Abstract
Invertebrates mount a sophisticated immune response with the potential to exhibit a form of immune memory through ‘priming’. Increased immune protection following early exposure to bacteria has been found both later in life (within generation priming) and in the next generation (transgeneration priming) in a number of invertebrates. However, it is unclear how general immune priming is and whether immune priming occurs in response to different parasites, including viruses. Here, using Plodia interpuctella (Lepidoptera) and its natural DNA virus, Plodia interpunctella granulosis virus, we find evidence for both within generation and transgeneration immune priming. Individuals previously exposed to low doses of virus, as well as the offspring of exposed individuals, are subsequently less susceptible to viral challenge. Relatively little is known about the mechanisms that underpin viral immunity but it is probable that the viral immune response is somewhat different to that of bacteria. We show that immune priming may, however, be a characteristic of both responses, mediated through different mechanisms, suggesting that immune memory may be a general phenomenon of insect immunity. This is important because immune priming may influence both host–parasite population and evolutionary dynamics.
Keywords: invertebrate immunity, immune priming, virus, Plodia interpunctella, PiGV
1. Introduction
Parasites, broadly defined to include both macroparasites and microparasites such as bacteria and viruses, have pronounced effects on host fitness and life history, and as a result help shape host evolution [1,2] population dynamics [3,4] and community structure [5–7]. Generally there will be an optimal level of immune defence against parasites determined by the associated costs of resistance and the risk of infection [2,8,9]. In nature, hosts are faced with attack from a range of different parasites, but in many circumstances they may be more likely to be repeatedly exposed to the same parasites either within one generation or across consecutive generations. The likelihood of such future exposure to a parasite will clearly determine the cost to benefit balance of eliciting an immune response and influence the type, specificity and length of the response.
The acquired immune system of vertebrates is well understood, and its primary role is to provide long lasting protection to microparasitic infections [10]. However, there are now a number of examples in invertebrates where previous exposure to parasites has led to increased protection on subsequent challenge (e.g. [11–15]). This increased or acquired protection against microparasitic infection in invertebrates following an initial exposure to the same parasite, a different parasite or an immune response elicitor has been termed ‘immune priming’. In some cases this protection seems to be broad. For example, previous exposure to lipopolysaccharides (LPS)-bacterial cell wall components, increased protection against a fungal parasite in the mealworm beetle, Tenebrio molitor [12]. However, there are a number of cases where the protection provided by the initial exposure is more pronounced when the parasite is of the same taxonomic type, species or even strain [13–15]. It is also increasingly apparent that exposure of mothers to parasites may influence offspring immunocompetence in invertebrates. For example, in Daphnia magna, offspring from mothers primed with the bacteria Pasteuria ramosa suffered less of a reduction in fitness, in terms of reproductive output, when subsequently infected with this bacteria [11]. This protection was also found to be specific, such that offspring exposed to the same parasite strain as their mother had a greater fitness advantage than offspring exposed to a different parasite to their mother. In addition, in the cabbage semilooper (Trichoplusia ni), offspring from mothers that had been raised on a bacteria-rich diet had an increased immune response in terms of immune enzyme activity, and the expression and transcription of immune-related proteins [16]. The phenomenon of transferring protection to parasites from mother to offspring in invertebrates is termed ‘transgenerational immune priming’. While most of these studies focus on maternal transgenerational immune priming, there is now evidence that paternal transgenerational immune priming can occur [17], which further highlights the need to understand how widespread the phenomenon is in nature.
