Abstract
Inbreeding and a consequent loss of genetic diversity threaten small, isolated populations. One mechanism by which genetically impoverished populations may become extinct is through decreased immunocompetence and higher susceptibility to parasites. Here, we investigate the relationship between immunity and inbreeding in bumblebees, using Hebridean island populations of Bombus muscorum. We sampled nine populations and recorded parasite prevalence and measured two aspects of immunity: the encapsulation response and levels of phenoloxidase (PO). We found that prevalence of the gut parasite Crithidia bombi was higher in populations with lower genetic diversity. Neither measure of immune activity was correlated with genetic diversity. However, levels of PO declined with age and were also negatively correlated with parasite abundance. Our results suggest that as insect populations lose heterozygosity, the impact of parasitism will increase, pushing threatened populations closer to extinction.
Keywords: inbreeding, social insects, heterozygosity, disease, immune defence
1. Introduction
Genetic diversity is crucial in maintaining the fitness of populations by allowing them to withstand short-term environmental perturbations and evolve in response to long-term environmental change [1]. Small, isolated populations are at risk from losing their genetic diversity, either over the long term, predominantly as a result of genetic drift [1] or over the short term as a result of inbreeding [2]. This can lead to inbreeding depression if there is a loss of reproductive fitness, usually owing to the increase in frequency of individuals homozygous for deleterious recessive alleles [3]. In turn, this may significantly increase the risk of local population extinctions [4–6].
One driver of extinction in genetically impoverished populations may be parasitism [7]. Inbreeding and increased homozygosity can increase both the prevalence of parasites at the population level and susceptibility to parasites at the individual level [1]. At the population level, a loss of genetic diversity owing to inbreeding reduces the capacity of the population to evolve in response to novel virulent parasite genotypes. The more genetically diverse a population is, the more likely it is that some individuals can resist a pathogen. Studies in vertebrates have supported this, showing that the genetic diversity of populations from a wide range of taxa is negatively correlated with pathogen prevalence (for example, [8,9]). At the individual level, lower heterozygosity may be associated with higher infection frequency and greater infection morbidity [10,11].
The studies that have addressed the effects of inbreeding on immunity and parasitism in invertebrates have demonstrated that the relationship is complex and can depend on host sex and genotype, as well as parasite species [12–14]. However, inbreeding can decrease invertebrate pathogen resistance at the individual level, either through the loss of specific resistance alleles [15], reduced defensive behaviour [16] or a lower efficacy of group level disease resistance [17]. At the population level, parasite transmission and the probability of infection is higher in inbred populations of some species [18,19]. However, correlations between heterozygosity and parasite prevalence are not universal [20].
It is important to understand the relationship between genetic diversity and parasitism in bumblebees as many species have suffered from significant population declines across their range, predominantly owing to the loss of habitats on which they depend [21]. The remaining populations of the rare species have become fragmented and genetically isolated. While common bumblebee species exhibit little genetic differentiation between populations [22,23], rare species appear to have much lower genetic diversity and considerable population subdivision [24]. One example is Bombus muscorum, which is a rare and declining bumblebee species in the UK, now predominantly found in the Western Isles of Scotland. Isolated island populations have substantially reduced genetic diversity [25]. Bombus muscorum shows markedly higher population structuring and isolation by distance than the coexisting Bombus jonellus, possibly because of its poor dispersal ability [26]. Hence, it is more susceptible to population isolation and inbreeding.
To date, only one study has tested how inbreeding in bumblebees influences immunity at the individual level. Gerloff et al. [27] found that one generation of sib-mating had no negative impact on the encapsulation response. Nevertheless, parasite load may be greater in locations where inbreeding is most acute as theory suggests that genetically diverse colonies of social insects have a selective advantage owing to higher parasite resistance [28,29]. This assumes that different host genotypes have varying susceptibility to different parasite strains, meaning that a parasitic infection is not likely to spread as rapidly or as far through a genetically heterogeneous colony [28,30]. Investigations with bumblebees using laboratory-reared colonies placed under field conditions have provided support for this theory. Genetically heterogeneous bumblebee colonies had significantly lower prevalence, load and species richness of a range of parasites compared with genetically homogeneous colonies [31,32].
Here, we aim to test the hypothesis that wild bumblebee populations that have a lower genetic diversity have a concomitant decrease in immunocompetence and an increase in parasite prevalence. We use the previously studied island populations of B. muscorum in the Western Isles of Scotland and measures of population genetic diversity are taken from Darvill et al. [25]. Immunocompetence was estimated by measuring two aspects of constitutive immunity; the encapsulation response and levels of the enzyme phenoloxidase (PO). The encapsulation response assay is a well-established method of measuring an insect's ability to respond to a foreign body and the synthetic implant provides a standardized challenge against which individual responses can be compared [33]. PO is a key component of the invertebrate immune system; it is stored as the inactive precursor pro-PO and activated when infection is detected [34]. Parasite prevalence was measured by dissecting bees and recording any parasitic infections present. These investigations allowed us to assess the impact of inbreeding on parasitism and immune parameters in wild insect populations.
2. Methods
Nine Hebridean islands off the west coast of Scotland were visited between 4 August and 20 August 2009 (Barra, Coll, Iona, Mingulay, North Uist, South Uist, Sandray, Staffa, Tiree). A total of 246 B. muscorum workers were collected, with a mean of 27.3 (range: 23–30) from each island. As samples were taken in the peak season for bumblebees, the numbers taken were unlikely to negatively impact the fitness of colonies. Collected bees were stored in hair curlers (Superdrug, UK) with access to sugar water (50% Attracker solution in distilled water, Koppert Biological Systems, The Netherlands).
