Abstract
Theory predicts that males should increase overall investment in ejaculate expenditure with increasing levels of sperm competition. Since ejaculate production is costly, we may expect males to tailor their reproductive investment according to anticipated levels of sperm competition. Here, we investigate plasticity in ejaculate investment in response to cues of population average levels of sperm competition in a promiscuous mammal, the bank vole (Myodes glareolus). We manipulated the social experience of experimental subjects during sexual development via differential exposure to the odour of rival males, to simulate conditions associated with relatively high or low average levels of sperm competition. Males exposed to a high level of competition developed larger major accessory reproductive glands (seminal vesicles) than those that experienced a low level of competition, suggesting that an increased investment in the production of copulatory plugs and/or mating rate may be beneficial at relatively high sperm competition levels. However, investment in sperm production, testis size and sperm motility were not altered according to social experience. Our findings emphasize the importance of non-sperm components of the ejaculate in mammalian postcopulatory sexual selection, and add to the growing evidence linking plasticity in reproductive traits to social cues of sperm competition.
Keywords: sexual selection, phenotypic plasticity, ejaculate production, seminal vesicle, copulatory plug, bank vole
1. Introduction
Sperm competition occurs when sperm from two or more males compete to fertilize a given set of ova [1] and is an important selection pressure in the evolution of diverse animal reproductive traits [2]. Sperm competition often follows a raffle principle [3], where the probability of male fertilization success increases according to the number of sperm transferred, and hence selects for increased sperm production [4,5]. The occurrence of sperm competition can be quantified at varying levels, from low risk (i.e. a low probability of female double-mating) to high intensity (i.e. many competing ejaculates) [3,4,6,7], and has been linked to variation in male reproductive traits across diverse species (e.g. [2]). For example, it is well established that testes are relatively large in those species for which levels of sperm competition are high [4,8], and that selection under sperm competition leads to increased testis size [9]. In mammals, high levels of sperm competition are also associated with relatively high rates of sperm production [10], high-quality ejaculates [11] and more frequent ejaculations [12].
In addition to well-established relationships with sperm production and quality, the influence of sperm competition in determining male investment in other ejaculate components such as seminal fluid proteins is currently a subject of increasing research interest. Evidence from comparative studies indicates that species in which levels of sperm competition are high may also have relatively large accessory reproductive glands, which function in the production of these proteins (e.g. [13,14], but see [15,16]). For example, high levels of sperm competition among rodents are associated with relatively large seminal vesicles, and relatively large copulatory plugs transferred during mating [14]. Similarly, in an experimental evolution study, Drosophila pseudoobscura males from highly promiscuous lines evolved larger accessory glands compared with those from monogamous lines [17], potentially as an adaptation for an increased mating rate [18].
Sperm competition theory predicts adaptive variation in male ejaculate investment within, as well as between, species [4,6,7]. Most attention to date has focused on exploring male responses to immediate cues of sperm competition risk or intensity such as the number of competitors present at the time of mating [6–8]. For example, male meadow voles (Microtus pennsylvanicus) increase their sperm allocation when ejaculating under an elevated risk of sperm competition [19] and human males produce ejaculates with a higher proportion of motile sperm [20]. The seminal fluid protein content of ejaculates may also vary adaptively according to the immediate sperm competition risk [21]. For instance, in Drosophila melanogaster, males increase their allocation of sex peptide and ovulin (two proteins of the seminal fluid) to females when a competitor is present in the environment [22].
As well as adjusting ejaculate allocation according to the immediate risk or intensity of sperm competition at the time of mating, males may also tailor their overall investment in ejaculate production in relation to population-average levels of sperm competition. However, such adaptive, plastic responses to average (or population) sperm competition risk or intensity have received relatively little attention. Currently, most available evidence comes from studies of sperm production in invertebrate taxa (e.g. [23–28], see also [29]). For example, flatworms (Macrostomum lignano) raised in large groups where the level of sperm competition is high have relatively larger testes and higher sperm production rates when compared with those raised in small groups where the level is lower [26,27]. Investigation of adaptive plasticity in accessory reproductive gland investment has to date received even less attention, despite growing interest in adaptive allocation of seminal fluid proteins under sperm competition (e.g. [21,22]).
Here, we investigate if males of a promiscuous small mammal, the bank vole, Myodes (formerly Clethrionomys) glareolus, tailor investment in ejaculate production according to cues of average sperm competition level experienced during sexual development. Population density is highly variable in natural populations of bank voles [30]. This is likely to affect average sperm competition levels because females mate multiply [31,32] and encounter more males with home ranges overlapping their own at high population density [33]. Male bank voles should therefore be sensitive to cues of average sperm competition level in their local environment and tailor investment in ejaculate production accordingly. Conspecific odours convey information concerning sperm competition risk and intensity [19,29,34] and are used for individual recognition in rodents [35], including bank voles [36]. We therefore predicted that frequent exposure to social odours of rival males would stimulate increased investment in ejaculate production by male bank voles. Specifically, our experiment was designed to test for evidence of adaptive plasticity in: (i) testis size and sperm production, (ii) sperm motility (as an index of ejaculate quality [37]), and (iii) seminal vesicle size, in response to cues of average sperm competition level. Our results emphasize the importance of non-sperm components of the ejaculate in mammalian sperm competition, and add to the growing evidence linking plasticity in reproductive traits to social cues of sperm competition.
