Abstract
In mammals, the neurotransmitter dopamine (DA) modulates a variety of behaviours, although DA function is mostly associated with motor control and reward. In insects such as the fruitfly, Drosophila melanogaster, DA also modulates a wide array of behaviours, ranging from sleep and locomotion to courtship and learning. How can a single molecule play so many different roles? Adaptive changes within the DA system, anatomical specificity of action and effects on a variety of behaviours highlight the remarkable versatility of this neurotransmitter. Recent genetic and pharmacological manipulations of DA signalling in Drosophila have launched a surfeit of stories—each arguing for modulation of some aspect of the fly's waking (and sleeping) life. Although these stories often seem distinct and unrelated, there are some unifying themes underlying DA function and arousal states in this insect model. One of the central roles played by DA may involve perceptual suppression, a necessary component of both sleep and selective attention.
Keywords: Drosophila, dopamine, arousal
1. The problem of arousal
One operational definition of arousal in mammals is increased motor activation, sensory responsiveness and emotional reactivity [1]. Applied to invertebrates, these categories might be more realistically combined under the common theme of behavioural responsiveness. However, even under this single definition for less complex animals, there are potential confounds depending on what behaviours are being measured. At one extreme, waking from sleep is a simple form of arousal—and invertebrates such as flies and bees do sleep [2–5]. While awake, however, responsiveness to one stimulus versus another is also a form of arousal, even though waking arousal has not necessarily increased. Thus, arousal can be a studied as an increase in behaviour in one case or attention-like selectivity of behaviour in another case [6]. What is the relationship between mechanisms inducing low-arousal states, such as sleep, and mechanisms increasing or suppressing responsiveness to stimuli in an awake animal? Are there multiple forms of arousal underlying different behaviours or is there one generalized arousal system that contributes to all behaviours? Researchers are increasingly turning to the fly model, Drosophila melanogaster, to tackle these complex problems. While the question of arousal has adopted multiple forms in Drosophila, at times depending on the behavioural paradigm being used, the answers have often converged on one molecule, dopamine (DA). Although DA signalling is tightly associated with mechanisms of olfactory learning and memory in the fly brain (for a recent review, see [7]), the neurotransmitter is also involved in setting responsiveness levels for a variety of other behaviours. There are only about 200 dopaminergic neurons in the fly brain (figure 1) [8,9], yet somehow these cells seem to control some aspect of every behaviour that has been measured in the fly, including sleep (see table 1 for the effect of various DA manipulations on fly behaviour and responsiveness). To understand the role of DA in setting arousal thresholds, we will proceed from studies of fly sleep to measures of behavioural responsiveness, and end with evidence for the role of DA in attention-like processes in insects.
Figure 1.
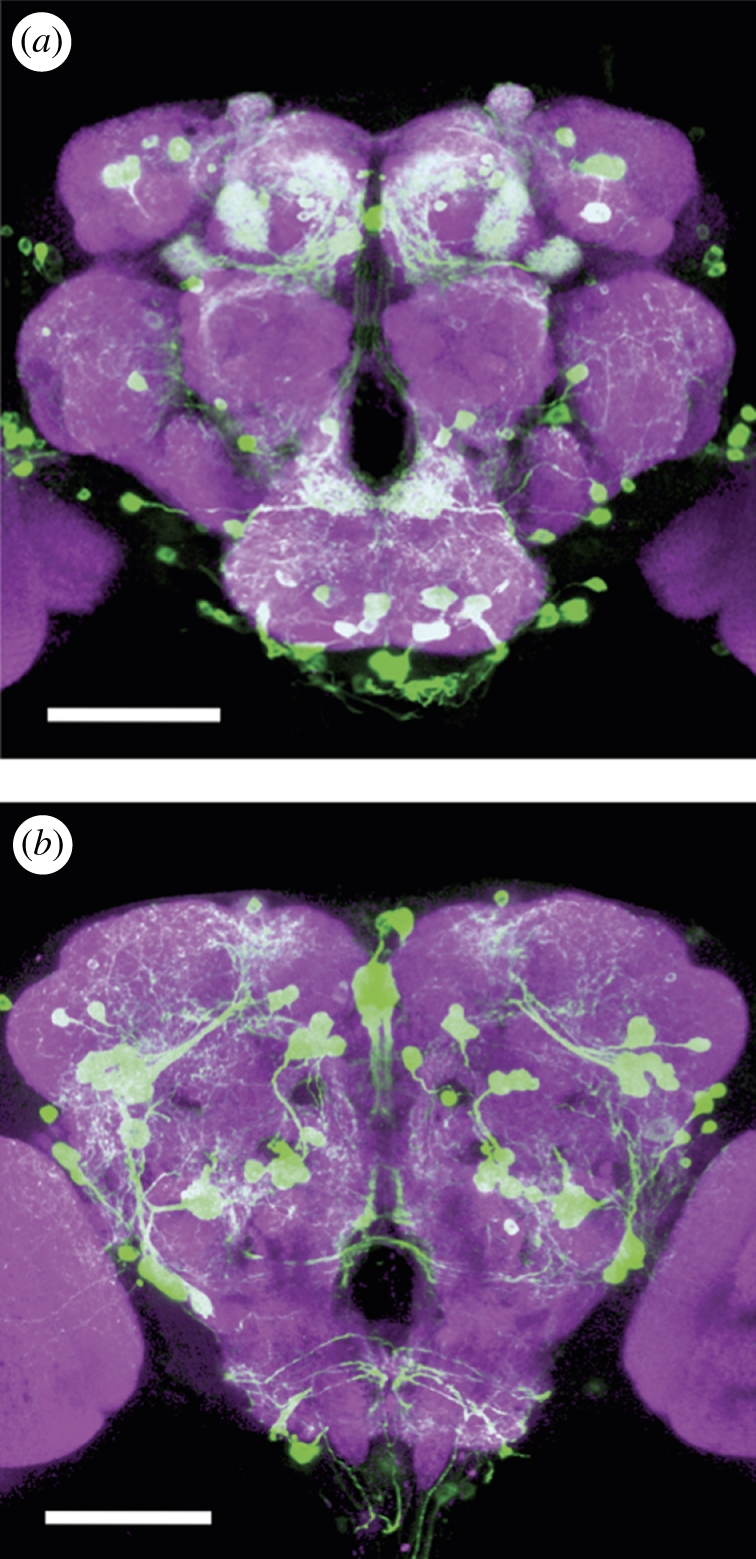
DA cells in the Drosophila brain. (a) An anterior view of DA neurons in the Drosophila brain. Labelling of DA cells and processes was achieved by a tyrosine-hydroxylase enhancer trap [24] driving expression of green fluorescent protein. (b) Posterior view. Images are reprinted with permission from Mao & Davis [9]. Scale bar, 100 µm.
