Abstract
Behaviour evolved before nervous systems. Various single-celled eukaryotes (protists) and the ciliated larvae of sponges devoid of neurons can display sophisticated behaviours, including phototaxis, gravitaxis or chemotaxis. In single-celled eukaryotes, sensory inputs directly influence the motor behaviour of the cell. In swimming sponge larvae, sensory cells influence the activity of cilia on the same cell, thereby steering the multicellular larva. In these organisms, the efficiency of sensory-to-motor transformation (defined as the ratio of sensory cells to total cell number) is low. With the advent of neurons, signal amplification and fast, long-range communication between sensory and motor cells became possible. This may have first occurred in a ciliated swimming stage of the first eumetazoans. The first axons may have had en passant synaptic contacts to several ciliated cells to improve the efficiency of sensory-to-motor transformation, thereby allowing a reduction in the number of sensory cells tuned for the same input. This could have allowed the diversification of sensory modalities and of the behavioural repertoire. I propose that the first nervous systems consisted of combined sensory-motor neurons, directly translating sensory input into motor output on locomotor ciliated cells and steering muscle cells. Neuronal circuitry with low levels of integration has been retained in cnidarians and in the ciliated larvae of some marine invertebrates. This parallel processing stage could have been the starting point for the evolution of more integrated circuits performing the first complex computations such as persistence or coincidence detection. The sensory-motor nervous systems of cnidarians and ciliated larvae of diverse phyla show that brains, like all biological structures, are not irreducibly complex.
Keywords: sensory-motor neuron, neural circuit evolution, ciliated larva, phototaxis
1. Introduction
Nervous systems enable organisms to receive sensory information from their external environment, process this information and regulate neurosecretory and motor systems. Although neurosecretory systems have deep ancestry in metazoan evolution [1,2], it is more probable that the first neurons with fast electrical signal propagation to evolve were controlling animal locomotion.
Two different types of locomotor systems are found in animals, one muscle-based, the other cilia-based. Muscle-based motor systems are present in all metazoans except sponges (eumetazoan). Ciliary locomotion, present in single-celled eukaryotes (protists) and also sponge larvae, is the more ancient form of locomotion. Since sponges represent the deepest branches of the metazoan tree ([3]; but see also [4]) and they lack a nervous system, it follows most parsimoniously that the first nervous systems evolved along the stem lineage leading to the eumetazoans (comprising cnidarians, ctenophores and bilaterians), all possessing a nervous system.
According to one possible scenario of animal origins, the first eumetazoans had a biphasic life cycle with a ciliated larval stage that evolved from a ciliated sponge-like larva [5]. A benthic adult with a radially symmetric body plan may also have been present. If ciliated larvae were as integral to the origin of eumetazoans as this scenario suggests, then it is conceivable that the first steps of nervous system evolution occurred in a ciliated stage and that the first neural circuits evolved to control locomotor cilia.
Cilia-driven locomotion is still prominent in many animal groups. With the exception of the ecdysozoans (nematodes, arthropods and others), the larval stages of diverse phyla use cilia for locomotion [6,7]. Ciliated larvae are widespread in cnidarians, lophotrochozoans (a major protostome group comprising annelids, molluscs and other marine phyla), and deuterostomes (echinoderms, hemichordates, cephalochordates). Sometimes the adult stages are also ciliated, as in ctenophores, acoels and rotifers.
Despite its wide phylogenetic distribution and importance, we know relatively little about the nervous control of ciliary locomotion. Our studies on the ciliated larvae of the marine annelid model Platynereis dumerilii have recently shown that these larvae have direct sensory-motor neurons and sensory-motor eyes ([8]; M. Conzelmann and G. Jékely 2010, unpublished). We argued that this circuitry represents an ancient evolutionary heritage from a very early stage of eye and brain evolution. If this is the case, then the study of the circuitry of cilia-based motors can give us novel insights into the very early stages of nervous system evolution.
Muscle-based motor systems are also very ancient and trace back to the stem eumetazoan [9]. The first smooth muscles may have evolved to steer a ciliated organism during tactic behaviours (e.g. photo- or gravitaxis) and to contract a sessile adult. True muscle-based locomotion may only have evolved later from such steering and body contracting muscles. Given the very different mechanics of muscle-based locomotion in cnidarians (polyp and medusa forms) and in bilaterians (worm-like body), complex muscle-based locomotory circuits may have evolved independently in the two groups (for a detailed discussion of the ancestral form of locomotion in cnidarians and the origin of striated muscles, see [9]).
2. Mechanisms of circuit evolution
Before discussing how the first cilia- and muscle-based motor circuits may have evolved, I first give an overview of the general principles governing neural circuit evolution.
Neuronal circuits evolve by the Darwinian processes of variation and selection. Given the extremely high costs of building and maintaining nervous systems [10–12], neutral evolutionary processes are arguably less prevalent during nervous system evolution than for example in genome evolution [13]. Selective optimization strongly shapes neuroanatomy. For example, the placement of the 11 ganglia in the nematode nervous system minimizes the total wiring length [14,15]. Similar rules apply to the layout of different cortical areas in mammalian brains [16].
The higher costs of information at higher performance also contribute to the optimization of nervous systems and severely penalize the evolution of overcapacity. In fly photoreceptors, for example, a fivefold increase in performance requires a 25-fold increase in energy consumption [17].
The high price and high benefits of information processing can also lead to rapid evolutionary optimization in olfactory systems. For example, the olfactory system of the morinda fruit specialist Drosophila sechellia has been grossly reorganized during the evolution of the species by the expansion of some olfactory sensilla at the expense of others [18].
Optimization reaches down to the subcellular level, with a strong drive, for example, to minimize axon diameters to save costs. This minimization, however, leads to increased channel noise, setting a lower limit of about 0.1 µm diameter for action potential-conducting axons [19]. When speed is important, axon miniaturization is not an option, since a non-myelinated axon with a smaller diameter has decreased conduction speed. A gain in conduction speed is sometimes so vital that it drives the evolution of giant axons [20].