The immune interactions between insects and their bacterial and fungal parasites are becoming increasingly well understood, while our knowledge of insect–virus interactions remains much more limited [18,19]. Potential mechanisms of viral resistance in insects may include essential defence processes such as RNA interference [20] and apoptosis [21]. There is a lack of generality and consistency in viral resistance mechanisms across insect taxa and there is debate as to whether the immune pathways and effectors which are responsible for clearing viral infections are similar (e.g. [22,23]) or different (e.g. [18,24,25]) to those important in antibacterial response. For example, Toll, an immune pathway involved in defence against Gram positive bacteria, is important in the response of Drosophila melanogaster to Drosophila X virus [23] and Aedes aegypti to dengue virus [22]. In D. melanogaster the Imd immune pathway, which is involved in the defence against Gram negative bacteria, has also been shown to be involved in antiviral immune responses [26]. However, haemolymph from D. melanogaster infected with Drosophila C virus contained none of the molecules which are the hallmark of the response to bacterial challenge [25]. Given that there may be differences between antibacterial and antiviral immune mechanisms, it is unclear whether the immune priming that occurs in response to bacterial exposure will also occur in response to viruses. Evidence for within generation immune priming to White spot syndrome virus in the crustaceans Penaeus monodon and Penaeus japonicus has been found [27,28] but within generation and transgeneration immune priming to viruses in insects has not been examined in detail. A greater understanding of insect–virus interactions, and antiviral resistance in particular is not only important for the control of human viral diseases vectored by insects including Dengue fever and West Nile Virus [29], but also because insect viruses are used as biocontrol agents and biopesticides [30].
Here, we assess whether early exposure to virus leads to immune priming either within or transgenerationally in an insect. We use the well-developed host–parasite laboratory model system, Plodia interpunctella (Lepidoptera) and its natural virus Plodia interpunctella granulosis virus (PiGV). In particular, we examine the effect of viral exposure in early life, and viral exposure in the previous generation on rates of subsequent infection after further challenge with the virus. We, to our knowledge, demonstrate for the first time in insects, that previous exposure to a low dose of live virus increases resistance to a lethal challenge both later in life and in the next generation.
2. Material and methods
(a). The insect–virus system
The Indian meal moth, P. interpunctella, is a pest of stored agricultural products, with a natural environment that is very similar to the one in which it is maintained in the laboratory. Insects were reared on a cereal-based diet consisting of 50 per cent Ready Brek, 30 per cent bran and 20 per cent rice, with 20 g yeast, 0.2 g sorbic acid, 0.2 g methyl paraben, 25 ml honey and 25 ml glycerol added to 100 g of cereal mix. Insects were kept at 27°C in a 16 L : 8 D regime. We used a naturally occurring DNA virus, PiGV, that infects larvae through the oral ingestion of viral particles. When the occlusion bodies enter the midgut their protein coat is dissolved, and virions are released into the midgut cavity and enter midgut epithelium cells. Secondary tissue infection occurs once the virus has passed through the midgut and virus proliferation in fat bodies and other tissues leads to cell lysis, tissue destruction and eventual host death. Infected individuals have a characteristic opaque white colour, and are easily distinguishable from healthy individuals. Once symptomatic, larvae die before pupation. Purified virus solution was produced by centrifugation of a homogenate of infected individuals [31], diluted in 75 per cent blue food dye in double distilled water with 5 per cent sucrose to the required viral concentration. Droplets of virus/dye solution were orally administered to the larvae using a droplet feeding method [32]. Only insects successfully inoculated (indicated by the presence of dye in half of the length of the gut) were used in the experiments. The same inoculation procedure was used for control larvae but using only the dye solution. Prior to the experiments a number of dose–response assays were carried out from which the lethal dose of 1 per cent (LD1) for second and third instar larvae and the lethal dose of 50 per cent (LD50) for third and fourth instar larvae were calculated.
