized effect size (SES). SES values above 1.96 (the upper dashed grey line) indicate significantly higher than expected beta functional diversity, or dissimilarity, given the observed beta species diversity. SES values below −1.96 (the lower dashed grey line) indicate significantly lower than the expected beta functional diversity, or dissimilarity, given the observed beta species diversity.
4. Discussion
The present study asked whether the turnover of tropical tree communities along an elevational gradient is deterministic or stochastic with respect to species function. To date, tropical tree community turnover has often been analysed using lists and abundances of species that are treated as functionally equivalent or binned into broad categorically defined functional groupings. Recently, species mean functional trait values have been used in two tropical forest plots to quantify whether the functional alpha diversity in forest subplots is higher or lower than expected given the species richness [8,10]. To date, this work has not addressed the turnover of tree species in these diverse systems and it has not explicitly dealt with the potentially high level of intraspecific variation in functional trait values. In this study, to our knowledge, we have presented the first analysis of functional beta diversity in tropical tree communities, and we have provided the first functional trait analysis of tropical tree communities that explicitly incorporates intraspecific trait variation. The functional beta diversity results showed a general non-random compositional turnover of function along the elevational gradient, suggesting a large role for deterministic processes in controlling community turnover along the gradient. Below, we discuss these results and their implications in more detail.
The primary goal of the present study was to ask whether the turnover of tropical tree communities is relatively deterministic or stochastic with respect to species function. To address this we quantified the functional beta diversity or dissimilarity between the forest plot at the lowest elevation to the other 14 forest plots at higher elevations using three different metrics (figure 2 and electronic supplementary material, figure S3). In general, the functional beta diversity increased with elevation using all three metrics (figure 2 and electronic supplementary material, figure S3). In other words, there was a clear distance decay in functional similarity from the lowest to the highest forest plot. Thus, the functional composition of the tree communities along the elevational gradient changes directionally for all of the traits studied.
Further analyses revealed that two of the metrics used were strongly correlated with species beta diversity, thereby making it difficult to determine whether functional turnover along the gradient was any different from that expected given the underlying species turnover. Consequently, it is tenuous to reject a stochastic model of community turnover and assembly from functional distance decay results alone and null model analyses are necessary.
The results from the null model analyses for all of the metrics were generally non-random with SESs often greater than 1.96 (figure 3). Thus, the functional composition of the communities along the elevational gradient turns over faster than expected given the species compositional turnover and changes in species abundance. The faster than expected turnover in function between communities could occur for two reasons. First, those species' that replace each other from one community to the next are functionally more divergent than expected given the species pool. For example, say that there was complete turnover in the species composition when transitioning from 300 to 900 m elevation. If the species at 900 m are a non-random subset of the species pool that is functionally more distant than expected at random from those found at 300 m, then this would indicate that the species turnover is not simply stochastic. Second, if there are substantial directional trends in population-level trait means along the environmental gradient, the functional turnover would be faster than that expected given the species turnover. While there is a high level of species turnover along the gradient studied (see the electronic supplementary material, figures S1 and S2), nowhere is there a complete turnover in species composition between forest plots, and a few species span the entire elevational gradient. We therefore suggest that both of the above processes probably generated the faster than expected functional turnover reported in this study. Further, both a functionally non-random replacement of species along a gradient and directional trends in population-level trait values along a gradient are evidence for deterministic species turnover and community assembly along the gradient.
The primary goal of the current study was to document patterns of functional turnover in tree communities along a substantial environmental gradient in the tropics. To achieve this goal, we implemented three metrics of functional beta diversity. In conducting the work we found that the first two metrics, Fsor and Dnn, were strongly related to a Bray–Curtis Distance while the third metric, Dpw, was not (see the electronic supplementary material, figure S4). Thus, the first two metrics may be considered more closely aligned with what is traditionally considered to be ‘beta diversity’ while the third metric may just serve as an alternative measure of similarity that cannot be easily fitted into a diversity-partitioning framework. As the topic of functional beta diversity becomes more broadly studied, detailed investigations into when and why these particular metrics and other metrics are preferred will be needed.
In summary, here we have provided, to our knowledge, the first functional beta diversity analyses of a series of tropical tree communities arrayed along a substantial ecological gradient, to address whether the compositional turnover between these communities is relatively stochastic or deterministic. We also present, to our knowledge, the first analysis of functional beta diversity that explicitly incorporates intraspecific variation in traits along a broad gradient. The results presented are largely non-random and the turnover of the functional composition of communities is generally faster than that expected along the gradient. The results are also consistent across all of the traits studied and each of the metrics used, which indicates that the compositional change along the gradient involves several axes of plant functional differentiation and is not just a shift in any one trait or axis. These results suggest the predominate role of deterministic factors promoting the compositional turnover of the tropical tree communities along the gradient and a lesser role for stochasticity.
5. Conclusions and future directions
Here, we have presented an initial test of whether or not the compositional similarity of tropical tree communities is non-random with respect to species function. The results show that tropical tree community similarity in our study system is indeed functionally non-random and supports a deterministic model of tropical tree community assembly and turnover. Future work should aim to repeat a similar approach across several gradients within and outside the tropics to determine whether or not similar distance decay relationships occur in other systems.