The huge benefits of processing information by neurons on the one hand and the high metabolic, wiring and signalling costs of nervous systems on the other, strongly influence nervous system evolution [10–12], and must have done so from the very beginnings of brain evolution.
In order to formulate meaningful hypotheses on the evolution of nervous circuits, these principles of nervous system optimization must be integrated into an evolutionary developmental (evo-devo) framework [21]. From an evo-devo perspective, mutational changes in an organism's developmental programme may generate variation in the nervous system during circuit evolution. This variation can manifest itself in neuron number and property, including neuron position, connectivity, sensory modality, excitability or plasticity. For example, total neuron number can change if alterations in the developmental programme affect the division of neuronal progenitors, leading to the duplication or elimination of certain neurons [18]. Duplications will result in identical or very similar neurons, given the similarity of their developmental programmes due to shared lineage and proximity. These neurons can subsequently diverge to form sister cell types [22]. The properties of neurons can change if mutations alter the neuronal differentiation programmes and lead to changes in the differentiation gene batteries. These changes are expected to show Darwinian gradualism, progressing by small steps in the elaboration, complexification or simplification of circuits.
Taking into consideration the above factors, I outline below a few hypothetical transition scenarios for the origin and early evolution of neural circuits in early eumetazoans and early bilaterians. The proposed scenarios are partly novel and partly based on some old ideas (e.g. [23]), that are further elaborated here based on modern data. My aim is to show how modern molecular and functional data on ciliated larvae and their circuits can influence our thinking about ancient circuit evolution.
3. The efficiency of sensory-to-motor transformation with and without a nervous system
The first question to be addressed is why the first neurons, communicating to target cells via synapses, appeared. A large diversity of single-celled eukaryotes (protists) show various forms of behaviour analogous to those found in animals. Since protists have no nervous system, a comparison of their behaviours to the same behaviours in animals could reveal the advantages of using neurons.
The behavioural repertoire of protists is rather limited, yet the individual behaviours that are present are remarkably sophisticated. Protists are capable of active predation [24], vectorial navigation such as phototaxis [25–27] or gravitaxis [28–30], and navigation along a gradient (chemotaxis; [31]). Simpler responses to mechanical [24] or light stimuli can also be present [32].
In single-celled protists it is the same cell that forms the sensory structures and also performs the motor functions (ciliary or amoeboid motility). Given that sensory structures need a minimum size to function (e.g. a certain number of photopigments to detect light), the costs of sensing a stimulus relative to the total energy budget of the organism will be higher for a protist than for a multicellular animal with specialized sensory cells. This higher relative cost will necessarily limit the repertoire of sensory inputs a protist can respond to (e.g. number of chemicals or wavelengths).
The best understood behaviour in protists is spiral vectorial phototaxis [33]. It can be found, among others, in the green alga Chlamydomonas [26], the excavate Euglena, the cryptophyte alga Cryptomonas [25], or in the ciliated spores of the chytrid fungi Allomyces [34]. An analogous spiral swimming phototactic strategy is found in the multicellular green alga Volvox [35], and also in the ciliated larvae of animals including sponges [36,37], cnidarians [38,39], annelids [8] and molluscs [40]. Some animal larvae, such as sponges [36,37] and some cnidarians [38], perform phototaxis without a nervous system, while others, including some cnidarians [39] and many bilaterians [8,40], perform phototaxis with a nervous system. Phototaxis therefore provides a very useful paradigm to assess the benefits of nervous systems in a comparative framework, even if we are comparing independent evolutionary innovations [33].
Comparison of various phototactic organisms shows that there is no major difference in the efficiency and precision of phototaxis. The single-celled Chlamydomonas [26], the multicellular Volvox [35] or the larvae of the annelid Platynereis [8] all orient very quickly and precisely along the light vector, usually within a few helical turns. What is very different between these organisms is the efficiency of sensory-to-motor transformation, estimated as the ratio of the number of sensory structures to the total number of cells being controlled. Assuming that the costs of building an efficient light-detecting device are similar for all organisms, the number of such devices divided by the total number of cells gives an estimate of the cost-efficiency of the behaviour.
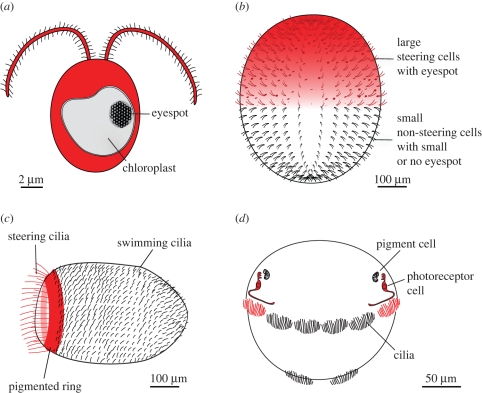
In a single-celled phototactic eukaryote, such as Chlamydomonas, the same cell senses the periodic light signal and executes the motor programme (1 : 1 ratio; figure 1). In the multicellular Volvox rousseletii, about half of the 5000 somatic cells have a sufficiently large eyespot to allow light detection. These cells, located in the anterior hemisphere of the alga, all contribute to phototactic steering (1 : 2 ratio; [35]). In the phototactic larvae of the sponge Amphimedon queenslandica a ring of ciliated photoreceptor cells (a few hundred cells) regulate phototaxis by bending their long, steering cilia. Other cells on the surface of the larva (a few thousand cells) have shorter cilia and contribute to larval locomotion (about 1 : 10 ratio). In the larvae of the annelid Platynereis, two directionally shaded photoreceptor neurons sense light and regulate steering by innervating the multiciliated cells (1 : 1000 ratio, figure 1, and see below).
Figure 1.
Comparison of the efficiency of sensory-to-motor transformation in (a) Chlamydomonas, (b) Volvox, (c) a sponge larva, and (d) an annelid larva. The parts of the organism involved in sensing and responding to the light stimulus are coloured red.