(b). Within generation immune priming to virus
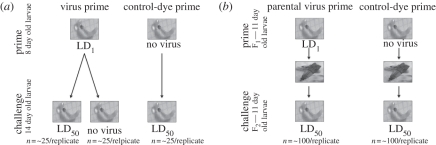
Experimental insects were established by placing 30 newly emerged adults from a large outbred stock population onto 40 g of food. Adults were left to mate and lay eggs. Second instar larvae (8 days) were collected from the food and starved for 2 h. Half of the larvae were orally primed with virus solution of a LD1 concentration while the other half were inoculated with control solution. Successfully inoculated larvae were given abundant food resources and virus primed and control primed larvae were kept separate. When the larvae reached the fourth instar (14 days) they were removed from the food, starved for 2 h then orally inoculated. No larvae showed viral symptoms at this stage. Virus primed and control primed larvae were kept separate and either inoculated with a LD50 virus solution or control solution. All larvae were then kept individually with abundant food resources after inoculation and examined for the presence of viral infection 7–8 days post challenge. The experiment was repeated in six blocks. The number of infected and non-infected larvae was recorded for each treatment group (figure 1a).
Figure 1.
Experimental design. (a) Within generation priming experiment. Second instar larvae (8 days) were collected from the food and starved. Half were orally primed with virus solution of concentration equivalent to LD1 and the other half were primed with control solution. When these larvae reached fourth instar (14 days) they were orally challenged with virus solution of concentration equivalent to LD50. To determine the level of infection that resulted from prime inoculation some larvae primed with virus were challenged with control solution. This figure outlines the procedure for one experimental block. This study was carried out in six blocks. (b) Transgeneration priming experiment. Third instar (11days) F1 generation larvae were removed from six containers established from the same large outbred insect stock, kept separately and starved. Larvae from three containers were primed with virus of concentration equivalent to LD1 and larvae from the other three containers were primed with a control solution. These primed larvae were left to develop and make six F2 generations. The small number of larvae that became infected following the virus prime treatment were removed immediately once they showed symptoms. Third instar (11 days) F2 generation larvae from each container were then challenged with virus solution of concentration equivalent to LD50. This figure outlines the procedure for one experimental replicate. This study was carried out in six blocks with three replicates of virus prime and three replicates of control prime per block.
(c). Transgeneration immune priming to virus
Experimental insects were established from newly emerged adults taken from a large outbred stock. Thirty adults were placed on 40 g of food in a container and left to mate and lay eggs. In total, six containers per block were established from the same large outbred stock. Third instar larvae (11 days) were taken from the food and starved for 2 h. Individuals from three containers were inoculated with a LD1 virus solution, while individuals from the remaining three containers were inoculated with control dye solution. Approximately 200 successfully inoculated larvae from each container were transferred to separate clean containers with abundant food resource after inoculation. Larvae were left to develop, pupate and emerge as adults. The small number of larvae that became infected as a result of the inoculation were removed immediately on presentation of symptoms. Upon emergence, thirty adults from each container were then transferred to separate clean containers with 40 g fresh food and allowed to mate and lay eggs (F2 generation). Third instar larvae (11 days) from each F2 container were picked out from the food, starved for 2 h and challenged with a LD50 virus solution. A number of F2 generation larvae from virus primed parents were orally inoculated with control solution and checked for symptoms to confirm that the virus could not pass vertically. Larvae were kept individually with abundant food resource after inoculation and examined for the presence of viral infection 7–8 days post-viral challenge. The number of infected and non-infected larvae was recorded for each treatment group. This experiment was repeated in six blocks (figure 1b).
(d). Statistical analysis
The effect of previous viral exposure on subsequent (same generation or next) susceptibility to viral challenge (proportion infection) was analysed using generalised linear models in R. Quasi-binomial errors were used for analysis of both experiments to correct for overdispersion. Experimental block did not explain a significant amount of variation, so statistics reported are from models with it excluded.
