Abstract
Animals are active at different times of the day and their activity schedules are shaped by competition, time-limited food resources and predators. Different temporal niches provide different light conditions, which affect the quality of visual information available to animals, in particular for navigation. We analysed caste-specific differences in compound eyes and ocelli in four congeneric sympatric species of Myrmecia ants, with emphasis on within-species adaptive flexibility and daily activity rhythms. Each caste has its own lifestyle: workers are exclusively pedestrian; alate females lead a brief life on the wing before becoming pedestrian; alate males lead a life exclusively on the wing. While workers of the four species range from diurnal, diurnal-crepuscular, crepuscular-nocturnal to nocturnal, the activity times of conspecific alates do not match in all cases. Even within a single species, we found eye area, facet numbers, facet sizes, rhabdom diameters and ocelli size to be tuned to the distinct temporal niche each caste occupies. We discuss these visual adaptations in relation to ambient light levels, visual tasks and mode of locomotion.
Keywords: temporal niches, compound eyes, ocelli, castes, workers, alates
1. Introduction
Animal activity schedules are shaped by competition, time-limited food resources and predators. In extreme cases, animals have become strictly diurnal or nocturnal in response to shifting selection pressures [1,2]. Different temporal niches, however, provide different conditions for information processing in fundamental behavioural tasks, such as navigation and predator avoidance. Light levels vary dramatically at different times of the day [3], which change the availability and the salience of visual cues. Animals cope with variation in ambient light intensities to a certain extent by increasing or decreasing the sensitivity of their visual system through pupil mechanisms (e.g. [4]) or even by using different classes of photoreceptors (e.g. vertebrate rods and cones). To extend or limit their range of operation to dimly lit environments, such as the deep sea or into the night, animals have evolved specific adaptations to cope with vision in low light (reviewed by [5,6]). The optical superposition compound eyes of Crustacea and Insecta are extreme examples of such modifications (e.g. [7,8]), but more subtle ones can also be found in many insects that possess apposition compound eyes, an eye design best suited for high light levels. To increase photon capture rates, apposition compound eyes become larger to accommodate larger lenses and larger rhabdoms (e.g. [6,9,10]). In addition, neural mechanisms serve to spatially and temporally integrate photoreceptor signals, thus increasing the signal-to-noise ratio at the expense of temporal and spatial resolution [11–13].
Substantial morphological and physiological adaptations are thus required to move from a day- to a night-active lifestyle and these have been documented in diverse and distantly related insect groups (reviewed by [8]). As a consequence, it has been argued that temporal niche partitioning could only be expected across distantly related species [14]. However, evidence from dung beetles [15], bees [16,17], wasps [18] and ants [19] indicates that closely related species can occupy different temporal niches and have evolved the necessary visual adaptations. Moreover, the fact that in many insects, the visual system is sexually dimorphic, with extreme cases among flies [20–22], butterflies [23] and bees [24,25], demonstrates that even within the same species, visual systems differ depending on visual tasks.
Ants have a well-defined caste system with workers, alate females and alate males. Caste-specificity in the visual system of ants, however, has largely remained unexplored (see, however, [26]), although the external morphological variation has been documented [27]. The differences in lifestyle and visual ecology between ant castes are quite extreme. Ant workers are sterile females and are exclusively pedestrian. Both diurnal and nocturnal workers use vision to navigate, to track targets and to avoid obstacles and predators, but remain in the dark nest during resting periods. Alate females experience diurnal or nocturnal light conditions when they fly from the nest for mating, following which they shed their wings, become pedestrian and lead the rest of their lives in the dark nest as the queen. They only use vision in a brief but crucial stage of their life to control flight, to navigate and to avoid obstacles and predators. Alate males also experience either diurnal or nocturnal light conditions when they leave the nest for mating. In addition to the vision-related tasks carried out by alate females, males also locate and track females and fight off competitors [27].
We ask here to what extent visual specializations occur both within and between species in a congeneric and sympatric group of Myrmecia ants in relation to the daily activity rhythms and the distinct lifestyles of each caste. Myrmecia have an unusual visual system compared with other ants. With more than 3000 facets in each eye, Myrmecia ants have the second largest eyes in the ant world [19,28] and are unusually responsive to moving visual targets [29].
2. Material and methods
(a). Activity schedule of workers
We located several nests for each of four Myrmecia species, Myrmecia croslandi, Myrmecia tarsata, Myrmecia nigriceps and Myrmecia pyriformis in Canberra, ACT, Australia. These ants are mostly sympatric and are often found foraging on Eucalyptus trees. The foraging activity schedule of workers ( ) of each species was recorded at three nests during 2007–2010. A 30 cm diameter reference circle around the nest entrance allowed us to count the number of outgoing and incoming foragers in 10 min bins for a 24 h period. Light-levels (M. pyriformis: [30]) and temperature (M. croslandi: Jayatilaka 2010, personal communication) regulate daily and seasonal foraging schedules, but workers remain faithful to their respective temporal niches throughout the year. Here, we report worker activity times averaged from three nests from December to March, since this corresponds to the period during which mating occurs.