The work has also successfully integrated intraspecific functional trait variation into the analyses. Accounting for this variation will become increasingly important as the scale of functional trait investigations into community structure broadens and even more so as functional beta diversity becomes increasingly measured across broad ecological gradients. Additional studies will be needed to quantify to what degree intraspecific variability will bias functional beta diversity analyses that use species-level mean trait values and how this bias may be enhanced or mitigated in systems with more or less variable species.
Acknowledgements
N.G.S. is supported by Michigan State University. An NSF grant (DEB-0080538) to the Institute for Tropical Ecosystem Studies, University of Puerto Rico and to the International Institute of Tropical Forestry, US Forest Service (US Department of Agriculture), as a part of the Long-Term Ecological Research Programme funded the original installations of the forest inventory plots. N.G.S. would like to thank Catherine Hulshof for her assistance in the field. N.G.S. would like to thank the NCEAS Working Group on Beta Diversity (NCEAS Project 12 437) for inspiration and conversations regarding this topic. We would like to thank Jess Zimmerman and Nick Brokaw for their logistical support and encouragement.
References
- 1.Gentry A. H. 1982. Patterns of neotropical plant species diversity. In Evolutionary biology, vol. 15 (eds Hecht M. K., Wallace B., Prance E. T.), pp. 1–84 New York, NY: Plenum Press [Google Scholar]
- 2.Gentry A. H. 1988. Changes in community diversity and floristic composition on environmental and geographical gradients. Ann. Miss. Bot. Gard. 75, 1–34 10.2307/2399464 (doi:10.2307/2399464) [DOI] [Google Scholar]
- 3.Pitman N. C. A., Terborgh J. W., Silman M. R., Percy Nunez V., Neill D. A., Ceron C. E., Palacios W. A., Aulestia M. 2001. Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology 82, 2101–2117 10.1890/0012-9658(2001)082[2101:DADOTS]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[2101:DADOTS]2.0.CO;2) [DOI] [Google Scholar]
- 4.Condit R., et al. 2002. Beta-diversity in tropical forest trees. Science 295, 666–669 10.1126/science.1066854 (doi:10.1126/science.1066854) [DOI] [PubMed] [Google Scholar]
- 5.Losos E. C., Leight E. G., Jr 2004. Tropical forest diversity and dynamism: findings from a large-scale plot network. Chicago, IL: University of Chicago Press [Google Scholar]
- 6.Hubbell S. P. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 7.McGill B. J. 2003. A test of the unified neutral theory of biodiversity. Nature 422, 881–885 10.1038/nature01583 (doi:10.1038/nature01583) [DOI] [PubMed] [Google Scholar]
- 8.Kraft N. J. B., Valencia R., Ackerly D. D. 2008. Functional traits and niche-based tree community assembly in an Amazonian forest. Science 322, 580–582 10.1126/science.1160662 (doi:10.1126/science.1160662) [DOI] [PubMed] [Google Scholar]
- 9.Swenson N. G., Enquist B. J., Thompson J., Zimmerman J. K. 2007. The influence of spatial and size scales on phylogenetic relatedness in tropical forest communities. Ecology 88, 1770–1780 10.1890/06-1499.1 (doi:10.1890/06-1499.1) [DOI] [PubMed] [Google Scholar]
- 10.Swenson N. G., Enquist B. J. 2009. Opposing assembly mechanisms in a neotropical dry forest: implications for phylogenetic and functional community ecology. Ecology 90, 2161–2170 10.1890/08-1025.1 (doi:10.1890/08-1025.1) [DOI] [PubMed] [Google Scholar]
- 11.McGill B. J., Enquist B. J., Weiher E., Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185 10.1016/j.tree.2006.02.002 (doi:10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 12.de Bello F., Thuiller W., Leps J., Choler P., Clement J. C., Macek P., Sebastià M. T., Lavorel S. 2009. Partitioning functional diversity reveals the scale and extent of trait convergence and divergence. J. Veg. Sci. 20, 475–486 10.1111/j.1654-1103.2009.01042.x (doi:10.1111/j.1654-1103.2009.01042.x) [DOI] [Google Scholar]
- 13.Laliberté E., Legendre P. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 10.1890/08-2244.1 (doi:10.1890/08-2244.1) [DOI] [PubMed] [Google Scholar]
- 14.Ricotta C., Burrascano S. 2009. Testing for differences in beta diversity with asymmetric dissimilarities. Ecol. Ind. 9, 719–724 10.1016/j.ecolind.2008.09.003 (doi:10.1016/j.ecolind.2008.09.003) [DOI] [Google Scholar]
- 15.Hardy O. J., Senterre B. 2007. Characterizing the phylogenetic structure of communities by an additive partitioning of phylogenetic diversity. J. Ecol. 95, 493–506 10.1111/j.1365-2745.2007.01222.x (doi:10.1111/j.1365-2745.2007.01222.x) [DOI] [Google Scholar]