Table 1.
Overview of Drosophila genes and manipulations affecting dopaminergic signalling. (NC, no change in activity.)
| endogenous arousal |
||||||
|---|---|---|---|---|---|---|
| DA manipulation or gene mutation | function or effect | sleepa | activityb | exogenous arousalc | reference | |
| dDA1d | dumb1 | D1-like receptor | upe | NC | down (odour learning) | [10,27] |
| dumb2f | up | down | up (air puffs) | [17,27] | ||
| dD2R | D2-like receptor | down | [20] | |||
| dDAT | fumin | DA transporter | down | up | up (mechanical) | [12] |
| VMAT | vesicular monoaminergic transporter: decrease DA | up | up | up (escape response) | [23] | |
| TH-Gal4/UAS-TNTg | decrease dopamine (constitutive) | NC | up (mechanical) | [24] | ||
| NC (odour startle) | [21] | |||||
| TH-Gal4/UAS-shibire | decrease dopamine (transient) | NC | down (odour learning) | [29,32] | ||
| down/NC (flight fixation) | [11,41] | |||||
| TH-Gal4/UAS-P2X2h | increase dopamine (transient) | up and downi | up (odour learning) | [22,33] | ||
| TH-Gal4/UAS-Trp1A | increase dopamine (transient) | up | NC (odour startle) | [21] | ||
| up (odour learning) | [8] | |||||
| TH-Gal4/ UAS-TH | increase dopamine | NC (males only) | up (courting) | [26] | ||
| ins-l (insomnia-like) | increase dopamine | down | up | up (mechanical, light) | [13,39] | |
| methamphetamine | increase dopamine | down | up | up (courting) | [11] | |
| down (visual response) | [11] | |||||
| cocaine | increase dopamine | down | up | down (air puffs) | [17] | |
| caffeine | activate DA receptors | down | up | [5,10] | ||
| 3-IYj | decrease dopamine | up | NC | down (visual learning) | [11,14] | |
aSleep amount.
bBaseline locomotor activity.
cBehavioural responsiveness to a stimulus.
dSynonyms DopR, DmDop1, DopR35F.
eLonger sleep episodes but no change in sleep amount.
fSynonym DopRf06276.
gTH, tyrosine hyroxylase, TNT, tetanus toxin.
hP2X2, ATP-activated purinoceptor.
iDepending on current activity level.
j3-IY, 3-iodo-tyrosine.
2. Endogenous arousal: sleep and wake
The regulation of sleep and wakefulness in Drosophila is tightly linked to DA. Among genes that affect sleep regulation, DA and genes involved in dopaminergic signalling have consistently been shown to directly affect the amount of sleep that flies get [10–15]. Indeed, there seems to be a simple relationship between DA and sleep amount, where more DA leads to less sleep, and less DA leads to more sleep [11,13,14]. This apparently simple function has been supported by several studies targeting DA signalling (table 1). These results therefore suggested that DA plays a single, arousing role in the fly brain. Pharmacological approaches using methamphetamine (METH) (which increases wakefulness in mammals by blocking the DA transporter and thereby increasing DA levels at the synapse [16]) indicated that the drug has a similar effect in Drosophila: flies exposed to METH have decreased and fragmented sleep. Similarly, other pharmacological interventions also point to the same trend: when DA signalling is increased by exposing flies to psycho-stimulants such as caffeine or cocaine, flies also sleep less [10,17].
Confirming a connection between wakefulness and DA in flies by using a completely different strategy, a recent study found that wild-type flies selected over several generations for decreased sleep (i.e. insomnia) had elevated levels of DA [13]. Similarly, a mutant in the DA transporter (dDAT), named fumin (fmn), was found to have shorter, fragmented sleep, and this was attributed to increased DA at the synapse [12]. The importance of DAT in regulating DA levels at the synapse, and consequently sleep amount, has been further confirmed by the identification of a new allele of dDAT in an independent genetic screen for short-sleeping mutants [15]. In line with these results, when DA action is compromised by feeding flies 3-iodo-tyrosine (an inhibitor of tyrosine hydroxylase, a rate-limiting enzyme in DA synthesis), or as a consequence of hypomorphic mutations in the DA receptor dDA1 (dumb1 and dumb2 alleles), flies sleep more [10,11,17].
