Abstract
Hosts are often infected by a variety of different parasites, leading to competition for hosts and coevolution between parasite species. There is increasing evidence that some vertically transmitted parasitic symbionts may protect their hosts from further infection and that this protection may be an important reason for their persistence in nature. Here, we examine theoretically when protection is likely to evolve and its selective effects on other parasites. Our key result is that protection is most likely to evolve in response to horizontally transmitted parasites that cause a significant reduction in host fecundity. The preponderance of sterilizing horizontally transmitted parasites found in arthropods may therefore explain the evolution of protection seen by their symbionts. We also find that protection is more likely to evolve in response to highly transmissible parasites that cause intermediate, rather than high, virulence (increased death rate when infected). Furthermore, intermediate levels of protection select for faster, more virulent horizontally transmitted parasites, suggesting that protective symbionts may lead to the evolution of more virulent parasites in nature. When we allow for coevolution between the symbiont and the parasite, more protection is likely to evolve in the vertically transmitted symbionts of longer lived hosts. Therefore, if protection is found to be common in nature, it has the potential to be a major selective force on host–parasite interactions.
Keywords: protection, symbionts, evolution, vertical transmission
1. Introduction
There is an increased recognition of the importance of mixed infections in the ecology and evolution of host–parasite interactions [1]. Conflicts are especially likely to occur between parasites that co-infect the same host when they require different life histories in the host to maximize their life cycle [2,3]. For example, parasites with complex life cycles that require different secondary hosts have a clear conflict, in that only one will be successfully transmitted [4,5]. Similarly, vertically and horizontally transmitted parasites co-infecting a host will have a clear conflict [6], which may have important evolutionary consequences to the evolution of parasite life histories [7]. Therefore, when parasites with conflicting host requirements are competing for the same host, they may derive a benefit from protecting the host from future infection by other parasites [8,9]. It is increasingly recognized that the evolution of protection of the host by one parasite to infection by another may occur in nature as a result of competition and conflict between parasite species [10].
Virulent vertically transmitted parasites face a challenge in persisting in nature because they have traits that decrease the fitness of infected individuals and thus decrease their own chance of being transmitted to the next generation. One well understood route by which they may persist is by causing an increase in infected individual's reproductive potential through feminization or male killing [11–14], which in turn leads to important ecological and evolutionary consequences [15]. More recently, it has been shown that persistence can also be favoured when vertically transmitted parasites provide protection from different horizontally transmitted parasites [8,16–18]. There is increasing evidence that this protection may be widespread in nature and mediated through direct effects that reduce the uptake or development/replication of a secondary horizontally transmitted parasite [10]. In particular, strains of vertically transmitted proteobacteria in aphid hosts interfere with the development of the larvae of parasitic wasps [2,19,20]. The protective symbiotic bacteria are considered to be both facultative, since they are not found in all host individuals, and parasitic, in that they cause reduced host fecundity [21]. Similarly, in the same pea aphid host, a different symbiotic bacteria Regella insecticola provides resistance to a fungal pathogen [18,20]. More recently, certain Wolbachia strains have been shown to provide resistance to RNA viruses in Drosophila melanogaster [22,23], while Spiroplasma protects D. neotestacea against the sterilizing effects of a parasitic nematode [16]. More generally, symbionts can also protect their hosts from predators by producing predator-deterrent toxins. For example, symbionts of Paederus beetles produce the polyketide toxin pederin, which confers protection from wolf spiders [24–26]. In addition to these direct interactions, there is also evidence that behavioural modifications by vertically transmitted parasites may lead to effective protection for the host [3,27]. Given this widespread evidence for protection in nature, there is a clear need to understand its evolutionary dynamics.
Our aim is to examine the evolutionary and coevolutionary dynamics between a protecting vertically transmitted symbiont and a virulent castrating horizontally transmitted parasite. We present a model that examines the evolution of protection based on the general ecological framework described by Jones et al. [8]. The two questions that we address are: (i) when is protection most likely to evolve? and (ii) what are the evolutionary implications to the horizontally transmitting parasites when faced with a protecting vertically transmitting symbiont? We therefore first examine which characteristics of the host and the horizontally transmitting parasite favour protection. In particular, we look at the role of castration, virulence and transmission rates in the horizontally transmitted parasite. Next we examine the evolution of the horizontally transmitted parasite in response to different levels of protection and finally develop a fully coevolutionary model that allows us to examine the importance of host lifespan in determining the evolution of protection.