These examples show that the building of multicellular bodies with specialized sensory-effector cells, but no neurons (Volvox and sponge larva) only provide a modest improvement in the efficiency of sensory-to-motor transformation. The use of a distinct sensor and effector, connected by a chemical synapse, however, provides a dramatic improvement of about two orders of magnitude in the annelid larva. Arguably, the use of chemical synapses with a potential for signal amplification provides a major advance in the efficiency of sensory-to-motor transformation. The release of many synaptic vesicles at synapses on the target cell can potentially lead to a large change in postsynaptic conductance even if the changes in presynaptic membrane potential are small. The strength of the synapse can be tuned during evolution in such a way that small changes in the sensory cell lead to large changes in the effector cell. This synapse-mediated signal amplification (together with the concentration of cilia, see below) allows a Platynereis larva to perform phototaxis using only two sensory cells as opposed to a similar sized Volvox colony that uses hundreds of sensory cells.
The example of phototaxis illustrates how neurons can provide very effective signal amplification, allowing dramatic reductions in the number of sensory structures specialized in one modality. With the advent of neurons a similar cost reduction may have occurred for other senses as well.
4. The evolution of the first neurons to regulate cilia
Sensory cells evolved in the first metazoans before the origin of bona fide neurons [36,37,41]. Sponge larvae have flask-shaped sensory cells (additional to their photoreceptors), embedded in an epithelial layer of polarized, ciliated cells [36]. These cells express orthologues of bilaterian neurogenic molecules, indicating that they may represent a protoneuron, an ancestral stage of neuronal cell-type evolution [41].
It is unclear how these putative sensory cells can influence the behaviour of the sponge larva. Since these cells have an apical cilium it is conceivable that sensory stimuli lead to the alterations in ciliary beating in a cell-autonomous manner, similar to the photoreceptors. These cells may also release unknown transmitters to regulate ciliary beating in neighbouring cells in a paracrine fashion. The sponge genome contains genes coding for neuropeptide processing enzymes and synaptic vesicle release proteins, however no neuropeptides have been identified [1]. Even if a local release of transmitters is possible in sponges, the effects of such signal transmission can only be local, since sponges have no gap junctions and no axons [1]. This means that in order to alter locomotion (e.g. swimming speed) of the whole larva, several of these local sensory-motor units must respond to the same sensory input. This restriction probably only allows a small number of sensory specializations for the larvae. Besides light, sponge sensory capacities probably include chemosensation, an important input during larval settlement [42]. The presence of approximately 130 rhodopsin-family G-protein coupled receptors (GPCRs) in the sponge genome also suggests the use of chemosensation [1]. These GPCRs originated by lineage-specific expansion, and many are single-exon genes forming clusters in the genome [1], reminiscent of lineage-specific putative chemosensory GPCRs in other animal groups [43]. It is, however, unclear how many of these sponge GPCRs are expressed in the larval stage.
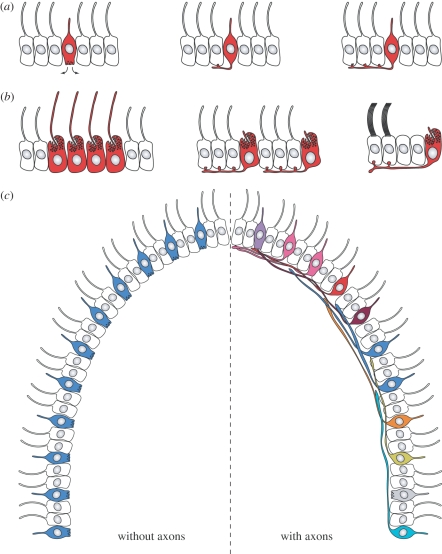
From such a sponge larva-like stage with autonomous epithelial sensory-motor cells, one can envisage a gradual transition into a minimally integrated sensory-motor nervous system controlling ciliary locomotion. In a ciliated larva the first sensory cells may have initially secreted neuropeptides or transmitters to regulate neighbouring ciliated cells, thereby altering swimming speed or direction. To channel signals more precisely and to increase the efficiency of signal transmission, these sensory cells may have developed basal processes and release sites with close contact to the neighbouring target cells (figure 2a). As these first synapses and axons evolved further, the sensory cells could have spread the amplified stimulus to more effector cells by budding off many en passant synapses in a row at the basal side of the ciliated epithelium.
Figure 2.
(a) Evolution of the first neurons to control ciliary locomotion from a sensory cell regulating its neighbouring cells by paracrine signals or (b) producing a cell-autonomous motor output with its cilia. (c) The evolution of long-range axonal contact of sensory cells to ciliated motor cells allowed the reduction of cells specialized in one input. This price reduction in sensing and behaviour allowed an increase in the number of senses.
Such a transformation probably also happened in the case of photoreceptors, which started as cell-autonomous sensory-motor units [36,38,44] and evolved into sensory-motor neurons regulating ciliary bands (figure 2b). We argued recently that the sensory-motor phototactic eyes, directly synapsing on ciliated cells in the larvae of the annelid Platynereis represent such an ancestral stage in eye circuit evolution [8].
Many neuropeptidergic sensory cells also directly innervate the ciliary band in Platynereis larvae and the released neuropeptides regulate ciliary beating (Conzelmann and Jékely, unpublished). Such sensory-motor peptidergic neurons can also be found in the ciliated larvae of cnidarians and echinoderms [45], and probably represent a very ancient neuron type (Conzelmann and Jékely, unpublished). The nervous system of the Platynereis larva, with its many direct sensory-motor neurons, represents a nervous system with low integration between the different sensory pathways.
Sensory-motor innervation of ciliary bands has also been found in the embryos of the pond snail, Helisoma trivolvis. Here sensory cells directly innervate the ciliated cells of the embryo and accelerate ciliary beating via the release of serotonin upon hypoxic stimulation in the egg capsule [46].
Similar to peptidergic sensory-motor cells, serotonergic neurons with sensory morphology also innervate ciliary bands in a wide range of ciliated larvae, both protostome and deuterostome [47]. Serotonin seems to play a general role in the upregulation of ciliary beat frequency (in mollusc [48], echinoderm [49,50] and annelid (G. Jékely unpublished) larvae), and the control of cilia by a sensory-motor serotonergic system may trace back to the common ancestor of protostomes and deuterostomes. Serotonin was also described in neurons of cnidarian planula larvae, but there is no evidence that it is involved in ciliary locomotion control [51].
