Abstract
How task specialization, individual task performance and within-group behavioural variation affects fitness is a longstanding and unresolved problem in our understanding of animal societies. In the temperate social spider, Anelosimus studiosus, colony members exhibit a behavioural polymorphism; females either exhibit an aggressive ‘asocial’ or docile ‘social’ phenotype. We assessed individual prey-capture success for both phenotypes, and the role of phenotypic composition on group-level prey-capture success for three prey size classes. We then estimated the effect of group phenotypic composition on fitness in a common garden, as inferred from individual egg-case masses. On average, asocial females were more successful than social females at capturing large prey, and colony-level prey-capture success was positively associated with the frequency of the asocial phenotype. Asocial colony members were also more likely to engage in prey-capture behaviour in group-foraging situations. Interestingly, our fitness estimates indicate females of both phenotypes experience increased fitness when occupying colonies containing unlike individuals. These results imply a reciprocal fitness benefit of within-colony behavioural variation, and perhaps division of labour in a spider society.
Keywords: animal personality, balancing selection, behavioural syndrome, cooperation, task specialization, social selection
1. Introduction
The global ecological success of social arthropods has classically been attributed to differential task performance by specialized individuals [1–3], and it is generally supposed that specialists are more efficient at their respective tasks than generalists ([1,4–6]; but see [7]). Perhaps the most extreme cases of task specialization are exhibited in eusocial insect societies (i.e. those showing discrete morphological castes). However, many societies, including humans [8,9], exhibit subdivision of labour without discrete morphological variants. Sex [10–12], body size [8,9,13–15], age [16–18], group size [19] and consistent individual differences in behaviour [5,20,21] have all been associated with specialization in animal societies. Taken together, whether by morphological castes or otherwise, within-group behavioural variation is commonly interpreted as adaptive for animal societies, particularly insects [1–3,22–28]. If within-group behavioural variation is indeed adaptive, this might provide a novel mechanism by which intraspecific behavioural variation, whether genetic or phenotypic, is promoted and maintained in social animal populations (reviewed in [20,29,30]).
Whether and how within-group behavioural variation is linked to individual- and group-level task performance is not highly resolved [6,7,31–35]; furthermore, there is a paucity of data that characterize whether or how selection acts on within-colony behavioural variation [26,27], particularly in societies lacking sterile castes. Are certain individuals innately more effective at performing various tasks in societies lacking castes? Are the more effective individuals, in fact, the ones performing those tasks? How do the phenotypic compositions of social groups influence individual- and group-level fitness? Addressing these questions is non-trivial in most social animal systems for several reasons: (i) group sizes are commonly large, and reliably quantifying a significant proportion of group members' behaviour is therefore difficult; (ii) replication (i.e. assaying many colonies) is resource-intensive; and (iii) attributing behavioural variation to particular factors (e.g. individual differences in ‘personality’, age and sex) can be exigent in prolonged, multi-generational animal societies. In an ideal model society, (i) there would be no more than a few dozen individuals, (ii) individual behaviour could be measured and tracked without significantly disturbing group social dynamics, (iii) colonies would naturally vary in their phenotypic composition (figure 1), (iv) experimental colonies of known phenotypic composition could be established in the laboratory and in the field, and (v) individual- and colony-level task performance and suitable fitness proxies could be estimated.
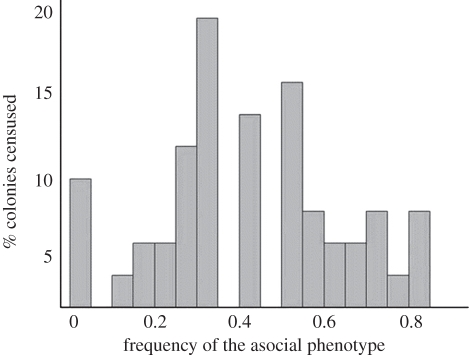
Figure 1.
Histogram of the phenotypic compositions of naturally occurring colonies (n = 50). The x-axis is the frequency of the asocial phenotype, and the y-axis is the percentage of colonies censused that exhibited each composition [44].
Spider societies offer a uniquely suited system with which to test hypotheses concerning the adaptive value of within-group behavioural variation, closely matching many of the preferred attributes listed above. Spider societies lack discrete morphological castes, and colony constituents cooperate together in shared web maintenance, prey capture and alloparental care [10–12]. In at least one species—the temperate social spider Anelosimus studiosus (Araneae, Theridiidae)—generations are relatively discrete [18,36]. Female A. studiosus feed their offspring for their first few instars (3–5 weeks), and in late summer mothers die. Offspring overwinter as late-instar juveniles and mature in spring the following year. With the exception of mating, males contribute little to colony function in spider societies [10–12,37]. Female A. studiosus exhibit a social behaviour polymorphism throughout the majority of their range [38], where they exhibit either an aggressive ‘asocial’ or a docile ‘social’ phenotype [39–43]. Perhaps, counterintuitively, both ‘social’ and ‘asocial’ females can be found in varying proportions both as singleton individuals and coalesced together in multi-female colonies [44,45], and naturally occurring colonies vary tremendously in the relative proportion of these two phenotypes [44]. In northern populations, colonies of A. studiosus may persist for several generations and form when females fail to disperse from their natal webs [37,40]. Males, in contrast, always disperse [43,46], and colonies of A. studiosus are therefore unlikely to be as inbred as are colonies of the inbred social spiders [11,12,47]. Interestingly, there is a significant relationship between colony size and composition, where larger colonies contain a higher frequency of the asocial phenotype [44].