) of each species was recorded at three nests during 2007–2010. A 30 cm diameter reference circle around the nest entrance allowed us to count the number of outgoing and incoming foragers in 10 min bins for a 24 h period. Light-levels (M. pyriformis: [30]) and temperature (M. croslandi: Jayatilaka 2010, personal communication) regulate daily and seasonal foraging schedules, but workers remain faithful to their respective temporal niches throughout the year. Here, we report worker activity times averaged from three nests from December to March, since this corresponds to the period during which mating occurs.
(b). Activity schedules of winged forms
We recorded nest departure and mating times of alate males (♂) and females (♀) of the four species during 2007–2010. Nuptial flights occurred usually following rain over 2–3 days between December and March. Typically, the sexual forms walk out of the nest, climb the nearest tree and fly off from the tips of branches (M. croslandi), or walk away (M. tarsata; see also [31]) or fly from the vicinity of the nest (M. nigriceps and M. pyriformis). Mating in all species occurred on hilltops. We recorded 18 matings in M. croslandi, six in M. tarsata and 23 in M. nigriceps. We did not witness mating in M. pyriformis, but are confident that alates did not leave the nest during the night (see also [32]).
(c). Mapping the distribution of facet lenses
We covered compound eyes with a thin layer of colourless nail polish to produce cornea replicas [25]. Once dry, the cornea replicas were carefully removed and flattened on a microscope slide by making incisions with a micro-scalpel. The replicas were photographed in a Zeiss light microscope. A custom-written program in Matlab (Richard Peters, La Trobe University) allowed us to mark each facet in the digital photographs of these replicas and determine their area. From this, we created maps of the facet array and determined the distribution of facet sizes.
(d). Histology
Ants were immobilized on ice, their mandibles removed and head capsules opened. Optimal retinal fixation was achieved by cutting the most ventral rim of the eye. Dissection occurred at the activity times of each caste and for nocturnal animals was carried out under red light. Specimens were fixed for 2 h in 2.5 per cent glutaraldehyde and 2 per cent paraformaldehyde in phosphate buffer (pH 7.2–7.5), followed by a series of buffer washes and post-fixation in 2 per cent OsO4 in distilled water for 2 h. Samples were then dehydrated in an ethanol series, transferred to acetone and embedded in Epoxy resin (FLUKA). Two-micron thick cross-sections of ommatidia from the medio-frontal region of the eye were cut on a Reichert Ultracut microtome using glass or diamond knives. Sections for light microscopy were stained with toluidine blue and digitally photographed in a Zeiss microscope.
Workers of all four species exhibit distinct size polymorphism [19] and we used the largest workers for morphometrics and histology. Body length, head width and diameter of the median ocellus were measured from photographs of preserved specimens (n = 5). Eye area was determined from the eye replicas of five animals of each caste using ImageJ (NIH, USA). Differences in morphometric measures within or between species were determined by an analysis of variance using JMP v. 8.0. We used specimens from our own collections and from those housed at the Australian National Insect Collection (ANIC, CSIRO, Canberra, Australia).
3. Results
(a). Activity schedules
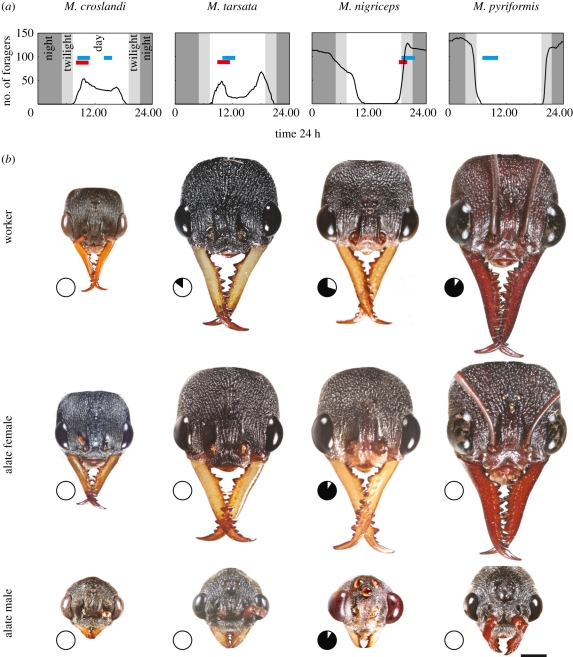
Daily rhythms of worker activity range from diurnal M. croslandi, diurnal-crepuscular M. tarsata, crepuscular-nocturnal M. nigriceps and nocturnal M. pyriformis (figure 1a; see also [19]). Except in M. pyriformis, mating periods in all species correspond to activity periods of workers. In M. pyriformis, workers leave the nest in a short time window during the evening twilight [30], forage throughout the night and return to the nest in another narrow time window during the morning twilight with little or no activity during the day. Mating, however, occurs during the day [32].
Figure 1.
Temporal niche partitioning both within and between species in Myrmecia ants. (a) Number of active workers averaged over three nests (black solid line) and the time at which sexual forms leave the nest (red bar) and mate (blue bar). The time at which winged forms of M. pyriformis leave the nest is unknown, but it is unlikely to happen at night. (b) Images of heads of the three castes of each species to scale. Pie-graph insets show the percentage of activity of each caste carried out during the day (white sectors) and at night (black sectors). Scale bar = 1 mm.