The sensory-motor nervous systems of ciliated larvae can be compared with the imaginary vehicles proposed by Valentino Braitenberg [52]. These vehicles have two motors (wheels) and any number of sensors that directly regulate the speed and direction of the motors (sensory-motor neurons). The vehicles can perform remarkably complex and dynamic behaviours with only a small number of sensors (simulated vehicles can be found at http://people.cs.uchicago.edu/~wiseman/vehicles/).
In many marine invertebrate larvae, locomotion is not based on wheels but on cilia, and does not happen in two dimensions, but follows a spiral trajectory with axial rotation in three dimensions [8,53]. These ciliary swimming vehicles can turn, speed up or slow down, simply through direct regulation by their sensory-motor neurons.
5. The evolution of ciliary bands in protostomes
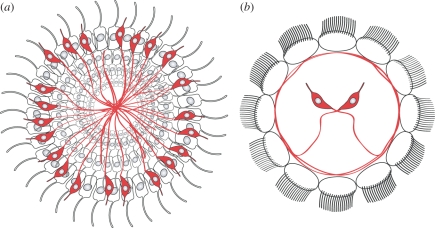
Ciliary locomotion and locomotory control was further optimized during the evolution of spiralians. Spiralians, a sub-group of lophotrochozoans comprising annelids, molluscs, entoprocts, nemerteans, and platyhelminths, have ciliary bands with multiciliated cells [6,7]. One driving force underlying the evolution of these multiciliated cells could have been the improvement of swimming efficiency. However, from a circuit evolution perspective, the concentration of ciliary motors into a few cells (e.g. two rows of 12 multiciliated cells in the annelid Platynereis; [8]) allows a very significant saving on wire length, cell number and synapse number. In a larva with monociliated cells, such as a cnidarian planula [54], a deuterostome tornaria [55] or a phoronid actinotroch larva [56] a sensory-motor neuron must innervate a large number of cells to achieve a sufficiently large motor response (e.g. speeding up the larva). In contrast, in a spiralian larva, a single neuron with a few synapses can generate a strong motor response (figure 3). For example, the axon of the Platynereis larval eye photoreceptors only forms a few en passant synapses on the adjacent multiciliated cell, but can generate a motor response strong enough to steer the whole larva.
Figure 3.
(a) Comparison of ciliary swimmers with monociliated cells and (b) multiciliated cells. The concentration of cilia in multiciliated cells allows a further reduction in the number of sensory cells required for one external input.
It would be very interesting to compare the nervous system of multiciliated and monociliated larvae (e.g. Platynereis and Owenia, an unusual polychaete with monociliated cells [57]) from this perspective. One prediction is that spiralian larvae will use fewer neurons tuned for the same input, and will probably have more senses and a more elaborate ciliary locomotory control than monociliated larvae, due to decreased costs.
The serotonergic nervous system seems to support this idea. In spiralian larvae the apical organ (a sensory organ specific for the larval stage [58]) generally contains only a few serotonergic cell bodies (about 2–4 [47]) that innervate the ciliary band with multiciliated cells [59]. In larvae that use monociliated cells for locomotion (echinoderms, phoronids, hemichordates), there are many more serotonergic cell bodies (e.g. 30–50 cells in a phoronid larva [59]) that project axons towards the monociliated ciliary bands [47].
6. The evolution of circuits for muscle contraction
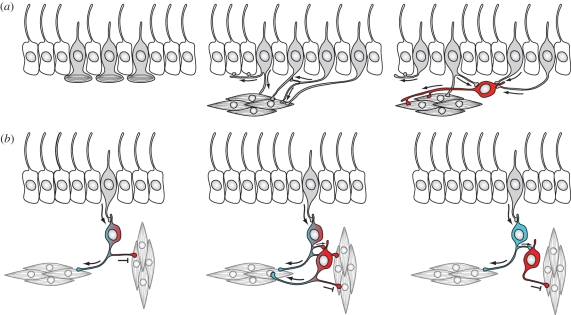
Muscle-based motor systems also evolved very early, and were probably already present in the stem eumetazoan lineage [9]. As argued above, the first function of muscle cells may have been whole body steering and contraction (as in some planula larvae and anthozoans), rather than locomotion. The first muscle cells and the first neurons may even have a common evolutionary origin from a myoepithelial sensory cell, as suggested by Mackie [23]. Such a myoepithelial cell may have evolved from the sponge-type epithelial sensory cell and may have modulated the contraction of its basal muscle fibres upon stimulation. The duplication of this cell led to a sensory cell type on the surface and a muscle cell type migrating deeper in the tissue. The two cell types could have remained connected electrically or via a chemical synapse (figure 4).
Figure 4.
(a) Evolution of muscle-controlling sensory-motor neurons from a myoepithelial cell by cell-type duplication and divergence. Originally, the motor control of muscles and cilia may have been linked in a ciliary swimming and muscle-based turning circuit. The integration of signals from more sensory cells evolved as some cells migrated deeper in the tissue (shown in red) and collected inputs from their sister cell type on the surface. The first dendrites could have evolved from the ‘sensory synapse’. (b) Evolution of a sign-reversing circuit by cell-type duplication and the segregation of an activatory and inhibitory transmitter. The circuit triggers the contraction of the longitudinal muscles and the relaxation of the circular muscles.
This scenario seems to be slightly at odds with the origin of the first neurons as regulators of ciliary locomotion, but the two can be reconciled by postulating the early separation of the two sensory-motor neuron populations. It is also possible that the earliest neurons regulated both cilia and muscles in a ciliary locomotor and steering circuit (e.g. for phototaxis).
In support of the neuro-myoepithelial cell scenario, direct sensory-motor neurons, innervating muscles, can still be observed in cnidarian nervous systems. They were described in the tentacles of Hydra [60–63] and the sea anemone Aiptasia [64]. These neurons trigger local muscle contractions.