What do the above manipulations of DA function do to an awake fly? Let us first consider locomotion, measured in the same infrared device most often used to quantify sleep [5,18] (but see [19] for a comparison with video tracking). In general, the same trend holds for locomotion: the DA receptor mutations decrease activity levels [17,20], while increased DA levels lead to hyperactive flies (table 1). Drugs that increase DA signalling, such as METH, cocaine or caffeine, all make flies hyperactive [10,11,17]. The fumin dDAT mutant, which sleeps less, is also hyperactive, as are the selected insomniac-like flies with increased DA levels [12,13]. Exciting dopaminergic circuits transiently (using transgenic ion channels) increases fly activity [21], although this also seems dependent on the animal's immediate behavioural state [22].
There are some exceptions to the trend relating increased DA with increased activity. One is a mutation in Drosophila vesicular monoamine transporter (dVMAT). dVMAT mutants have decreased DA head content, but contrary to expectations, have increased locomotion [23]. A proposed explanation is that the general absence of all amines during development, including DA, may result in adaptive changes at the post-synaptic level for multiple circuits, leading to increased locomotion effects unrelated to general arousal phenotypes. This is supported by other studies showing increased excitability in transgenic animals with chronically decreased or abolished DA [24,25]. Thus, increased arousal assessed by behavioural output can in some cases be a direct consequence of increased dopaminergic signalling, while in others, a consequence of post-synaptic adaptations resulting from long-term developmental changes in DA release.
3. Exogenous arousal: behavioural responsiveness
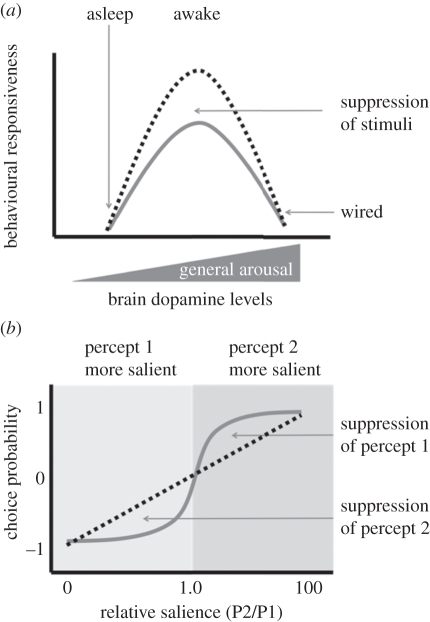
The simple linear function relating DA levels with increased arousal seems to fall apart when behavioural responsiveness to a specific stimulus, rather than mere activity, is examined more closely (table 1). At first, a survey of results fulfils expectations: fumin and other DAT mutants, which are hyperactive and sleep less, are also hyper-responsive to mechanical stimuli (i.e. they have decreased arousal thresholds) [12]. The insomnia-like (ins-l) flies are also hyper-responsive to a geotactic response and to a light pulse [13]. Increasing DA content in the brains of male flies increases sexual arousal and leads to shorter latency to court [11,26]. These results suggest that increased responsiveness, increased activity and increased DA are all correlated. However, such linearity between DA and arousal does not fit all findings. For example, METH-induced hyperactivity can make flies less responsive to visual stimuli [11], and flies with downregulated expression of dDA1 learn poorly [27]—two examples where either increasing or decreasing DA signalling impairs more specific forms of exogenous arousal. Such data suggested that DA's effects are nonlinear [28]. In this view, there exists an optimal general arousal level where either increases or decreases in DA away from this optimum would compromise behavioural performance (figure 2a).
Figure 2.
Models of DA function. (a) The inverted U model. Increasing levels of DA increase general arousal, measured by locomotion activity, in a linear manner (grey wedge on the x-axis). Along this axis, behavioural responsiveness changes nonlinearly, best described by an ‘inverted U’ (grey line). Thus, there exists a level of general arousal producing optimal responsiveness for multiple behaviours. Acute manipulations of DA signalling increase behavioural responsiveness (decrease arousal thresholds) to maladaptive levels in awake flies (black dashed line). However, the same manipulations do not change responsiveness as much at the extremes of the general arousal continuum, such as following METH treatment (wired). (b) The sigmoidal model. DA enables suppression of a competing stimulus or percept up to a threshold level of relative salience, whereupon DA mechanisms switch to suppressing the alternate percept (grey line). Without functional DA in the relevant circuit, choice behaviour reflects a linear combination of the competing percepts (black dashed line), and responsiveness to the competing stimulus is thus increased (arousal thresholds are decreased), without the adaptive value of suppressing one or the other.
But what is the relationship, if any, between general arousal and responsiveness to specific stimuli? A recent study by Lebestky et al. [17] looking at exogenous arousal in great detail found that a DA receptor mutant, dumb2 (a mutant allele of DopR or dDA1) is more responsive to air puff stimuli, suggesting that defects in DA signalling increase behavioural responsiveness. Since the same mutant also sleeps more, this study proposed that sleep and responsiveness might be separately regulated. The authors showed that DA's specificity of action is achieved through anatomical expression of the dDA1 receptor, where dDA1 in one part of the fly brain, the central complex, plays an important role in controlling responses to environmental stressors (air puffs), while the same receptor in another part, probably the mushroom bodies, influences endogenous arousal (sleep). An anatomical separation between endogenous and exogenous arousal seems likely because either behaviour (sleep and responsiveness) in mutant flies is rescued by dDA1 receptor expression in entirely different brain structures. Such anatomical localization of an arousal phenotype was also recently shown in ethanol-induced hyperactivity, which seems to be regulated by dDA1 expression in the central complex [21]. Lebestky et al. go on to propose a compartmentalized view of DA function, where behavioural responsiveness, endogenous arousal and also learning and memory would exist as separate manifestations of this neuromodulator's multiple roles. Even within one role, such as learning and memory, it appears that dopamineric effects can be spatially segregated among distinct synapses, as shown in a recent aversive odour learning study [8]. Together, these studies suggest that DA is playing a dynamic role in setting responsiveness levels to different stimuli in addition to a general arousing role.