On the day of capture, bees were subjected to an encapsulation assay using an abiotic implant, following the methods of König & Schmid-Hempel [33]. Each bee was anaesthetized with CO2, placed under a dissecting microscope and secured with pins so that its ventral side was exposed. A fine sterile pin was used to make an incision in the inter-segmental membrane between the second and the third sternite. A nylon implant (diameter 0.16 mm, mean length 1.44 ± 0.012 mm) was inserted through this incision, where it would be exposed to the circulating haemolymph. After 4 h, the bee was freeze-killed in liquid nitrogen, before being stored in a −80°C freezer for later examination. A temperature data logger (Tinytag, Gemini data loggers, UK) recorded the ambient temperature during each assay and a mean was calculated for each 4 h implant period.
Before dissection and examination, each bee's abdomen was separated from its thorax and defrosted on ice. The implant was dissected out and mounted onto a slide using Eukitt (Electron Microscopy Sciences, USA). The degree of encapsulation was then measured by viewing the implant on a light table, with constant background illumination. A picture of the implant was taken and the mean grey value calculated using Image J software (US National Institutes of Health, MD, USA). This value was then subtracted from a control value (the mean grey value for an implant that had not been placed in a bee) to give a value for the encapsulation response [33].
The abdomen was inspected for the presence of the tracheal mite Locustacarus buchneri and any other macroparasites. The gut (excluding the honey sac) was then removed and homogenized in 200 µl of insect ringer solution (9.1 g NaCl; 0.52 g KCl; 1.2 g CaCl2ċ2H2O; 0.8 g MgCl2ċH2O; made to 1000 ml with distilled water). Ten microlitres of homogenate was examined at 400× magnification to determine the presence/absence of the microparasites Crithidia bombi, Nosema bombi and Apicystis bombi. If present, a further sample was examined on a haemocytometer and the number of cells in the two 0.1 µl grids was counted. The intensity of infection was recorded as the mean number of cells in 0.1 µl of gut homogenate.
The width of the thorax was measured using electronic digital callipers and the bee's age was estimated by assessing the extent of wing wear, using a four-point scale (modified from Mueller & Wolfmueller [35]). The PO activity assay was adapted from Brown et al. [36]. The thoraces were homogenized in 300 µl phosphate-buffered saline (PBS: 8.74 g NaCl; 1.78 g Na2HPO4ċ2H2O; 1000 ml distilled water; pH 6.5) before being centrifuged at 15.7g (4°C for 10 min). The supernatant was used to measure the concentration of the active PO as well as the total PO (proPO plus the active PO). Reaction mixtures for the active PO measurements contained 20 µl of the thorax supernatant, 140 µl distilled water, 20 µl PBS and 20 µl l-DOPA solution (4 mg ml−1 distilled water). For the total PO measurements, reaction mixtures contained 20 µl of the thorax supernatant, 120 µl distilled water, 20 µl PBS, 20 µl l-DOPA solution and 20 µl bovine α-chymotrypsin solution (Sigma, C4129; 2.1 mg ml−1 distilled water) and were incubated for 5 min at room temperature. The reaction was allowed to proceed at 30°C for 40 min in a microplate reader (Versamax, Molecular Devices, USA). Absorbance readings were taken every 10 s at 480 nm and analysed using SOFTmaxPRO 4.0 software (Molecular Devices, USA). Enzyme activity was measured as the slope (Vmax value) of the reaction curve during the linear phase of the reaction. Eight replicate assays were performed on each bee, four of total PO (including chymotrypsin) and four of active PO (without chymotrypsin). All measures of PO were corrected for bee size as approximated by the thorax width cubed.
(a). Statistical analysis
Data were analysed in R, v. 2.7.2 [37]. Conservative population-level analyses were first carried out using each population as a replicate and employing Pearson's correlations to investigate relationships between measures of genetic diversity, effective population size and parasite prevalence. The measures of genetic diversity (heterozygosity and allelic richness) were taken from Darvill et al. [25] and were based on nine microsatellite loci, of which one was monomorphic and a second almost so. Effective population size estimates were computed using Colony v. 2.0 [38,39]. In-depth individual-based analyses followed and as causal relationships between variables are unknown, a series of models were used, exchanging the dependent variable to explore all relationships. Population-level heterozygosity was used in individual analyses as a number of studies have shown that it is a more accurate predictor of heterozygosity than using individual measures based on a relatively small number of loci [40].
Binomial generalized linear models were used to investigate determinants of parasite prevalence (C. bombi and L. buchneri, respectively). Because of overdispersion in the L. buchneri prevalence data, a quasi-binomial model was used. Zero-inflated negative binomial models (ZINB) were used to investigate the variables influencing parasite abundance (both the number of C. bombi cells per 0.1 µl gut homogenate and the number of adult L. buchneri present in the abdomen). General linear models were used to investigate the variables influencing both the levels of PO and the encapsulation response. Both these response variables were Box–Cox-transformed to fulfil the assumptions of normality. In all models, island heterozygosity, population size, individual thorax width, wing wear, PO (total PO divided by thorax width cubed) and the load of the other parasite were entered as covariates. All statistical tests were two-tailed and all two-way interactions were investigated. Models were selected and simplified according to Akaike Information Criterion. Only significant interactions are presented here. Means are recorded as ± their standard errors throughout.
3. Results
Three species of parasite were detected: the gut trypanosome C. bombi, the tracheal mite L. buchneri and a conopid fly Physocephala sp. Crithidia bombi was detected at very high frequency in all island populations: prevalence ranged from 77% to 100%. Locustacarus buchneri was also detected in all populations, with a prevalence ranging from 3% to 53% (table 1). Larvae of Physocephala sp. were detected at very low prevalence (3%) and on only two islands (Staffa = two infected bees, Iona = six infected bees) and no further analysis was carried out for this parasite. No N. bombi or A. bombi were detected.
Table 1.