From this stage of organization subsequent evolution could have led to neurons that collected sensory inputs from different sensory cells and established long-range contacts with muscles. This could have allowed economization on wire length, and could have combined cells with the same sensory modalities into minimally integrated circuits. At an intermediate stage the sensory cells may have kept synaptic contacts with the effector cells, but may also have contacted a dedicated motoneuron that provided the dominant input in a feed-forward circuit. These cells may have evolved into persistence or coincidence detectors to integrate signals over time or from numerous sensory cells [65].
From an evo-devo point of view, such integrated circuits could have evolved when some of the sensory cells migrated deeper in the tissue but kept their contacts with the surface sensory cells and with the musculature (figure 4a). The striking molecular and functional similarities between the sensory endings of neurons (the ‘sensory synapse’) and postsynaptic sites on neuron–neuron synapses [66] suggest that the postsynaptic sites and the dendrites of these second-order neurons could have evolved from the ciliated endings of their sensory cell precursors.
Neural circuits representing such a stage are present in cnidarians. In the sea anemone Aiptasia, epithelial sensory cells, besides synapsing directly on muscles, also synapse on a ganglion cell that has long-range contacts with the nerve plexus. The long-range contacts of the ganglion cells allow the generation of coordinated movements [64]. Such dual innervation by sensory neurons of a motor organ and a motoneuron represents an important intermediate stage between sensory-motor neurons and more complex circuits.
A further development in nervous system circuitry could have coincided with the evolution of more elaborate musculature, comprising both the circular and the longitudinal muscles of a radially symmetric animal. Since these muscles work antagonistically, their proper regulation by the nervous system requires sign reversing circuits. These circuits may have evolved gradually from a ganglion motor cell that first used two transmitters—one inhibitory, one excitatory—and innervated two sets of muscles with different receptors. Following this scenario, once this cell duplicated, the two sister cells could have stayed in synaptic contact. The upstream cell gradually lost the inhibitory transmitter and kept the excitatory one, while the downstream cell kept the inhibitory transmitter. The synaptic targets of the two cells were reorganized in parallel such that the upstream cell lost its contacts to the muscle fibre to be inhibited, and the downstream cell lost its contact to the muscles to be activated by the circuit (figure 4b). A hypothetical network such as this could have functioned normally throughout these evolutionary transitions.
Circuit evolution in some of the crown cnidarians resulted in the development of rapid motor networks that mediate fast muscle contractions during an escape response. In these circuits, motoneurons form a network connected by gap junctions, allowing the spread of currents to large motor areas. These networks are controlled by fast giant axons, such as in the hydrozoan Aglantha [67], that receive input from mechanosensory cells.
Another possible optimization from a radially symmetric stage with a diffused sensory and motor system is the concentration of some of the sensory cells. Compared with a diffused arrangement this would allow a saving on wire length and increased sensitivity. Sensory cell concentration can be observed in some cnidarians, such as the box jellyfish that have rhopalia with advanced visual eyes and statocysts [68]. It was this type of concentrated sensory cell arrangement that became prominent in bilaterians, coinciding with the origin of a centralized brain.
7. Outlook
Our knowledge about the early evolution of neural circuits is still fragmentary. In order to compare ancestrally simple circuits and reconstruct their evolution we will have to address the problem from both a cell-type evolution perspective [69] and a functional neural circuit perspective [8]. Even though general developmental patterning [70,71] and maybe even large-scale circuitry [72] can be compared between animals as distantly related as protostomes and deuterostomes, the size and complexity of traditional terrestrial model organism brains make detailed comparisons of circuits difficult. Studies in nematodes may also largely misrepresent early brain evolution, given that these animals have a highly evolved and optimized nervous system. An emerging research direction focuses on comparative studies of neuronal circuitry in diverse marine larvae. Even though the evolutionary origin and ancient versus modern origin of larvae is still debated [73,74], our results support the view that the larval eyes and brain of annelids (and probably other spiralian) represent more closely an ancestral stage of brain evolution [8]. Comparing circuits in spiralian larval nervous systems to the circuits of cnidarian larvae and the ciliated larvae of certain marine deuterostomes (e.g. hemichordates) is a promising research strategy with which to begin the reconstruction of the earliest circuits and their subsequent evolution.
Footnotes
One contribution to a Special Feature ‘Information processing in miniature brains’.
