Abstract
As in human societies, social learning may play an important role in shaping individual and group characteristics in other mammals. Here, we review research on non-primate mammals, concentrating on work at our long-term meerkat study site, where longitudinal data and field experiments have generated important insights into the role of social learning under natural conditions. Meerkats live under high predation pressure and occupy a difficult foraging niche. Accordingly, pups make extensive use of social information in learning to avoid predation and obtain food. Where individual learning is costly or opportunities are lacking, as in the acquisition of prey-handling skills, adults play an active role in promoting learning through teaching. Social learning can also cause information to spread through groups, but our data suggest that this does not necessarily result in homogeneous, group-wide traditions. Moreover, traditions are commonly eroded by individual learning. We suggest that traditions will only persist where there are high costs of deviating from the group norm or where skill development requires extensive time and effort. Persistent traditions could, theoretically, modify selection pressures and influence genetic evolution. Further empirical studies of social learning in natural populations are now urgently needed to substantiate theoretical claims.
Keywords: culture, development, evolution, mammals, social learning, traditions
1. Introduction
In human societies, the ability to learn from others (‘social learning’) promotes the development of individual skills and shapes the behaviour of groups, giving rise to varied local cultures [1]. Understanding the extent to which social learning has similar effects in other species is one of the most fundamental questions in the life sciences. Theoretical models suggest that social learning may have major ecological and evolutionary implications, promoting the spread of adaptive information within groups and between generations, dissociating behavioural traits from ecological conditions and modifying selection pressures [2–4]. Furthermore, comparative studies of social learning are critical for understanding the biological basis of human culture [5–7].
In recognition of these implications, social learning has become a major research topic in recent years. Studies in captivity have revealed mechanisms of social learning across a range of taxa and shown that information can spread across chains of individuals and diffuse through groups, forming group-level behavioural characteristics or traditions [8]. However, patterns of social learning in artificial groups of animals in close proximity to one another, with freely available food and no predation pressure may not adequately reflect those found in nature. Descriptive, observational studies of animals in their natural environment provide greater ecological validity, but cannot generate unequivocal evidence that social learning shapes individual or group behaviour [9]. Consequently, the role of social learning in nature remains rather poorly understood.
In this review, we synthesize existing knowledge of the importance of social learning in wild animal societies, with a focus on non-primate mammals (primates [10,11], birds [12] and fish [13] are topics of other contributions in this issue, and we discuss research on these and other taxa where relevant). We concentrate particularly on research at our long-term field site of cooperatively breeding meerkats (Suricata suricatta), where access to multiple groups of individually recognizable, habituated animals has allowed us to obtain detailed records of individual development and enabled experimental tests of social learning. We then turn our attention to two key questions: can socially transmitted traditions influence evolution; and can traditions themselves evolve?
2. Social learning and individual development
Young animals whose own skills are poorly developed may often benefit from the knowledge of more experienced individuals. Theory predicts a reliance on vertical or oblique transmission (from parents or other adults to offspring) when different generations experience similar environmental challenges, whereas horizontal transmission is favoured when environmental change is faster than generation time [14]. Consequently, horizontal transmission may allow the rapid spread of innovations and improve the efficiency of individuals foraging at ephemeral food patches, while vertical transmission may promote the development of fundamental skills. Indeed, there is now evidence that many key behavioural determinants of fitness, including the ability to avoid predators, obtain food and select mates are determined in part by social learning in early life.
(a). Predator avoidance
A plethora of experiments have shown that naive animals can acquire a fear of novel predators as a result of exposure to the fearful responses of conspecifics (see [15] for a review). However, these experiments seldom consider social learning as a developmental process contributing to skill acquisition by the young, and no study has yet demonstrated that social learning affects anti-predator behaviour in wild mammals (though see [16,17] for experiments on learned enemy avoidance in wild birds and fish).
Despite a lack of unequivocal evidence, social learning is likely to play a role in the development of anti-predator responses in many mammals. Inflexible anti-predator responses under tight genetic control are unlikely to be adaptive if predation risk varies in time and space, and learning about predators through direct experience is extremely dangerous. The high costs of individual learning should therefore favour a reliance on social information [2]. Young animals, whose small size, poor motor skills and lack of experience make them especially vulnerable to predators, may be particularly likely to benefit from attending to the anti-predator behaviour of older individuals.