3. Results
(a). Within generation
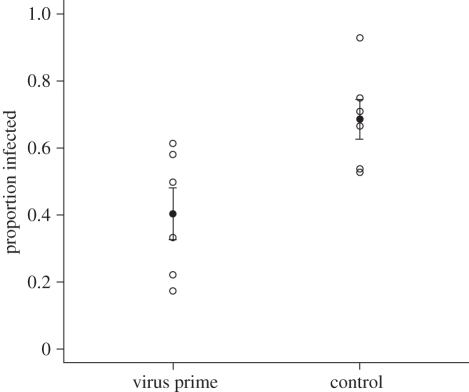
Previous exposure to a low dose of virus significantly reduced the susceptibility of insects to a subsequent lethal virus challenge (F1,10 = 9.22, p = 0.013; figure 2). Thus we provide evidence that immune priming with virus confers lasting protection against virus challenge later in life. Higher than expected levels of infection from the initial low virus exposure was found in one of the six replicates of the treatment group: virus prime, dye challenge; but when this replicate was removed the effect of priming treatment is stronger (F1,10 = 13.35, p < 0.01). Mortality was negligible and no insects exposed to only control dye solution became infected, which demonstrates that there was no contamination throughout the experiment.
Figure 2.
Within generation priming, by exposure to low level virus, significantly reduced susceptibility of insects to a lethal virus challenge (F1,10 = 9.22, p = 0.013). Open circles represent replicates, filled circles represent means ± s.e.
(b). Transgeneration
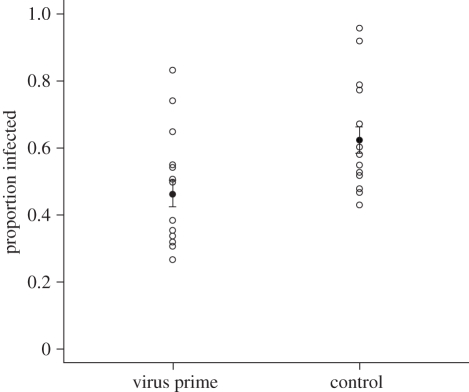
Offspring from parents exposed to a low dose of virus were less susceptible to viral challenge when compared to offspring from parents exposed to control dye solution (F1,32 = 7.13, p = 0.012; figure 3). Exposing parents to virus confers protection in offspring challenged with the same virus, providing evidence for transgenerational immune priming. One replicate from one of the experimental blocks was removed from the analysis as no F2 generation larvae were produced. We examined the F3 generation in order to rule out the possibility of selection for resistance and found that the transgeneration priming effect only lasted one generation. There was no significant difference in viral infection between F3 larvae originating from virus primed F1s and F3 larvae originating from control F1s (F1,32 = 0.81, p = 0.37), confirming that the effect seen in the F2 generation was not a result of selection. Variation between replicates in the small number of individuals that become infected and had to be removed following virus priming did not explain variation in infection following viral challenge (F1,32 = 0.0014, p = 0.97). Mortality was negligible and there was no vertical transmission and no contamination (data not shown).
Figure 3.
Transgenerational immune priming, by exposing parents to low level virus, significantly reduced the susceptibility of offspring to lethal virus challenge (F1,32 = 7.13, p = 0.012). Open circles represent replicates, filled circles represent means ± s.e.
4. Discussion
We have demonstrated both within generation and transgenerational immune priming with a DNA virus in its natural insect host. Exposure to a low dose of virus (not leading to a systemic infection) reduces subsequent susceptibility to a lethal viral challenge both later in life and in the offspring of exposed parents. There have been a number of studies demonstrating that immune priming provides protection to bacteria both within generation (e.g. [14,15]) and transgenerationally (e.g. [11,33,34]). However, immune priming to viruses is not well studied with only very limited evidence for its existence in invertebrates [28]. This is, to our knowledge, the first study to investigate and report this phenomenon in response to a virus in an insect. The immune responses to viruses in invertebrates may be different to the immune response to other parasites but this study suggests that priming may be a general phenomenon of the invertebrate immune system.