Population means for host genetic diversity, parasite prevalence and immunocompetence measures. The figures in parentheses are the standard errors for genetic diversity, PO and encapsulation and the 95% CI for parasite prevalence. Measures for heterozygosity and allelic richness are taken from Darvill et al. [25].
| island | n |
Bombus muscorum genetic diversity |
effective population size | Crithidia bombi prevalence | Locustacarus bombi prevalence | total PO (Vmax) | active PO (Vmax) | encapsulation (grey value) | |
|---|---|---|---|---|---|---|---|---|---|
| heterozygosity | allelic richness | ||||||||
| Barra | 30 | 0.393 (0.11) | 3.10 (0.66) | 59 | 0.90 (0.78–1.02) | 0.30 (0.14–0.46) | 7.92 (1.10) | 2.33 (0.47) | 36.29 (3.17) |
| Coll | 28 | 0.499 (0.09) | 3.46 (0.69) | 66 | 0.93 (0.83–1.03) | 0.54 (0.36–0.72) | 2.54 (0.45) | 0.89 (0.18) | 19.00 (1.84) |
| Iona | 29 | 0.554 (0.09) | 4.22 (0.91) | 68 | 0.79 (0.63–0.95) | 0.03 (−0.03–0.09) | 4.05 (0.57) | 1.25 (0.22) | 25.49 (4.18) |
| Mingulay | 27 | 0.374 (0.12) | 2.99 (0.64) | 46 | 0.93 (0.83–1.03) | 0.15 (0.01–0.29) | 7.19 (1.12) | 2.55 (0.36) | 27.71 (3.55) |
| N. Uist | 23 | 0.404 (0.11) | 3.32 (0.66) | 81 | 1.00 | 0.30 (0.12–0.48) | 4.99 (0.65) | 1.48 (0.25) | 22.95 (2.90) |
| S. Uist | 23 | 0.404 (0.11) | 3.32 (0.66) | 81 | 0.91 (0.79–1.03) | 0.39 (0.19–0.59) | 5.49 (0.83) | 1.62 (0.28) | 30.53 (4.14) |
| Sandray | 27 | 0.367 (0.11) | 3.05 (0.63) | 39 | 1.00 | 0.44 (0.26–0.62) | 5.79 (0.65) | 2.39 (0.49) | 16.05 (1.35) |
| Staffa | 30 | 0.484 (0.09) | 3.33 (0.51) | 52 | 0.77 (0.59–0.95) | 0.23 (0.07–0.39) | 3.76 (0.48) | 0.87 (0.14) | 22.29 (2.59) |
| Tiree | 29 | 0.499 (0.09) | 3.27 (0.55) | 79 | 0.89 (0.77–1.01) | 0.27 (0.11–0.43) | 5.46 (0.61) | 1.53 (0.18) | 26.42 (2.46) |
(a). Population level results
Pearson correlations revealed general relationships between measures of host genetic diversity and parasite prevalence. There was a negative relationship between heterozygosity and the prevalence of both parasites; this correlation was only significant in the case of Crithidia bombi (L. buchneri: r = −0.338, p = 0.374; C. bombi: r = −0.67, p = 0.048, figure 1). Heterozygosity and allelic richness were tightly correlated (r = 0.815, p = 0.007) and heterozygosity had better explanatory power in the analyses and hence was used in subsequent tests as the measure of genetic diversity. As effective population size had no correlation with parasite prevalence, it was excluded from the subsequent in-depth analysis of parasite prevalence.
Figure 1.
Relationship between Crithidia bombi prevalence and heterozygosity of host population. Each point represents an island population. Islands with higher heterozygosity had significantly lower prevalence of C. bombi (p = 0.003; table 2).
The negative correlation between C. bombi prevalence and host population heterozygosity remained significant in the detailed analysis that took other explanatory variables into account (Z = −2.99, p = 0.003). Age, as measured by wing wear, also significantly influenced C. bombi prevalence, with populations having a greater mean age more likely to be highly infected (Z = 2.02, p = 0.043). No other variable significantly affected the prevalence of C. bombi (table 2). No variable significantly affected the prevalence of Locustacarus bombi (table 2).
Table 2.
Output of binomial generalized linear model (GLM) and quasi-binomial GLM for the prevalence of C. bombi and L. buchneri, respectively. Degrees of freedom are given in parentheses. Significant results are highlighted in bold.
|
C. bombi |
L. buchneri |
|||||||
|---|---|---|---|---|---|---|---|---|
| coefficient estimate | s.e. | Z | p | coefficient esimate | s.e. | Z | p | |
| heterozygosity of population | −12.65 | 4.22 | −2.99 (1) | 0.003 | −9.01 | 5.17 | −1.74 (1) | 0.132 |
| date | 0.048 | 0.052 | 0.815 (1) | 0.415 | 0.053 | 0.051 | 1.04 (1) | 0.348 |
| age | 1.37 | 0.679 | 2.02 (1) | 0.043 | −0.156 | 1.83 | −0.085 (1) | 0.940 |
| bee size | −2.81 | 2.90 | −0.697 (1) | 0.334 | −2.36 | 2.08 | −1.13 (1) | 0.320 |
| encapsulation | −0.089 | 0.052 | −1.72 (1) | 0.086 | 0.014 | 0.081 | 0.171 (1) | 0.875 |
| phenoloxidase | −20.49 | 261.56 | −0.078 (1) | 0.938 | −48.67 | 29.67 | −1.64 (1) | 0.152 |
| L. buchneri prevalence | −6.91 | 4.73 | −1.46 (1) | 0.145 | — | — | — | — |
| C. bombi prevalence | — | — | — | — | 0.587 | 27.86 | 0.021 (1) | 0.987 |
(b). Trends across individuals in parasite abundance
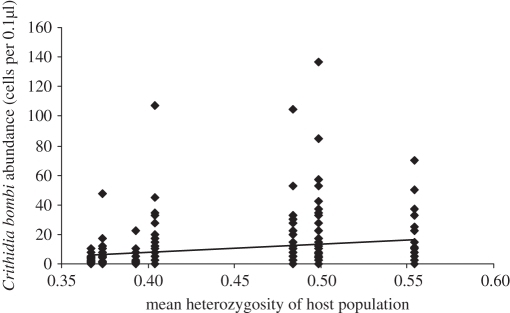
Here we use the parameter abundance to represent the number of parasites infecting a bee, including those bees that were uninfected. Crithidia bombi abundance was significantly positively correlated with both heterozygosity in the local host population and host population size, while it was negatively correlated with PO levels (table 3 and figure 2). Abundance of C. bombi was positively correlated with that of L. buchneri, and this effect was amplified in bees that also had high levels of PO (there was a significant positive interaction between the two factors). There was also a significant interaction between the effects of the abundance of L. buchneri and bee size: large bees with a high L. buchneri load had lower C. bombi loads than expected. Age and size also interacted with abundance of C. bombi, with older, larger bees having lower parasite loads (table 3).