(b). Variations in body and eye size
Alate females are larger than workers and males, except in M. tarsata (M. croslandi: p < 0.001, F2,14 = 32.46; M. tarsata: p = 0.088, F2,14 = 2.997; M. nigriceps: p < 0.001, F2,14 = 94.96; M. pyriformis: p < 0.001, F2,14 = 32.30; figures 1b and 2a). Body length of all castes increases from the diurnal M. croslandi to the nocturnal M. pyriformis (activity schedules indicated by pie-graph pictograms in figure 1b and following figures). Irrespective of their activity time, male ants have significantly smaller heads compared with their respective workers and alate females (figures 1b and 2b). Out of all species, only the diurnal M. croslandi has a relatively larger head compared with the crepuscular and nocturnal species (figure 2b).
Figure 2.
Morphometrics of the different castes of four Myrmecia ant species. (a) Body length and (b) head width relative to body length in workers ( , blue), alate females (♀, red) and alate males (♂, green). Means ± s.d., n = 5 for each caste. Otherwise conventions as before.
, blue), alate females (♀, red) and alate males (♂, green). Means ± s.d., n = 5 for each caste. Otherwise conventions as before.
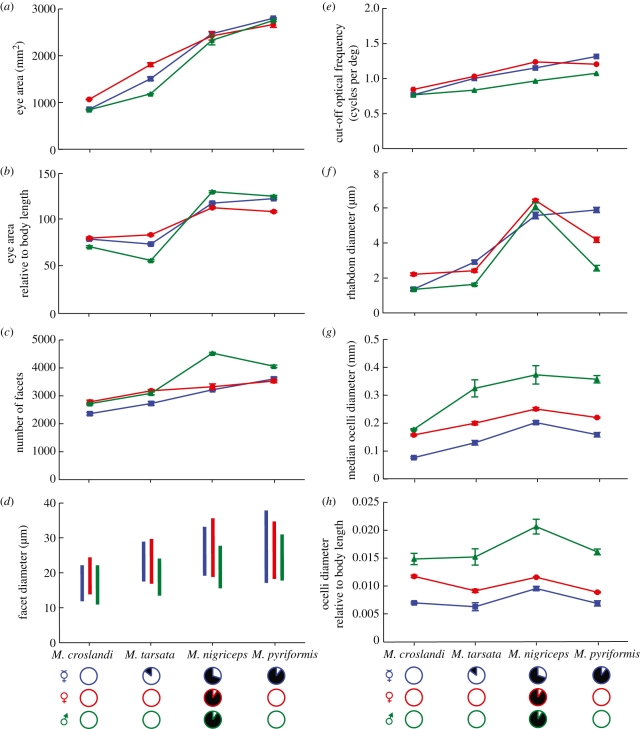
Eye area increases in all castes from day- to night-active species. Eye areas between castes are different in the diurnal and diurnal-crepuscular species (M. croslandi: p < 0.001, F2,14 = 289.1; M. tarsata: p < 0.001, F2,14 = 170.8), but similar in crepuscular-nocturnal and nocturnal species (M. nigriceps: p = 0.235, F2,14 = 2.128; M. pyriformis: p = 0.084, F2,14 = 6.309; figures 3a and 4a). Eye area is smallest in castes of M. croslandi, which have the smallest body size and largest in M. pyriformis, which have the largest body (figures 2a and 4a). However, body size alone does not explain the variation in eye area. For instance, males of M. tarsata are nearly twice the length of males of M. croslandi (figure 2a), but have a smaller relative eye area (figure 4b). Differences between castes within species also indicate that eye area does not increase linearly with body size (compare figures 2a and 4b). For instance, in M. nigriceps, males have the smallest body length, smallest head size and smallest eyes among all castes, but have the largest relative eye area. Eye area does not scale linearly with head size either. For instance, male ants have the smallest heads in all species (figure 2b), but their relative eye area is either slightly smaller than the alate female and workers (in M. croslandi and M. tarsata) or similar (in M. nigriceps and M. pyriformis; figure 4b).
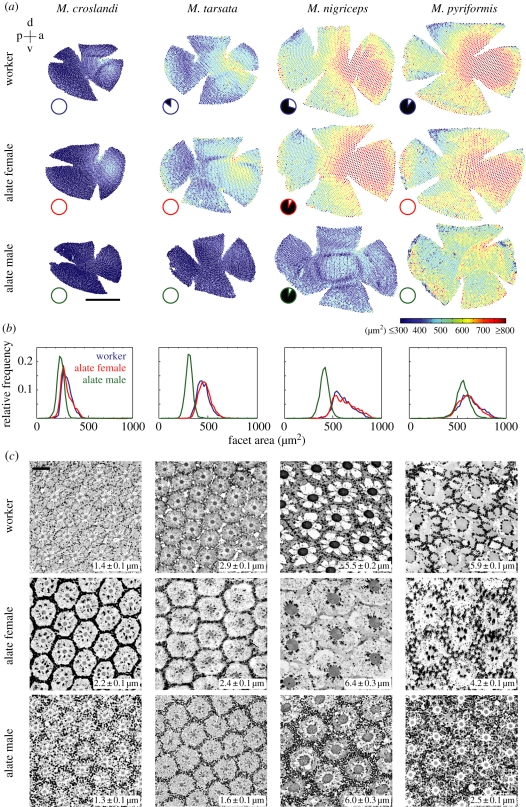
Figure 3.