References
- 1.Srivastava M., et al. 2010. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 466, 720–726 10.1038/nature09201 (doi:10.1038/nature09201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tessmar-Raible K. 2007. The evolution of neurosecretory centers in bilaterian forebrains: insights from protostomes. Semin. Cell Dev. Biol. 18, 492–501 [DOI] [PubMed] [Google Scholar]
- 3.Philippe H., et al. 2009. Phylogenomics revives traditional views on deep animal relationships. Curr. Biol. 19, 706–712 10.1016/j.cub.2009.02.052 (doi:10.1016/j.cub.2009.02.052) [DOI] [PubMed] [Google Scholar]
- 4.Dunn C. W., et al. 2008. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 10.1038/nature06614 (doi:10.1038/nature06614) [DOI] [PubMed] [Google Scholar]
- 5.Degnan S. M., Degnan B. M. 2006. The origin of the pelagobenthic metazoan life cycle: what's sex got to do with it? Integr. Comp. Biol. 46, 683–690 10.1093/icb/icl028 (doi:10.1093/icb/icl028) [DOI] [PubMed] [Google Scholar]
- 6.Nielsen C. 2004. Trochophora larvae: cell-lineages, ciliary bands, and body regions. 1. Annelida and Mollusca. J. Exp. Zool. B Mol. Dev. Evol. 302, 35–68 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen C. 2005. Trochophora larvae: cell-lineages, ciliary bands and body regions. 2. Other groups and general discussion. J. Exp. Zool. B Mol. Dev. Evol. 304, 401–447 [DOI] [PubMed] [Google Scholar]
- 8.Jékely G., Colombelli J., Hausen H., Guy K., Stelzer E., Nédélec F., Arendt D. 2008. Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399 10.1038/nature07590 (doi:10.1038/nature07590) [DOI] [PubMed] [Google Scholar]
- 9.Seipel K., Schmid V. 2005. Evolution of striated muscle: jellyfish and the origin of triploblasty. Dev. Biol. 282, 14–26 10.1016/j.ydbio.2005.03.032 (doi:10.1016/j.ydbio.2005.03.032) [DOI] [PubMed] [Google Scholar]
- 10.Laughlin S. B., Sejnowski T. J. 2003. Communication in neuronal networks. Science 301, 1870–1874 10.1126/science.1089662 (doi:10.1126/science.1089662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niven J. E., Laughlin S. B. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804 10.1242/jeb.017574 (doi:10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 12.Chittka L., Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008 10.1016/j.cub.2009.08.023 (doi:10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 13.Lynch M., Conery J. S. 2003. The origins of genome complexity. Science 302, 1401–1404 10.1126/science.1089370 (doi:10.1126/science.1089370) [DOI] [PubMed] [Google Scholar]
- 14.Cherniak C. 1994. Component placement optimization in the brain. J. Neurosci. 14, 2418–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherniak C. 1995. Neural component placement. Trends Neurosci. 18, 522–527 10.1016/0166-2236(95)98373-7 (doi:10.1016/0166-2236(95)98373-7) [DOI] [PubMed] [Google Scholar]
- 16.Klyachko V. A., Stevens C. F. 2003. Connectivity optimization and the positioning of cortical areas. Proc. Natl Acad. Sci. USA 100, 7937–7941 10.1073/pnas.0932745100 (doi:10.1073/pnas.0932745100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niven J. E., Anderson J. C., Laughlin S. B. 2007. Fly photoreceptors demonstrate energy-information trade-offs in neural coding. PLoS Biol. 5, e116. 10.1371/journal.pbio.0050116 (doi:10.1371/journal.pbio.0050116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekker T., Ibba I., Siju K. P., Stensmyr M. C., Hansson B. S. 2006. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr. Biol. 16, 101–109 10.1016/j.cub.2005.11.075 (doi:10.1016/j.cub.2005.11.075) [DOI] [PubMed] [Google Scholar]
- 19.Faisal A. A., White J. A., Laughlin S. B. 2005. Ion-channel noise places limits on the miniaturization of the brain's wiring. Curr. Biol. 15, 1143–1149 10.1016/j.cub.2005.05.056 (doi:10.1016/j.cub.2005.05.056) [DOI] [PubMed] [Google Scholar]
- 20.Hartline D. K., Colman D. R. 2007. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr. Biol. 17, R29–R35 10.1016/j.cub.2006.11.042 (doi:10.1016/j.cub.2006.11.042) [DOI] [PubMed] [Google Scholar]
- 21.Erwin D. H., Davidson E. H. 2009. The evolution of hierarchical gene regulatory networks. Nat. Rev. Genet. 10, 141–148 10.1038/nrg2499 (doi:10.1038/nrg2499) [DOI] [PubMed] [Google Scholar]
- 22.Arendt D., Denes A. S., Jekely G., Tessmar-Raible K. 2008. The evolution of nervous system centralization. Phil. Trans. R. Soc. B 363, 1523–1528 10.1098/rstb.2007.2242 (doi:10.1098/rstb.2007.2242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackie G. O. 1970. Neuroid conduction and the evolution of conducting tissues. Q. Rev. Biol. 45, 319–332 10.1086/406645 (doi:10.1086/406645) [DOI] [PubMed] [Google Scholar]
- 24.Hara R., Asai H. 1980. Electro-physiological responses of Didinium-Nasutum to Paramecium capture and mechanical stimulation. Nature 283, 869–870 10.1038/283869a0 (doi:10.1038/283869a0) [DOI] [Google Scholar]
- 25.Watanabe M., Furuya M. 1974. Action spectrum of phototaxis in a cryptomonad alga, Cryptomonas sp. Plant Cell Physiol. 15, 413–420 [Google Scholar]
- 26.Witman G. B. 1993. Chlamydomonas phototaxis. Trends Cell Biol. 3, 403–408 10.1016/0962-8924(93)90091-E (doi:10.1016/0962-8924(93)90091-E) [DOI] [PubMed] [Google Scholar]
- 27.Wolken J. J. 1977. Euglena: the photoreceptor system for phototaxis. J. Eurkaryotic Microbiol. 24, 518–522 10.1111/j.1550-7408.1977.tb01004.x (doi:10.1111/j.1550-7408.1977.tb01004.x) [DOI] [PubMed] [Google Scholar]