2. Material and methods
(a). Subjects
Male bank voles (n = 28) used in this experiment were F3 descendants of 29 wild-caught animals (15 males and 14 females) trapped in Cheshire (UK) within a 1 km2 area. Subjects were weaned at approximately 22 days, passive integrated transponder tagged for individual identification and housed individually in M3 cages (48 × 11.5 × 12 cm, North Kent Plastic Cages Ltd, UK) for the duration of the experiment. Subjects allocated to two treatment groups (‘low’ versus ‘high’ competition) did not differ in body mass (‘low’: 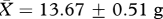 , ‘high’:
, ‘high’: 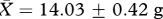 , t26 = 0.60, p = 0.55) or age (‘low’:
, t26 = 0.60, p = 0.55) or age (‘low’: 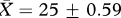 days, ‘high’:
days, ‘high’: 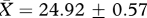 days, t26 = −0.09, p = 0.93) at the start of the experiment. The presence of brothers of the same family was balanced between the two treatments and males placed in the same enclosure were unrelated. Body mass was also monitored during the experiment, with further measures taken in weeks 5 and 11 using an electronic balance. One subject died during week 3 of the experiment and was replaced by another F3 male unrelated to the male already present in the enclosure. In addition to the experimental subjects, eight sexually mature males and 21 sexually mature females from the same population were used to provide social odours for the experimental treatments. These animals were maintained in a separate room, with males housed in individual M3 cages and females housed as unrelated trios in MB1 cages (45 × 28 × 13 cm, North Kent Plastic Cages Ltd, UK). All cages contained Corn cob Absorb 10/14 substrate and paper-wool nest material. In both rooms, animals were maintained on a reversed photoperiod (16 L : 8 D cycle, lights on at 1700 h), and at a temperature of 21 ± 1°C. Food and water were provided ad libitum (LabDiet 5002 Certified Rodent Diet, Purina Mills, St Louis, MO, USA).
days, t26 = −0.09, p = 0.93) at the start of the experiment. The presence of brothers of the same family was balanced between the two treatments and males placed in the same enclosure were unrelated. Body mass was also monitored during the experiment, with further measures taken in weeks 5 and 11 using an electronic balance. One subject died during week 3 of the experiment and was replaced by another F3 male unrelated to the male already present in the enclosure. In addition to the experimental subjects, eight sexually mature males and 21 sexually mature females from the same population were used to provide social odours for the experimental treatments. These animals were maintained in a separate room, with males housed in individual M3 cages and females housed as unrelated trios in MB1 cages (45 × 28 × 13 cm, North Kent Plastic Cages Ltd, UK). All cages contained Corn cob Absorb 10/14 substrate and paper-wool nest material. In both rooms, animals were maintained on a reversed photoperiod (16 L : 8 D cycle, lights on at 1700 h), and at a temperature of 21 ± 1°C. Food and water were provided ad libitum (LabDiet 5002 Certified Rodent Diet, Purina Mills, St Louis, MO, USA).
(b). Manipulation of social experience
Subject males in two treatment groups received contrasting social experience for a period of 10 weeks shortly after weaning. During this time, subjects in the ‘high’ competition group (n = 14) received weekly contact with social cues from four different males, whereas those in the ‘low’ competition group (n = 14) received contact with social cues from only one other male. At the end of the 10 week period, at age 14 weeks, all subjects were sexually mature (see also [38]) with mature sperm in storage, consistent with previous findings for this species reared under laboratory conditions [39].
Exposure to social odours other than those presented as part of the experimental treatment was minimized by placing subjects' cages within larger high-sided enclosures (1.2 × 1.2 m) within an environmentally controlled room. Two males from the same treatment group were kept within 14 such enclosures, with control for spatial position according to treatment group. On a weekly basis, subject males received in their home cage a small amount of substrate (approximately 12.5 g) collected from the cage of the other male in their enclosure. On three other days of the week, males from the ‘high’ competition group received an odour stimulus (i.e. approximately 12.5 g soiled substrate) from one of the three external males, whereas males from the ‘low’ competition group received an equivalent quantity of clean substrate to control for potential effects of cage handling. In the ‘high’ competition group, each male from the same enclosure consistently received substrate from the same three external males. To provide these odours, we used eight males maintained for this purpose (see §2a) with a different combination used for each enclosure.