In all of our models, the key assumption is that protection is costly for the vertically transmitted symbiont. Without costs, we would expect protection, when it evolves to be fixed in symbiont populations. Initially we assume that there is a trade-off between protection and the virulence (defined as increased death rate) that the vertically transmitted parasite causes, such that strains of the symbiont that give higher protection also cause more damage to their host. This trade-off is likely to arise if symbionts conferring protection impose a greater metabolic load on their host and therefore cause more damage. Although there are, without doubt costs to certain secondary facultative symbionts [28–30], a direct link between protection and increased virulence in vertically transmitted parasites has not been measured. It is also conceivable that increased protection occurs through the upregulation of the host immune system and that this leads to immunopathology [31] causing increased damage to the host. We therefore also examine a trade-off in which symbionts that are better at protecting have reduced vertical transmission. As of yet, this trade-off has not been the focus of empirical investigation, but it is possible that protective strains have lower overall growth rates and thus poorer transmission. In both cases we show that the level of protection depends critically on the characteristics of both the host and the horizontally transmitting parasite and the epidemiological feedbacks that arise.
2. Modelling
We divide the host population into four groups: (i) density of the susceptible host population (with neither symbiont nor parasite infection), X; (ii) density of the protected host population (infected with a vertically transmitted symbiont), V; (iii) density of the infected host population (with a horizontally transmitted parasite), Yx; and (iv) density of the hosts infected by both the symbiont and the parasite, Yv. The population dynamics can be represented by the following system of differential equations:
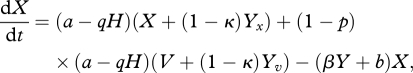 |
2.1 |
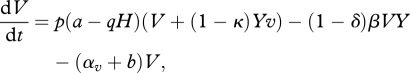 |
2.2 |
| 2.3 |
| 2.4 |
where H = X + V + Yx + Yv and Y = Yx + Yv.
Hosts are born at rate a, and have a natural death rate b, with density dependence from the total host population acting on the birth rate via the crowding parameter, q. Hosts experience an additional death rate (virulence) owing to infection by the vertically transmitted symbiont, αv. A proportion, p (where 0 ≤ p < 1) of the offspring from hosts infected with the symbiont are born infected through vertical transmission (therefore (1 − p) are born into the susceptible class). The parasite has transmission coefficient, β, but hosts infected with the symbiont experience protection from parasite transmission, δ ∈ [0,1]. Hosts infected with the horizontally transmitted parasite experience an increased death rate αy and potentially a reduced rate of reproduction owing to the level of castration, κ, by the parasite (where 0 ≤ κ ≤ 1). (A schematic diagram that represents the population dynamical equations (2.1–2.4) is shown in the electronic supplementary material, online appendix.)
(a). The evolution of protection in symbionts
We first examine how the level of protection, δ, will evolve in response to horizontally transmitted parasites with different characteristics. We first assume that protection is costly for the symbiont in that strains that produce higher protection cause more damage to their host and therefore impose a saturating trade-off between protection and the virulence that the symbiont causes. We restrict our analysis to a part of the parameter space, where a stable equilibrium with positive density for all host classes exists and is denoted by (Xr, Vr, YXr, YVr). The techniques of adaptive dynamics [32] are then used to examine the invasion of rare mutants. The invasion exponent, Im, of a given mutant with parameters (δm, αvm), attempting to invade a resident strain with parameters (δr, αvr) at equilibrium can be determined by considering the determinant of the resident-mutant Jacobian matrix at the resident equilibrium [33].
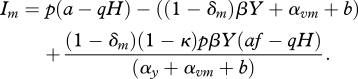 |
2.5 |
The invasion exponent is a proxy for the mutant fitness and when Im > 0, it implies that the mutant strain can invade and increase from low density in the resident environment. Adaptive dynamics techniques [32] can be applied to work out the position of evolutionary singular points (where the fitness gradient is zero) and the evolutionary behaviour at these singular points. Singular points can be evolutionarily stable (ES), whereby when at the fixed point no nearby types can invade, or convergently stable (CS), which means they evolve towards the singular point (if starting nearby). As a consequence of the saturating trade-off chosen in this study, the evolutionary singular point for a fixed set of parameters is both ES and CS and therefore an evolutionary attractor. (Further details on the derivation of the invasion exponent and the evolutionary singular point are shown in the electronic supplementary material, online appendix.)
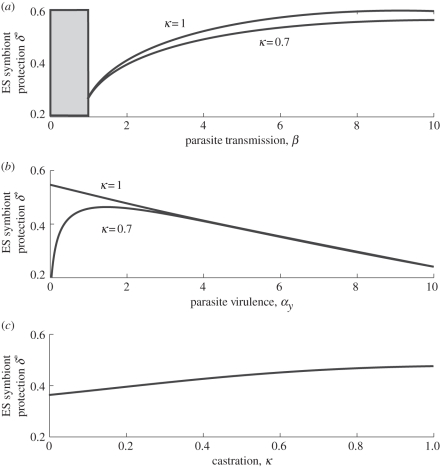
With symbiont protection (δ) linked to increased virulence (αv), we find that the higher the transmission of the virulent parasite the higher the coevolutionarily stable strategy (CSS) protection in the symbiont that evolves (figure 1a). However, this effect saturates such that increases at high levels of transmission tend to have only a relatively small effect (figure 1a). When faced with a horizontally transmitted parasite that completely castrates the host, less protection is selected for the higher the virulence of the parasite (figure 1b). However, at lower levels of castration, intermediate values of virulence are selected for the greatest protection (figure 1b). Finally we see higher levels of castration in the parasite selection for higher protection (figure 1c).