- 28.Häder D. P. 1999. Gravitaxis in unicellular microorganisms. Adv. Space Res. 24, 843–850 10.1016/S0273-1177(99)00965-5 (doi:10.1016/S0273-1177(99)00965-5) [DOI] [PubMed] [Google Scholar]
- 29.Krause M., Bräucker R. 2009. Gravitaxis of Bursaria truncatella: electrophysiological and behavioural analyses of a large ciliate cell. Eur. J. Protistol. 45, 98–111 10.1016/j.ejop.2008.09.001 (doi:10.1016/j.ejop.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 30.Streb C., Richter P., Ntefidou M., Lebert M., Hader D. P. 2002. Sensory transduction of gravitaxis in Euglena gracilis. J. Plant Physiol. 159, 855–862 10.1078/0176-1617-00769 (doi:10.1078/0176-1617-00769) [DOI] [Google Scholar]
- 31.Kaupp U. B., Kashikar N. D., Weyand I. 2008. Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 70, 93–117 10.1146/annurev.physiol.70.113006.100654 (doi:10.1146/annurev.physiol.70.113006.100654) [DOI] [PubMed] [Google Scholar]
- 32.Saranak J., Foster K. W. 2005. Photoreceptor for curling behavior in Peranema trichophorum and evolution of eukaryotic rhodopsins. Eukaryot. Cell 4, 1605–1612 10.1128/EC.4.10.1605-1612.2005 (doi:10.1128/EC.4.10.1605-1612.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jékely G. 2009. Evolution of phototaxis. Phil. Trans. R. Soc. B 364, 2795–2808 10.1098/rstb.2009.0072 (doi:10.1098/rstb.2009.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saranak J., Foster K. W. 1997. Rhodopsin guides fungal phototaxis. Nature 387, 465–466 10.1038/387465a0 (doi:10.1038/387465a0) [DOI] [PubMed] [Google Scholar]
- 35.Ueki N., Matsunaga S., Inouye I., Hallmann A. 2010. How 5000 independent rowers coordinate their strokes in order to row into the sunlight: phototaxis in the multicellular green alga Volvox. BMC Biol. 8, 103. 10.1186/1741-7007-8-103 (doi:10.1186/1741-7007-8-103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leys S. P., Degnan B. M. 2001. Cytological basis of photoresponsive behavior in a sponge larva. Biol. Bull. 201, 323–338 10.2307/1543611 (doi:10.2307/1543611) [DOI] [PubMed] [Google Scholar]
- 37.Maldonado M., Durfort M., McCarthy D. A., Young C. M. 2003. The cellular basis of photobehavior in the tufted parenchymella larva of demosponges. Mar. Biol. 143, 427–441. 10.1007/s00227-003-1100-1 (doi:10.1007/s00227-003-1100-1) [DOI] [Google Scholar]
- 38.Nordström K., Wallén R., Seymour J., Nilsson D. 2003. A simple visual system without neurons in jellyfish larvae. Proc. R. Soc. Lond. B 270, 2349–2354 10.1098/rspb.2003.2504 (doi:10.1098/rspb.2003.2504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plickert G., Schneider B. 2004. Neuropeptides and photic behavior in Cnidaria. Hydrobiologia 530, 49–57 [Google Scholar]
- 40.Barile P., Stoner A., Young C. 1994. Phototaxis and vertical migration of the queen conch (Strombus gigas linne) veliger larvae. J. Exp. Mar. Biol. Ecol. 183, 147–162 [Google Scholar]
- 41.Richards G. S., Simionato E., Perron M., Adamska M., Vervoort M., Degnan B. M. 2008. Sponge genes provide new insight into the evolutionary origin of the neurogenic circuit. Curr. Biol. 18, 1156–1161 10.1016/j.cub.2008.06.074 (doi:10.1016/j.cub.2008.06.074) [DOI] [PubMed] [Google Scholar]
- 42.Avila E., Carballo J. L. 2006. Habitat selection by larvae of the symbiotic sponge Haliclona caerulea (Hechtel, 1965) (Demospongiae, Haplosclerida). Symbiosis 41, 21–29 [Google Scholar]
- 43.Raible F., Tessmar-Raible K., Arboleda E., Kaller T., Bork P., Arendt D., Arnone M. I. 2006. Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 300, 461–475 10.1016/j.ydbio.2006.08.070 (doi:10.1016/j.ydbio.2006.08.070) [DOI] [PubMed] [Google Scholar]
- 44.Arendt D., Hausen H., Purschke G. 2009. The ‘division of labour' model of eye evolution. Phil. Trans. R. Soc. B 364, 2809–2817 10.1098/rstb.2009.0104 (doi:10.1098/rstb.2009.0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beer A. J., Moss C., Thorndyke M. 2001. Development of serotonin-like and SALMFamide-like immunoreactivity in the nervous system of the sea urchin Psammechinus miliaris. Biol. Bull. 200, 268–280 10.2307/1543509 (doi:10.2307/1543509) [DOI] [PubMed] [Google Scholar]
- 46.Kuang S., Doran S. A., Wilson R. J., Goss G. G., Goldberg J. I. 2002. Serotonergic sensory-motor neurons mediate a behavioral response to hypoxia in pond snail embryos. J. Neurobiol. 52, 73–83 10.1002/neu.10071 (doi:10.1002/neu.10071) [DOI] [PubMed] [Google Scholar]
- 47.Hay-Schmidt A. 2000. The evolution of the serotonergic nervous system. Proc. R. Soc. Lond. B 267, 1071–1079 10.1098/rspb.2000.1111 (doi:10.1098/rspb.2000.1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braubach O. R., Dickinson A. J., Evans C. C., Croll R. P. 2006. Neural control of the velum in larvae of the gastropod, Ilyanassa obsoleta. J. Exp. Biol. 209, 4676–4689 10.1242/jeb.02556 (doi:10.1242/jeb.02556) [DOI] [PubMed] [Google Scholar]
- 49.Soliman S. 1983. Pharmacological control of ciliary activity in the young sea urchin larva. Effects of monoaminergic agents. Comp. Biochem. Physiol. C 76, 181–191 10.1016/0742-8413(83)90061-0 (doi:10.1016/0742-8413(83)90061-0) [DOI] [PubMed] [Google Scholar]
- 50.Wada Y., Mogami Y., Baba S. 1997. Modification of ciliary beating in sea urchin larvae induced by neurotransmitters: beat-plane rotation and control of frequency fluctuation. J. Exp. Biol. 200, 9–18 [DOI] [PubMed] [Google Scholar]