To stimulate the development of normal sexual physiology and behaviour, all subjects also received exposure to female odours once a week during the experiment [40]. Exposure to female odours was achieved by transferring approximately 12.5 g of freshly collected substrate from a cage containing three sexually mature females (see above) to each subject male's home cage. Subject males always received odours from the same female cage, and each female cage was used to provide odour for four different male subjects, balanced between the two treatment groups.
(c). Reproductive organs: testis and seminal vesicle mass
Subject males were killed humanely using an overdose of halothane and dissected in week 11, their paired testes and seminal vesicles were weighed, and testes were frozen for subsequent analysis (see below). All masses and sperm measures (see below) were recorded blind to treatment group.
(d). Epididymal sperm count and sperm motility measures
Immediately after dissection, the left epididymis was removed for analysis of sperm motility measures, with the cauda isolated in a Petri dish containing 150 µl of Biggers Whitten Whittingham (BWW) medium solution [41]. Using a scalpel blade, 10 incisions were performed in the cauda and the sperm were allowed to disperse for 1 min before adding another 250 µl of BWW solution. All 400 µl of BWW solution was next transferred to an Eppendorf tube and maintained in a water bath at 37°C, while the right epididymis was dissected following a protocol identical to Ramm & Stockley [29] in order to count the number of epididymal sperm. During the time the haemocytometer was left to stand, the sperm motility analysis was performed. After 15 min in the 37°C water bath, 10 µl of the solution produced from the left epididymis (see above) was placed on a microscope slide under a coverslip. The slide was placed under a microscope (Leica DM1000) on a Microstat heated stage (Brunel Microscopes) set at 37°C. Swimming sperm were recorded at 20X magnification using a Flea2 camera (FL2-03S2M-C, Point Grey Research, Inc.), no more than 30 min after the start of the dissection. The duration of each recording was 2 s (75 frames s−1, 150 frames in total for each recording).
Recordings were analysed using the Computer-Assisted Sperm Analysis (CASA) plugin [42] for ImageJ software (v. 1.38x, http://rsbweb.nih.gov/ij/). We quantified estimates of: (i) curvilinear velocity (VCL, µm s−1), which estimates the velocity point-to-point along the trajectory, (ii) average path velocity (VAP, µm s−1), which estimates the point-to-point velocity over a constructed smooth path, and (iii) straight line velocity (VSL, µm s−1), which estimates the velocity point-to-point along a straight line. The threshold values for excluding static sperm were set up as 25 µm s−1 for VCL, 20 µm s−1 for VAP and 3 µm s−1 for VSL. To compare sperm motility between males from the two competition groups, we used two recordings of swimming sperm for each individual. First, we analysed each recording twice to test for repeatability of the measures taken by the CASA plugin on the same recording. These measures were highly repeatable for each variable (e.g. for VAP: intraclass coefficient of correlation: ICC = 0.98, F1,55 = 141.61, p < 0.001) and were therefore averaged. Next, we tested repeatability of the measures between the two different recordings for each subject, using the average value of each recording previously calculated. These measures were also highly repeatable (e.g. for VAP: ICC = 0.96, F1,27 = 51.23, p < 0.001) and were therefore averaged to obtain a mean value of each sperm motility trait.
(e). Estimates of daily sperm production
Daily sperm production rate was based on spermatid head counts from testicular homogenates [43,44] following the protocol described by Ramm & Stockley [29]. Because the timing of spermatogenesis in bank voles is known (total duration: 31 ± 0.7 days) [45], a static measure of sperm cells at the homogenization-resistant stage of spermatogenesis can be converted into a dynamic estimate of daily sperm production (sperm produced by the testis per day).
(f). Statistical analysis
Since the three descriptors of sperm velocity (VCL, VAP, VSL) were highly correlated, a principal component analysis of the variance–covariance matrix of these three log-transformed variables was used to reduce the number of parameters in subsequent analyses. The first principal component summarizing multivariate motility variation explained 96.28 per cent of the variance and had an eigenvalue of 2.88. The loadings of the three velocity measurements on this first factor were: 0.97 (VCL); 0.99 (VAP); 0.98 (VSL). The factor score is thus the single variable used in the subsequent analyses to represent sperm motility (hereafter called ‘sperm motility factor’).
Raw data were log-transformed when they did not fulfil normality conditions (assessed by Kolmogorov–Smirnov tests). Independent t-tests compared reproductive traits between treatment groups. These were performed for all individuals (n = 28) and also using enclosure mean values (n = 14) to control for potential non-independence of individual subjects owing to the shared enclosures. All tests are two-tailed and were conducted using SPSS 16.0. Data are presented as means ± s.e.m.