The influence of asocial females on colony function is presently unresolved. Some data indicate that asocial females might act as social parasites within mixed-phenotype colonies, consuming a disproportionately large amount of food and even attacking other colony members [41,44]. However, asocial individuals are also more aggressive towards predators, prey and mates, and are generally more active than social females [38,41–44]. Because of the pronounced behavioural differences between social and asocial females, there exists a potential for differential task performance within mixed-phenotype colonies, and thus asocial females might contribute to colony function in some significant way. For instance, asocial females might act as a ‘soldier’ behavioural type within multi-female webs, or contribute more to prey capture or colony defence.
In this study, we consider individual and group-level prey-capture success in A. studiosus, and estimate the potential fitness effects of within-group behavioural variation. We determine (i) whether individual prey-capture success is associated with behavioural phenotype, (ii) whether there is a phenotypic bias in prey-capture behaviour in mixed-phenotype colonies, (iii) whether the phenotypic composition of colonies influences their ability to capture prey of various sizes and (iv) whether individuals experience increased fitness in mixed versus monomorphic multi-female colonies in a common garden experiment. We choose to emphasize prey-capture behaviour in the present study because the wealth of evidence suggests that spider societies are promoted and maintained by improved prey-capture efficiency, particularly of large high-value prey items, which are otherwise unobtainable for singleton individuals [11,12,14,48–56].
2. Methods
(a). Collection and laboratory maintenance
Colonies of late-instar juvenile A. studiosus were collected along riparian habitats in east Tennessee by placing garbage bags over the colonies and trimming the supporting branches. Colonies were then transported back to the laboratory and individual juveniles were isolated in 59 ml opaque plastic cups. Singleton juveniles were fed a mixed maintenance diet of termite workers and one-week-old crickets. Females were checked twice weekly for molts and upon maturity their social phenotype was assessed using the protocol described below. Females of known phenotype were then either (i) run through a single prey-capture efficiency trial, or (ii) assigned to a cohort of size-matched females for use in staged group prey-capture trials or our common garden experiment. Multi-female colonies were established after the protocol of Pruitt & Reichert [44]. Multi-female colonies were housed in 490 ml clear plastic enclosures containing a ball of tangled poultry wiring to facilitate web construction.
(b). Inter-individual distance test
To determine females' social tendency, two females of unknown social tendency were individually marked with fluorescent powder and placed in the centre of clear plastic containers (13 × 13.5 × 2.5 cm). After 24 h of settling time, we measured the distance between them. All females that exhibited an inter-individual distance greater than zero were run through a second confirmatory test with a known highly social female (i.e. one that previously exhibited an inter-individual distance score of 0). This is because more aggressive females that demand space may chase away social females. Females that aggregated in the same corner were categorized as ‘social’ and females that settled in opposite or adjacent corners in the second confirmatory test were ‘asocial’. Females' inter-individual distance scores are both repeatable and exhibit an additive genetic component to the behaviour ([38,41,43]; Pruitt & Reichert 2008, unpublished data).
(c). Individual prey-capture success
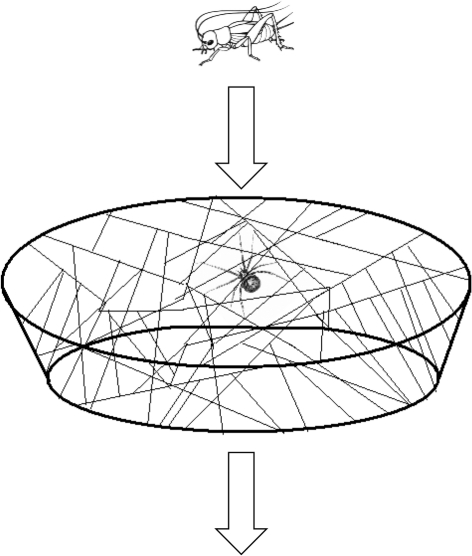
Females used in individual prey-capture trials were used in only one trial. Trials occurred in resident female webs. Before trials were initiated, the top of the female's enclosure was removed and the bottom surface was cut-off. Thus, prey items had the potential to jump/walk out of the top of the web or crawl directly through it (figure 2). Three size classes of crickets were used, and crickets were size-matched relative to the test female's body mass (i.e. small spiders received proportionately smaller crickets). The size classes were small (50 ± 5%), medium (150 ± 5%) and large (250 ± 5%). Before weighing a prey item, its rear legs were snipped along points of natural leg autonomization using a pair of surgical scissors; this procedure necessarily lengthens web retention time, which is particularly important for large prey items. Prey items were then dropped 1–2 cm over the web in a central position, and the test female's response and capture success was observed (figure 2).