In meerkats, social influences play a clear role in responses to humans. Wild meerkats normally flee upon sighting a human, so initial attempts to habituate groups at our study site to human observers took well over a year. However, once the first groups were habituated, all the pups born into them were unafraid of people (T. Clutton-Brock 1994, unpublished data). Social learning may also aid the development of mobbing behaviour, used by meerkats when encountering threats such as snakes. Pups are less likely than adults to mob snakes, but more likely to mob non-threatening Cape ground squirrels (Xerus inauris), suggesting that mobbing may be shaped by experience. Adults show heightened responses to snakes when pups are present. Although this probably reflects the greater need to drive away snakes when vulnerable pups are present, mobbing may additionally provide pups with relatively safe opportunities to learn about the characteristics of the threat [18].
Circumstantial evidence suggests that social learning also facilitates the development of meerkats' responses to alarm calls. Meerkats live in open habitats under high predation pressure and, like many primates, use functionally referential alarm calls, with predator-specific calls eliciting distinct responses [19]. While adults respond rapidly and appropriately to alarms (e.g. running to bolt-holes in response to aerial predator alarms), pups react more slowly and generally run to the nearest adult [20]. Pups may therefore gain opportunities to learn appropriate responses by following their elders. Interestingly, female pups, who spend more time near adults, are faster to react than males and are more likely to show adult-like responses [20]. Whether this results from greater opportunities for social learning is unclear, but a similar pattern is found in chimpanzees (Pan troglodytes), where sex differences in the development of termite fishing have been attributed to differences in offspring's attentiveness to mothers [21].
Meerkats' use of alarm calls is also likely to be influenced by social learning. In common with other young mammals (e.g. vervet monkeys, Chlorocebus aethiops [22]), meerkat pups often alarm inappropriately, for example producing terrestrial alarm calls in response to aerial predators or alarming in response to non-threatening birds. Differences in pup and adult call use are not adaptive responses to age-related differences in vulnerability, as predators that are more threatening to pups than adults do not elicit more alarm calling from pups. Rather, pups begin responding appropriately to common predators at an earlier age than to rarer predators, suggesting that alarm call usage improves with experience [23]. Given that adults' responses provide the only means for pups to learn associations between predators and call types, social learning is likely to be important.
Together, these findings suggest that meerkats' predator-avoidance skills are strongly linked to social information acquired in early life. Similar effects are likely to be common in small mammals with substantial periods of parental care. For instance, in Belding's ground squirrels (Spermophilus beldingi), the presence of mothers is strongly related to the development of pups' ability to discriminate between alarm and non-alarm calls [24]. Nevertheless, a role for social learning does not negate the importance of genetic effects and individual experience. Rather, it is likely that selection tailors animals with certain predispositions for responding to predators, which are then refined by social and asocial learning processes. In support of this view, experiments suggest that naive monkeys can learn to fear snakes more easily than flowers [25]. Similarly, passerines cross-fostered between species develop relatively weak responses to the alarm calls of their own species, suggesting that genetically controlled templates are refined through learning [26].
(b). Foraging
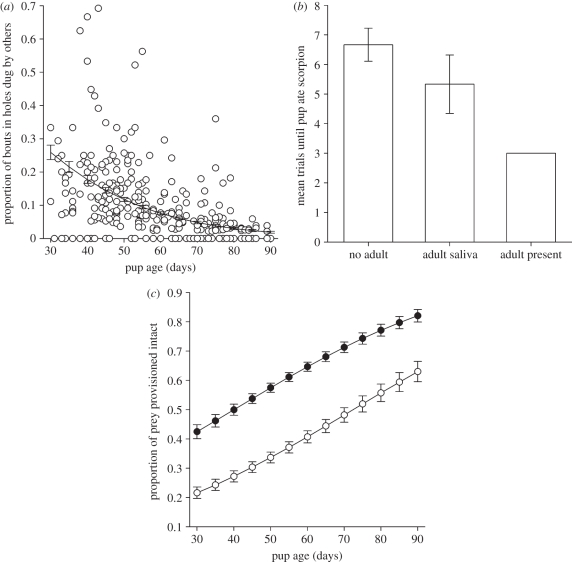
Species with complex foraging techniques or generalist diets commonly have long periods of parental care [27], and an offspring's transition to nutritional independence is likely to rely heavily on information acquired from adults. In meerkats, social interactions help pups to learn where to look for food, what to eat and how to handle difficult prey (figure 1).