3. Results
Contrary to predictions based on sperm competition theory, males from the ‘high’ competition treatment group did not have significantly higher sperm production rates than those in the ‘low’ competition group. That is, there was no significant difference between treatment groups in epididymal sperm counts (high: 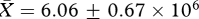 , low:
, low: 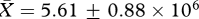 , t26 = −0.72, p = 0.48), estimated numbers of sperm produced daily (high:
, t26 = −0.72, p = 0.48), estimated numbers of sperm produced daily (high: 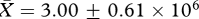 , low:
, low: 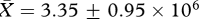 , t26 = 0.78, p = 0.44) or testis mass (high:
, t26 = 0.78, p = 0.44) or testis mass (high: 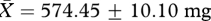 , low:
, low: 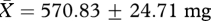 , t26 = 0.35, p = 0.73; figure 1a). Similarly, we found no evidence that males in the ‘high’ competition group produced higher quality ejaculates than those in the ‘low’ competition group, since the sperm motility factor was not significantly different between males from the two treatment groups (high:
, t26 = 0.35, p = 0.73; figure 1a). Similarly, we found no evidence that males in the ‘high’ competition group produced higher quality ejaculates than those in the ‘low’ competition group, since the sperm motility factor was not significantly different between males from the two treatment groups (high: 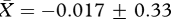 , low:
, low: 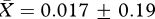 , t26 = 0.09, p = 0.93).
, t26 = 0.09, p = 0.93).
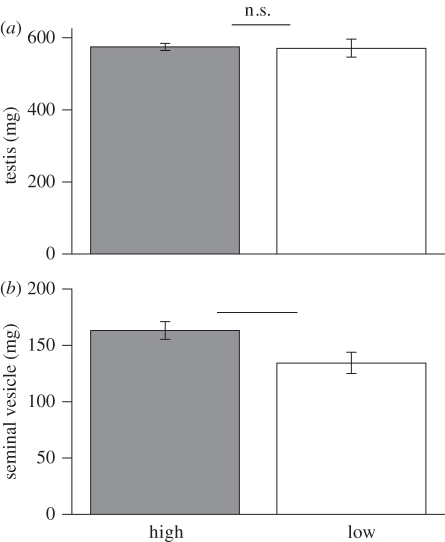
Figure 1.
Comparison of reproductive organ mass for male bank voles from the ‘high’ and the ‘low’ competition groups. (a) Testis mass did not significantly differ between males for the two competition groups (t26 = 0.35, p = 0.73). (b) Males from the ‘high’ competition group had significantly heavier seminal vesicles than those from the ‘low’ competition group (t26 = 2.45, p = 0.02). Bars represent mean ± s.e.m. n.s., non-significant.
In contrast to the results for sperm production and motility, we found a significant effect of treatment group on male investment in accessory reproductive glands. That is, males from the ‘high’ competition group had significantly heavier seminal vesicles than those from the ‘low’ competition group (high: 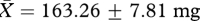 , low:
, low: 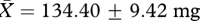 , t26 = 2.45, p = 0.02; figure 1b), as predicted if an increase in the production of seminal fluid proteins is favoured under sperm competition. Each of these results, including the significant effect of treatment group on seminal vesicle mass, remains qualitatively unchanged when the variables are compared using independent t-tests based on enclosure means (table 1).
, t26 = 2.45, p = 0.02; figure 1b), as predicted if an increase in the production of seminal fluid proteins is favoured under sperm competition. Each of these results, including the significant effect of treatment group on seminal vesicle mass, remains qualitatively unchanged when the variables are compared using independent t-tests based on enclosure means (table 1).
Table 1.
Comparison of reproductive traits for male bank voles from the ‘low’ and the ‘high’ competition groups based on enclosure means.
| group | mean | s.e.m | d.f | t | p | |
|---|---|---|---|---|---|---|
| body mass (g) | low | 22.91 | 0.33 | 12 | 0.13 | 0.90 |
| high | 23.02 | 0.56 | 12 | |||
| testis mass (mg) | low | 570.83 | 26.11 | 12 | 0.26 | 0.80 |
| high | 574.45 | 6.38 | 12 | |||
| seminal vesicle mass (mg) | low | 134.40 | 7.48 | 12 | 2.28 | 0.02 |
| high | 163.26 | 6.98 | 12 | |||
| epididymal sperm count (×106) | low | 5.61 | 1.00 | 12 | 0.86 | 0.40 |
| high | 6.06 | 0.32 | 12 | |||
| daily sperm production (×106) | low | 3.35 | 0.38 | 12 | −0.65 | 0.53 |
| high | 3.00 | 0.16 | 12 |
Although males from the ‘high’ competition group had significantly heavier seminal vesicles than those in the ‘low’ competition group, we found no evidence for an influence of experimental treatment group on the growth rate or body mass of subjects. There was no significant difference in the body mass of subjects in the ‘low’ and ‘high’ competition groups either at the middle (week 5: high: 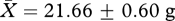 , low:
, low: 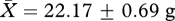 , t26 = 0.51, p = 0.62) or end of the experiment (week 11: high:
, t26 = 0.51, p = 0.62) or end of the experiment (week 11: high: 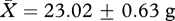 , low:
, low: 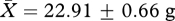 , t26 = −0.14, p = 0.89). Similarly, a repeated-measures analysis of variance incorporating all three body mass measurements (start, middle, end) revealed no effect of treatment group on body mass (F2,26 = 0.29, p = 0.75), and including body mass as a covariate in general linear models to explore effects of treatment group on male reproductive traits gives similar results to those presented above without control for body mass (e.g. testis mass: F1,26 = 0.01, p = 0.90; seminal vesicles mass: F1,26 = 5.60, p = 0.03).