Properties of the compound eyes of workers, alate females and males of Myrmecia ants. (a) Eye maps, (b) facet size distribution and (c) cross-sections through the ommatidia in the medio-frontal region at the level of the distal rhabdom. (a) Eye maps are to scale with colour indicating the facet area (see inset colour bar; scale bar, 0.5 mm). See top left inset for orientation: p, posterior; d, dorsal; a, anterior; v, ventral; (b) Relative frequency distribution of facet area for a worker, alate female and alate male of each species. (c) Rhabdoms are the dark circular structures at the centre of each ommatidium. Numbers in the inset show mean rhabdom diameters (±s.d.; n = 10 for each caste). Scale bar = 10 µm. Otherwise conventions as before.
Figure 4.
Compound eye properties of different castes of Myrmecia ants. (a) Absolute eye area; (b) eye area relative to body length; (c) number of ommatidia; (d) facet diameters (bar indicates the total range of facet diameters in each caste); (e) optical cut-off frequency νco = A/λ, where A = facet diameter (the largest facet diameter was used), λ = wavelength (0.5 µm); (f) rhabdom diameter; (g) diameter of the median ocellus and (h) ocellus diameter relative to body length. All values are means ± s.d. with n = 5 for workers ( , blue), alate females (♀, red) and alate males (♂, green). Otherwise conventions as before.
, blue), alate females (♀, red) and alate males (♂, green). Otherwise conventions as before.
(c). Compound eye properties
Facet numbers differ between castes in all four species (M. croslandi: p < 0.001, F2,14 = 63.39; M. tarsata: p < 0.001, F2,14 = 90.37; M. nigriceps: p < 0.001, F2,14 = 641.3; M. pyriformis: p < 0.001, F2,14 = 122.5; figures 3a and 4c). Both facet numbers (figure 4c) and facet sizes (figures 3b and 4d) increase gradually from day- to night-active species. Males of M. nigriceps, the only males that are active in dim light, have nearly 50 per cent more facets than the workers of their own species and have the largest number of facets among all castes and species. The frequency distribution of facet sizes has a long tail in the workers and alate females, which is absent in males (figure 3b). This is perhaps a reflection of the large facets found in the medio-frontal region of workers and alate females, a region that is less developed in the flying males (figure 3a). The largest facets in male ants are distributed around the ventral, anterior and posterior region of the eye. In all species, the largest facet sizes of males are smaller than in conspecific workers and alate females (figure 4d). Among castes in all species, the largest facets are found in alate females, except in M. pyriformis. In M. pyriformis, the largest facets are present in the workers, which is the only caste in this species to be active at night. The larger the facets, the potentially better the resolving power and also the more light that is captured by the eye. The cut-off optical frequency is the finest resolvable spatial frequency transmitted by lenses, which is the reciprocal of the Airy disc half-width [33]. Based on facet sizes, males have the poorest optical resolving power among conspecifics (figure 4e).
The wider the rhabdoms (light sensitive portion of photoreceptors) the more light is captured. Hence, as expected, all diurnal castes have small rhabdoms and nocturnal castes have large rhabdoms. The large differences in rhabdom size between castes of the same species correlate with their respective activity times. Workers of M. pyriformis that are nocturnal have wider rhabdoms (5.9 µm) than their strictly diurnal males (2.5 µm) and alate females (4.2 µm; figures 3c and 4f). A similar, but less exaggerated, pattern is seen in M. tarsata, where diurnal-crepuscular workers have wider rhabdoms than their strictly diurnal males and alate females (figure 4f).
Workers, alate females and males of all four Myrmecia species have ocelli. The ocelli of alate females are more similar to the pedestrian workers than to the flying males. Irrespective of the time of activity, the flying males have the largest median ocellus (figures 1b and 4g) with the night-flying male of M. nigriceps having the largest (figure 4g,h). Differences both within and between castes indicate that ocelli size does not increase linearly with body size (figure 4h). For instance, alate females have the largest body size, but small ocelli, while males with similar or smaller body size than alate females have the largest ocelli. The day-flying males of M. croslandi, M. tarsata and M. pyriformis have different body lengths (figure 2a), but similar relative ocelli size. The night-flying male M. nigriceps, in contrast, has the largest ocelli, indicating that time of activity also determines the size of ocelli (figure 4h). Comparison of visual structures relative to head size would exaggerate the investment made by males owing to their reduced head size (figure 2b).
4. Discussion
We have shown here that both between congeneric sympatric species and within species, animals can occupy different light environments and possess visual adaptations that are finely tuned to their respective temporal niches.
Compound eye specializations for different light environments are well documented in insects with apposition eyes [9,17–19] and superposition eyes [15]. However, to the best of our knowledge, this study provides the first evidence of such specializations to occur within a single species. Workers of M. pyriformis begin activity during evening twilight, remain active throughout the night and return to the nest during the morning twilight [30]. However, mating in this species occurs only during the day. Myrmecia pyriformis castes thus experience different light intensities and the night-active workers possess more and larger facets (figures 3a and 4d) and wider rhabdoms (figure 4f) than do the alate females and males. In M. tarsata, workers are active until after sunset whereas mating occurs only in the day. Hence workers of M. tarsata are equipped with rhabdoms that are nearly twice the size as those in males (figures 3c and 4f).