- 16.Hardy O. J., Jost L. 2008. Interpreting and estimating measures of community phylogenetic structuring. J. Ecol. 96, 849–852 10.1111/j.1365-2745.2008.01423.x (doi:10.1111/j.1365-2745.2008.01423.x) [DOI] [Google Scholar]
- 17.Zimmerman J. K., Everham E. M., Waide R. B., Lodge D. J., Taylor C. M., Brokaw N. V. L. 1994. Responses of tree species to hurricane winds in subtropical wet forest in Puerto Rico: implications for tropical tree life histories. J. Ecol. 82, 911–922 [Google Scholar]
- 18.Weiher E., Clarke G. D. P., Keddy P. A. 1998. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 81, 309–322 10.2307/3547051 (doi:10.2307/3547051) [DOI] [Google Scholar]
- 19.Swenson N. G., Enquist B. J. 2007. Ecological and evolutionary determinants of a key plant functional trait: wood density and its community-wide variation across latitude and elevation. Am. J. Bot. 91, 451–459 [DOI] [PubMed] [Google Scholar]
- 20.Cornwell W. K., Ackerly D. D. 2009. Community assembly and shifts in the distribution of trait values across an environmental gradient in coastal California. Ecol. Monogr. 79, 109–126 10.1890/07-1134.1 (doi:10.1890/07-1134.1) [DOI] [Google Scholar]
- 21.Swenson N. G., Weiser M. D. 2010. Plant geography upon the basis of functional traits: an example from eastern North American trees. Ecology 91, 2234–2241 10.1890/09-1743.1 (doi:10.1890/09-1743.1) [DOI] [PubMed] [Google Scholar]
- 22.Westoby M. 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199, 213–227 10.1023/A:1004327224729 (doi:10.1023/A:1004327224729) [DOI] [Google Scholar]
- 23.Weiher E., Van der Werf A., Thompson K., Roderick M., Garnier E., Eriksson O. 1999. Challenging Theophrastus: a common core list of plant traits for functional ecology. J. Veg. Sci. 10, 609–620 10.2307/3237076 (doi:10.2307/3237076) [DOI] [Google Scholar]
- 24.Westoby M., Falster D. S., Moles A. T., Vesk P. A., Wright I. J. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 33, 125–159 10.1146/annurev.ecolsys.33.010802.150452 (doi:10.1146/annurev.ecolsys.33.010802.150452) [DOI] [Google Scholar]
- 25.Lavorel S., Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 16, 545–556 10.1046/j.1365-2435.2002.00664.x (doi:10.1046/j.1365-2435.2002.00664.x) [DOI] [Google Scholar]
- 26.Hulshof C. M., Swenson N. G. 2010. Variation in leaf functional trait values within and across individuals and species: an example from a Costa Rican dry forest. Funct. Ecol. 24, 217–223 10.1111/j.1365-2435.2009.01614.x (doi:10.1111/j.1365-2435.2009.01614.x) [DOI] [Google Scholar]
- 27.Brown S., Lugo A. E., Silander S., Liegel L. 1983. Research history and opportunities in the Luquillo experimental forest. General Technical Report No. SO-44. U.S. Department of Forestry, Southern Experiment Station, New Orleans,132 pp
- 28.Barone J. A., Thomlinson J., Anglada Cordero P., Zimmerman J. K. 2008. Metacommunity structure of tropical forest along an elevational gradient in Puerto Rico. J. Trop. Ecol. 24, 525–534 [Google Scholar]
- 29.Wright I. J., et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827 10.1038/nature02403 (doi:10.1038/nature02403) [DOI] [PubMed] [Google Scholar]
- 30.Chave J., Coomes D., Jansen S., Lewis S., Swenson N. G., Zanne A. E. 2009. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 10.1111/j.1461-0248.2009.01285.x (doi:10.1111/j.1461-0248.2009.01285.x) [DOI] [PubMed] [Google Scholar]
- 31.Bailey I. W., Sinnott E. W. 1916. The climatic distribution of certain types of angiosperm leaves. Am. J. Bot. 3, 24–39 10.2307/2435109 (doi:10.2307/2435109) [DOI] [Google Scholar]
- 32.Moles A. T., Warton D. I., Warman L., Swenson N. G., Laffan S. W., Zanne A. E., Pitman A., Hemmings F. A., Leishman M. R. 2009. Global patterns in plant height. J. Ecol. 97, 923–932 10.1111/j.1365-2745.2009.01526.x (doi:10.1111/j.1365-2745.2009.01526.x) [DOI] [Google Scholar]
- 33.Cornelissen J. H. C., et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 355–380 10.1071/BT02124 (doi:10.1071/BT02124) [DOI] [Google Scholar]
- 34.Petchey O. L., Gaston K. J. 2002. Functional diversity (FD), species richness, and community composition. Ecol. Lett. 4, 402–411 [Google Scholar]
- 35.Bryant J. B., Lamanna C., Morlon H., Kerkhoff A. J., Enquist B. J., Green J. L. 2008. Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc. Natl Acad. Sci. USA 105, 7774–7778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gotelli N. J., Graves G. R. 1996. Null models in ecology. Washington, DC: Smithsonian Institution Press [Google Scholar]