, t26 = −0.14, p = 0.89). Similarly, a repeated-measures analysis of variance incorporating all three body mass measurements (start, middle, end) revealed no effect of treatment group on body mass (F2,26 = 0.29, p = 0.75), and including body mass as a covariate in general linear models to explore effects of treatment group on male reproductive traits gives similar results to those presented above without control for body mass (e.g. testis mass: F1,26 = 0.01, p = 0.90; seminal vesicles mass: F1,26 = 5.60, p = 0.03).
4. Discussion
Our results show that male bank voles altered investment in the size of a major accessory reproductive gland, the seminal vesicles, in response to cues of average sperm competition level, but did not adjust investment in sperm production or motility. Our study thus provides evidence for phenotypic plasticity of accessory reproductive gland size in response to sperm competition, and adds to growing evidence that non-sperm components of the ejaculate can play an important role in postcopulatory sexual selection [22,46].
In most mammals studied, the seminal vesicles are a major source of seminal fluid proteins (review in Poiani [47]) that often coagulate to form mating plugs in the female reproductive tract after copulation [13,47]. Recent studies highlight the likely functional significance of such plugs in mammalian sperm competition. For example, comparative studies of rodents reveal that higher levels of sperm competition are associated with relatively large seminal vesicles and mating plugs [14], a faster rate of molecular evolution among seminal vesicle-expressed genes [48], and a higher molecular mass of SVS II, a key protein involved in plug formation [49]. Although the precise function of mammalian copulatory plugs is uncertain, roles in promoting sperm transport and/or reducing the rate of female remating in the context of sperm competition appear likely [14,47]. Hence, it is possible that the increased investment in seminal vesicles of male bank voles in our study may function to permit the production of larger copulatory plugs, which seem likely to promote male fertilization success under elevated sperm competition [14].
Another potential benefit that larger accessory glands may confer is the ability to maintain an increased mating rate (e.g. [12,18], but see [50]). Even without an overall increase in the number of sperm transferred, repeated ejaculation may be beneficial to spread the delivery of sperm transferred to the same female under competitive conditions [12,51], and/or to spread delivery of fewer sperm per ejaculate to more females under conditions of high sperm competition intensity [5]. Hence it is possible that the increased investment in seminal vesicles shown by male bank voles in our ‘high’ competition group may function to promote more frequent ejaculation, but with a reduced sperm investment per ejaculate when compared with males in the ‘low’ competition group, as predicted by the ‘intensity’ model of sperm competition [5,6]. Consistent with the findings presented here, in a recent experimental evolution study, male D. pseudoobscura from promiscuous lines evolved larger accessory glands and had a higher mating rate when compared with males from monogamous lines, but did not evolve larger testes [17]. Therefore, in some species, seminal fluid proteins available for ejaculate allocation may constrain mating rates to a greater extent than the number of sperm available [17,52].
That male bank voles in our study did not increase sperm production rates in response to cues of high average levels of sperm competition contrasts with the recent findings for house mice (Mus musculus domesticus) [29]. Moreover, we found no evidence of plasticity in sperm motility according to social environment in bank voles. This contrasts with the growing evidence of adaptive plasticity in sperm phenotype among other taxa [53,54]; however, in line with the discussion above, it is important to recognize that our measurements were made in the absence of seminal fluid proteins, which might influence sperm motility within the female reproductive tract [47]. Nonetheless, previous studies have found evidence of intraspecific variation in mammalian sperm motility parameters related to individual quality using comparable methodology [55].
In conclusion, our study demonstrates evidence of adaptive plasticity in seminal vesicle size according to sexual competition in a promiscuous mammal. This new finding highlights the importance of non-sperm ejaculate components in rodent sperm competition (see also [48,49]) and the significance of sperm competition-induced phenotypic plasticity for explaining intraspecific variation in vertebrate reproductive traits [29,54].
Acknowledgements
This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society guidelines for the Use of Animals in Research, the legal requirements of the country in which the work was carried out and all institutional guidelines. No specific licences were required for this work.