Figure 2.
Diagram of singleton trials assessing the individual capture efficiencies of social versus asocial females.
(d). Phenotype composition and colony-level capture success
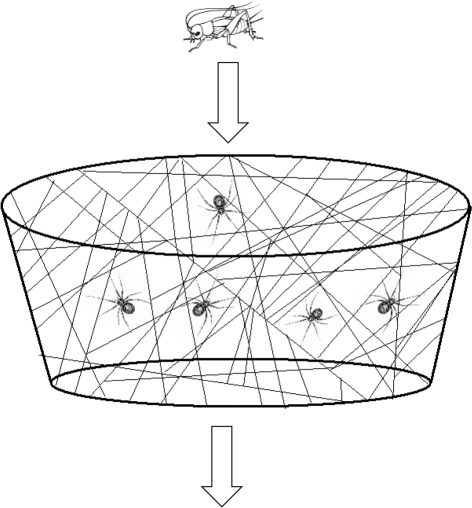
Our experimental colonies were composed of six size-matched (±5% body mass), randomly mated and individually marked females of known social tendency. Females were assigned to experimental colonies within one week of their maturation and painted with a unique pair of coloured dots using fast-drying modelling paint. Experimental colonies were constructed for three phenotypic compositions: 100 per cent social; 50 per cent social and 50 per cent asocial; and 100 per cent asocial. Colonies were housed in 473 ml clear plastic cups and provided a maintenance diet of two 2-week-old crickets once a week. Each colony (n = 57) was tested for each prey size class two days after a routine feeding, and the order of testing was randomized among colonies. As above, we used three prey size classes in these trials (small, 50 ± 5%; medium, 150 ± 5%; large, 250 ± 5%), and the rear legs of the prey were removed before weighing. The prey item was then dropped from 1–2 cm above the web at a randomly selected point above the colony (figure 3). We dropped the prey item from various points above the colony to avoid potential spatial biases. We recorded (i) which individuals engaged in prey capture and (ii) whether the prey item was successfully subdued.
Figure 3.
Diagram of multi-female trials assessing group capture efficiencies for varying phenotypic composition.
(e). Individual and colony-level fitness in a common garden
To assess the effect of phenotypic composition on relative fitness within and among colonies, we established 20 colonies of each of the three phenotypic compositions described above. Our experimental colonies were maintained between 22 and 24°C in the laboratory under a natural light–dark photo period. Colonies were presented with a single prey item daily, and the prey size was alternated between small and medium-sized prey among feedings. Preliminary data suggested that large prey escape too frequently to reliably maintain laboratory colonies containing only social females. Females increase slowly in body mass post-maturity and dramatically shrink in size after parturition. Because of the size of egg cases relative to females, parturition events are easily identified in A. studiosus. Post-parturition females were encouraged to momentarily abandon their egg cases by prodding them gently with a probe. Egg cases were weighed and returned to their mothers. We used egg-case mass as a proxy for fitness in our analyses because egg-case mass is highly correlated with female body condition and the number of offspring produced in spiders [57–59].
(f). Statistical methods
We used χ2 statistics to test for differences in individual prey-capture efficiency among phenotypes and for phenotypic bias in prey-capture behaviour in staged mixed-phenotype foraging events. To test the effect of colony composition on prey-capture efficiency, we used logistic regression with capture success as a binomial response variable (main effects: phenotypic composition, prey size, phenotypic composition × prey size). The order in which prey sizes were presented was randomized among colonies, and thus we blocked by colony ID in our analysis.
We used general linear models to compare female egg-case masses among our three phenotypic compositions (100% AS, 50% AS 50% S, 100% S). First we tested for differences in egg-case masses among the three phenotypic compositions irrespective of females' phenotype (n = 20 replicate colonies of each composition; six females per colony). Next, we tested for a difference in egg-case mass between social and asocial females within the mixed treatment. For both analyses, we use individuals' starting mass as a covariate and colony ID as a random effect in our model.
3. Results
The effect of female phenotype on individual capture efficiency differed among prey sizes (total nasocial = 120, nsocial = 93; table 1). The capture efficiency of social and asocial females were statistically indistinguishable for small prey (χ2 = 0.74, d.f. = 1, p = 0.39); however, asocial females were more likely to capture medium-sized prey (χ2 = 3.93, d.f. = 1, p = 0.047). Too few large prey items were captured to permit a reliable comparison.
Table 1.