Figure 1.
The evolutionarily stable (ES) level of protection, δ*, owing to the symbiont (and symbiont virulence via the trade-off with protection) against (a) parasite transmission, the grey area denotes where the parasite was unable to persist in the system, (b) parasite virulence and (c) the level of castration caused by the parasite. The parameters are a = 5, b = 1, p = 0.95, q = 1. The trade-off is defined as αv = 5δ4 and when not varied in the figures αy = 2 and β = 3.
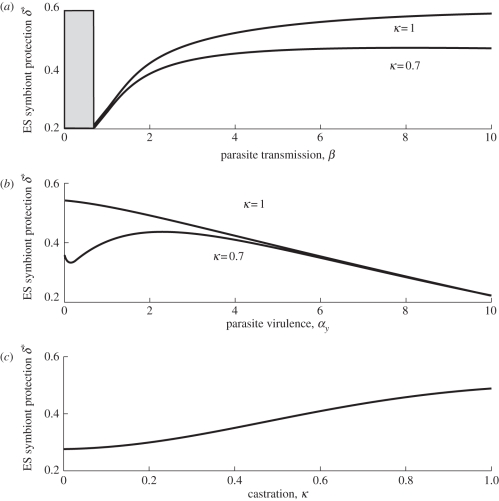
We next carry out the same analysis but this time we assume that the cost to protection comes from reduced transmission ability for the symbiont. Assuming a saturating trade-off between protection (δ) and the vertical transmission rate of the symbiont (p), we find strikingly similar results as and when the cost is through increased virulence (above). Again, higher protection in the symbiont is selected for in response to parasites with higher transmission (figure 2a), which significantly reduce host reproduction (figure 2c). Maximum protection is selected for at intermediate levels of virulence unless the horizontal parasite completely castrates the host, when minimum virulence maximizes protection (figure 2b). Overall, whether the costs to protection for the symbiont are paid through increased damage to the host, or a reduced ability to transmit, high protection will be selected for in response to competing for hosts with a highly transmissible parasite with intermediate virulence that causes significant reductions in host reproduction. If the horizontally transmitted parasite completely castrates the host, however, more protection is selected for if it has a minimal effect on host death rate.
Figure 2.
ES level of protection, δ*, owing to the symbiont (and symbiont transmission via the trade-off with protection) against (a) parasite transmission, the grey area denotes where the parasite was unable to persist in the system, (b) parasite virulence and (c) the level of castration caused by the parasite. The parameters are a = 5, b = 1, αv = 0.1, q = 1. The trade-off is defined as δ = (1−p)0.4 and when not varied in the figures αy = 2 and β = 3.
(b). Evolution of a parasite in the presence of a protecting symbiont
The presence of the vertically transmitted symbiont will clearly affect the evolution of the horizontally transmitted parasite. We have examined the evolutionary implication to the parasite of competing for hosts with vertically transmitted parasites that do not provide protection [7]. Here, we consider how different levels of protection by the symbiont might select for parasite transmission and virulence. We assume that there is a trade-off between the transmission and virulence of the parasite (following classical theory [34,35]). This well-established assumption rests on the idea that increased within-host growth rates of the parasite cause damage and therefore increased virulence, but that the higher growth rate increases transmission [36–39]. We assume a saturating trade-off, and determine the invasion exponent of a mutant parasite strain with parameters (βm, αym), attempting to invade a resident strain with parameters (βr, αyr), which is at the equilibrium (Xr, Vr, YXr, YVr). The invasion exponent is given by
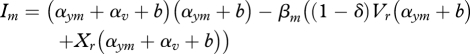 |
2.6 |
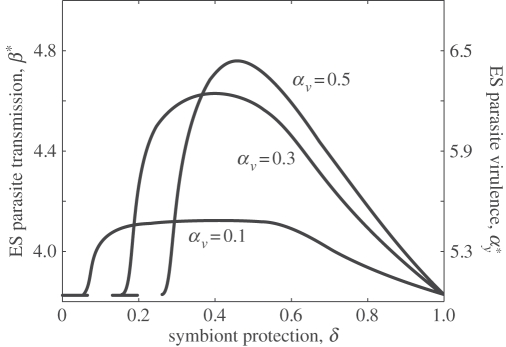
and, as carried out previously, can be used to determine the position and evolutionary behaviour at singular points (the choice of a saturating trade-off produces an evolutionary attracting singular point). Parasite transmission and virulence peak at intermediate levels of symbiont protection and then decrease as protection becomes very efficient (figure 3). This threshold, above which an increase in protection by the symbiont causes selection for reduced levels of parasite transmission and virulence, arises because when there is very high protection there are fewer susceptibles for the parasite to infect.