We thank all members of the Mammalian Behaviour and Evolution Group for useful feedback and discussion, A. Davidson, J. Fick, R. Humphries, S. Jopson and J. Waters for their help in conducting the experiment, and J. Wilson-Leedy and S. Koyama for advice on sperm motility measurements. We also thank two anonymous referees and T. Pizzari for providing helpful comments on the original manuscript. J.F.L. and S.A.R. were supported by a grant from the Leverhulme Trust to P.S. (F/00025/W).
References
- 1.Parker G. A. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567 10.1111/j.1469-185X.1970.tb01176.x (doi:10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Birkhead T. R., Møller A. P. 1998. Sperm competition and sexual selection. London, UK: Academic Press [Google Scholar]
- 3.Parker G. A., Pizzari T. In press Sperm competition and ejaculate economics. Biol. Rev. 10.1111/j.1469-185X.2010.00140.x (doi:10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 4.Parker G. A. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 3–49 London, UK: Academic Press [Google Scholar]
- 5.Parker G. A., Ball M. A. 2005. Sperm competition, mating rate and the evolution of testis and ejaculate sizes: a population model. Biol. Lett. 1, 235–238 10.1098/rsbl.2004.0273 (doi:10.1098/rsbl.2004.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker G. A., Ball M. A., Stockley P., Gage M. J. G. 1996. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. Lond. B 263, 1291–1297 10.1098/rspb.1996.0189 (doi:10.1098/rspb.1996.0189) [DOI] [Google Scholar]
- 7.Parker G. A., Ball M. A., Stockley P., Gage M. J. G. 1997. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. Lond. B 264, 1793–1802 10.1098/rspb.1997.0249 (doi:10.1098/rspb.1997.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedell N., Gage M. J. G., Parker G. A. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 10.1016/S0169-5347(02)02533-8 (doi:10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 9.Hosken D. J., Ward P. I. 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13 10.1046/j.1461-0248.2001.00198.x (doi:10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 10.Ramm S. A., Stockley P. 2010. Sperm competition and sperm length influence the rate of mammalian spermatogenesis. Biol. Lett. 6, 219–221 10.1098/rsbl.2009.0635 (doi:10.1098/rsbl.2009.0635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Møller A. P. 1988. Ejaculate quality, testes size and sperm competition in primates. J. Hum. Evol. 17, 479–488 10.1016/0047-2484(88)90037-1 (doi:10.1016/0047-2484(88)90037-1) [DOI] [Google Scholar]
- 12.Stockley P., Preston B. T. 2004. Sperm competition and diversity in rodent copulatory behaviour. J. Evol. Biol. 17, 1048–1057 10.1111/j.1420-9101.2004.00742.x (doi:10.1111/j.1420-9101.2004.00742.x) [DOI] [PubMed] [Google Scholar]
- 13.Dixson A. F. 1998. Sexual selection and evolution of the seminal vesicles in primates. Folia Primatol. 69, 300–306 10.1159/000021643 (doi:10.1159/000021643) [DOI] [PubMed] [Google Scholar]
- 14.Ramm S. A., Parker G. A., Stockley P. 2005. Sperm competition and the evolution of male reproductive anatomy in rodents. Proc. R. Soc. B 272, 949–955 10.1098/rspb.2004.3048 (doi:10.1098/rspb.2004.3048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baer B., Boomsma J. J. 2004. Male reproductive investment and queen mating-frequency in fungus-growing ants. Behav. Ecol. 15, 426–432 10.1093/beheco/arh025 (doi:10.1093/beheco/arh025) [DOI] [Google Scholar]
- 16.Vahed K. 2006. Larger ejaculate volumes are associated with a lower degree of polyandry across bushcricket taxa. Proc. R. Soc. B 273, 2387–2394 10.1098/rspb.2006.3593 (doi:10.1098/rspb.2006.3593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crudgington H. S., Fellows S., Badcock N. S., Snook R. R. 2009. Experimental manipulation of sexual selection promotes greater male mating capacity but does not alter sperm investment. Evolution 63, 926–938 10.1111/j.1558-5646.2008.00601.x (doi:10.1111/j.1558-5646.2008.00601.x) [DOI] [PubMed] [Google Scholar]