- 51.McCauley D. W. 1997. Serotonin plays an early role in the metamorphosis of the hydrozoan Phialidium gregarium. Dev. Biol. 190, 229–240 10.1006/dbio.1997.8698 (doi:10.1006/dbio.1997.8698) [DOI] [PubMed] [Google Scholar]
- 52.Braitenberg V. 1984. Vehicles: experiments in synthetic psychology. Cambridge, MA: MIT Press [Google Scholar]
- 53.Jennings H. S. 1901. On the significance of the spiral swimming of organisms. Am. Nat. 35, 369–378 10.1086/277922 (doi:10.1086/277922) [DOI] [Google Scholar]
- 54.Yuan D., Nakanishi N., Jacobs D. K., Hartenstein V. 2008. Embryonic development and metamorphosis of the scyphozoan Aurelia. Dev. Genes Evol. 218, 525–539 10.1007/s00427-008-0254-8 (doi:10.1007/s00427-008-0254-8) [DOI] [PubMed] [Google Scholar]
- 55.Nielsen C., Hay-Schmidt A. 2007. Development of the enteropneust Ptychodera flava: ciliary bands and nervous system. J. Morphol. 268, 551–570 10.1002/jmor.10533 (doi:10.1002/jmor.10533) [DOI] [PubMed] [Google Scholar]
- 56.Hay-Schmidt A. 1989. The nervous-system of the actinotroch larva of Phoronis-Muelleri (Phoronida). Zoomorphology 108, 333–351 10.1007/BF00312274 (doi:10.1007/BF00312274) [DOI] [Google Scholar]
- 57.Smart T. I., Von Dassow G. 2009. Unusual development of the mitraria larva in the polychaete Owenia collaris. Biol. Bull. 217, 253–268 [DOI] [PubMed] [Google Scholar]
- 58.Nielsen C. 2005. Larval and adult brains. Evol. Dev. 7, 483–489 10.1111/j.1525-142X.2005.05051.x (doi:10.1111/j.1525-142X.2005.05051.x) [DOI] [PubMed] [Google Scholar]
- 59.Hay-Schmidt A. 1990. Distribution of catecholamine-containing, serotonin-like and neuropeptide fmrfamide-like immunoreactive neurons and processes in the nervous-system of the Actinotroch larva of Phoronis-Muelleri (Phoronida). Cell Tissue Res. 259, 105–118 10.1007/BF00571435 (doi:10.1007/BF00571435) [DOI] [Google Scholar]
- 60.Westfall J. A. 1973. Ultrastructural evidence for a granule-containing sensory-motor-interneuron in Hydra littoralis. J. Ultrastruct. Res. 42, 268–282 10.1016/S0022-5320(73)90055-5 (doi:10.1016/S0022-5320(73)90055-5) [DOI] [PubMed] [Google Scholar]
- 61.Westfall J. A., Kinnamon J. C. 1978. A second sensory-motor-interneuron with neurosecretory granules in Hydra. J. Neurocytol. 7, 365–379 10.1007/BF01176999 (doi:10.1007/BF01176999) [DOI] [PubMed] [Google Scholar]
- 62.Westfall J. A., Wilson J. D., Rogers R. A., Kinnamon J. C. 1991. Multifunctional features of a gastrodermal sensory cell in Hydra: three-dimensional study. J. Neurocytol. 20, 251–261 10.1007/BF01235543 (doi:10.1007/BF01235543) [DOI] [PubMed] [Google Scholar]
- 63.Grimmelikhuijzen C. J., Westfall J. A. 1995. The nervous systems of cnidarians. The nervous system of invertebrates: an evolutionary and comparative approach, vol. 72 (eds Breidbach O., Kutsch W.), pp. 7–24 Basel, Switzerland: Birkhäuser [Google Scholar]
- 64.Westfall J. A., Elliott C. F., Carlin R. W. 2002. Ultrastructural evidence for two-cell and three-cell neural pathways in the tentacle epidermis of the sea anemone Aiptasia pallida. J. Morphol. 251, 83–92 10.1002/jmor.1075 (doi:10.1002/jmor.1075) [DOI] [PubMed] [Google Scholar]
- 65.Kashtan N., Itzkovitz S., Milo R., Alon U. 2004. Topological generalizations of network motifs. Phy. Rev. E 70, (doi:10.1103/PhysRevE.70.031909) [DOI] [PubMed] [Google Scholar]
- 66.Shaham S. 2010. Chemosensory organs as models of neuronal synapses. Nat. Rev. Neurosci. 11, 212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackie G. O. 2004. Central neural circuitry in the jellyfish Aglantha: a model ‘simple nervous system’. Neurosignals 13, 5–19 10.1159/000076155 (doi:10.1159/000076155) [DOI] [PubMed] [Google Scholar]
- 68.Garm A., Ekström P., Boudes M., Nilsson D. E. 2006. Rhopalia are integrated parts of the central nervous system in box jellyfish. Cell Tissue Res. 325, 333–343 10.1007/s00441-005-0134-8 (doi:10.1007/s00441-005-0134-8) [DOI] [PubMed] [Google Scholar]
- 69.Arendt D. 2008. The evolution of cell types in animals: emerging principles from molecular studies. Nat. Rev. Genet. 9, 868–882 10.1038/nrg2416 (doi:10.1038/nrg2416) [DOI] [PubMed] [Google Scholar]
- 70.Denes A. S., Jékely G., Steinmetz P. R., Raible F., Snyman H., Prud'homme B., Ferrier D. E., Balavoine G., Arendt D. 2007. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 129, 277–288 10.1016/j.cell.2007.02.040 (doi:10.1016/j.cell.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 71.Lowe C. J. 2008. Molecular genetic insights into deuterostome evolution from the direct-developing hemichordate Saccoglossus kowalevskii. Phil. Trans. R. Soc. B 363, 1569–1578. 10.1098/rstb.2007.2247 (doi:10.1098/rstb.2007.2247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erclik T., Hartenstein V., Lipshitz H., Mcinnes R. 2008. Conserved role of the vsx genes supports a monophyletic origin for bilaterian visual systems. Curr. Biol. 18, 1–10. 10.1016/j.cub.2008.07.076 (doi:10.1016/j.cub.2008.07.076) [DOI] [PubMed] [Google Scholar]
- 73.Nielsen C. 2008. Six major steps in animal evolution: are we derived sponge larvae? Evol. Dev. 10, 241–257 10.1111/j.1525-142X.2008.00231.x (doi:10.1111/j.1525-142X.2008.00231.x) [DOI] [PubMed] [Google Scholar]
- 74.Raff R. A. 2008. Origins of the other metazoan body plans: the evolution of larval forms. Phil. Trans. R. Soc. B 363, 1473–1479 10.1098/rstb.2007.2237 (doi:10.1098/rstb.2007.2237) [DOI] [PMC free article] [PubMed] [Google Scholar]