We use a natural host–virus combination, challenge the insects through the natural route of infection and use live infectious virus. We expose the hosts to a very low dose of virus through oral inoculation and subsequently challenge those primed hosts, or offspring of primed hosts, by oral inoculation with the same viral stock at a higher concentration. We therefore build on previous studies where immune priming has been found in response to heat-killed pathogens (e.g. [14]) or immune elicitors (e.g. [12]). The obvious advantage of using heat-killed pathogens or immune elicitors is that it ensures the absence of live pathogen, which may alter the immune response in the insect on subsequent pathogen challenge. It also means that there is little chance of selecting the host for increased immune function over one generation. Our approach however, directly examines a natural host–pathogen interaction by priming with live virus using the natural route of infection. The likelihood of live virus from the initial exposure still being present in the midgut of the primed insects is very minimal, given that two instars of development occur between the initial priming exposure and the subsequent challenge in the within generation study. In addition, no difference was found in susceptibility between F3 insects from virus primed F1s and F3 insects from control F1s, showing that the protection only lasted a single generation. Therefore it is unlikely that we selected for increased viral resistance in the transgeneration priming experiment. Our results suggest that the reduced susceptibility to lethal viral challenge both within and across generations is owing to immune priming.
It is possible that the effect seen both within a generation and across generations is a result of multiple components of the immune response, however the specific mechanisms that underpin immune priming in invertebrates are not well understood. There is, however, some evidence to suggest that phagocytosis by haemocytes may play a role in the specificity of immune responses in invertebrates and protection against bacteria gained from immune priming. For example, protection against bacteria resulting from previous exposure was linked to an increase in phagocytic activity in both Drosophila [13] and the woodlouse, Porcellio scaber [35]. The mechanisms involved in immune priming to viruses are even less well understood. A greater knowledge of the components that confer resistance to viruses in insects, in general, will give insight into the specific immune mechanisms or pathways that may lead to immune priming. The mechanisms conferring transgenerational resistance through immune priming are even more intriguing, and even less understood. Elevated antibacterial activity in Bombus terrestris offspring following priming of queens was shown to be dependent on factors transferred to the offspring in the egg, and not based on rearing [36]. In principle, exposure of mothers to parasites may speed up the production of immune components in offspring and/or increase the efficiency of immune components in offspring. In theory this could be the result of transmission of immune proteins or RNA from parents to offspring, but as yet there is little experimental evidence of the mechanisms that underlie the phenotypic responses that we measure.
While many studies have found evidence for immune memory in invertebrates, it is not ubiquitous. For example, studies of mosquitoes have found that priming the melanization response, an immune defence important in malarial parasite infections, did not increase the melanization response in offspring [37]. Within generation and transgenerational priming is likely to be a plastic trait dependent on specific ecological and evolutionary conditions [38]. It may also be dependent on the host or pathogen life history and the specific host–pathogen combination. Immune priming has been demonstrated in B. terrestris (e.g. [15,34]). This is a social insect and therefore immune priming may be more beneficial as repeated exposure to the same pathogen is very probable. In addition, the life history of the parasite will also be important. Here, the pathogen used is an obligate killer and therefore the cost of infection is high and will lead to strong selection pressure for resistance mechanisms in general.
Immune priming may be costly both at the individual and population level. Long lasting protection against one pathogen strain may result in selection for different strains of the parasite with different effects on the host [39]. For example, Sadd & Schmid-Hempel [40] found that offspring were more resistant when exposed to the same parasite as their mothers, but that these same offspring had increased susceptibility to different pathogens. This suggests that priming may be specific and costly in terms of resistance to other parasites, and that there may be trade-offs between resistance to different pathogens. Further work on immune priming in invertebrates needs to examine both the costs and specificity of immune priming in more detail. It is also interesting that the specificity of immune priming seems to vary between host–pathogen combinations. For example, priming by LPS in T. molitor protects against fungal infection [12], while in other interactions protection is highly specific even down to the strain of the pathogen (e.g. [11,14]). This difference in specificity may indicate that immune protection is owing to a range of different mechanisms that are not necessarily mutually exclusive.