It is thus likely that DA's action is not equal throughout the brain. Indeed, recent imaging of DA neurons in Drosophila (figure 1) supports this view by identifying 13 distinct DA subgroups as well as clearly different types of projection patterns throughout the fly brain [8,9,21]. Even the mushroom bodies, which had previously been identified as a general target of DA neurons [29], now appear to include a number of anatomically distinct target regions (the calyx, the vertical lobes, the horizontal lobes), and even each region is subdivided into clusters receiving input from distinct DA subtypes [8,9]. It seems logical to conclude that such compartmentalization of DA clusters and post-synaptic effects is the most obvious way that one molecule can exert distinct effects for multiple forms of arousal.
4. Another view: dA and suppression effects
However, a re-examination of behavioural effects following DA manipulations could still present some common ground towards explaining DA's effects on fly behaviour, at least for the awake state. If, instead of considering the peculiarities of each behavioural assay, one just considers the notion of arousal thresholds—how responsive is the animal to stimuli?—then many results begin to follow a common trend. In general, compromising DA function in either direction (up or down) appears to decrease arousal thresholds (make flies more responsive to lower intensity stimuli). Some results support this view unambiguously: DA receptor or transporter mutations all produced a decrease in arousal thresholds (to air puffs, light or mechanical stimuli) in awake flies, regardless of what is happening to sleep or activity levels [12,13,17,30]. Similarly, chronic silencing of DA neurons caused prolonged locomotor hyper-excitability after a startling stimulus [24]. Increases in DA caused by METHs [11] or transgenic methods [26] resulted in increased responsiveness to courtship cues. A synthesis of these results paints the following picture: general arousal, regulated by DA levels, sets the performance level (i.e. responsiveness) along a nonlinear threshold landscape (figure 2a, grey line), but a defect in the local DA environment produces a maladaptive increase in responsiveness in awake flies (figure 2a, dashed black line).
How might classical conditioning fit into this scenario? Demonstrations of associative learning and memory involve changing responsiveness to competing stimuli by associating one of these with punishment or reward. Therefore, failure to learn could also involve inappropriate setting of arousal thresholds. The role of DA as the negative valence cue in aversive odour learning is well established. In a landmark study, Schwaerzel et al. [29] showed that flies fail to associate odours with electric shocks when DA neurons are transiently silenced. Similarly, olfactory learning is also impaired when DA1 receptors are mutant [27] and by chronically increasing DA [31]. In another pioneering study, the activity of DA neurons was altered following a protocol mimicking odour-shock learning in a restricted optical imaging preparation [32]. At first, these observations do seem to imply that learning and memory are part of a distinct DA mechanism tied to aversive stimuli. However, could it also be possible that tinkering with the fly DA system might lower arousal thresholds to alter behavioural responsiveness during the course of a learning experiment? For example, by compromising DA function, flies in a learning paradigm may be responding to a greater variety of non-predictive and possibly irrelevant stimuli (i.e. context) that would otherwise be suppressed in a normal DA environment. With lowered arousal thresholds, it might be not surprising that several studies show flies to be less able to make the correct associations between two stimuli following DA manipulations: the irrelevant context—a multitude of competing sensations—might not be suppressed anymore.
As a counterargument to this, some of the strongest evidence that DA plays a specific role in aversive olfactory learning can be found in recent studies where TH-Gal4 (i.e. DA) neurons were transiently activated in place of electric shocks [8,33]. These studies showed that components of the DA circuit are sufficient to communicate an aversive stimulus in association with odours in adult flies. A similar result was also found for odour learning in fly larvae [34], suggesting that transient DA activation probably carries negative valence cues, and thereby arguing against the idea of a more general stimulus suppression role for the neurotransmitter. Still, there remains the possibility that transient activation of local DA circuits increases arousal thresholds to (i.e. suppresses) an associated odour, and that it is this change that shapes subsequent choice behaviour. It may be interesting to revisit fly olfactory learning along the perspective of dynamic arousal thresholds rather than valence, since it is now clear that DA is not restricted to aversive reinforcement [27,35,36].
Arousal thresholds are of course also set by physiological context, such as starvation. Since the first days of Drosophila memory research, it was understood that starved flies often performed better in behavioural assays than well-fed flies. Two recent appetitive learning studies have provided a DA-based explanation for this observation, which again matches well with the concept of altered arousal thresholds [35,37]. Blocking output from only six DA neurons innervating the MB ‘heel’ releases memory performance in otherwise satiated flies, while activating these DA neurons suppressed memory performance in otherwise starved flies [35]. These effects might be reconsidered as manipulations of arousal thresholds, with hunger as a physiological context: satiated flies normally have higher thresholds, and decreasing these via compromised DA function resets fly responsiveness to levels seen in starved animals. In addition, the fact that these studies used appetitive (sugar) learning rather than aversive (electric shocks) shows again that DA's role in fly learning is not confined to situations associated with a negative valence.