Figure 1.
(a) Young pups often dig in holes dug by adults. (Reproduced with permission from [28].) (b) Young pups learn to eat dead scorpions more rapidly if they see helpers eating scorpions than if exposed to dead scorpions (unadulterated or covered in adult saliva) when alone. (Reproduced with permission from [38].) (c) Adults increasingly provision pups with live, intact prey items as they grow older (open circles, scorpions; filled circles, other mobile prey). (Reproduced with permission from [47].)
Animals in heterogeneous environments typically target their foraging attempts towards particular microhabitats. Such preferences are major determinants of the foraging niche, and may thereby influence species coexistence and speciation (see [12]). Where habitats vary temporally or spatially, individual preferences must be moulded by experience. Meerkats' development of microhabitat preferences involves the integration of information acquired through personal experience and social cues obtained from adults [28]. Adult meerkats preferentially forage at the base of sparsely distributed vegetation. Pups initially show no such preference and their foraging attempts are highly unproductive, but become more productive and adult-like as they get older. This transition rests largely on learning through direct experience of the profitability of different microhabitats (cf. [29]), but pups may also use cues obtained by foraging near adults and digging in holes already created by adults (figure 1a). Digging in the existing holes offers few nutritional rewards, but may allow pups to obtain olfactory cues from prey removed by the original hole-digger, thereby raising the probability that a pup would dig in similar locations in future [28].
In many species, including rodents, dogs and fowl, social interactions can also facilitate learning of suitable items to incorporate into the diet [30]. This is particularly important in generalist species where the costs of learning by sampling novel items may be high owing to the presence of potentially toxic items. The varied diets of rats (Rattus spp.) are especially well-studied in this context [31]. Here, laboratory studies show that social influences on food preferences begin in the womb, as traces of mothers' food are transported across the placenta into the foetal blood system, influencing pups' food choices after birth [32]. Similarly, food preferences may be transmitted from mothers to nursing offspring through milk [33]. Social learning processes continue into adulthood, as naive individuals show preferences for food associated with odours detected on the conspecifics' breath [34]. Similar effects have been found in other mammals, e.g. mice, Mus musculus [35], and dogs, Canis familiaris [36].
Numerous studies suggest that social information helps young mammals to learn what to eat in the wild [30,37], although most evidence is inconclusive. The most direct evidence comes from meerkats, where pups are neophobic towards unfamiliar foods, but will incorporate them into their diet after interacting with older group members. In field experiments, young pups refused to eat hard-boiled egg (used at the study site to entice meerkats onto balances for the collection of weight data), but rapidly learned to eat it after exposure to adults eating egg [38]. Most pups were similarly reticent to eat dead scorpions (a common prey type in the meerkat diet), but were attracted to the sight of adults eating scorpions, causing them to sample the prey and subsequently incorporate it into their diet [38] (figure 1b).
The most complex element of foraging behaviour is typically the ability to handle and process food types such as items encased in hard coverings or live, mobile prey. The difficulty in perfecting these skills through individual learning alone may favour a reliance on social learning from more experienced individuals. Developmental studies suggest that social learning may be important in the development of extractive foraging, hunting and tool use in primates, carnivores, rodents and cetaceans [37,39]. For instance, bottlenose dolphin (Tursiops sp.) calves spend many years in close proximity to their mothers, and longitudinal data reveal strong correlations between certain maternal foraging tactics, including the use of sponges as foraging tools, and the acquisition of same skills by calves [40]. These data strongly suggest that social learning is involved in the development of foraging skills, but definitive experimental support is still lacking. Stronger evidence is provided by Terkel and colleagues, who found that black rats (Rattus rattus) in recently planted forests of Jerusalem pine in Israel had learned to extract seeds from pine cones. Subsequent laboratory experiments showed that experience of completing the stripping of cones started by others facilitated learning in naive individuals and that only young rats raised by dams that could strip cones learned to do so efficiently themselves [41]. Here, a simple mechanism of social learning allowed the rats to enter a previously unoccupied niche.