Figure 3.
ES level of parasite transmission, β* (and virulence, 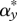 via the trade-off) at different virulences and protection levels of the symbiont. The parameters are a = 5, b = 1, p = 0.99, q = 1, κ = 1. The trade-off is defined as αy = δ1.2.
via the trade-off) at different virulences and protection levels of the symbiont. The parameters are a = 5, b = 1, p = 0.99, q = 1, κ = 1. The trade-off is defined as αy = δ1.2.
(c). Coevolution of a protecting symbiont and horizontally transmitting parasite
We next examine the evolution of protection when both the parasite and the symbiont can coevolve. The approach is to use the invasion conditions for the evolution of parasite and symbiont in isolation (equations (2.5) and (2.6)) and plot the position of the CSS at the intersection of the singular points for the parasite and symbiont. In all cases, a saturating trade-off between protection and symbiont virulence and parasite transmission and virulence is assumed and which leads to a coevolutionarily attracting singular point (the intersection of two CSSs).
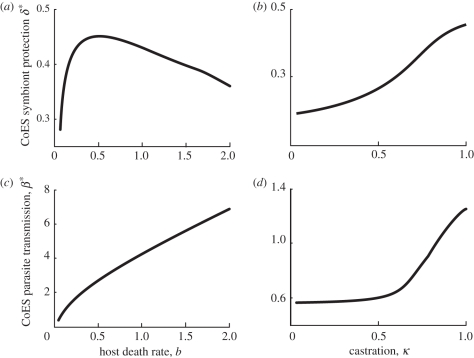
We show that transmission and virulence in the parasite increase as host death rate increases (which equates to a reduced average lifespan for the host; figure 4a). If the host lifespan is short, it is important for the parasite to invest in transmission (with the associated increase in virulence). In contrast, the symbiont responds to the increase in host death rate by initially increasing protection owing to the corresponding increase in transmission and virulence of the parasite. As host death rate increases further (which reduces the overall force of the parasite infection), the symbiont evolves towards lower virulence and protection. Increasing levels of host castration by the parasite leads to selection for higher virulence in both the parasite and symbiont (figure 4b). The increase in castration drives the increase in protection and (and virulence) in the symbiont as was seen in figure 1c. The increase in castration has no direct effect on the evolved level of parasite transmission (equation (2.6)); however, its evolution is driven through the effect of castration on the symbiont. This interaction leads to a small increase in the CSS parasite transmission.
Figure 4.
The coevolutionarily stable (CoES) level of symbiont protection (a,b) and parasite transmission (c,d), against (a) the host death rate and (b) the level of parasite castration. Trade-offs between parasite transmission and virulence, and symbiont protection and virulence are assumed. When not varied in the figures, the parameters are a = 5, b = 0.1, κ = 1, q = 1 and the trade-offs are αy = β1.2 and αv = 5δ4.
3. Discussion
Our models predict that protection in symbionts will be selected for by fast-transmitting, castrating parasites with low to intermediate virulence. We would therefore expect the evolution of protection in vertically transmitting symbionts when they are in competition with horizontally transmitting parasites that have strong effects on host fecundity. Given the number of horizontally transmitted arthropod parasites that effectively sterilize their hosts, we would expect to find protection conferred by vertically transmitted arthropod symbionts. Indeed, there is evidence of protection from a few key systems, including pea-aphid parasitoids [2,19] and fungi [21]. Our results suggest that horizontally transmitting parasites that act to reduce fecundity, rather than increase mortality, act to select for increased protection by symbionts. Therefore, we may be less likely to see protection in systems, where the horizontally transmitting parasite acts predominantly to increase the mortality of the host. That said, although often overlooked, there may be sublethal effects on fecundity of even the most virulent diseases [40] and therefore protection may often be selected for. This further emphasizes the importance of examining the effects of disease on fecundity [41] and that these effects may have very important evolutionary implications [42].