Percentage of prey items of various sizes captured by asocial (total n = 120) and social females (total n = 93).
| prey size | asocial (n = 40) | social (n = 31) |
|---|---|---|
| small | 75.0 | 58.10 |
| medium | 47.5 | 19.40 |
| large | 7.5 | 3.22 |
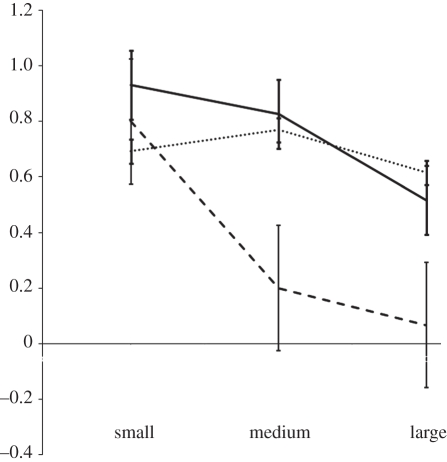
Our logistic regression model detected significant main effects of prey size (χ2 = 21.62, d.f. = 2, p < 0.001) and colony phenotypic composition (χ2 = 19.67, d.f. = 2, p < 0.001) on the probability of prey capture (figure 4). Irrespective of phenotypic composition, prey-capture success was negatively associated with prey size. The capture efficiencies of our three phenotypic compositions were, as in singleton trials, indistinguishable for small prey (χ2 = 1.42, d.f. = 2, p = 0.49), but differences among compositions were detected for medium (χ2 = 6.59, d.f. = 2, p = 0.037) and large prey (χ2 = 6.29, d.f. = 2, p = 0.043). In the mixed phenotypic composition, prey-capture behaviour was largely biased towards asocial colony members for medium (χ2 = 6.23, d.f. = 1, p = 0.0123) and large prey (χ2 = 7.20, d.f. = 1, p = 0.007), but no phenotypic bias was detected for small prey items (χ2 = 1.80, d.f. = 1, p = 0.179; table 2).
Figure 4.
The interaction between the phenotypic composition of social groups and prey size on prey-capture success. Vertical error bars represent standard error of the mean. Solid line, asocial; dotted line, mixed; dashed line, social.
Table 2.
Proportion of prey items captured in group prey-capture trials by social and asocial females.
| prey size | number of captures | asocial | social |
|---|---|---|---|
| small | 20 | 0.65 | 0.35 |
| medium | 19 | 0.80 | 0.20 |
| large | 13 | 0.85 | 0.15 |
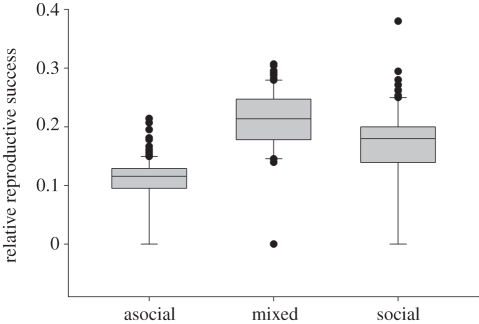
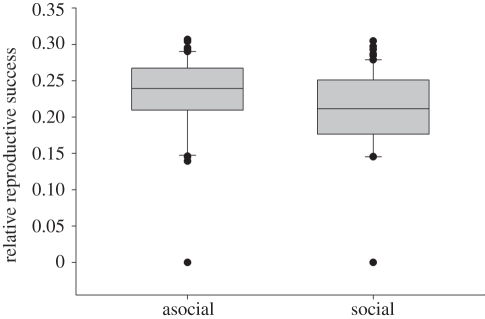
Our common garden experiment detected significant effects of females' initial starting mass (F1,54 = 85.03, p < 0.001) and colony phenotypic composition (F2,53 = 18.27, p < 0.001) on individual fitness (figure 5). However, we failed to detect a significant interaction between individuals' starting mass and colony composition (F2,54 = 0.19, p = 0.83). Irrespective of phenotype, larger females and those occupying mixed-phenotype colonies tended to produce larger egg cases. Within mixed phenotype colonies, we failed to detect a significant fitness difference between social versus asocial colony members (F1,119 = 1.83, p = 0.18; figure 6); however, individuals' starting mass exhibited a significant effect (F1,198 = 16.65, p < 0.001).
Figure 5.
Box plots of the relative reproductive success of females (egg-case mass/female starting mass), irrespective of phenotype, in the three experimental phenotypic compositions of social group.
Figure 6.
Box plots of the relative reproductive success (egg-case mass/female starting mass) of asocial versus social females in mixed phenotype colonies.
4. Discussion
It is commonly suggested that task specialization, increased individual efficiency and within-group phenotypic variation have together driven the ecological success of social arthropods [1–3]. However, some recent evidence suggests that these supposed driving factors may, in fact, be inaccurate in some instances, and in some cases the individuals performing tasks are not necessarily the most efficient at it ([6,7]; A. Dornhaus 2010, personal communication). Our data from a socially polymorphic spider reveals a phenotypic bias in task performance between social versus asocial colony members. Asocial females, which are generally more aggressive than social females [41–43], were more likely to engage in prey-capture behaviour in mixed-phenotype foraging bouts. Asocial females were also more efficient at capturing larger prey items in isolation; concordantly, their presence facilitates increased capture success of large prey in group-foraging situations. Thus, at least in the case of prey-capture behaviour, the most individually efficient colony members are those most commonly engaging in the task. We also provide evidence that both social and asocial females experience fitness benefits in the presence of unlike individuals. Taken together, our data suggest that there is a positive association between task specialization, individual- and group-level task efficiency, and within-group behavioural variation in A. studiosus. These findings are generally consistent with longstanding models from the social insect literature, which posit an adaptive significance of within-group behavioural variation ([1–3,6]; but see [7]).