Although social influences on skill development are common, until recently it was generally thought that non-human animals never actively facilitate learning in others through teaching. This view stemmed from the anthropocentric assumption that teaching requires the capacity to understand the knowledge states of others and intentionally endeavour to correct their ignorance [42–44]. In contrast, the evolutionary approach promoted by Caro & Hauser [45] treats teaching as a functional category of behaviour whereby knowledgeable individuals incur short-term costs to promote learning in others. From this point of view, teaching is not contingent on particular cognitive mechanisms and may be favoured by selection if opportunities for individual learning are low or involve severe costs and passive social learning is ineffective [39]. For instance, meerkat pups seldom find mobile prey items and so lack opportunities to refine their prey-handling skills. Moreover, incompetent attempts to handle certain prey types, such as scorpions, may be dangerous and, as the development of the motor skills involved in prey-capture requires repeated practice [46], simply watching others is ineffective. Consequently, adults teach pups by providing them with otherwise unavailable opportunities to handle live prey. Young pups are primarily given dead or disabled prey items and are gradually introduced to live, intact prey as they grow older (figure 1c). Provisioning pups with live prey that might escape is costly to adults, but experiments show that pups' skills improve as a result of handling practice [47]. Other clear examples of teaching occur in pied babblers (Turdoides bicolor), cooperatively breeding birds where helpers teach nestlings to associate particular calls with food [48], and in the ant Temnothorax albipennis where knowledgeable individuals teach colony members routes to food [49]. In the context of foraging skills, there is strong evidence that many solitary-hunting mammalian carnivores, particularly felids, teach their young to hunt in a similar manner to meerkats, by providing them with live prey [45]. Weaker evidence is also found in raptorial birds, cetaceans and primates (reviewed in [39]).
(c). Mate selection
Where selecting a mate involves substantial costs, individuals may benefit from copying others. Female lek-breeding mammals, for instance, may reduce the risks of predation and harassment by following other females between territories [50]. In some fish and birds, females avoid the costs of mate assessment by preferentially affiliating with males chosen by other females. If such socially induced preferences generalize to other, phenotypically similar males [51,52], they can generate long-term effects on mate choice. As well as influencing mate choice, social learning may affect the development of secondary sexual characteristics. In song-birds, for example, young males commonly learn to match their song to that of adults in the vicinity and females learn to prefer song types they hear in early life [53]. This may cause lifelong mating preferences, assortative mating and perhaps even speciation [54,55]. Such downstream effects of social learning may be less common in mammals, where mate choice relies heavily on olfactory and morphological signals which are unaffected by learning, and where male coercion often masks the effects of female choice [56]. Nevertheless, developmental effects of social learning on mate choice may occur in certain species, particularly in bats and cetaceans that, like passerines, are capable of vocal learning and use vocalizations to attract mates [57,58].
3. Social learning and group behaviour
Can social learning cause information to spread through groups of non-human animals, forming traditions akin to those of human societies? Much of the debate surrounding this question has centred on semantic issues concerning what precisely constitutes a tradition. The broad definition proposed by Fragaszy & Perry [59]—‘a distinctive behaviour pattern shared by two or more individuals in a social unit, which persists over time and that new practitioners acquire in part through socially aided learning’—is widely accepted, but its requirement for persistence is ambiguous and could arguably exclude the short-term trends and fads that are so prevalent in human societies. Some authors would further stipulate a requirement that socially learned traits must become the norm within a group and endure across generations [60]. Conflicting views on the distinction between traditions and culture further complicate the issue. Some authors equate the terms [61], others treat culture as a collection of traditions (leaving open the question of how many traditions make a culture) [62] and yet others reserve the term ‘culture’ for traditions that signal group membership [63], or which increase in complexity over time [64].