Moving away from olfactory paradigms to vision, DA's possible role in suppressing responses to stimuli is supported by a recent study on the effects of sleep deprivation on visual learning. Learning in the aversive phototaxic suppression assay [38,39] requires suppression of a simple reflex attracting flies to light paired with quinine. This learned suppression phenotype is impaired following sleep deprivation, which downregulates dDA1 receptor expression, but is rescued with a variety of treatments that return DA signalling to higher levels [14]. Thus, an optimal DA environment is required for learned suppression of a phototaxic reflex.
How dynamic might such a DA effect on stimulus suppression be? The role of DA in setting arousal thresholds for competing stimuli has been perhaps best demonstrated in studies of visual learning in the flight arena. In this paradigm, tethered flies demonstrate their visual choices by controlling the angular position of competing objects with their wing beat dynamics (see [40] for a visual explanation). This remarkable paradigm allows for a much more precise readout of individual fly behaviour than other learning assays (because torque behaviour of the tethered flies is continuously monitored) as well as excellent control of the stimuli presented to the fly in the arena. The set-up allows researchers to ask precisely which aspects of the conditioned stimulus (colour, shape, background) are being ‘ignored’ or suppressed as context and which are being selected as relevant to learning. Such studies have shown that without DA, flies perform less well in this paradigm [41] and, crucially, are less able to disambiguate competing cues [42]. Transient DA silencing causes choice behaviour to become a linear function of combined stimulus parameters rather than the ‘winner-takes-all’ behaviour inherent in sigmoidal functions (figure 2b). To put these results in the same language we use to discuss arousal here, transiently blocking DA release decreases arousal thresholds to stimuli; the role of DA is therefore to suppress less salient or contextual cues and thereby gate the selection of salient features. The main conclusion regarding the role of DA in this entirely different paradigm is therefore potentially similar as for other behavioural studies: to set arousal thresholds. When DA signalling is compromised (either increased or decreased), arousal thresholds in awake animals are decreased and responsiveness to possibly irrelevant stimuli is thereby increased to a maladaptive level. These visual learning studies lend support to the notion that DA in Drosophila may be involved in dynamically setting arousal thresholds among competing stimuli.
Understanding the role of DA in setting arousal thresholds begs the question: what then controls the relatively few hundred DA neurons [9,21] in the adult fly brain? DA neurons appear to receive inputs from a variety of neurons including even the same ones that they target, suggesting that both pre- and post-synaptic functions may be quite proximal. The problem of understanding how DA neurons dynamically modulate arousal thresholds is somewhat similar to the problem of understanding how selective attention might work. Selective attention describes the experience-dependent stimulus suppression dynamics that allow an animal to make adaptive choices at the right time [6,43,44]. How experience, or memory, modulates stimulus suppression dynamics is unclear, just as the question of what modulates DA is unclear. Feedback mechanisms seem to be required for resolving this problem, and, indeed, DA circuits seem to be good candidates for uncovering the architecture required for attention-like mechanisms. Many DA neurons extend projections over very long distances relative to the size of the fly brain, and a given neuropil may be innervated by multiple DA clusters projecting from various sources [9]. Interestingly, there is to date only one published example of a system that might influence DA neuron function in Drosophila, and that is neuropeptide F, which is involved in the aforementioned motivation/appetitive learning circuit [35]. If DA circuits are indeed involved in selective attention, then they would appear to require some input from neurons that have been associated with memory formation. For fly vision, that would be input from the central complex [45,46], for olfaction, from the mushroom bodies [47].
5. Selective attention
The idea that simple animals such as insects may be endowed with selective attention remains controversial, although this is being supported by a growing body of behavioural and electrophysiological research. Before discussing the evidence for dopaminergic control of attention in flies, it is necessary to convince ourselves that attention-like processes are present in such miniature brains. To best address attention in the insect brain requires devising experiments measuring the effect of competition on perceptual load. Tethered fly experiments in the flight arena showed, for example, that introducing a competing static bar reduced optomotor responsiveness to a periodically moving bar by half, suggesting that fly attention was equally divided (in time) between the two competing visuals [48]. More recent work on honeybees reached a similar conclusion that some perceptual resources in insects might be partitioned serially in time, as in human attention [49]. This last study found that increasing the number of visual distractors (coloured discs) increased the decision time for bees to home in on a rewarded colour, suggesting the insects were performing an attention-like serial search. Such experiments in flies and bees strongly suggest that a larger brain is not required for behavioural flexibility and selective attention [6,50].
Selective attention is a cognitive process [43] with characteristic neural signatures in mammals, such as gamma-band (20–80 Hz) oscillations [51], and so if present in insects, it should also be associated with neural correlates in the tiny insect brain. Indeed, visual competition experiments in Drosophila have uncovered 20–30 Hz activity associated with salience [52], as well as selection and suppression dynamics of this neural signature of visual attention [44,53]. Together with the aforementioned behavioural data, these results provide good evidence that suppression mechanisms in the insect brain are dynamic and tuned to the immediate requirements of a constantly changing salience environment.
The immediate question that follows from the arguments put forth in this review is whether DA's role in setting arousal thresholds also applies to the dynamic requirements of selective attention. So far, the evidence is quite sparse but nevertheless tantalizing. First, transiently attenuating DA release in Drosophila was found to also attenuate the 20–30 Hz response to visual salience [11]. Then, feeding flies methylphenidate (a drug acting on DA signalling [54]) rescued 20–30 Hz responsiveness as well as selection/suppression dynamics in mutant flies [44]. Finally, the behavioural competition experiments in the flight arena discussed earlier [42] revealed that transient attenuation of DA release impaired flies' ability to suppress competing cues or visual context (figure 2b). Context generalization and selective attention may be viewed as two sides of the same coin: each are concerned with increasing responsiveness to a feature while decreasing responsiveness to the associated surround. The observation that DA enables this in flies supports the view that arousal thresholds are dynamically set by the neurotransmitter, and that DA modulates selective attention in Drosophila.