Generally protection is more likely to evolve in response to parasites with high transmission rates. This makes intuitive sense since more individuals are likely to become challenged with co-infection and we would expect a higher realized benefit from protection. Less intuitive, perhaps, is the result that very high parasite virulence tends to select for lower levels of protection. At maximum castration, the relationship between parasite virulence and symbiont protection is close to negatively linear (figures 1b and 2b with κ = 1). This is because, as castration decreases, the cost of being co-infected decreases, and therefore at low parasite virulence, the level of evolved protection also decreases (figures 1b and 2b with κ = 0.7). The selection for lower protection at higher parasite virulence is thus due to a reduction in horizontally transmitted parasite prevalence in the population as the infectious period is reduced. In other words, the cost of protection is no longer worth paying since the challenge of co-infection is less common. As virulence falls, prevalence rises and co-infection is more likely to occur, subsequently increasing the benefit of protection. On the other hand, at very low virulence and low castration, there is relatively little cost to actually being co-infected and hence selection for protection falls again.
We have also examined how the presence of a protective symbiont will affect the evolution of a shared horizontally transmitting parasite. The main result is that it is intermediate/high levels of protection that select for higher virulence and transmission. There is an obvious interest in what determines the virulence of parasites and particularly in what leads to the evolution of fast acute pathogens that cause high virulence in their host. The potential for cryptic symbiotic parasites to select for high virulence in co-infecting hosts has recently been examined in detail theoretically [7]. Here we build on these results by showing that if these symbionts offer protection to the host then selection for high parasite virulence may be further enhanced. A consequence of the evolution of protection in the symbiont may therefore lead to be the evolution of a more deadly horizontally transmitted parasite for the host. Specifically, fewer hosts will become infected, but those that do are more likely to die. The possibility that cryptic symbionts (which seem to be ubiquitous in arthropods; [10]) have selected for the high numbers of virulent parasites found in arthropod populations thus deserves further attention.
In our coevolutionary models, we find that short-lived hosts select for more virulent horizontally transmitted parasites, but less-virulent and less-protective symbionts. An increase in virulence and transmission of parasite in short-lived hosts is expected from previous theories [43–45]. Vertically transmitted symbionts show a decrease in the evolution of protection in short-lived hosts as it becomes less important. In particular, it is less important because the high virulence of the horizontal parasite reduces the infectious period and therefore the prevalence of infection, and therefore less advantage to protection. The implication of this is that we predict the evolution of protection in relatively longer lived hosts challenged by less-virulent horizontally transmitting parasites.
Throughout, we assume that the parasites and symbionts evolve at similar rates, and we do not consider the evolution of resistance in the host. Protection by the symbiont may be seen in some sense to act as a defence mechanism for the host, leading to the idea of adaptation via symbiosis [16]. This may be particularly important for longer lived hosts, in which the host is unable to evolve resistance at a similar rate to the parasite's response. It is conceivable that in some cases a protective symbiont, that potentially evolves very rapidly, may be the major component of defence in the host towards fast-evolving virulent parasites. Furthermore, symbionts that have evolved the ability to circumvent host defences in order to be maintained in host lineages, may also tend to be the ones that evolve the ability to mimic the defences. For example, bacteria have been found to be capable of producing antimicrobial compounds that protect their hosts from pathogens [46,47]. In effect, these bacterial symbionts may act as a fast-evolving surrogate immune system. Another limitation of our model is that we do not allow parasite-infected hosts to recover which intuitively is likely to reduce selection for protection. Future work in a framework that allows hosts to recover would allow the evolutionary dynamics of protection via faster clearance and reduced virulence (tolerance) [42,48] to be examined. The details of where protection acts may have important consequences for its evolution and more generally there are likely to be subtle interactions between selection acting indirectly on symbiotic protection and directly on host defence [49]. We hope that this model can provide a baseline on which a theory that examines the evolution of the combination of protection through symbiosis and direct host defence can be built.
The view that many vertically transmitted symbionts, and indeed other parasites, protect the hosts that they infect from further infection is becoming increasingly accepted [50]. With the advance of molecular techniques for describing the plethora of vertical parasites that went hitherto unnoticed [51], a key question is emerging as to how these many parasites persist and transmit in a shared host population. One clear possibility is that they offer host protection. Our models predict that protection is most likely to evolve in vertically transmitting symbionts that infect longer lived hosts that are challenged with castrating and fast-transmitting parasites. In addition, many horizontally transmitting diseases including insect viruses have been shown to have interfering as well as additive effects on each other's virulence and transmission when co-infecting the same host [52–54]. The evolutionary dynamics of protection between parasites with the same transmission mode also need to be understood. Taken as a whole, our results emphasize that more empirical evidence is needed that not only examines whether particular parasites are protective, but also whether there are any costs associated with protection, and if there are, where do they act. As these data become available, a meta-analysis would allow the predictions of the theory to be examined in detail.
Acknowledgements
The project was funded by an NERC studentship, A.W. is supported by a Royal Society of Edinburgh and Scottish Government Research Support Fellowship, while M.B. is supported by a Leverhulme Trust Fellowship.