In our study, we did not find compound eye features or ocelli size to scale in proportion to body or head size, as they do in other ants [34] and generally across species [35–38]. Larger insects tend to have more ommatidia per eye, larger facets (and hence higher overall sensitivity) and smaller interommatidial angles, resulting in higher visual resolution [17,36,39–42]. However, in Camponotus pennsylvanicus castes have similar head sizes but winged forms have more facets than workers, suggesting a non-allometric relationship driven by visual processing needs associated with flight and/or mating [43]. In fire ants, the number of facets in the winged males (870 facets) and alate females (589 facets) is much greater than in workers (92 facets), while workers have larger facets (20 µm) than the flying alates (13 µm; [26]). Our study thus confirms that there are within-species differences in selective pressures on visual adaptations for different ambient light intensities (day–night activity), for different modes of locomotion (pedestrian–flight) and for different tasks (male–female–worker) that lead to clear deviations from simple proportional scaling relationships with body or head size (see also [35]).
This is also true when we consider the ocelli of Myrmecia ants. As in other insects [17,40,44,45], ocelli are larger in night-active species. In the leafcutter ants (genus Atta), ocelli are larger in night-flying (0.28 mm diameter) than in day-flying species (0.19 mm), scaling with body size [34]. Regardless of whether ocelli provide celestial compass information [46] and/or function as horizon detectors for head stabilization [47] there thus appears to be a need to increase light sensitivity in ocelli. It is less clear why there should be a sexual dimorphism in ocellar size in flying forms of Myrmecia ants, in contrast to what has been found in the leafcutter ants [34]. The only difference in visual tasks we can identify between males and alate females is that males may have to visually locate, track and intercept the alate female. Does this task generate additional selective pressure for increased light sensitivity and a better signal-to-noise ratio in ocellar photoreceptors and interneurons that allow faster or more reliable control of head orientation?
Our results also raise the question why pedestrian worker ants should need ocelli. Ocelli are particularly common in individually foraging ant species (see http://antweb.org). One possibility is that ocelli provide celestial compass information [46] and may provide this information faster [48] than the polarization sensitive dorsal rim of the compound eye [49].
(a). Adaptations, costs and trade-offs
We found that males of M. pyriformis, M. croslandi and M. tarsata are all active during the day and thus have smaller facet sizes, but an increased number of them to increase sampling resolution. However, as ant species and castes become more night active, rather than increasing the diameter of facets, the number of facets increases, a pattern that has also been found in carpenter bees [16] and other ants [34,50]. This observation raises a number of questions regarding the relative costs of modifying different compound eye components that affect light sensitivity. We know that eyes are costly because the construction, maintenance and operation of photoreceptors are energy consuming, and more so at low light intensities [51]. However, it remains unclear what the relative costs are of producing more ommatidia and of pooling signals, compared with making facets and rhabdoms larger. What contributions do more ommatidia make to the overall size, carrying weight and energy consumption of eyes compared with larger ones with larger facets and/or larger rhabdoms?
The optical adaptations in night-active worker ants involve a threefold increase in rhabdom diameter, a nearly twofold increase in facet diameter and a doubling of rhabdom length [19]. We unfortunately could not measure the latter in the alates, but for the workers of the species we studied, the values as measured in the medio-frontal eye region are 170 µm for M. croslandi, 250 µm for M. tarsata, 300 µm for M. nigriceps, 400 µm for M. pyriformis (major worker) and 260 µm for M. pyriformis (minor worker; from the electronic supplementary material in Greiner et al. [19]). Together, these modifications provide a 27-fold increase in optical sensitivity [19]. This improvement in optical sensitivity is modest considering that light levels at night are up to 100 million times dimmer than day light levels [3] and thus on its own cannot explain how animals are able to operate at night (see also [52]). As suggested before (e.g. [11–13,53]), ants thus require spatial and temporal integration of receptor signals to improve the signal-to-noise ratio at low light levels. Given the severe pressure on making information processing energy efficient [51] it will be of general interest to compare the physiology of photoreceptors and visual interneurons in the alates—with a visual system fit for the control of flight—with that of the pedestrian workers in both day- and night-active Myrmecia ants. Given the fact that photoreceptors and visual interneurons in different species of insects are exquisitely tuned to flight-speed [54], flight style [55] and ambient light conditions [6,56] we would expect to find differences not only in photoreceptor speed, but also in the spatio-temporal properties of lamina and lobula-plate neurons between species and between alates and worker castes. It is of additional interest that in ants, the same genome produces visual systems for both a life on the wing and for a pedestrian mode of locomotion.
Acknowledgements
We are grateful for facilities provided by the Centre for Advanced Microscopy at The Australian National University, Canberra and the Australian National Insect Collection (ANIC), CSIRO, Canberra. We thank Bob Taylor and Steve Shattuck for taxonomic advice, Doekele Stavenga, Eric Warrant and the late Ross Crozier for discussions and encouraging comments from two referees. We acknowledge funding support from the Centre for Visual Sciences, from the Australian Research Council's Discovery Project (DP0986606) and Centre of Excellence Schemes, from the German Science Foundation and from the Private University of Liechtenstein.