In our common garden experiment, females of both phenotypes experienced increased fitness in mixed-phenotype colonies. Previous data demonstrated that asocial females consume a disproportionately large amount of prey and generally behave aggressively towards conspecifics [41–44], leading to the hypothesis that asocial females act as social parasites in mixed-phenotype colonies, and thus impede overall colony function. In prior work, we found the ‘social parasite’ hypothesis to be somewhat supported by the finding that group-level prey extraction efficiency was positively associated with the social phenotype in staged mixed-phenotype foraging bouts [44]. On the contrary, our fitness data from the present study appear to contradict our previous hypothesis—and perhaps the use of the terms ‘social’ and ‘asocial’—because we previously failed to consider the role of prey-capture success. Granted, the fitness data collected here are from an artificial scenario (no predation, parasitism, web damage, etc.), but females of both phenotypes produced relatively larger egg cases when occupying behaviourally diverse colonies. We propose that asocial females might capture more resources than they can reasonably consume, and therefore afford social females more feeding opportunities than they might experience in purely social colonies. How asocial females benefit from mixed-phenotype colonies remains an issue of more delicate speculation. One possibility is that asocial females, when numerically prevalent, impede one another's ability to feed and expend significant amounts of energy-resolving agonistic disputes. Taken together, the wealth of evidence presented here suggests that asocial females contribute at least as much as social individuals during prey-capture events, and therefore they are in some sense more ‘social’ than social females (i.e. they appear to contribute more in this ecologically important context). Thus, these new data support the need for a revision in the terminology used to describe these two behavioural types in A. studiosus, and we propose that the terms ‘docile’ and ‘aggressive’ more accurately describe the behaviour of ‘social’ and ‘asocial’ females, respectively.
Our results also provide a potential adaptive explanation for the positive association between colony size and frequency of the asocial phenotype [44]. Large multi-female colonies exhibit a reduced surface area to volume ratio, and their capture surface per colony member is therefore much lower than small colonies. Interestingly, several lines of research have suggested that large groups can overcome this scaling constraint by subduing larger and larger prey [56]. Our data on group prey-capture efficiencies suggest that larger colonies contain higher frequencies of the asocial phenotype [44], and the presence of asocial females helps facilitate increased prey-capture success of large, colony-sustaining prey items (figure 4). Thus, it could be suggested that asocial females are essential for the persistence of large multi-female colonies, which might otherwise be incapable of subduing large enough prey to resolve their scaling constraint [56].
A recurring theme in evolutionary biology is how variation is maintained in the face of optimization (i.e. the concept that evolution eliminates all but the most ‘fit’ of variants). One important mechanism by which behavioural variation could be favoured and maintained in social animals is by complementary phenotypes, where the strategies of some individuals are favoured and/or facilitated by the presence of other, unlike strategies. In some cases, the interactions of these phenotypes might not be mutually beneficial. For instance, in the case of intra-specific social parasitism, parasites benefit by the persistence of cooperative individuals, but the cooperative individuals are burdened by the interaction. Alternatively, phenotypes might reciprocally benefit from an interaction, and mixed phenotype compositions might emerge as an important mechanism of balancing selection in animal societies (e.g. [27,28]). We present evidence for such a mutualism in A. studiosus, where genetically influenced behavioural phenotypes (here relabelled as ‘docile’ and ‘aggressive’ from social and asocial, respectively) appear to experience increased fitness by associating with unlike individuals ([40,43]; figures 5 and 6). Whether and how individuals of various behavioural tendencies identify unlike individuals and the mechanisms behind the phenotypic assemblage of colonies are, unfortunately, largely unknown in our system. However, there is at least some preliminary evidence to suggest that social and asocial females might exhibit distinct chemical signatures, which could permit chemical discrimination [43].
Acknowledgements
We would like to thank Ben Fitzpatrick for his general statistical advice, and Kyle Demes, Anna Dornhaus and Jennifer Fewell for their comments, which aided in developing our manuscript. We are particularly indebted to the board member, editor and two anonymous reviewers for their assistance in improving the quality of our work.