References
- 1.Read A. F., Taylor L. H. 2001. The ecology of genetically diverse infections. Science 292, 1099–1102 10.1126/science.1059410 (doi:10.1126/science.1059410) [DOI] [PubMed] [Google Scholar]
- 2.Oliver K. M., Russell J. A., Moran N. A., Hunter M. S. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA 100, 1803–1807 10.1073/pnas.0335320100 (doi:10.1073/pnas.0335320100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas F., Fauchier J., Lafferty K. D. 2002. Conflict of interest between a nematode and a trematode in an amphipod host: test of the ‘sabotage’ hypothesis. Behav. Ecol. Sociobiol. 51, 296–301 10.1007/s00265-001-0442-2 (doi:10.1007/s00265-001-0442-2) [DOI] [Google Scholar]
- 4.Cezilly F., Gregoire A., Bertin A. 2000. Conflict between co-occuring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology 120, 625–630 [DOI] [PubMed] [Google Scholar]
- 5.Lafferty K. D. 1999. The evolution of trophic transmission. Parasitology 15, 111–115 [DOI] [PubMed] [Google Scholar]
- 6.Rigaud T., Haine E. R. 2005. Conflict between co-occuring parasites as a confounding factor in manipulation studies? Behav. Process. 68, 259–262 10.1016/j.beproc.2004.09.005 (doi:10.1016/j.beproc.2004.09.005) [DOI] [PubMed] [Google Scholar]
- 7.Jones E. O., White A., Boots M. 2010. The evolutionary implications of conflict between parasites with different transmission modes. Evolution 64, 2408–2416 10.1111/j.1558-5646.2010.00992.x (10.1111/j.1558-5646.2010.00992.x) [DOI] [PubMed] [Google Scholar]
- 8.Jones E. O., White A., Boots M. 2007. Interference and the persistence of vertically transmitted parasites. J. Theor. Biol. 246, 10–17 10.1016/j.jtbi.2006.12.007 (doi:10.1016/j.jtbi.2006.12.007) [DOI] [PubMed] [Google Scholar]
- 9.Lively C. M., Clay K., Wade M. J., Fuqua C. 2005. Competitive co-existence of vertically and horizontally transmitted parasites. Evol. Ecol. Res. 7, 1183–1190 [Google Scholar]
- 10.Hurst G. D. D., Darby A. C. 2009. The inherited microbiota of arthropods, and their importance in understanding resistance and immunity. In Insect infection and immunity. Evolution, ecology, and mechanisms (eds Rolff J., Reynolds S.), pp. 119–136 New York, NY: Oxford University Press [Google Scholar]
- 11.Hurst G. D. D., Purvis E., Sloggett J., Majerus M. 1994. The effect of infection with male-killing Rickettsia on the demography of female Adalia punctala L. (two-spot ladybird). Heredity 73, 309–316 10.1038/hdy.1994.138 (doi:10.1038/hdy.1994.138) [DOI] [Google Scholar]
- 12.Hurst G. D. D., Jiggins F. M. 2000. Male-killing bacteria in insects: mechanisms, incidence and implications. Emerg. Infect. Dis. 6, 329–336 10.3201/eid0604.000402 (doi:10.3201/eid0604.000402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst L. D. 1993. The incidences, mechanisms and evolution of cytoplasmic sex-ratio distorters in animals. Biol. Rev. Camb. Philos. Soc. 68, 121–193 [Google Scholar]
- 14.Terry R. S., Smith J. E., Dunn A. M. 1998. Impact of a novel feminizing microsporidium on its crustacean host. J. Eukaryot. Microbiol. 45, 497–501 10.1111/j.1550-7408.1998.tb05106.x (doi:10.1111/j.1550-7408.1998.tb05106.x) [DOI] [Google Scholar]
- 15.Riegler M., O'Neill S. L. 2007. Evolutionary dynamics of insect symbiont associations. Trends Ecol. Evol. 22, 625–627 10.1016/j.tree.2007.08.013 (doi:10.1016/j.tree.2007.08.013) [DOI] [PubMed] [Google Scholar]
- 16.Jaenike J., Unckless R., Cockburn S. N., Boelio L. M., Perlman S. J. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329, 212–215 10.1126/science.1188235 (doi:10.1126/science.1188235) [DOI] [PubMed] [Google Scholar]
- 17.Lipsitch M., Nowak M. A., Ebert D., May R. M. 1995. The population dynamics of vertically and horizontally transmitted parasites. Proc. R. Soc. Lond. B 260, 321–327 10.1098/rspb.1995.0099 (doi:10.1098/rspb.1995.0099) [DOI] [PubMed] [Google Scholar]
- 18.Regniere J. 1984. Vertical transmission of diseases and population dynamics of insects with discrete generations: a model. J. Theor. Biol. 107, 287–301 10.1016/S0022-5193(84)80029-6 (doi:10.1016/S0022-5193(84)80029-6) [DOI] [Google Scholar]