Table 3.
Factors affecting C. bombi and L. buchneri abundance. The response variables are the number of C. bombi cells per 0.1 µl and the number of adult L. buchneri per bee, respectively. Coefficient estimates and standard errors are taken from the zero-inflated negative binomial model output. Log-likelihood ratio tests provide χ2 and p-values for each term. Significant results are highlighted in bold.
| source |
C. bombi abundance |
L. buchneri abundance |
||||||
|---|---|---|---|---|---|---|---|---|
| coefficient estimate | s.e. | χ2 | p | coefficient estimate | s.e. | χ2 | p | |
| heterozygosity of population | 4.67 | 1.33 | 10.12 | 0.001 | −2.96 | 2.71 | 1.16 | 0.282 |
| population size | 0.021 | 0.006 | 12.96 | <0.001 | 0.010 | 0.013 | 0.560 | 0.454 |
| date | −0.02 | 0.029 | 0.467 | 0.494 | −0.049 | 0.029 | 2.79 | 0.095 |
| PO | −59.49 | 22.26 | 6.74 | 0.009 | −18.94 | 6.36 | 8.55 | 0.003 |
| L. bombi load | 0.881 | 0.391 | 5.60 | 0.018 | −0.008 | 0.01 | 1.70 | 0.192 |
| bee size | 0.216 | 0.609 | 0.126 | 0.722 | 0.391 | 0.235 | 2.75 | 0.097 |
| age | 0.601 | 0.608 | 0.968 | 0.325 | −0.089 | 0.141 | 0.402 | 0.526 |
| encapsulation | 0.004 | 0.049 | 0.008 | 0.931 | 0.006 | 0.009 | 0.433 | 0.510 |
| age × size | −0.051 | 0.016 | 9.43 | 0.002 | — | — | — | — |
| L. bombi load × size | −0.193 | 0.077 | 6.98 | 0.008 | — | — | — | — |
| L. bombi load × PO | 4.36 | 1.83 | 6.54 | 0.011 | — | — | — | — |
Figure 2.
The relationship between Crithidia bombi abundance and heterozygosity of host population. Bees from populations with higher heterozyosity had higher parasite abundance (p = 0.001; table 3).
Abundance of L. buchneri, in contrast, was only significantly predicted by levels of PO (table 3), with higher infection intensities observed in bees with lower levels of PO.
(c). Trends across individuals in immune parameters
A total of 233 nylon implants were successfully dissected out of the bees. The encapsulation response showed large variation and the degree of greyness on the implants ranged from 0.73 units to 83.73 units, with a mean of 25.50 ± 1.05 units. This melanization neither showed any correlation with the length of the implant (r = 0.032, p = 0.616) nor with the ambient temperature during the 4 h assay (r = 0.066, p = 0.298). The encapsulation response had no relationship with population level heterozygosity but was significantly predicted by levels of PO; bees with higher levels of PO showed greater encapsulation responses. The population size and date also significantly influenced the encapsulation response; smaller populations had lower responses and the response also declined over the sampling period (table 4).
Table 4.
Output for general linear models for the encapsulation response and levels of total phenoloxidase. Degrees of freedom are given in parentheses. Significant results are highlighted in bold.
| encapsulation |
phenoloxidase |
|||
|---|---|---|---|---|
| F | p | F | p | |
| heterozygosity of population | 1.41 (1,226) | 0.236 | 28.07 (1,222) | <0.001 |
| population size | 5.23 (1,226) | 0.023 | 0.25 (1,221) | 0.62 |
| date | 10.35 (1,226) | 0.001 | 4.07 (1,222) | 0.045 |
| age | 2.66 (1,226) | 0.104 | 5.03 (1,222) | 0.002 |
| bee size | 0.234 (1,225) | 0.629 | 11.26 (1,222) | <0.001 |
| C. bombi load | 0.475 (1,225) | 0.491 | 6.14 (1,222) | 0.014 |
| L. bombi load | 0.009 (1,224) | 0.922 | 32.38 (1,222) | <0.001 |
| encapsulation | — | — | 4.17 (1,222) | 0.042 |
| phenoloxidase | 4.23 (1,226) | 0.041 | — | — |
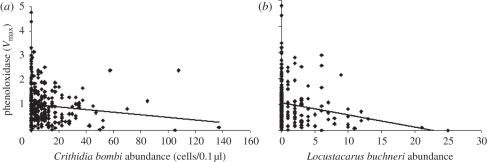
Total and active PO showed a strong positive correlation (r = 0.816, p < 0.0001), so only total PO was used in all analyses. Levels of total PO, in contrast to the encapsulation response, were significantly predicted by a large number of variables. Lower levels of PO were found in bees from populations with higher heterozygosity (table 4). Levels of PO were also found to decline with age (figure 3). Additionally, larger bees were found to have higher levels of volume-corrected PO. As expected from previous analyses, bees with higher infection intensities of C. bombi and L. buchneri had lower levels of PO (figure 4).