References
- 1.Kappeler P. M., Erkert H. G. 2003. On the move around the clock: correlates and determinants of cathemeral activity in wild redfronted lemurs (Eulemur fulvus rufus). Behav. Ecol. Sociobiol. 54, 359–369 10.1007/s00265-003-0652-x (doi:10.1007/s00265-003-0652-x) [DOI] [Google Scholar]
- 2.Mukhin A., Grinkevich V., Helm B. 2009. Under cover of darkness: nocturnal life of diurnal birds. J. Biol. Rhyth. 24, 225–231 10.1177/0748730409335349 (doi:10.1177/0748730409335349) [DOI] [PubMed] [Google Scholar]
- 3.Johnsen S., Kelber A., Warrant E. J., Sweeney A. M., Widder W. A., Lee R. L., Hernández-Andrés J. 2006. Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. J. Exp. Biol. 209, 789–800 10.1242/jeb.02053 (doi:10.1242/jeb.02053) [DOI] [PubMed] [Google Scholar]
- 4.Jonson A. C. J., Land M. F., Osorio D. C., Nilsson D. E. 1998. Relationships between pupil working range and habitat luminance in flies and butterflies. J. Comp. Physiol. A 182, 1–9 10.1007/s003590050152 (doi:10.1007/s003590050152) [DOI] [Google Scholar]
- 5.Warrant E. J. 2004. Vision in the dimmest habitats on earth. J. Comp. Physiol. A 190, 765–789 10.1007/s00359-004-0546-z (doi:10.1007/s00359-004-0546-z) [DOI] [PubMed] [Google Scholar]
- 6.Warrant E. J. 2008. Seeing in the dark: vision and visual behaviour in nocturnal bees and wasps. J. Exp. Biol. 211, 1737–1746 10.1242/jeb.015396 (doi:10.1242/jeb.015396) [DOI] [PubMed] [Google Scholar]
- 7.Land M. F., Fernald R. D. 1992. The evolution of eyes. Annu. Rev. Neurosci. 15, 1–29 10.1146/annurev.ne.15.030192.000245 (doi:10.1146/annurev.ne.15.030192.000245) [DOI] [PubMed] [Google Scholar]
- 8.Land M. F., Nilsson D. E. 2002. Animal eyes. New York, NY: Oxford University Press [Google Scholar]
- 9.Frederiksen R., Warrant E. J. 2008. Visual sensitivity in the crepuscular owl butterfly Caligo memnon and the diurnal blue morpho Morpho peleides: a clue to explain the evolution of nocturnal apposition eyes? J. Exp. Biol. 211, 844–851 10.1242/jeb.012179 (doi:10.1242/jeb.012179) [DOI] [PubMed] [Google Scholar]
- 10.Greiner B. 2006. Adaptations for nocturnal vision in insect apposition eyes. Int. Rev. Cytol. 250, 1–46 10.1016/S0074-7696(06)50001-4 (doi:10.1016/S0074-7696(06)50001-4) [DOI] [PubMed] [Google Scholar]
- 11.Greiner B., Ribi W. A., Warrant E. J. 2005. A neural network to improve dim-light vision? Dendritic fields of first-order interneurons in the nocturnal bee Megalopta genalis. Cell Tissue Res. 322, 313–320 10.1007/s00441-005-0034-y (doi:10.1007/s00441-005-0034-y) [DOI] [PubMed] [Google Scholar]
- 12.van Hateren J. H. 1993. Three modes of spatiotemporal preprocessing by eyes. J. Comp. Physiol. A 172, 583–591 [DOI] [PubMed] [Google Scholar]
- 13.Warrant E. J. 1999. Seeing better at night: life style, eye design and the optimum strategy of spatial and temporal summation. Vis. Res. 39, 1611–1630 10.1016/S0042-6989(98)00262-4 (doi:10.1016/S0042-6989(98)00262-4) [DOI] [PubMed] [Google Scholar]
- 14.Kronfeld-Schor N., Dayan T. 2003. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 153–181 10.1146/annurev.ecolsys.34.011802.132435 (doi:10.1146/annurev.ecolsys.34.011802.132435) [DOI] [Google Scholar]
- 15.McIntyre P., Caveney S. 1998. Superposition optics and the time of flight in onitine dung beetles. J. Comp. Physiol. A 183, 45–60 10.1007/s003590050233 (doi:10.1007/s003590050233) [DOI] [Google Scholar]
- 16.Somanathan H., Borges R. M., Warrant E. J. 2008. Visual ecology of Indian carpenter bees I: light intensities and flight activity. J. Comp. Physiol. A 194, 97–107 10.1007/s00359-007-0291-1 (doi:10.1007/s00359-007-0291-1) [DOI] [PubMed] [Google Scholar]
- 17.Somanathan H., Kelber A., Borges R., Wallen R., Warrant E. J. 2009. Visual ecology of Indian carpenter bees II: adaptations of eyes and ocelli to nocturnal and diurnal lifestyles. J. Comp. Physiol. A 195, 571–583 10.1007/s00359-009-0432-9 (doi:10.1007/s00359-009-0432-9) [DOI] [PubMed] [Google Scholar]
- 18.Greiner B. 2006. Visual adaptations in the night-active wasp Apoica pallens. J. Comp. Neurol. 495, 255–262 10.1002/cne.20882 (doi:10.1002/cne.20882) [DOI] [PubMed] [Google Scholar]