An excessive focus on semantic issues may risk creating artificial dichotomies and obscuring central ecological and evolutionary issues. First, classifying traits as cultural (i.e. socially learned) or non-cultural is problematic because phenotypes are often shaped by combinations of genetic predispositions, epigenetic effects on gene regulation, and information acquired through individual exploration and social learning. Moreover, although social learning is commonly assumed to homogenize group behaviour [65], recent studies suggest that social learning may sometimes maintain behavioural heterogeneity [66–68]. Rather than fixating on terminology, we must determine the extent to which social learning shapes behavioural phenotypes and their stability at individual and group levels. Current attempts to address these issues have been limited by a heavy reliance on laboratory studies. In the wild, the popular ethnographic method (or method of exclusion) for identifying traditions by comparing the behaviour of geographically separated groups is generally unable to exclude genetic or ecological explanations for group differences [9]. Field experiments are therefore critical to bridge the divide between captive and observational studies.
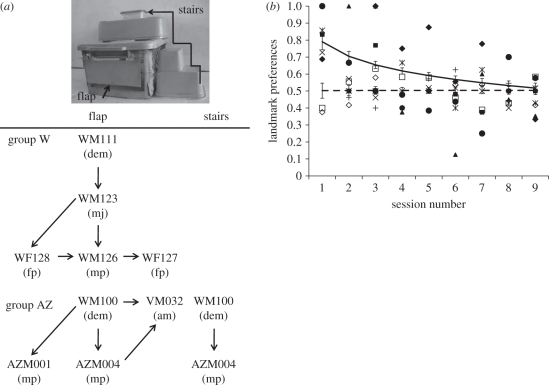
To date, only a handful of field experiments have examined the spread of information through natural groups (reviewed in [8]). One powerful approach is to examine whether individuals translocated between groups subsequently adopt the behaviour of their new groups. Such experiments have generated strong evidence for socially learned mating sites and foraging routes in reef fish ([69,70], but are unlikely to be feasible with other vertebrates). With meerkats, we have used an alternative approach: training individual ‘demonstrators’ out of sight of the rest of the group (while foraging or babysitting) to perform a task, and then examining the adoption of demonstrators' behaviour by others. These experiments have generated a number of important insights. For instance, they show that the social transmission of information need not result in the adoption of uniform, group-wide traditions. In one experiment, we seeded six groups with demonstrators trained to obtain food from an experimental apparatus using one of two techniques, while a further three groups with no demonstrators served as controls for individual learning. Individuals in control groups were unlikely to obtain food, but in experimental groups, demonstrators' techniques preferentially spread to other group members. However, not all individuals learned. In particular, pups were more attentive than adults to demonstrators' behaviour, were more likely to follow demonstrators and scrounge scraps of food and consequently were more likely to learn (note that scrounging has variable effects on social learning depending on levels of social tolerance and task complexity [68]). This suggests that individuals' attentiveness, rather than their learning abilities, governs the spread of information (a similar effect is found in wild vervet monkeys, where selective attention to female demonstrators governs patterns of transmission of novel skills [71]). Moreover, a number of meerkats learned to use the technique on which their demonstrator was not trained, and this technique also spread to others, leading to the coexistence of alternative techniques within groups (figure 2a). A lack of stable behavioural homogeneity in groups need not, therefore, imply a lack of social transmission [68].
Figure 2.
(a) Meerkats could obtain food from the experimental apparatus either by going through the flap or by climbing up the stairs and breaking a paper lid. In group W, the flap technique was transmitted from the trained demonstrator to other individuals. The flap technique also spread in group AZ, but here the demonstrator also discovered the stairs technique and was observed by a pup, who subsequently adopted the technique. dem, demonstrator; m, male; f, female; a, adult; j, juvenile; p, pup. (Reproduced with permission from [68].) (b) Meerkats in control groups with no demonstrators showed no preference for either of the two equally rewarding landmarks; meerkats in experimental groups showed an initial preference for landmarks used by demonstrators, but this collapsed over time. Symbols are mean preferences for each group per session (open, control groups; filled, experimental groups); solid and dotted lines show combined means ± s.e. for experimental and control groups, respectively. (Reproduced with permission from [76].)