Figure 3.
Mean levels of PO for each age group, with wing wear as a proxy for age. Bars represent the least-square means and their standard errors as predicted from the generalized linear model. PO was found to significantly decline with age (p = 0.002; table 4).
Figure 4.
The relationship between phenoloxidase and abundance of (a) C. bombi and (b) L. buchneri. Levels of phenoxidase significantly declined with increasing abundance of C. bombi (p = 0.014; table 4) and L. buchneri (p < 0.001; table 4).
4. Discussion
This study is the first to demonstrate a relationship between the genetic diversity of natural bee populations and the prevalence of parasites. Bombus muscorum populations with lower levels of heterozygosity had a higher prevalence of the gut parasite C. bombi. This field-based study using wild bumblebee populations supports previous laboratory and experimental work that found genetic heterogeneity within colonies to be negatively correlated with parasitic infections in social insects [32,41,42]. Additionally, high loads of the ectoparasitic mite have been found on the invasive Bombus terrestris in Tasmania, which is inbred owing to small numbers of founding queens [43].
There are two mechanisms that might result in low heterozygosity causing increased parasite prevalence. Inbred individuals may have low immunocompetence, resulting in greater susceptibility to infection and, secondly, parasite infections may be able to spread faster through populations with lower genetic diversity. We measured two immune system parameters—PO levels and the encapsulation response. We found no evidence to suggest that bees from less heterozygous populations had inferior immune activity. However, it is possible that other immune system components may suffer negatively from inbreeding. Our results are consistent with the theory that population genetic homogeneity leads to higher parasite prevalence [28,30]. The theory assumes that host genotypes differ in their ability to resist different parasite strains, which is certainly true for C. bombi, as several studies have demonstrated a strong genetic component to the susceptibility of bumblebees to this parasite using cross-infection experiments, genetic analysis and quantitative trait locus mapping [44–46].
Higher parasite prevalence in more genetically depauperate populations has been found in a number of other species, particularly vertebrates, for example Whiteman et al. [47]. This relationship has not been so commonly studied in invertebrates but experimental work has shown that a lower genetic diversity increases the probability of parasitic infection in Daphnia magna and the freshwater snail Lymnaea stagnalis [18,19]. Thus parasitism may be a mechanism that increases the risk of extinction in small, isolated and inbred populations [7]. This is particularly relevant in the case of bumblebees because of their recent population declines in Europe, North America, Japan and China [21]. Populations of rare species are becoming fragmented and isolated, which has led to a decline in their genetic diversity [24].
It is likely that higher prevalence of bumblebee parasites reduces fitness and increases mortality in inbred populations. Crithidia bombi increases the mortality rate of food-stressed worker bees by up to 50 per cent [48] and reduces worker foraging efficiency [49]. Infection also reduces the fitness of colony-founding queens by 40 per cent [50,51], which could have a severe impact on declining populations. Relatively little research has been conducted on L. buchneri but limited data suggest that heavy infections might be associated with lethargy and reduced foraging [52]. Parasitic infection may also have indirect effects on fitness simply by stimulating the immune system; C. bombi infection elicits PO production [36] and L. buchneri infection triggers a melanization response by the host (P. R. Whitehorn 2009, personal observation). Colonies whose workers are immune challenged may have lower reproductive output, an effect exacerbated by harsh environmental conditions [53,54].
Interestingly, while bees in inbred populations were more likely to be infected with C. bombi, these populations also had lower mean parasite abundance. This could reflect the inability of inbred bees to survive high levels of infection meaning that high spore loads were not observed. A small but significant positive correlation was observed between the parasite load of C. bombi and L. buchneri. This may suggest that some individual bees are more generally susceptible to parasitic infection or that the two parasite species act synergistically.
Levels of PO were shown to decline with increasing bee age. A decline with age in both the encapsulation response and PO has been reported for bumblebees under laboratory conditions [55,56], but this is the first study to suggest that such immune senescence occurs in the wild. Levels of PO were also negatively correlated with the load of both parasite species. This is in agreement with a study by Siva-Jothy et al. [57], who found that PO levels became negatively correlated with gut parasite burden in a damselfly, after an acute immune challenge with a nylon implant. This suggests that a similar trade-off may be occurring in bumblebees; those infected with C. bombi may be unable to upregulate PO when subjected to the nylon implant we inserted. An alternative explanation for the negative correlation between PO and parasite load would be that more intense infections are able to establish in bees with lower immune capacity [58]. A negative correlation between heterozygosity and PO was also observed, but it seems likely that this is owing to the tight correlation between PO and C. bombi abundance. Levels of PO were positively correlated with the encapsulation response. As the enzyme was measured after the implant had been inserted, the correlation reflects the involvement of PO in the immune cascade that results in encapsulation [34].
In conclusion, this study has demonstrated that low genetic diversity in B. muscorum populations is associated with a higher prevalence of parasites, although we detected no associated loss of immunocompetence. This supports theories that suggest population genetic homogeneity enables parasites to spread to higher prevalence. Inbreeding negatively affects a range of fitness traits in insects; our current data suggest that elevated parasitism may pose an additional threat to isolated populations.
Acknowledgements
We thank Calum Brown, Katheryn Leggart and SAMS, Oban, for help with the fieldwork and we are grateful to James Weir for technical support. Thanks also to Olivier Lepais for Ne estimates and two anonymous reviewers for comments on the manuscript. P.R.W. was funded by a NERC studentship.