Although mechanistically very different to vertebrate adaptive immunity, our work suggests that the insect innate immune system has the capacity to adapt in response to previous encounters with a virus. The fact that immune priming occurs in response to both viruses and bacteria in insects suggests that similar evolutionary pressures have shaped these responses, even though they may involve different components of the immune system. Immune priming may have many wider implications, such as altering the dynamics of host and pathogen populations and the interaction between co-infecting pathogens within a population. It may also be important when considering the long term success of using viral pathogens as biological control agents and when predicting the severity of viral disease outbreak.
Acknowledgements
We thank V. Spencer and E. Boardman for help with insect rearing and G. H. Long for comments on the manuscript. This work was supported by the Natural Environment Research Council (NERC).
References
- 1.Anderson R. M., May R. M. 1981. The population-dynamics of micro-parasites and their invertebrate hosts. Phil. Trans. R. Soc. Lond. B 291, 451–524 10.1098/rstb.1981.0005 (doi:10.1098/rstb.1981.0005) [DOI] [Google Scholar]
- 2.Boots M., Begon M. 1993. Trade-offs with resistance to a granulosis-virus in the Indian meal moth, examined by a laboratory evolution experiment. Funct. Ecol. 7, 528–534 10.2307/2390128 (doi:10.2307/2390128) [DOI] [Google Scholar]
- 3.Hudson P. J., Dobson A. P., Newborn D. 1998. Prevention of population cycles by parasite removal. Science 282, 2256–2258 10.1126/science.282.5397.2256 (doi:10.1126/science.282.5397.2256) [DOI] [PubMed] [Google Scholar]
- 4.Pedersen A. B., Greives T. J. 2008. The interaction of parasites and resources cause crashes in a wild mouse population. J. Anim. Ecol. 77, 370–377 10.1111/j.1365-2656.2007.01321.x (doi:10.1111/j.1365-2656.2007.01321.x) [DOI] [PubMed] [Google Scholar]
- 5.Hatcher M. J., Dick J. T. A., Dunn A. M. 2006. How parasites affect interactions between competitors and predators. Ecol. Lett. 9, 1253–1271 10.1111/j.1461-0248.2006.00964.x (doi:10.1111/j.1461-0248.2006.00964.x) [DOI] [PubMed] [Google Scholar]
- 6.Lafferty K. D., Dobson A. P., Kuris A. M. 2006. Parasites dominate food web links. Proc. Natl Acad. Sci. USA 103, 11 211–11 216 10.1073/pnas.0604755103 (doi:10.1073/pnas.0604755103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood C. L., Byers J. E., Cottingham K. L., Altman I., Donahue M. J., Blakeslee A. M. H. 2007. Parasites alter community structure. Proc. Natl Acad. Sci. USA 104, 9335–9339 10.1073/pnas.0700062104 (doi:10.1073/pnas.0700062104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoang A. 2001. Immune response to parasitism reduces resistance of Drosophila melanogaster to desiccation and starvation. Evolution 55, 2353–2358 [DOI] [PubMed] [Google Scholar]
- 9.Kraaijeveld A. R., Godfray H. C. J. 1997. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389, 278–280 10.1038/38483 (doi:10.1038/38483) [DOI] [PubMed] [Google Scholar]
- 10.Murphy K. M., Travers P., Walport M. 2007. Janeway's immunobiology, 7th edn. New York, NY: Garland Science [Google Scholar]
- 11.Little T. J., O'Connor B., Colegrave N., Watt K., Read A. F. 2003. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 13, 489–492 10.1016/S0960-9822(03)00163-5 (doi:10.1016/S0960-9822(03)00163-5) [DOI] [PubMed] [Google Scholar]