6. Conclusions
The original evolutionary advantage of a nervous system was probably to coordinate movement in rapid response to a variety of transient environmental stimuli. Following this breakthrough, it seems that the subsequent evolution of brains has been largely about selecting and suppressing responses to stimuli in an ‘intelligent’ way. DA may have provided an early solution here, by flexibly setting arousal thresholds for different circuits. Of course, DA does not act alone to regulate behavioural responsiveness in the insect brain; there are counteracting neuronal systems, such as serotonin and octopamine, when it comes to punishment–reward processing [29] or sleep–wake cycles [55]. However, an ancient role for DA in setting arousal thresholds seems likely, and evidence can be found in animals with even simpler nervous systems, such as the roundworm Caenorhabditis elegans. Mechanosensation (the response to touch) in C. elegans was found to be modulated by DA in a positive feedback circuit tied to food availability [56]. This suggested that the roundworm's relative sensitivity to environmental stimuli (such as a predator's touch) required integration of experience (food context) with sensory input via a DA feedback circuit. Although this is not quite selective attention, one could imagine how such dynamic regulation of arousal thresholds may have set the stage for the evolution of attention-like mechanisms. One open question is how such a coordinator of arousal thresholds might have evolved into the more specialized reward system we associate with DA action in mammals [57,58].
While general arousal may indeed be a linear function of DA levels in Drosophila, it is possible that attention-like suppression effects in flies require precisely timed patterns of DA activity regulating coherence of firing among neuronal groups, and such a temporal distinction (tonic DA levels versus phasic DA dynamics) may eventually explain how selective attention and sleep/wake may be subserved by a common molecule. Which DA circuits are associated with attention-like processes versus general arousal, and how these might differ in DA release patterns, should be resolved with electrical recordings or optical imaging from DA neurons [32]. Yet, why the emergence of selective attention in animals should have coincided with a daily need for sleep will probably remain a mystery until we understand both the function of sleep and the mechanisms regulating arousal thresholds. Ten years on from the discovery of fly sleep, Drosophila research is now at the forefront of these endeavours.
Footnotes
One contribution to a Special Feature ‘Information processing in miniature brains'.
References
- 1.Pfaff D., Banavar J. R. 2007. A theoretical framework for CNS arousal. Bioessays 29, 803–810 10.1002/bies.20611 (doi:10.1002/bies.20611) [DOI] [PubMed] [Google Scholar]
- 2.Campbell S. S., Tobler I. 1984. Animal sleep: a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8, 269–300 10.1016/0149-7634(84)90054-X (doi:10.1016/0149-7634(84)90054-X) [DOI] [PubMed] [Google Scholar]
- 3.Hendricks J. C., Finn S. M., Panckeri K. A., Chavkin J., Williams J. A., Sehgal A., Pack A. I. 2000. Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 10.1016/S0896-6273(00)80877-6 (doi:10.1016/S0896-6273(00)80877-6) [DOI] [PubMed] [Google Scholar]
- 4.Kaiser W., Steiner-Kaiser J. 1983. Neuronal correlates of sleep, wakefulness and arousal in a diurnal insect. Nature 301, 707–709 10.1038/301707a0 (doi:10.1038/301707a0) [DOI] [PubMed] [Google Scholar]
- 5.Shaw P. J., Cirelli C., Greenspan R. J., Tononi G. 2000. Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 10.1126/science.287.5459.1834 (doi:10.1126/science.287.5459.1834) [DOI] [PubMed] [Google Scholar]
- 6.Van Swinderen B. 2005. The remote roots of consciousness in fruit-fly selective attention? Bioessays 27, 321–330 10.1002/bies.20195 (doi:10.1002/bies.20195) [DOI] [PubMed] [Google Scholar]
- 7.Waddell S. 2010. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 33, 457–464 10.1016/j.fins.2010.07.001 (doi:10.1016/j.fins.2010.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aso Y., Siwanowicz I., Bracker L., Ito K., Kitamoto T., Tanimoto H. 2010. Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 20, 1445–1451 10.1016/j.cub.2010.06.048 (doi:10.1016/j.cub.2010.06.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao Z., Davis R. L. 2009. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front. Neural Circuits 3, 5. 10.3389/neuro.04.005.2009 (doi:10.3389/neuro.04.005.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andretic R., Kim Y. C., Jones F. S., Han K. A., Greenspan R. J. 2008. Drosophila D1 dopamine receptor mediates caffeine-induced arousal. Proc. Natl Acad. Sci. USA 105, 20 392–20 397 10.1073/pnas.0806776105 (doi:10.1073/pnas.0806776105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andretic R., Van Swinderen B., Greenspan R. J. 2005. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15, 1165–1175 10.1016/j.cub.2005.05.025 (doi:10.1016/j.cub.2005.05.025) [DOI] [PubMed] [Google Scholar]