References
- 1.Oster G. F., Wilson E. O. 1978. Caste and ecology in the social insects. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 2.Wilson E. O. 1987. Causes of ecological success: the case of the ants. J. Anim. Ecol. 56, 1–9 10.2307/4795 (doi:10.2307/4795) [DOI] [Google Scholar]
- 3.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Belknap Press [Google Scholar]
- 4.Calabi P., Traniello J. F. A. 1989. Social organization in the ant Pheidole dentata: physical and temporal caste ratios lack ecological correlates. Behav. Ecol. Sociobiol. 24, 69–78 10.1007/BF00299638 (doi:10.1007/BF00299638) [DOI] [Google Scholar]
- 5.Beshers S. N., Fewell J. H. 2001. Models of division of labor in social insects. Ann. Rev. Entomol. 46, 413–440 10.1146/annurev.ento.46.1.413 (doi:10.1146/annurev.ento.46.1.413) [DOI] [PubMed] [Google Scholar]
- 6.Chittka L., Muller H. 2009. Learning, specialization, efficiency and task allocation in social insects. Commun. Integr. Biol. 2, 151–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dornhaus A. 2008. Specialization does not predict individual efficiency in an ant. PLoS Biol. 6, e285. 10.1371/journal.pbio.0060285 (doi:10.1371/journal.pbio.0060285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argyris C. 1973. Personality and organization. New York, NY: Harper & Brothers [Google Scholar]
- 9.Schneider B. 1975. Organization climates: an essay. Pers. Psychol. 78, 447–479 10.1111/j.1744-6570.1975.tb01386.x (doi:10.1111/j.1744-6570.1975.tb01386.x) [DOI] [Google Scholar]
- 10.Buskirk R. E. 1981. Sociality in Arachnida. In Social insects (ed. Hermann H. R.), pp. 281–367 New York, NY: Academic Press [Google Scholar]
- 11.Avilés L. 1997. Causes and consequences of cooperation and permanent sociality in spiders. In The evolution of social behaviour in insects and arachnids (eds Choe J. C., Crespi B.), pp. 476–498 Cambridge, UK: Cambridge University Press [Google Scholar]
- 12.Lubin Y., Bilde T. 2007. The evolution of sociality in spiders. Adv. Study Behav. 27, 83–145 10.1016/S0065-3454(07)37003-4 (doi:10.1016/S0065-3454(07)37003-4) [DOI] [Google Scholar]
- 13.Vollrath F. 1986. Eusociality and extraordinary sex ratios in the spider Anelosimus eximius (Araneae: Theridiidae). Behav. Ecol. Sociobiol. 18, 283–287 10.1007/BF00300005 (doi:10.1007/BF00300005) [DOI] [Google Scholar]
- 14.Rypstra A. L. 1993. Prey size, social competition and the development of reproductive division of labor in social spider groups. Am. Nat. 142, 868–880 10.1086/285577 (doi:10.1086/285577) [DOI] [Google Scholar]
- 15.Ebert D. 1998. Asymmetry in relation to body weight and hunger in the tropical social spider Anelosimus eximius (Araneae, Theridiidae). J. Arachnol. 26, 70–80 [Google Scholar]
- 16.Christenson T. E. 1984. Behaviour of colonial and solitary spiders of the theridiid species Anelosimus eximius. Anim. Behav. 32, 725–734 10.1016/S0003-3472(84)80148-7 (doi:10.1016/S0003-3472(84)80148-7) [DOI] [Google Scholar]
- 17.Vollrath F. 1986. Environment, reproduction and the sex ratio of the social spider Anelosimus eximius (Araneae: Theridiidae). J. Arachnol. 14, 267–281 [Google Scholar]
- 18.Jones T. C., Parker P. G. 2002. Delayed dispersal benefits both mother and offspring in the cooperative spider Anelosimus studiosus (Araneae: Theridiidae). Behav. Ecol. 13, 142–148 10.1093/beheco/13.1.142 (doi:10.1093/beheco/13.1.142) [DOI] [Google Scholar]
- 19.Jeanson R., Fewell J. H., Gorelick R., Bertram S. M. 2007. Emergence of increased division of labor as a function of group size. Behav. Ecol. Sociobiol. 62, 289–298 10.1007/s00265-007-0464-5 (doi:10.1007/s00265-007-0464-5) [DOI] [Google Scholar]
- 20.Sih A., Bell A. M., Johnson J. C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 21.Bolnick D. I., Svanback R., Fordyce J. A., Yang L. H., Davis J. M., Hulsey C. D., Forister M. L. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 [DOI] [PubMed] [Google Scholar]
- 22.Porter S. D., Tschinkel W. R. 1985. Fire ant polymorphism: the ergonomics of brood production. Behav. Ecol. Sociobiol. 16, 323–336 10.1007/BF00295545 (doi:10.1007/BF00295545) [DOI] [Google Scholar]
- 23.Porter S. D., Tschinkel W. R. 1985. Fire ant polymorphism: factors affecting worker size. Ann. Entomol. Soc. Am. 78, 381–386 [Google Scholar]
- 24.Schmid-Hempel P. 1992. Worker castes and adaptive demography. J. Evol. Biol. 5, 1–12 10.1046/j.1420-9101.1992.5010001.x (doi:10.1046/j.1420-9101.1992.5010001.x) [DOI] [Google Scholar]