Our experiments suggest that the uniformity and stability of non-human animal traditions depend principally on the balance between social and individual learning. Where low-cost opportunities for individual learning are readily available, socially learned traditions are unlikely to persist (see also [72–75]). This is illustrated by a second experiment, which examined whether naive meerkats would adopt the preferences of demonstrators trained to obtain rewards from one of two adjacent and equally rewarding landmarks of distinctive shape and colour [76]. Here, social learning promoted an initial bias towards the landmark used by demonstrators, generating arbitrary traditions within groups. However, having learned that one landmark was profitable, individuals began exploring the other and learned that it provided equal rewards, so the tradition collapsed over time. This contrasts with human societies, where conformity to group norms and punishment of transgressors can maintain arbitrary traditions, regardless of the profitability of alternative options [1,77].
In non-human animals, traditions may only persist when the net benefits of switching to alternative patterns of behaviour are low. Traditional foraging routes or food preferences, for example, may persist because the severe costs of leaving the safety of the group or sampling unknown foods outweigh any benefits. This may explain the persistence of group differences in emergence times in our meerkat population. Here, extensive gene flow precludes genetic differentiation between groups. Nevertheless, some groups consistently emerged from their sleeping burrows later in the morning than others for more than a decade, despite complete turnovers in group membership and the influx of immigrants. These differences do not appear to be driven by environmental factors, as group territories overlap, the same burrows are often used by different groups, and emergence times are unrelated to territory quality. Moreover, strong group effects remained even after accounting for ecological and meteorological factors in multi-factorial analyses. Group differences may therefore constitute local traditions maintained because individuals face large risks if they emerge from the burrow at a different time to the rest of the group [78].
Traditions may also persist if skills take extensive time and effort to perfect. If skill development is difficult and protracted, individuals may benefit from sticking with the first technique they learned, even if alternative techniques could be equally productive. This may explain the maintenance of complex food extraction skills, such as tool-use techniques in chimpanzee populations, negating the need to invoke human-like conformity to social norms (cf. [77]). For instance, in banded mongooses (Mungos mungo), communally breeding relatives of meerkats where pups form exclusive associations with one adult escort, pups take several months to learn to open encased food items by biting them or smashing them against anvils. Müller & Cant [66] experimentally presented escorts with food-filled plastic eggs and found that many escorts showed stable preferences for either biting or smashing. Pups tended to adopt their escorts' preferences and maintain them into adulthood, suggesting that the difficulty in perfecting techniques favours the maintenance of learned skills. However, both techniques coexisted within groups, providing further evidence that social learning can maintain within-group heterogeneity (see also [67]).
4. Broader implications
(a). Can social learning affect evolution?
Theoretical models suggest that by allowing organisms to alter their environments and enter new niches, socially transmitted traits can modify selection pressures and thereby influence genetic evolution [2–4]. Empirical evidence is rapidly accumulating for human populations, with a recent survey identifying over 100 candidate genes, involved in a range of physiological, morphological and behavioural traits, which may have been influenced by culturally modified selective pressures [79]. However, data from other species are lacking. Among the most likely cases are those where social learning may affect sexual selection by influencing the development of secondary sexual traits and mate choice. For instance, in passerines and cetaceans where songs are learned, they must, by definition, be learned from others. As male song is commonly involved in female mate choice, female preferences for song dialects from their local area could theoretically cause pre-zygotic isolation between populations, ultimately leading to speciation [54,55]. Explicit empirical tests of this prediction have yet to be conducted.
Social learning may also modify selection pressures through its long-term effects on foraging behaviour and habitat use. In humans, the invention of dairy farming resulted in the spread of alleles for lactose tolerance in pastoralist populations [80]. At our meerkat study site, social learning has resulted in a simple tradition of hard-boiled egg eating [38] which could, in theory, generate similar selective pressures for effective hard-boiled egg digestion over many generations. Social learning could also play an important role in the expansion of populations into new habitats. For example, the social transmission of innovative methods of food acquisition and mating site preferences may have played an important role in the expansion of many animals into urban areas. Subsequent exposure to new dangers, new food types and light regimes distorted by street lamps over many generations could well result in urban populations diverging genetically from their rural counterparts. Unfortunately, information on the role of social learning in urbanization is lacking. Indeed, excluding humans, the only well-documented case of niche invasion facilitated by social learning is in black rats, where social transmission of pine-cone stripping allowed invasion of pine forests [41]. Genetic comparisons of current forest populations with their counterparts outside the forest would provide an excellent test of gene–culture coevolution. Empirical analyses such as these are critical if we are to move beyond pure speculation as to the evolutionary implications of social learning.