- 19.Ferrari J., Darby A. C., Daniell T. J., Godfray C. J., Douglas A. E. 2004. Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol. Entomol. 29, 60–65 10.1111/j.1365-2311.2004.00574.x (doi:10.1111/j.1365-2311.2004.00574.x) [DOI] [Google Scholar]
- 20.Oliver K. M., Campos J., Moran N. A., Hunter M. S. 2007. Population dynamics of defensive symbionts in aphids. Proc. R. Soc. B 275, 293–299 10.1098/rspb.2007.1192 (doi:10.1098/rspb.2007.1192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarborough C. L., Ferrari J., Godfray C. J. 2005. Aphid protected from pathogen by endosymbiont. Science 310, 1781. 10.1126/science.1120180 (doi:10.1126/science.1120180) [DOI] [PubMed] [Google Scholar]
- 22.Hedges L. M., Brownlie J. C., O'Neill S. L., Johnson K. N. 2008. Wolbachia and virus protection in insects. Science 322, 702–702 10.1126/science.1162418 (doi:10.1126/science.1162418) [DOI] [PubMed] [Google Scholar]
- 23.Teixeira L., Ferreira A., Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, 2753–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellner R. L. L., Dettner K. 1996. Differential efficacy of toxin pederin in deterring potential arthropod predators of Paederus (Coloptera, Staphylinidae) offspring. Oecologia 107, 300. [DOI] [PubMed] [Google Scholar]
- 25.Kellner R. L. L. 1999. What is the basis of pederin polymorphism in Paederus riparius rove beetles? The endosymbiotic hypothesis. Entomol. Exp. Appl. 93, 41–49 10.1023/A:1003842927497 (doi:10.1023/A:1003842927497) [DOI] [Google Scholar]
- 26.Kellner R. L. L. 2001. Suppression of pederin biosynthesis through antibiotic elimination of endosymbionts in Paederus sabaeus. J. Insect Physiol. 47, 475–483 10.1016/S0022-1910(00)00140-2 (doi:10.1016/S0022-1910(00)00140-2) [DOI] [PubMed] [Google Scholar]
- 27.Haine E. R., Boucansaud K., Rigaud T. 2005. Conflict between parasites with different transmission strategies infecting an amphipod host. Proc. R. Soc. B 272, 2505–2510 10.1098/rspb.2005.3244 (doi:10.1098/rspb.2005.3244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y. P., Zhao Y., Hammond J., Hsu H. T., Evans J., Feldlaufer M. 2004. Multiple virus infections in the honey bee and genome divergence of honey bee viruses. J. Invertebr. Pathol. 87, 84–93 10.1016/j.jip.2004.07.005 (doi:10.1016/j.jip.2004.07.005) [DOI] [PubMed] [Google Scholar]
- 29.Oliver K. M., Moran N. A., Hunter M. S. 2006. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc. R. Soc. B 273, 1273–1280 10.1098/rspb.2005.3436 (doi:10.1098/rspb.2005.3436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell J. A., Moran N. A. 2006. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. R. Soc. B 273, 603–610 10.1098/rspb.2005.3348 (doi:10.1098/rspb.2005.3348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham A. L., Allen J. E., Read A. F. 2005. Evolutionary causes and consequences of immunopathology. Ann. Rev. Ecol. Evol. Syst. 36, 373–397 10.1146/annurev.ecolsys.36.102003.152622 (doi:10.1146/annurev.ecolsys.36.102003.152622) [DOI] [Google Scholar]
- 32.Geritz S. A. H., Kisdi E., Meszena G., Metz J. A. J. 1998. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol. Ecol. Res. 12, 35–57 10.1023/A:1006554906681 (doi:10.1023/A:1006554906681) [DOI] [Google Scholar]
- 33.Miller M. R., White A., Boots M. 2005. The evolution of host resistance: tolerance and control as distinct strategies. J. Theor. Biol. 236, 198–207 10.1016/j.jtbi.2005.03.005 (doi:10.1016/j.jtbi.2005.03.005) [DOI] [PubMed] [Google Scholar]
- 34.Anderson R. M., May R. M. 1982. Coevolution of hosts and parasites. Parasitology 85, 411–421 10.1017/S0031182000055360 (doi:10.1017/S0031182000055360) [DOI] [PubMed] [Google Scholar]
- 35.Bremermann H. J., Thieme H. R. 1989. A competitive exclusion principle for pathogen virulence. J. Math. Biol. 27, 179–190 [DOI] [PubMed] [Google Scholar]