References
- 1.Frankham R., Ballou J. D., Briscoe D. A. 2010. Introduction to conservation genetics. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Keller L. F., Waller D. M. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 10.1016/S0169-5347(02)02489-8 (doi:10.1016/S0169-5347(02)02489-8) [DOI] [Google Scholar]
- 3.Charlesworth B., Charlesworth D. 1999. The genetic basis of inbreeding depression. Genet. Res. 74, 329–340 10.1017/S0016672399004152 (doi:10.1017/S0016672399004152) [DOI] [PubMed] [Google Scholar]
- 4.Frankham R. 2005. Genetic and extinction. Biol. Conserv. 126, 131–140 10.1016/j.biocon.2005.05.002 (doi:10.1016/j.biocon.2005.05.002) [DOI] [Google Scholar]
- 5.O'Grady J. J., Brook B. W., Reed D. H., Ballou J. D., Tonkyn D. W., Frankham R. 2006. Realistic levels of inbreeding depression strongly affect extinction risk in wild populations. Biol. Conserv. 133, 42–51 10.1016/j.biocon.2006.05.016 (doi:10.1016/j.biocon.2006.05.016) [DOI] [Google Scholar]
- 6.Reed D. H., Nicholas A. C., Stratton G. E. 2007. Genetic quality of individuals impacts population dynamics. Anim. Conserv. 10, 275–283 10.1111/j.1469-1795.2007.00120.x (doi:10.1111/j.1469-1795.2007.00120.x) [DOI] [Google Scholar]
- 7.de Castro F., Bolker B. 2005. Mechanisms of disease-induced extinction. Ecol. Lett. 8, 117–126 10.1111/j.1461-0248.2004.00693.x (doi:10.1111/j.1461-0248.2004.00693.x) [DOI] [Google Scholar]
- 8.Pearman P. B., Garner T. W. J. 2005. Susceptibility of Italian agile frog populations to an emerging strain of Ranavirus parallels population genetic diversity. Ecol. Lett. 8, 401–408 10.1111/j.1461-0248.2005.00735.x (doi:10.1111/j.1461-0248.2005.00735.x) [DOI] [Google Scholar]
- 9.Hedrick P. W., Kim T. J., Parker K. M. 2001. Parasite resistance and genetic variation in the endangered Gila topminnow. Anim. Conserv. 4, 103–109 10.1017/S1367943001001135 (doi:10.1017/S1367943001001135) [DOI] [Google Scholar]
- 10.Coltman D. W., Pilkington J. G., Smith J. A., Pemberton J. M. 1999. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution 53, 1259–1267 10.2307/2640828 (doi:10.2307/2640828) [DOI] [PubMed] [Google Scholar]
- 11.Acevedo-Whitehouse K., Spraker T. R., Lyons E., Melin S. R., Gulland F., Delong R. L., Amos W. 2006. Contrasting effects of heterozygosity on survival and hookworm resistance in California sea lion pups. Mol. Ecol. 15, 1973–1982 10.1111/j.1365-294X.2006.02903.x (doi:10.1111/j.1365-294X.2006.02903.x) [DOI] [PubMed] [Google Scholar]
- 12.Stevens L., Yan G. Y., Pray L. A. 1997. Consequences of inbreeding on invertebrate host susceptibility to parasitic infection. Evolution 51, 2032–2039 10.2307/2411025 (doi:10.2307/2411025) [DOI] [PubMed] [Google Scholar]
- 13.Rantala M. J., Roff D. A. 2007. Inbreeding and extreme outbreeding cause sex differences in immune defence and life history traits in Epirrita autumnata. Heredity 98, 329–336 10.1038/sj.hdy.6800945 (doi:10.1038/sj.hdy.6800945) [DOI] [PubMed] [Google Scholar]
- 14.Haag C. R., Sakwinska O., Ebert D. 2003. Test of synergistic interaction between infection and inbreeding in Daphnia magna. Evolution 57, 777–783 [DOI] [PubMed] [Google Scholar]
- 15.Spielman D., Brook B. W., Briscoe D. A., Frankham R. 2004. Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv. Genet. 5, 439–448 10.1023/B:COGE.0000041030.76598.cd (doi:10.1023/B:COGE.0000041030.76598.cd) [DOI] [Google Scholar]
- 16.Luong L. T., Heath B. D., Polak M. 2007. Host inbreeding increases susceptibility to ectoparasitism. J. Evol. Biol. 20, 79–86 10.1111/j.1420-9101.2006.01226.x (doi:10.1111/j.1420-9101.2006.01226.x) [DOI] [PubMed] [Google Scholar]
- 17.Calleri D. V., Reid E. M., Rosengaus R. B., Vargo E. L., Traniello J. F. A. 2006. Inbreeding and disease resistance in a social insect: effects of heterozygosity on immunocompetence in the termite Zootermopsis angusticollis. Proc. R. Soc. B 273, 2633–2640 10.1098/rspb.2006.3622 (doi:10.1098/rspb.2006.3622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puurtinen M., Hytönen M., Knott K. E., Taskinen J., Nissinen K., Kaitala V. 2004. The effects of mating system and genetic variability on susceptibility to trematode parasites in a freshwater snail, Lymnaea stagnalis. Evolution 58, 2747–2753 10.1554/04-465 (doi:10.1554/04-465) [DOI] [PubMed] [Google Scholar]
- 19.Ebert D., Altermatt F., Lass S. 2007. A short term benefit for outcrossing in a Daphnia metapopulation in relation to parasitism. J. R. Soc. Interface 4, 777–785 10.1098/rsif.2007.0232 (doi:10.1098/rsif.2007.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trouve S., Degen L., Renaud F., Goudet J. 2003. Evolutionary implications of a high selfing rate in the freshwater snail Lymnaea truncatula. Evolution 57, 2303–2314 10.1554/02-452 (doi:10.1554/02-452) [DOI] [PubMed] [Google Scholar]