- 19.Greiner B., Narendra A., Reid S. F., Dacke M., Ribi W. A., Zeil J. 2007. Eye structure correlates with distinct foraging-bout timing in primitive ants. Curr. Biol. 17, R879–R880 10.1016/jcub.2007.08.015 (doi:10.1016/jcub.2007.08.015) [DOI] [PubMed] [Google Scholar]
- 20.Franceschini N. 1984. Chromatic organization and sexual dimorphism of the fly retinal mosaic. In Photoreceptors (eds Borsellino A., Cervetto L.), pp. 319–350 New York, NY: Plenum Press [Google Scholar]
- 21.Land M. F. 1989. Variations in the structure and design of compound eyes. In Facets of vision (eds Stavenga D. G., Hardie R. C.), pp. 90–111 Berlin, Germany: Springer [Google Scholar]
- 22.Zeil J. 1983. Sexual dimorphism in the visual-system of flies—the compound eyes and neural superposition in Bibionidae (Diptera). J. Comp. Physiol. 150, 379–393 10.1007/BF00605027 (doi:10.1007/BF00605027) [DOI] [Google Scholar]
- 23.Lau T. F., Gross E. M., Meyer-Rochow V. B. 2007. Sexual dimorphism and light/dark adaptation in the compound eyes of male and female Acentria ephemerella (Lepidoptera: Pyraloidea: Crambidae). Eur. J. Entomol. 104, 459–470 [Google Scholar]
- 24.Menzel J. G., Wunderer H., Stavenga D. G. 1991. Functional morphology of the divided compound eye of the honeybee drone (Apis mellifera). Tissue Cell 23, 525–535 10.1016/0040-8166(91)90010-Q (doi:10.1016/0040-8166(91)90010-Q) [DOI] [PubMed] [Google Scholar]
- 25.Ribi W. A., Engels E., Engels W. 1989. Sex and caste specific eye structures in stingless bees and honeybees (Hymenoptera: Trigonidae, Apidae). Entomol. Gen. 14, 233–242 [Google Scholar]
- 26.Baker G. T., Ma P. W. K. 2006. Morphology and number of ommatidia in the compound eyes of Solenopsis invicta, Solenopsis richteri, and their hybrid (Hymenoptera: Formicidae). Zool. Anz. 245, 121–125 10.1016/j.jcz.2006.06.001 (doi:10.1016/j.jcz.2006.06.001) [DOI] [Google Scholar]
- 27.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Belknap Press, Harvard University Press [Google Scholar]
- 28.Gronenberg W. 2008. Structure and function of ant (Hymenoptera: Formicidae) brains: strength in numbers. Myrm. News 11, 25–36 [Google Scholar]
- 29.Via S. 1977. Visually mediated snapping in the bulldog ant: a perceptual ambiguity between size and distance. J. Comp. Physiol. A 121, 33–51 10.1007/BF00614179 (doi:10.1007/BF00614179) [DOI] [Google Scholar]
- 30.Narendra A., Reid S. F., Hemmi J. M. 2010. The twilight zone: light intensity trigers activity in primitive ants. Proc. R. Soc. B 277, 1531–1538 10.1098/rspb.2009.2324 (doi:10.1098/rspb.2009.2324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McReavery S. J. 1948. Some observation on Myrmecia tarsata Smith. Proc. Linn. Soc. NSW. 73, 137–141 [Google Scholar]
- 32.Tepper J. G. O. 1882. Observation about the habits of some South Australian ants. Trans. Proc. R. Soc. S. Austr. 5, 24–26 106–107 [Google Scholar]
- 33.Land M. F. 1997. Visual acuity in insects. Annu. Rev. Entomol. 42, 147–177 10.1146/annurev.ento.42.1.147 (doi:10.1146/annurev.ento.42.1.147) [DOI] [PubMed] [Google Scholar]
- 34.Moser J. C., Reeve J. D., Bento J. M. S., Lucia T. M. C. D., Cameron R. S., Heck N. M. 2004. Eye size and behaviour of day- and night-flying leafcutting ant alates. J. Zool. Lond. 264, 69–75 10.1017/S0952836904005527 (doi:10.1017/S0952836904005527) [DOI] [Google Scholar]
- 35.Brooke M. de. L., Hanley S., Laughlin S. B. 1999. The scaling of eye size with body mass in birds. Proc. R. Soc. Lond. B 266, 405–412 10.1098/rspb.1999.0652 (doi:10.1098/rspb.1999.0652) [DOI] [Google Scholar]
- 36.Jander U., Jander R. 2002. Allometry and resolution of bee eyes (Apoidea). Arthr. Struc. Dev. 30, 179–193 10.1016/S1467-8039(01)00035-4 (doi:10.1016/S1467-8039(01)00035-4) [DOI] [PubMed] [Google Scholar]
- 37.Land M. F. 1980. Comparative physiology and evolution of vision in invertebrates. In Handbook of sensory physiology, vol. VII/6B (ed. Autrum H.), pp. 471–592 Berlin, Germany: Springer [Google Scholar]
- 38.Snyder A. W., Menzel R. 1975. Photoreceptor optics. Heidelberg, Germany: Springer [Google Scholar]