- 36.De Roode J. C., Yates A. J., Altizer S. 2008. Virulence-transmission trade-offs and population divergence in virulence in a naturally-occurring butterfly parasite. Proc. Natl Acad. Sci. USA 105, 7489–7494 10.1073/pnas.0710909105 (doi:10.1073/pnas.0710909105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebert D. 1998. Evolution: experimental evolution of parasites. Science 282, 1432–1435 10.1126/science.282.5393.1432 (doi:10.1126/science.282.5393.1432) [DOI] [PubMed] [Google Scholar]
- 38.Mackinnon M. J., Read A. F. 1999. Genetic relationships between parasite virulence and transmission in the rodent malaria Plasmodium chabaudi. Evolution 52, 689–703 [DOI] [PubMed] [Google Scholar]
- 39.Mackinnon M. J., Read A. F. 1999. Selection for high and low virulence in the malaria parasite Plasmodium chabaudi. Proc. R. Soc. Lond. B 266, 741–748 10.1098/rspb.1999.0699 (doi:10.1098/rspb.1999.0699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boots M., Norman R. 2000. Sublethal infection and the population dynamics of host–microparasite interactions. J. Anim. Ecol. 69, 517–524 10.1046/j.1365-2656.2000.00417.x (doi:10.1046/j.1365-2656.2000.00417.x) [DOI] [Google Scholar]
- 41.Dobson A. P., Hudson P. J. 1992. Regulation and stability of a free-living host–parasite system: Trichostrongylus tenuis in Red Grouse. J. Anim. Ecol. 61, 487–498 [Google Scholar]
- 42.Best A., White A., Boots M. 2010. Resistance is futile: tolerance can explain why parasites don't always castrate their hosts. Evolution 64, 348–357 10.1111/j.1558-5646.2009.00819.x (doi:10.1111/j.1558-5646.2009.00819.x) [DOI] [PubMed] [Google Scholar]
- 43.Gandon S., Jansen V. A. A., van Baalen M. 2001. Host life-history and the evolution of parasite virulence. Evolution 12, 35–57 [DOI] [PubMed] [Google Scholar]
- 44.Restif O., Koella J. C. 2003. Shared control of epidemiological traits in a coevolutionary model of host–parasite interactions. Am. Nat. 161, 827–836 10.1086/375171 (doi:10.1086/375171) [DOI] [PubMed] [Google Scholar]
- 45.van Baalen M., Sabelis M. W. 1995. The dynamics of multiple infections and the evolution of virulence. Am. Nat. 146, 881–910 10.1086/285830 (doi:10.1086/285830) [DOI] [Google Scholar]
- 46.Gil-Turnes S., Hay M. E., Fenical W. 1989. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 247, 116–118 [DOI] [PubMed] [Google Scholar]
- 47.Gil-Turnes S., Fenical W. 1992. Embryos of Homarus americanus are protected by epibitic bacteria. Biol. Bull. 182, 105–108 10.2307/1542184 (doi:10.2307/1542184) [DOI] [PubMed] [Google Scholar]
- 48.Best A., White A., Boots M. 2008. Maintenance of host variation in tolerance to pathogens and parasites. Proc. Natl Acad. Sci. 105, 20 786–20 791 10.1073/pnas.0809558105 (doi:10.1073/pnas.0809558105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boots M., Best A., Miller M. R., White A. 2009. The role of ecological feedbacks in the evolution of host defence: what does theory tell us? Phil. Trans. R. Soc. B 364, 27–36 10.1098/rstb.2008.0160 (doi:10.1098/rstb.2008.0160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haine E. R. 2007. Symbiont-mediated protection. Proc. R. Soc. B 275, 353–361 10.1098/rspb.2007.1211 (doi:10.1098/rspb.2007.1211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terry R. S., et al. 2004. Widespread vertical transmission and associated host sex-ratio distortion within the eukaryotic phylum Microspora. Proc. R. Soc. Lond. B 271, 1783–1789 10.1098/rspb.2004.2793 (doi:10.1098/rspb.2004.2793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bird F. T. 1959. Polyhedrosis and granulosis viruses causing single and double infections in the spruce budworm, Choristoneura fumiferana (Clemens). J. Insect Pathol. 1, 406–430 [Google Scholar]
- 53.Tanada Y. 1959. Synergism between two viruses of the army worm, Pseudaletia unipuncta (Haworth) (Lepidoptera, Nocturnidae). J. Insect Pathol. 1, 215–231 [Google Scholar]
- 54.Tanada Y. 1985. A synopsis of studies on the synergistic property of an insect baculovirus: a tribute to Edward A. Steinhaus (Founders Lecture). J. Invertebr. Pathol. 45, 125–138 [Google Scholar]