- 12.Kume K., Kume S., Park S. K., Hirsh J., Jackson F. R. 2005. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25, 7377–7384 10.1523/JNEUROSCI.2048-05.2005 (doi:10.1523/JNEUROSCI.2048-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seugnet L., Suzuki Y., Thimgan M., Donlea J., Gimbel S. I., Gottschalk L., Duntley S. P., Shaw P. J. 2009. Identifying sleep regulatory genes using a Drosophila model of insomnia. J. Neurosci. 29, 7148–7157 10.1523/JNEUROSCI.5629-08.2009 (doi:10.1523/JNEUROSCI.5629-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seugnet L., Suzuki Y., Vine L., Gottschalk L., Shaw P. J. 2008. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr. Biol. 18, 1110–1117 10.1016/j.cub.2008.07.028 (doi:10.1016/j.cub.2008.07.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu M. N., Koh K., Yue Z., Joiner W. J., Sehgal A. 2008. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep 31, 465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisor J. P., Nishino S., Sora I., Uhl G. H., Mignot E., Edgar D. M. 2001. Dopaminergic role in stimulant-induced wakefulness. J. Neurosci. 21, 1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebestky T., Chang J. S., Dankert H., Zelnik L., Kim Y. C., Han K. A., Wolf F. W., Perona P., Anderson D. J. 2009. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron 64, 522–536 10.1016/j.neuron.2009.09.031 (doi:10.1016/j.neuron.2009.09.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber R., Hill S. L., Holladay C., Biesiadecki M., Tononi G., Cirelli C. 2004. Sleep homeostasis in Drosophila melanogaster. Sleep 27, 628–639 [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman J. E., Raizen D. M., Maycock M. H., Maislin G., Pack A. I. 2008. A video method to study Drosophila sleep. Sleep 31, 1587–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Draper I., Kurshan P. T., McBride E., Jackson F. R., Kopin A. S. 2007. Locomotor activity is regulated by D2-like receptors in Drosophila: an anatomic and functional analysis. Dev. Neurobiol. 67, 378–393 10.1002/dneu.20355 (doi:10.1002/dneu.20355) [DOI] [PubMed] [Google Scholar]
- 21.Kong E. C., et al. 2010. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE 5, e9954. 10.1371/journal.pone.0009954 (doi:10.1371/journal.pone.0009954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima S. Q., Miesenbock G. 2005. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152 10.1016/j.cell.2005.02.004 (doi:10.1016/j.cell.2005.02.004) [DOI] [PubMed] [Google Scholar]
- 23.Simon A. F., et al. 2009. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics 181, 525–541 10.1534/genetics.108.094110 (doi:10.1534/genetics.108.094110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friggi-Grelin F., Coulom H., Meller M., Gomez D., Hirsh J., Birman S. 2003. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54, 618–627 10.1002/neu.10185 (doi:10.1002/neu.10185) [DOI] [PubMed] [Google Scholar]
- 25.Li H., Chaney S., Roberts I. J., Forte M., Hirsh J. 2000. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr. Biol. 10, 211–214 10.1016/S0960-9822(00)00340-7 (doi:10.1016/S0960-9822(00)00340-7) [DOI] [PubMed] [Google Scholar]
- 26.Liu T., Dartevelle L., Yuan C., Wei H., Wang Y., Ferveur J. F., Guo A. 2008. Increased dopamine level enhances male–male courtship in Drosophila. J. Neurosci. 28, 5539–5546 10.1523/JNEUROSCI.5290-07.2008 (doi:10.1523/JNEUROSCI.5290-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y. C., Lee H. G., Han K. A. 2007. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. 27, 7640–7647 10.1523/JNEUROSCI.1167-07.2007 (doi:10.1523/JNEUROSCI.1167-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birman S. 2005. Arousal mechanisms: speedy flies don't sleep at night. Curr. Biol. 15, R511–R513 10.1016/j.cub.2005.06.032 (doi:10.1016/j.cub.2005.06.032) [DOI] [PubMed] [Google Scholar]
- 29.Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10 495–10 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsh J., Riemensperger T., Coulom H., Iche M., Coupar J., Birman S. 2010. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr. Biol. 20, 209–214 10.1016/j.cub.2009.11.037 (doi:10.1016/j.cub.2009.11.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S., Yin Y., Lu H., Guo A. 2008. Increased dopaminergic signaling impairs aversive olfactory memory retention in Drosophila. Biochem. Biophys. Res. Commun. 370, 82–86 10.1016/j.bbrc.2008.03.015 (doi:10.1016/j.bbrc.2008.03.015) [DOI] [PubMed] [Google Scholar]
- 32.Riemensperger T., Voller T., Stock P., Buchner E., Fiala A. 2005. Punishment prediction by dopaminergic neurons in Drosophila. Curr. Biol. 15, 1953–1960 10.1016/j.cub.2005.09.042 (doi:10.1016/j.cub.2005.09.042) [DOI] [PubMed] [Google Scholar]
- 33.Claridge-Chang A., Roorda R. D., Vrontou E., Sjulson L., Li H., Hirsh J., Miesenbock G. 2009. Writing memories with light-addressable reinforcement circuitry. Cell 139, 405–415 10.1016/j.cell.2009.08.034 (doi:10.1016/j.cell.2009.08.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroll C., et al. 2006. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 10.1016/j.cub.2006.07.023 (doi:10.1016/j.cub.2006.07.023) [DOI] [PubMed] [Google Scholar]