- 21.Williams P. H., Osborne J. L. 2009. Bumblebee vulnerability and conservation world-wide. Apidologie 40, 367–387 10.1051/apido/2009025 (doi:10.1051/apido/2009025) [DOI] [Google Scholar]
- 22.Estoup A., Solignac M., Cornuet J. M., Goudet J., Scholl A. 1996. Genetic differentiation of continental and island populations of Bombus terrestris (Hymenoptera: Apidae) in Europe. Mol. Ecol. 5, 19–31 10.1111/j.1365-294X.1996.tb00288.x (doi:10.1111/j.1365-294X.1996.tb00288.x) [DOI] [PubMed] [Google Scholar]
- 23.Widmer A., Schmid-Hempel P. 1999. The population genetic structure of a large temperate pollinator species, Bombus pascuorum (Scopoli) (Hymenoptera: Apidae). Mol. Ecol. 8, 387–398 10.1046/j.1365-294X.1999.00584.x (doi:10.1046/j.1365-294X.1999.00584.x) [DOI] [PubMed] [Google Scholar]
- 24.Ellis J. S., Knight M. E., Darvill B., Goulson D. 2006. Extremely low effective population sizes, genetic structuring and reduced genetic diversity in a threatened bumblebee species, Bombus sylvarum (Hymenoptera: Apidae). Mol. Ecol. 15, 4375–4386 10.1111/j.1365-294X.2006.03121.x (doi:10.1111/j.1365-294X.2006.03121.x) [DOI] [PubMed] [Google Scholar]
- 25.Darvill B., Ellis J. S., Lye G. C., Goulson D. 2006. Population structure and inbreeding in a rare and declining bumblebee, Bombus muscorum (Hymenoptera: Apidae). Mol. Ecol. 15, 601–611 10.1111/j.1365-294X.2006.02797.x (doi:10.1111/j.1365-294X.2006.02797.x) [DOI] [PubMed] [Google Scholar]
- 26.Darvill B., O'Connor S., Lye G. C., Waters J., Lepais O., Goulson D. 2010. Cryptic differences in dispersal lead to differential sensitivity to habitat fragmentation in two bumblebee species. Mol. Ecol. 19, 53–63 10.1111/j.1365-294X.2009.04423.x (doi:10.1111/j.1365-294X.2009.04423.x) [DOI] [PubMed] [Google Scholar]
- 27.Gerloff C. U., Ottmer B. K., Schmid-Hempel P. 2003. Effects of inbreeding on immune response and body size in a social insect, Bombus terrestris. Funct. Ecol. 17, 582–589 10.1046/j.1365-2435.2003.00769.x (doi:10.1046/j.1365-2435.2003.00769.x) [DOI] [Google Scholar]
- 28.Sherman P. W., Seeley T. D., Reeve H. K. 1988. Parasites, pathogens, and polyandry in social Hymenoptera. Am. Nat. 131, 602–610 10.1086/284809 (doi:10.1086/284809) [DOI] [PubMed] [Google Scholar]
- 29.van Baalen M., Beekman M. 2006. The costs and benefits of genetic heterogeneity in resistance against parasites in social insects. Am. Nat. 167, 568–577 10.1086/501169 (doi:10.1086/501169) [DOI] [PubMed] [Google Scholar]
- 30.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press [Google Scholar]
- 31.Liersch S., Schmid-Hempel P. 1998. Genetic variation within social insect colonies reduces parasite load. Proc. R. Soc. Lond. B 265, 221–225 10.1098/rspb.1998.0285 (doi:10.1098/rspb.1998.0285) [DOI] [Google Scholar]
- 32.Baer B., Schmid-Hempel P. 2001. Unexpected consequences of polyandry for parasitism and fitness in the bumblebee, Bombus terrestris. Evolution 55, 1639–1643 10.1554/0014-3820(2001)055[1639:UCOPFP]2.0.co;2 (doi:10.1554/0014-3820(2001)055[1639:UCOPFP]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 33.König C., Schmid-Hempel P. 1995. Foraging activity and immunocompetence in workers of the bumble bee, Bombus terrestris L. Proc. R. Soc. Lond. B 260, 225–227 10.1098/rspb.1995.0084 (doi:10.1098/rspb.1995.0084) [DOI] [Google Scholar]
- 34.Soderhall K., Cerenius L. 1998. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 10, 23–28 10.1016/S0952-7915(98)80026-5 (doi:10.1016/S0952-7915(98)80026-5) [DOI] [PubMed] [Google Scholar]
- 35.Mueller U. G., Wolfmueller B. 1993. A method for estimating the age of bees: age-dependent wing wear and coloration in the Wool-Carder bee Anthidium manicatum (Hymenoptera, Megachilidae). J. Insect Behav. 6, 529–537 10.1007/BF01049530 (doi:10.1007/BF01049530) [DOI] [Google Scholar]
- 36.Brown M. J. F., Moret Y., Schmid-Hempel P. 2003. Activation of host constitutive immune defence by an intestinal trypanosome parasite of bumble bees. Parasitology 126, 253–260 10.1017/S0031182002002755 (doi:10.1017/S0031182002002755) [DOI] [PubMed] [Google Scholar]
- 37.R Development Core Team. 2008 R: A language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing. See http://www.R-project.org . [Google Scholar]
- 38.Wang J. L. 2009. A new method for estimating effective population sizes from a single sample of multilocus genotypes. Mol. Ecol. 18, 2148–2164 10.1111/j.1365-294X.2009.04175.x (doi:10.1111/j.1365-294X.2009.04175.x) [DOI] [PubMed] [Google Scholar]
- 39.Jones O. R., Wang J. L. 2010. COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 10, 551–555 10.1111/j.1755-0998.2009.02787.x (doi:10.1111/j.1755-0998.2009.02787.x) [DOI] [PubMed] [Google Scholar]