- 18.Rogers D. W., Chapman T., Fowler K., Pomiankowski A. 2005. Mating-induced reduction in accessory reproductive organ size in the stalk-eyed fly Cyrtodiopsis dalmanni. BMC Evol. Biol. 5, 37. 10.1186/1471-2148-5-37 (doi:10.1186/1471-2148-5-37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.delBarco-Trillo J., Ferkin M. H. 2004. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature 431, 446–449 10.1038/nature02845 (doi:10.1038/nature02845) [DOI] [PubMed] [Google Scholar]
- 20.Kilgallon S. J., Simmons L. W. 2005. Image content influences men's semen quality. Biol. Lett. 1, 253–255 10.1098/rsbl.2005.0324 (doi:10.1098/rsbl.2005.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron E., Day T., Rowe L. 2007. Sperm competition and the evolution of ejaculate composition. Am. Nat. 169, E158–E172 10.1086/516718 (doi:10.1086/516718) [DOI] [PubMed] [Google Scholar]
- 22.Wigby S., Sirot L. K., Linklater J. R., Buehner N., Calboli F. C. F., Bretman A., Wolfner M., Chapman T. 2009. Seminal fluid protein allocation and male reproductive success. Curr. Biol. 19, 751–757 10.1016/j.cub.2009.03.036 (doi:10.1016/j.cub.2009.03.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gage M. J. G. 1995. Continuous variation in reproductive strategy as an adaptive response to population-density in the moth Plodia interpunctella. Proc. R. Soc. Lond. B 261, 25–30 10.1098/rspb.1995.0112 (doi:10.1098/rspb.1995.0112) [DOI] [Google Scholar]
- 24.Oppliger A., Hosken D. J., Ribi G. 1998. Snail sperm production characteristics vary with sperm competition risk. Proc. R. Soc. Lond. B 265, 1527–1534 10.1098/rspb.1998.0468 (doi:10.1098/rspb.1998.0468) [DOI] [Google Scholar]
- 25.Stockley P., Seal N. J. 2001. Plasticity in reproductive effort of male dung flies (Scatophaga stercoraria) as a response to larval density. Funct. Ecol. 15, 96–102 10.1046/j.1365-2435.2001.00496.x (doi:10.1046/j.1365-2435.2001.00496.x) [DOI] [Google Scholar]
- 26.Schärer L., Ladurner P. 2003. Phenotypically plastic adjustment of sex allocation in a simultaneous hermaphrodite. Proc. R. Soc. Lond. B 270, 935–941 10.1098/rspb.2002.2323 (doi:10.1098/rspb.2002.2323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schärer L., Vizoso D. B. 2007. Phenotypic plasticity in sperm production rate: there's more to it than testis size. Evol. Ecol. 21, 295–306 10.1007/s10682-006-9101-4 (doi:10.1007/s10682-006-9101-4) [DOI] [Google Scholar]
- 28.Bjork A., Dallai R., Pitnick S. 2007. Adaptive modulation of sperm production rate in Drosophila bifurca, a species with giant sperm. Biol. Lett. 3, 517–519 10.1098/rsbl.2007.0219 (doi:10.1098/rsbl.2007.0219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramm S. A., Stockley P. 2009. Adaptive plasticity of mammalian sperm production in response to social experience. Proc. R. Soc. B 276, 745–751 10.1098/rspb.2008.1296 (doi:10.1098/rspb.2008.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansson L., Henttonen H. 1985. Gradients in density variations of small rodents: the importance of latitude and snow cover. Oikos 67, 394–402 [DOI] [PubMed] [Google Scholar]
- 31.Ratkiewicz M., Borkowska A. 2000. Multiple paternity in the bank vole (Clethrionomys glareolus): field and experimental data. Mamm. Biol. 65, 6–14 [Google Scholar]
- 32.Klemme I., Eccard J. A., Ylönen H. 2006. Do female bank voles (Clethrionomys glareolus) mate multiply to improve on previous mates? Behav. Ecol. Sociobiol. 60, 415–421 10.1007/s00265-006-0181-5 (doi:10.1007/s00265-006-0181-5) [DOI] [Google Scholar]
- 33.Bujalska G. 1973. The role of spacing behaviour among females in the regulation of reproduction in the bank vole. J. Reprod. Fertil. 19, 465–474 [PubMed] [Google Scholar]
- 34.delBarco-Trillo J., Ferkin M. H. 2006. Male meadow voles respond differently to risk and intensity of sperm competition. Behav. Ecol. 17, 581–585 10.1093/beheco/ark001 (doi:10.1093/beheco/ark001) [DOI] [Google Scholar]
- 35.Hurst J. L., Beynon R. J. 2004. Scent wars: the chemobiology of competitive signalling in mice. BioEssays 26, 1288–1298 10.1002/bies.20147 (doi:10.1002/bies.20147) [DOI] [PubMed] [Google Scholar]
- 36.Radwan J., Tkacz A., Kloch A. 2008. MHC and preferences for male odour in the bank vole. Ethology 114, 827–833 10.1111/j.1439-0310.2008.01528.x (doi:10.1111/j.1439-0310.2008.01528.x) [DOI] [Google Scholar]