- 12.Moret Y., Siva-Jothy M. T. 2003. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. Lond. B 270, 2475–2480 10.1098/rspb.2003.2511 (doi:10.1098/rspb.2003.2511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham L. N., Dionne M. S., Shirasu-Hiza M., Schneider D. S. 2007. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3, e26. 10.1371/journal.0030026 (doi:10.1371/journal.0030026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth O., Sadd B. M., Schmid-Hempel P., Kurtz J. 2009. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc. R. Soc. B 276, 145–151 10.1098/rspb.2008.1157 (doi:10.1098/rspb.2008.1157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadd B. M., Schmid-Hempel P. 2006. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr. Biol. 16, 1206–1210 10.1016/j.cub.2006.04.047 (doi:10.1016/j.cub.2006.04.047) [DOI] [PubMed] [Google Scholar]
- 16.Freitak D., Heckel D. G., Vogel H. 2009. Dietary-dependent trans-generational immune priming in an insect herbivore. Proc. R. Soc. B 276, 2617–2624 10.1098/rspb.2009.0323 (doi:10.1098/rspb.2009.0323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth O., Joop G., Eggert H., Hilbert J., Daniel J., Schmid-Hempel P., Kurtz J. 2010. Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum. J. Anim. Ecol. 79, 403–413 10.1111/j.1365-2656.2009.01617.x (doi:10.1111/j.1365-2656.2009.01617.x) [DOI] [PubMed] [Google Scholar]
- 18.Imler J., Elftherianos I. 2009. Drosophila as a model for studying antiviral defences. In Insect infection and immunity (eds Rolff J., Reyolds S. E.), pp. 49–68 Oxford, UK: Oxford University Press [Google Scholar]
- 19.Strand M. R. 2008. The insect cellular immune response. Insect Sci. 15, 1–14 [Google Scholar]
- 20.Wang X. H., Aliyari R., Li W. X., Li H. W., Kim K., Carthew R., Atkinson P., Ding S. W. 2006. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312, 452–454 10.1126/science.1125694 (doi:10.1126/science.1125694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke T. E., Clem R. J. 2003. Insect defenses against virus infection: the role of apoptosis. Int. Rev. Immunol. 22, 401–424 10.1080/08830180305215 (doi:10.1080/08830180305215) [DOI] [PubMed] [Google Scholar]
- 22.Xi Z. Y., Ramirez J. L., Dimopoulos G. 2008. The Aedes aegypti Toll pathway controls dengue virus infection. PLoS Pathog. 4, e1000098. 10.1371/journal.ppat.1000098 (doi:10.1371/journal.ppat.1000098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zambon R. A., Nandakumar M., Vakharia V. N., Wu L. P. 2005. The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl Acad. Sci. USA 102, 7257–7262 10.1073/pnas.0409181102 (doi:10.1073/pnas.0409181102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dostert C., Jouanguy E., Irving P., Troxler L., Galiana-Arnoux D., Hetru C., Hoffmann J. A., Imler J. L. 2005. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 6, 946–953 10.1038/ni1237 (doi:10.1038/ni1237) [DOI] [PubMed] [Google Scholar]
- 25.Sabatier L., Jouanguy E., Dostert C., Zachary D., Dimarcq J. L., Bulet P., Imler J. L. 2003. Pherokine-2 and -3: two Drosophila molecules related to pheromone/odor-binding proteins induced by viral and bacterial infections. Eur. J. Biochem. 270, 3398–3407 10.1046/j.1432-1033.2003.03725.x (doi:10.1046/j.1432-1033.2003.03725.x) [DOI] [PubMed] [Google Scholar]
- 26.Costa A., Jan E., Sarnow P., Schneider D. 2009. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE 4, 37436. 10.1371/journal.pone.0007436 (doi:10.1371/journal.pone.0007436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witteveldt J., Vlak J. M., Van Hulten M. C. W. 2004. Protection of Penaeus monodon against white spot syndrome virus using a WSSV subunit vaccine. Fish Shellfish Immunol. 16, 571–579 10.1016/j.fsi.2003.09.006 (doi:10.1016/j.fsi.2003.09.006) [DOI] [PubMed] [Google Scholar]