- 35.Krashes M. J., DasGupta S., Vreede A., White B., Armstrong J. D., Waddell S. 2009. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427 10.1016/j.cell.2009.08.035 (doi:10.1016/j.cell.2009.08.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selcho M., Pauls D., Han K. A., Stocker R. F., Thum A. S. 2009. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS ONE 4, e5897. 10.1371/journal.pone.0005897 (doi:10.1371/journal.pone.0005897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colomb J., Kaiser L., Chabaud M. A., Preat T. 2009. Parametric and genetic analysis of Drosophila appetitive long-term memory and sugar motivation. Genes Brain Behav. 8, 407–415 10.1111/j.1601-183X.2009.00482.x (doi:10.1111/j.1601-183X.2009.00482.x) [DOI] [PubMed] [Google Scholar]
- 38.Le Bourg E., Buecher C. 2002. Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim. Learn. Behav. 30, 330–341 [DOI] [PubMed] [Google Scholar]
- 39.Seugnet L., Suzuki Y., Stidd R., Shaw P. J. 2009. Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav. 8, 377–389 10.1111/j.1601-183X.2009.00483.x (doi:10.1111/j.1601-183X.2009.00483.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brembs B. 2008. Operant learning of Drosophila at the torque meter. JoVE 16, 2006–2008 10.3791/731 (doi:10.3791/731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye Y., Xi W., Peng Y., Wang Y., Guo A. 2004. Long-term but not short-term blockade of dopamine release in Drosophila impairs orientation during flight in a visual attention paradigm. Eur. J. Neurosci. 20, 1001–1007 10.1111/j.1460-9568.2004.03575.x (doi:10.1111/j.1460-9568.2004.03575.x) [DOI] [PubMed] [Google Scholar]
- 42.Zhang K., Guo J. Z., Peng Y., Xi W., Guo A. 2007. Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science 316, 1901–1904 10.1126/science.1137357 (doi:10.1126/science.1137357) [DOI] [PubMed] [Google Scholar]
- 43.Bichot N. P., Desimone R. 2006. Finding a face in the crowd: parallel and serial neural mechanisms of visual selection. Prog. Brain Res. 155, 147–156 10.1016/S0079-6123(06)55009-5 (doi:10.1016/S0079-6123(06)55009-5) [DOI] [PubMed] [Google Scholar]
- 44.Van Swinderen B., Brembs B. 2010. Attention-like deficit and hyperactivity in a Drosophila memory mutant. J. Neurosci. 30, 1003–1014 10.1523/JNEUROSCI.4516-09.2010 (doi:10.1523/JNEUROSCI.4516-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu G., Seiler H., Wen A., Zars T., Ito K., Wolf R., Heisenberg M., Liu L. 2006. Distinct memory traces for two visual features in the Drosophila brain. Nature 439, 551–556 10.1038/nature04381 (doi:10.1038/nature04381) [DOI] [PubMed] [Google Scholar]
- 46.Pan Y., Zhou Y., Guo C., Gong H., Gong Z., Liu L. 2009. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn. Mem. 16, 289–295 10.1101/lm.1331809 (doi:10.1101/lm.1331809) [DOI] [PubMed] [Google Scholar]
- 47.Keene A. C., Waddell S. 2007. Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 8, 341–354 10.1038/nrn2098 (doi:10.1038/nrn2098) [DOI] [PubMed] [Google Scholar]
- 48.Heisenberg M., Wolf R. 1984. Vision in Drosophila: genetics of microbehavior. Studies of brain function, vol. 12 Berlin, Germany: Springer-Verlag [Google Scholar]
- 49.Spaethe J., Tautz J., Chittka L. 2006. Do honeybees detect colour targets using serial or parallel visual search? J. Exp. Biol. 209, 987–993 10.1242/jeb.02124 (doi:10.1242/jeb.02124) [DOI] [PubMed] [Google Scholar]
- 50.Chittka L., Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008 10.1016/j.cub.2009.08.023 (doi:10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 51.Uhlhaas P. J., Singer W. 2006. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52, 155–168 10.1016/j.neuron.2006.09.020 (doi:10.1016/j.neuron.2006.09.020) [DOI] [PubMed] [Google Scholar]
- 52.Van Swinderen B., Greenspan R. J. 2003. Salience modulates 20–30 Hz brain activity in Drosophila. Nat. Neurosci. 6, 579–586 10.1038/nn1054 (doi:10.1038/nn1054) [DOI] [PubMed] [Google Scholar]
- 53.Van Swinderen B. 2007. The attention span of a fly. Fly 1, 187–189 [Google Scholar]
- 54.Iversen S. D., Iversen L. L. 2007. Dopamine: 50 years in perspective. Trends Neurosci. 30, 188–193 10.1016/j.tins.2007.03.002 (doi:10.1016/j.tins.2007.03.002) [DOI] [PubMed] [Google Scholar]
- 55.Crocker A., Shahidullah M., Levitan I. B., Sehgal A. 2010. Identification of a neural circuit that underlies the effects of octopamine on sleep : wake behavior. Neuron 65, 670–681 10.1016/j.neuron.2010.01.032 (doi:10.1016/j.neuron.2010.01.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kindt K. S., Quast K. B., Giles A. C., De S., Hendrey D., Nicastro I., Rankin C. H., Schafer W. R. 2007. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55, 662–676 10.1016/j.neuron.2007.07.023 (doi:10.1016/j.neuron.2007.07.023) [DOI] [PubMed] [Google Scholar]
- 57.Mirenowicz J., Schultz W. 1996. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 379, 449–451 10.1038/379449a0 (doi:10.1038/379449a0) [DOI] [PubMed] [Google Scholar]
- 58.Ungless M. A., Magill P. J., Bolam J. P. 2004. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303, 2040–2042 10.1126/science.1093360 (doi:10.1126/science.1093360) [DOI] [PubMed] [Google Scholar]