- 25.Anderson C., Ratnieks F. L. W. 1999. Task partitioning in insect societies. I. Effect of colony size on queuing delay and colony ergonomic efficiency. Am. Nat. 154, 521–535 10.1086/303255 (doi:10.1086/303255) [DOI] [PubMed] [Google Scholar]
- 26.Mattila H. R., Seeley T. D. 2007. Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317, 362–364 10.1126/science.1143046 (doi:10.1126/science.1143046) [DOI] [PubMed] [Google Scholar]
- 27.Burns J. G., Dyer A. G. 2008. Diversity of speed-accuracy strategies benefits social insects. Curr. Biol. 18, R953–R954 10.1016/j.cub.2008.08.028 (doi:10.1016/j.cub.2008.08.028) [DOI] [PubMed] [Google Scholar]
- 28.Muller H., Chittka L. 2008. Animal personalities: the advantage of diversity. Curr. Biol. 20, R961–R963 10.1016/j.cub.2008.09.001 (doi:10.1016/j.cub.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 29.Nettle D. 2006. The evolution of personality variation in humans and other animals. Am. Psychol. 61, 622–631 10.1037/0003-066X.61.6.622 (doi:10.1037/0003-066X.61.6.622) [DOI] [PubMed] [Google Scholar]
- 30.Bell A. M. 2007. Future directions in behavioural syndromes research. Proc. R. Soc. B 274, 755–761 10.1098/rspb.2006.0199 (doi:10.1098/rspb.2006.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trumbo S. T., Robinson G. E. 1997. Learning and task interference by corpse-removal specialists in honey bee colonies. Ethology 103, 966–975 10.1111/j.1439-0310.1997.tb00138.x (doi:10.1111/j.1439-0310.1997.tb00138.x) [DOI] [Google Scholar]
- 32.Trumbo S. T., Huang Z. Y., Robinson G. E. 1997. Division of labor between undertaker specialists and other middle-aged workers in honey bee colonies. Behav. Ecol. Sociobiol. 41, 151–163 10.1007/s002650050374 (doi:10.1007/s002650050374) [DOI] [Google Scholar]
- 33.Waibel M., Floreano D., Magnenat S., Keller L. 2006. Division of labour and colony efficiency in social insects: effects of interactions between genetic architecture, colony kin structure and rate of perturbations. Proc. R. Soc. B 273, 1815–1823 10.1098/rspb.2006.3513 (doi:10.1098/rspb.2006.3513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeanson R., Clark R. M., Holbrook C. T., Bertram S. M., Fewell J. H., Kukuk P. F. 2008. Division of labor and socially induced changes in response thresholds in associations of solitary halictine bees. Anim. Behav. 7, 593–602 10.1016/j.anbehav.2008.04.007 (doi:10.1016/j.anbehav.2008.04.007) [DOI] [Google Scholar]
- 35.Langridge E. A., Sendova-Franks A. B., Franks N. R. 2008. How experienced individuals contribute to an improvement in collective performance in ants. Behav. Ecol. Sociobiol. 62, 447–456 10.1007/s00265-007-0472-5 (doi:10.1007/s00265-007-0472-5) [DOI] [Google Scholar]
- 36.Jones T. C., Parker P. G. 2000. Costs and benefits of foraging associated with delayed dispersal in the spider Anelosimus studiosus (Araneae: Theridiidae). J. Arachnol. 28, 61–69 10.1636/0161-8202(2000)028[0061:CABOFA]2.0.CO;2 (doi:10.1636/0161-8202(2000)028[0061:CABOFA]2.0.CO;2) [DOI] [Google Scholar]
- 37.Furey R. E. 1998. Two cooperatively social populations of the theridiid spider Anelosimus studiosus in a temperate region. Anim. Behav. 55, 727–735 10.1006/anbe.1997.0648 (doi:10.1006/anbe.1997.0648) [DOI] [PubMed] [Google Scholar]
- 38.Pruitt J. N., Riechert S. E., Iturralde G., Vega M., Fitzpatrick B. M., Avilés L. 2010. Population differences in behaviour are explained by shared within-population trait correlations. J. Evol. Biol. 23, 748–756 10.1111/j.1420-9101.2010.01940.x (doi:10.1111/j.1420-9101.2010.01940.x) [DOI] [PubMed] [Google Scholar]
- 39.Jones T. C., Riechert S. E., Dalrymple S., Parker P. G. 2007. Fostering model explains environmental variation in levels of sociality in a spider system. Anim. Behav. 73, 195–204 10.1016/j.anbehav.2006.06.006 (doi:10.1016/j.anbehav.2006.06.006) [DOI] [Google Scholar]
- 40.Riechert S. E., Jones T. C. 2008. Phenotypic variation in the behaviour of the spider, Anelosimus studiosus, facilitates shift from single female to multiple female nests in colder environments. Anim. Behav. 75, 1893–1902 10.1016/j.anbehav.2007.10.033 (doi:10.1016/j.anbehav.2007.10.033) [DOI] [Google Scholar]
- 41.Pruitt J. N., Riechert S. E., Jones T. C. 2008. Behavioral syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim. Behav. 76, 871–879 10.1016/j.anbehav.2008.05.009 (doi:10.1016/j.anbehav.2008.05.009) [DOI] [Google Scholar]