- 28.Wu J. L., Nishioka T., Mori K., Nishizawa T., Muroga K. 2002. A time-course study on the resistance of Penaeus japonicus induced by artificial infection with white spot syndrome virus. Fish Shellfish Immunol. 13, 391–403 10.1006/fsim.2002.0414 (doi:10.1006/fsim.2002.0414) [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Vargas I., Scott J. C., Poole-Smith B. K., Franz A. W. E., Barbosa-Solomieu V., Wilusz J., Olson K. E., Blair C. D. 2009. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito's RNA interference pathway. PLoS Pathog. 5, e1000299. 10.1371/journal.ppat.1000299 (doi:10.1371/journal.ppat.1000299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinsley T. W. 1977. Viruses and biological-control of insect pests. Bioscience 27, 659–661 10.2307/1297549 (doi:10.2307/1297549) [DOI] [Google Scholar]
- 31.Smith I. R. L., Crook N. E. 1988. In vivo isolation of Baculovirus genotypes. Virology 166, 240–244 10.1016/0042-6822(88)90165-1 (doi:10.1016/0042-6822(88)90165-1) [DOI] [PubMed] [Google Scholar]
- 32.Boots M., Begon M. 1994. Resource limitation and the lethal and sub-lethal effects of a viral pathogen in the Indian meal moth. Ecol. Entomol. 19, 319–326 10.1111/j.1365-2311.1994.tb00248.x (doi:10.1111/j.1365-2311.1994.tb00248.x) [DOI] [Google Scholar]
- 33.Moret Y. 2006. ‘Trans-generational immune priming': specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B 273, 1399–1405 10.1098/rspb.2006.3465 (doi:10.1098/rspb.2006.3465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadd B. M., Kleinlogel Y., Schmid-Hempel R., Schmid-Hempel P. 2005. Trans-generational immune priming in a social insect. Biol. Lett. 1, 386–388 10.1098/rsbl.2005.0369 (doi:10.1098/rsbl.2005.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth O., Kurtz J. 2009. Phagocytosis mediates specificity in the immune defence of an invertebrate, the woodlouse Porcellio scaber (Crustacea: Isopoda). Dev. Comp. Immunol. 33, 1151–1155 10.1016/j.dci.2009.04.005 (doi:10.1016/j.dci.2009.04.005) [DOI] [PubMed] [Google Scholar]
- 36.Sadd B. M., Schmid-Hempel P. 2007. Facultative but persistent transgenerational immunity via the mother's eggs in bumblebees. Curr. Biol. 17, R1046–R1047 10.1016/j.cub.2007.11.007 (doi:10.1016/j.cub.2007.11.007) [DOI] [PubMed] [Google Scholar]
- 37.Voordouw M. J., Lambrechts L., Koella J. 2008. No maternal effects after stimulation of the melanization response in the yellow fever mosquito Aedes aegypti. Oikos 117, 1269–1279 10.1111/j.0030-1299.2008.16741.x (doi:10.1111/j.0030-1299.2008.16741.x) [DOI] [Google Scholar]
- 38.Little T. J., Kraaijeveld A. R. 2004. Ecological and evolutionary implications of immunological priming in invertebrates. Trends Ecol. Evol. 19, 58–60 10.1016/j.tree.2003.11.011 (doi:10.1016/j.tree.2003.11.011) [DOI] [PubMed] [Google Scholar]
- 39.Kurtz J. 2004. Memory in the innate and adaptive immune systems. Microb. Infect. 6, 1410–1417 10.1016/j.micinf.2004.10.002 (doi:10.1016/j.micinf.2004.10.002) [DOI] [PubMed] [Google Scholar]
- 40.Sadd B. M., Schmid-Hempel P. 2009. A distinct infection cost associated with trans-generational priming of antibacterial immunity in bumble-bees. Biol. Lett. 5, 798–801 10.1098/rsbl.2009.0458 (doi:10.1098/rsbl.2009.0458) [DOI] [PMC free article] [PubMed] [Google Scholar]