- 37.Gomendio M., Malo A. F., Garde J., Roldan E. R. S. 2007. Sperm traits and male fertility in natural populations. Reproduction 134, 19–29 10.1530/REP-07-0143 (doi:10.1530/REP-07-0143) [DOI] [PubMed] [Google Scholar]
- 38.Ylönen H., Horne T. J., Luukkonen M. 2004. Effect of birth and weaning mass on growth, survival and reproduction in the bank vole. Evol. Ecol. Res. 6, 433–442 [Google Scholar]
- 39.Kruczek M. 1986. Seasonal effects on sexual maturation of male bank voles (Clethrionomys glareolus). J. Reprod. Fertil. 76, 83–89 10.1530/jrf.0.0760083 (doi:10.1530/jrf.0.0760083) [DOI] [PubMed] [Google Scholar]
- 40.Vandenbergh J. G. 1971. The influence of the social environment on sexual maturation in male mice. J. Reprod. Fertil. 24, 383–390 [DOI] [PubMed] [Google Scholar]
- 41.Koyama S., Kamimura S. 1999. Lowered sperm motility in subordinate social status of mice. Physiol. Behav. 65, 665–669 10.1016/S0031-9384(98)00205-4 (doi:10.1016/S0031-9384(98)00205-4) [DOI] [PubMed] [Google Scholar]
- 42.Wilson-Leedy J. G., Ingermann R. L. 2007. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 67, 661–672 [DOI] [PubMed] [Google Scholar]
- 43.Amann R. P. 1970. Sperm production rates. In The testis, vol. 1, development, anatomy and physiology (eds Johnson A. D., Gomes W. R., Vandermark N. L.), pp. 433–482 New York, NY: Academic Press [Google Scholar]
- 44.Seung H., Wolfe G., Rocca M. 2003. Performing a testicular spermatid head count. Curr. Protocols Toxicol. (Suppl. 16), 1671–1676 [DOI] [PubMed] [Google Scholar]
- 45.Grocock C. A., Clarke J. R. 1976. Duration of spermatogenesis in vole (Microtus agrestis) and bank vole (Clethrionomys glareolus). J. Reprod. Fertil. 47, 133–135 [DOI] [PubMed] [Google Scholar]
- 46.Fricke C., Wigby S., Hobbs R., Chapman T. 2009. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. J. Evol. Biol. 22, 275–286 10.1111/j.1420-9101.2008.01638.x (doi:10.1111/j.1420-9101.2008.01638.x) [DOI] [PubMed] [Google Scholar]
- 47.Poiani A. 2006. Complexity of seminal fluid: a review. Behav. Ecol. Sociobiol. 60, 289–310 10.1007/s00265-006-0178-0 (doi:10.1007/s00265-006-0178-0) [DOI] [Google Scholar]
- 48.Ramm S. A., Oliver P., Ponting C. P., Stockley P., Emes R. D. 2008. Sexual selection and the adaptive evolution of mammalian ejaculate proteins. Mol. Biol. Evol. 25, 207–219 10.1093/molbev/msm242 (doi:10.1093/molbev/msm242) [DOI] [PubMed] [Google Scholar]
- 49.Ramm S. A., McDonald L., Hurst J. L., Beynon R. J., Stockley P. 2009. Comparative proteomics reveals evidence for evolutionary diversification of rodent seminal fluid and its functional significance in sperm competition. Mol. Biol. Evol. 26, 189–198 10.1093/molbev/msn237 (doi:10.1093/molbev/msn237) [DOI] [PubMed] [Google Scholar]
- 50.Reuter M., Linklater J. R., Lehmann L., Fowler K., Chapman T., Hurst G. D. D. 2008. Adaptation to experimental alterations of the operational sex ratio in populations of Drosophila melanogaster. Evolution 62, 401–412 10.1111/j.1558-5646.2007.00300.x (doi:10.1111/j.1558-5646.2007.00300.x) [DOI] [PubMed] [Google Scholar]
- 51.Parker G. A. 1984. Sperm competition and the evolution of animal mating strategies. In Sperm competition and the evolution of animal mating systems (ed. Smith R. L.), pp. 1–60 London, UK: Academic Press [Google Scholar]
- 52.Hihara F. 1981. Effects of the male accessory gland secretion on oviposition and remating in females of Drosophila melanogaster. Zoo. Mag. 90, 307–316 [Google Scholar]
- 53.Crean A. J., Marshall D. J. 2008. Gamete plasticity in a broadcast spawning marine invertebrate. Proc. Natl Acad. Sci. USA 91, 9277–9281 10.1073/PNAS.0806590105 (doi:10.1073/PNAS.0806590105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Immler S., Pryke S. R., Birkhead T. R., Griffith S. C. 2010. Pronounced within-individual plasticity in sperm morphometry across social environments. Evolution 64, 1634–1643 10.1111/j.1558-5646.2009.00924.x (doi:10.1111/j.1558-5646.2009.00924.x) [DOI] [PubMed] [Google Scholar]
- 55.Malo A. F., Roldan E. R. S., Garde J., Soler A. J., Gomendio M. 2005. Antlers honestly advertise sperm production and quality. Proc. R. Soc. B 272, 149–157 10.1098/rspb.2004.2933 (doi:10.1098/rspb.2004.2933) [DOI] [PMC free article] [PubMed] [Google Scholar]