- 42.Pruitt J. N., Riechert S. E. 2009. Male mating preference is associated with risk of pre-copulatory cannibalism in a socially polymorphic spider. Behav. Ecol. Sociobiol. 63, 1573–1580 10.1007/s00265-009-0751-4 (doi:10.1007/s00265-009-0751-4) [DOI] [Google Scholar]
- 43.Pruitt J. N., Riechert S. E. 2009. Sex matters: sexually dimorphic fitness consequences of a behavioral syndrome. Anim. Behav. 78, 175–181 10.1016/j.anbehav.2009.04.016 (doi:10.1016/j.anbehav.2009.04.016) [DOI] [Google Scholar]
- 44.Pruitt J. N., Riechert S. E. 2009. Frequency-dependent success of cheaters during foraging bouts might limit their spread within colonies of a socially polymorphic spider. Evolution 63, 2966–2973 10.1111/j.1558-5646.2009.00771.x (doi:10.1111/j.1558-5646.2009.00771.x) [DOI] [PubMed] [Google Scholar]
- 45.Jones T. C., Pruitt J. N., Riechert S. E. In press Reproductive success in a socially polymorphic spider: social individuals experience depressed reproductive success in isolation. Ecol. Entomol. [Google Scholar]
- 46.Albo M. J., Viera C., Costa F. G. 2007. Pseudocopulation and male–male conflict elicited by subadult females of the subsocial spider Anelosimus studiosus (Theridiidae). Behaviour 144, 1217–1234 10.1163/156853907781890896 (doi:10.1163/156853907781890896) [DOI] [Google Scholar]
- 47.Duncan S. E., Riechert S. E., Fitzpatrick B. M., Fordyce J. A. 2010. Relatedness and genetic structure in a socially polymorphic population of the spider Anelosimus studiosus. Mol. Ecol. 4, 810–818 10.1111/j.1365-294X.2010.04523.x (doi:10.1111/j.1365-294X.2010.04523.x) [DOI] [PubMed] [Google Scholar]
- 48.Ward P. I., Enders M. M. 1985. Conflict and co-operation in the group feeding of the social spider Stegodyphus mimosarum. Behaviour 94, 167–182 10.1163/156853985X00325 (doi:10.1163/156853985X00325) [DOI] [Google Scholar]
- 49.Ward P. I. 1986. Prey availability increases less quickly than nest size in the social spider Stegodyphus mimosarum. Behaviour 97, 213–225 10.1163/156853986X00603 (doi:10.1163/156853986X00603) [DOI] [Google Scholar]
- 50.Rypstra A. L., Tirey R. S. 1991. Prey size, prey perishability and group foraging in a social spider. Oecologia 86, 25–30 10.1007/BF00317384 (doi:10.1007/BF00317384) [DOI] [PubMed] [Google Scholar]
- 51.Pasquet A., Krafft B. 1992. Cooperation and prey capture efficiency in a social spider, Anelosimus eximius (Araneae, Theridiidae). Ethology 90, 121–133 10.1111/j.1439-0310.1992.tb00826.x (doi:10.1111/j.1439-0310.1992.tb00826.x) [DOI] [Google Scholar]
- 52.Whitehouse M. E. A., Lubin Y. 2005. The functions of societies and the evolution of group living: spider societies as a test case. Biol. Rev. Camb. Phil. Soc. 80, 347–361 10.1017/S1464793104006694 (doi:10.1017/S1464793104006694) [DOI] [PubMed] [Google Scholar]
- 53.Powers K. S., Avilés L. 2007. The role of prey size and abundance in the geographical distribution of spider sociality. J. Anim. Ecol. 76, 995–1003 10.1111/j.1365-2656.2007.01267.x (doi:10.1111/j.1365-2656.2007.01267.x) [DOI] [PubMed] [Google Scholar]
- 54.Purcell J., Avilés L. 2007. Smaller colonies and more solitary living mark higher elevation populations of a social spider. J. Anim. Ecol. 76, 590–597 10.1111/j.1365-2656.2007.01228.x (doi:10.1111/j.1365-2656.2007.01228.x) [DOI] [PubMed] [Google Scholar]
- 55.Guevara J., Avilés L. 2007. Multiple sampling techniques confirm differences in insect size between low and high elevations that may influence levels of spider sociality. Ecology 88, 2015–2023 10.1890/06-0995.1 (doi:10.1890/06-0995.1) [DOI] [PubMed] [Google Scholar]
- 56.Yip E. C., Powers K. S., Avilés L. 2008. Cooperative capture of large prey solves scaling challenge faced by spider societies. Proc. Natl Acad. Sci. USA 105, 11 818–11 822 10.1073/pnas.0710603105 (doi:10.1073/pnas.0710603105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petersen B. 1950. The relationship between the size of mother and number of eggs and young in some spiders and its significance for the evolution of size. Experientia 6, 96–98 10.1007/BF02153369 (doi:10.1007/BF02153369) [DOI] [Google Scholar]
- 58.Kessler A. 1971. Relationship between egg production and food consumption in species of the genus Pardosa (Lycosidae, Araneae) under experimental conditions of food abundance and food storage. Oecologia 8, 93–109 10.1007/BF00345629 (doi:10.1007/BF00345629) [DOI] [PubMed] [Google Scholar]
- 59.Foelix R. F. 1996. Biology of spiders. New York, NY: Oxford University Press [Google Scholar]