- 39.Barlow H. B. 1952. The size of ommatidia in apposition eyes. J. Exp. Biol. 29, 667–674 [Google Scholar]
- 40.Kelber A., Warrant E. J., Pfaff M., Wallen R., Theobald J. C., Wcislo W. T., Raguso R. A. 2006. Light intensity limits foraging activity in nocturnal and crepuscular bees. Behav. Ecol. 17, 63–72 10.1093/beheco/arj001 (doi:10.1093/beheco/arj001) [DOI] [Google Scholar]
- 41.Spaethe J., Chittka L. 2003. Interindividual variation of eye optics and single object resolution in bumblebees. J. Exp. Biol. 206, 3447–3453 10.1242/jeb.00570 (doi:10.1242/jeb.00570) [DOI] [PubMed] [Google Scholar]
- 42.Wehner R. 1981. Spatial vision in arthropods. In Handbook of sensory physiology, vol. VII/6B (ed. Autrum H.), pp. 287–616 Berlin, Germany: Springer [Google Scholar]
- 43.Klotz J. H., Reid B. L., Gordon W. C. 1992. Variation of ommatidia number as a function of worker size in Camponotus pennsylvanicus (DeGeer) (Hymenoptera, Formicidae). Insect Soc. 39, 233–236 10.1007/BF01249297 (doi:10.1007/BF01249297) [DOI] [Google Scholar]
- 44.Kerfoot W. B. 1967. Correlation between ocellar size and the foraging activities of bees (Hymenoptera; Apoidea). Am. Nat. 101, 65–70 10.1086/282470 (doi:10.1086/282470) [DOI] [Google Scholar]
- 45.Warrant E. J., Kelber A., Wallén R., Wcislo W. 2006. Ocellar optics in nocturnal and diurnal bees and wasps. Arthr. Struc. Dev. 35, 293–305 10.1016/j.asd.2006.08.012 (doi:10.1016/j.asd.2006.08.012) [DOI] [PubMed] [Google Scholar]
- 46.Fent K., Wehner R. 1985. Ocelli—a celestial compass in the desert ant Cataglyphis. Science 228, 192–194 10.1126/science.228.4696.192 (doi:10.1126/science.228.4696.192) [DOI] [PubMed] [Google Scholar]
- 47.Berry R., van Kleef J., Stange G. 2007. The mapping of visual space by dragonfly lateral ocelli. J. Comp. Physiol. A 193, 495–513 10.1007/s00359-006-0204-8 (doi:10.1007/s00359-006-0204-8) [DOI] [PubMed] [Google Scholar]
- 48.Parsons M. M., Krapp H. G., Laughlin S. B. 2006. A motion-sensitive neurone responds to signals from the two visual systems of the blowfly, the compound eyes and ocelli. J. Exp. Biol. 209, 4464–4474 10.1242/jeb.02560 (doi:10.1242/jeb.02560) [DOI] [PubMed] [Google Scholar]
- 49.Labhart T., Meyer E. 1999. Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Micro. Res. Tech. 47, 368–379 (doi:10.1002/(SICI)1097-0029(19991215)47:6<368::AID-JEMT2>3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 50.Coody C. J., Watkins J. F. 1986. The correlation of eye size with circadian flight periodicity of Nearctic army ant males of the genus Neivamyrmex (Hymenoptera, Formicidae, Ecitoninae). Tex. J. Sci. 38, 3–7 [Google Scholar]
- 51.Niven J. E., Anderson J. C., Laughlin S. B. 2007. Fly photoreceptors demonstrate energy-information trade-offs in neural coding. PLoS Biol. 5, e16. 10.1371/journal.pbio.0050116 (doi:10.1371/journal.pbio.0050116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greiner B., Ribi W. A., Warrant E. J. 2004. Retinal and optical adaptations for nocturnal vision in the halictid bee Megalopta genalis. Cell Tissue Res. 316, 377–390 10.1007/s00441-004-0883-9 (doi:10.1007/s00441-004-0883-9) [DOI] [PubMed] [Google Scholar]
- 53.Theobald J. C., Greiner B., Wcislo W. T., Warrant E. J. 2006. Visual summation in night-flying sweat bees: a theoretical study. Vis. Res. 46, 2298–2309 10.1016/j.visres.2006.01.002 (doi:10.1016/j.visres.2006.01.002) [DOI] [PubMed] [Google Scholar]
- 54.Laughlin S. B., Weckström A. 1993. Fast and slow photoreceptors—a comparative study of the functional diversity of coding and conductances in the Diptera. J. Comp. Physiol. A 172, 593–609 10.1007/BF00213682 (doi:10.1007/BF00213682) [DOI] [Google Scholar]
- 55.O'Carroll D. C., Bidwell N. J., Laughlin S. B., Warrant E. J. 1996. Insect motion detectors matched to visual ecology. Nature 382, 63–66 10.1038/382063a0 (doi:10.1038/382063a0) [DOI] [PubMed] [Google Scholar]
- 56.Frederiksen R., Wcislo W. T., Warrant E. J. 2008. Visual reliability and information rate in the retina of a nocturnal bee. Curr. Biol. 18, 349–353 10.1016/j.cub.2008.01.057 (doi:10.1016/j.cub.2008.01.057) [DOI] [PubMed] [Google Scholar]