- 40.Pemberton J. 2004. Measuring inbreeding depression in the wild: the old ways are the best. Trends Ecol. Evol. 19, 613–615 10.1016/j.tree.2004.09.010 (doi:10.1016/j.tree.2004.09.010) [DOI] [PubMed] [Google Scholar]
- 41.Hughes W. O. H., Boomsma J. J. 2004. Genetic diversity and disease resistance in leaf-cutting ant societies. Evolution 58, 1251–1260 10.1554/03-546 (doi:10.1554/03-546) [DOI] [PubMed] [Google Scholar]
- 42.Seeley T. D., Tarpy D. R. 2007. Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. B 274, 67–72 10.1098/rspb.2006.3702 (doi:10.1098/rspb.2006.3702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen G. R., Seeman O. D., Schmid-Hempel P., Buttermore R. E. 2007. Low parasite loads accompany the invading population of the bumblebee, Bombus terrestris in Tasmania. Insect. Soc. 54, 56–63 10.1007/s00040-007-0908-y (doi:10.1007/s00040-007-0908-y) [DOI] [Google Scholar]
- 44.Imhoof B., Schmid-Hempel P. 1998. Patterns of local adaptation of a protozoan parasite to its bumblebee host. Oikos 82, 59–65 10.2307/3546917 (doi:10.2307/3546917) [DOI] [Google Scholar]
- 45.Schmid-Hempel P., Puhr K., Kruger N., Reber C., Schmid-Hempel R. 1999. Dynamic and genetic consequences of variation in horizontal transmission for a microparasitic infection. Evolution 53, 426–434 10.2307/2640779 (doi:10.2307/2640779) [DOI] [PubMed] [Google Scholar]
- 46.Wilfert L., Gadau J., Baer B., Schmid-Hempel P. 2007. Natural variation in the genetic architecture of a host–parasite interaction in the bumblebee Bombus terrestris. Mol. Ecol. 16, 1327–1339 10.1111/j.1365-294X.2007.03234.x (doi:10.1111/j.1365-294X.2007.03234.x) [DOI] [PubMed] [Google Scholar]
- 47.Whiteman N. K., Matson K. D., Bollmer J. L., Parker P. G. 2006. Disease ecology in the Galapagos Hawk (Buteo galapagoensis): host genetic diversity, parasite load and natural antibodies. Proc. R. Soc. B 273, 797–804 10.1098/rspb.2005.3396 (doi:10.1098/rspb.2005.3396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown M. J. F., Loosli R., Schmid-Hempel P. 2000. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 91, 421–427 10.1034/j.1600-0706.2000.910302.x (doi:10.1034/j.1600-0706.2000.910302.x) [DOI] [Google Scholar]
- 49.Otterstatter M. C., Gegear R. J., Colla S. R., Thomson J. D. 2005. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behav. Ecol. Sociobiol. 58, 383–389 10.1007/s00265-005-0945-3 (doi:10.1007/s00265-005-0945-3) [DOI] [Google Scholar]
- 50.Brown M. J. F., Schmid-Hempel R., Schmid-Hempel P. 2003. Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. J. Anim. Ecol. 72, 994–1002 10.1046/j.1365-2656.2003.00770.x (doi:10.1046/j.1365-2656.2003.00770.x) [DOI] [Google Scholar]
- 51.Yourth C. P., Brown M. J. F., Schmid-Hempel P. 2008. Effects of natal and novel Crithidia bombi (Trypanosomatidae) infections on Bombus terrestris hosts. Insect. Soc. 55, 86–90 10.1007/s00040-007-0974-1 (doi:10.1007/s00040-007-0974-1) [DOI] [Google Scholar]
- 52.Husband R. W., Sinha R. N. 1970. A revision of genus Locustacarus with a key to genera of family Podapolipidae (Acarina). Ann. Entomol. Soc. Am. 63, 1152 [Google Scholar]
- 53.Moret Y., Schmid-Hempel P. 2001. Entomology: immune defence in bumble-bee offspring. Nature 414, 506. 10.1038/35107138 (doi:10.1038/35107138) [DOI] [PubMed] [Google Scholar]
- 54.Moret Y., Schmid-Hempel P. 2004. Social life-history response to individual immune challenge of workers of Bombus terrestris L.: a possible new cooperative phenomenon. Ecol. Lett. 7, 146–152 10.1046/j.1461-0248.2003.00561.x (doi:10.1046/j.1461-0248.2003.00561.x) [DOI] [Google Scholar]
- 55.Doums C., Moret Y., Benelli E., Schmid-Hempel P. 2002. Senescence of immune defence in Bombus workers. Ecol. Entomol. 27, 138–144 10.1046/j.1365-2311.2002.00388.x (doi:10.1046/j.1365-2311.2002.00388.x) [DOI] [Google Scholar]
- 56.Moret Y., Schmid-Hempel P. 2009. Immune responses of bumblebee workers as a function of individual and colony age: senescence versus plastic adjustment of the immune function. Oikos 118, 371–378 10.1111/j.1600-0706.2008.17187.x (doi:10.1111/j.1600-0706.2008.17187.x) [DOI] [Google Scholar]
- 57.Siva-Jothy M. T., Tsubaki Y., Hooper R. E., Plaistow S. J. 2001. Investment in immune function under chronic and acute immune challenge in an insect. Physiol. Entomol. 26, 1–5 10.1046/j.1365-3032.2001.00206.x (doi:10.1046/j.1365-3032.2001.00206.x) [DOI] [Google Scholar]
- 58.Nigam Y., Maudlin I., Welburn S., Ratcliffe N. A. 1997. Detection of phenoloxidase activity in the hemolymph of tsetse flies, refractory and susceptible to infection with Trypanosoma brucei rhodesiense. J. Invertebr. Pathol. 69, 279–281 10.1006/jipa.1996.4652 (doi:10.1006/jipa.1996.4652) [DOI] [PubMed] [Google Scholar]