(b). Do non-human animal traditions evolve?
Human traditions are thought to evolve according to principles that are much in common with Darwinian evolution, with each generation building upon the innovations of the last [64,81]. Apart from bird and cetacean song, however, the traditions of other species do not seem to show such incremental changes (though see [82] and [83] for tentative suggestions in the tool use of chimpanzees and New Caledonian crows, Corvus moneduloides). Kendal et al. [84] have suggested that social learning strategies such as copying others whose behaviour yields higher rewards than one's own may allow individuals to converge on fitness-maximizing behaviour over repeated iterations, thus promoting cumulative cultural evolution (see also [13]). However, although such strategies may allow sticklebacks in laboratory experiments to choose the best foraging patches [84], there is little direct evidence that they can allow the elaboration of technical innovations as seen in humans.
One common argument for the lack of cumulative culture in non-humans is that human culture is underpinned by higher fidelity mechanisms of social learning than those prevalent in other species. One such mechanism is imitation, which was commonly thought to be rare or absent in non-human animals [64,85]. However, laboratory experiments have now generated evidence for imitation in a number of species [86]. Moreover, the fidelity of information transmission by imitation may not be as great as previously supposed [72], and in experiments on humans cumulative cultural evolution was observed in the absence of opportunities for imitation [87].
The ability to facilitate learning in others by teaching has also been suggested to be a uniquely human trait allowing high-fidelity information transmission and promoting cumulative cultural change [64,85]. However, we now know that teaching is found across a range of taxa [39]. It now seems likely that neither imitation nor teaching per se provide the basis for cultural evolution. Rather, certain cognitive mechanisms that humans incorporate into some of their imitative and pedagogical activities may be important [88,89]. For instance, non-human teaching is restricted to particular adaptive contexts (e.g. facilitating acquisition of hunting skills) and is not involved in the transmission of innovations [39,90]. In contrast, humans' capacity for mental state attribution, joint attention and foresight may allow teachers to recognize and correct their pupils' ignorance, demonstrate novel actions, and thereby facilitate the transmission and improvement of cultural inventions across a range of contexts [91,92] (see also [88,93]).
5. Conclusions
Information transmitted between individuals is likely to have important effects in many mammal societies. In African elephant (Loxodonta africana) herds, for example, matriarchs are thought to act as repositories of social knowledge, and their removal may lead to decreases in the per capita reproductive success of remaining group members [94]. It is likely that many of the traits that behavioural ecologists model as genetically controlled adaptations, including anti-predator behaviour, foraging skills and social strategies, are to some extent shaped by social learning. However, the role of social learning remains poorly understood in wild mammal groups, and many common assumptions remain to be verified. For instance, great apes are often assumed to rely more heavily than other animals on socially transmitted information [60,95], but the strongest experimental evidence that social learning influences individual and group behaviour in wild mammals is found in social carnivores [38,47,66,68,76]. This may simply reflect the greater tractability of small carnivores for field experiments, but claims of ape cultural supremacy nevertheless remain premature. Similarly, claims of cumulative traditions and evolutionary impacts of social learning currently amount to little more than tentative suggestions lacking in empirical support. Long-term field studies, incorporating field experiments and novel statistical techniques [6] provide the means to revealing the true importance of social learning in nature.
Acknowledgements
We thank Marta Manser for discussion and support, Katherine McAuliffe and Nichola Raihani for comments on the manuscript and Pembroke College, Cambridge for funding.
Footnotes
One contribution of 26 to a Discussion Meeting Issue ‘Culture evolves’.
References
- 1.Richerson P. J., Boyd R. 2005. Not by genes alone: how culture transformed human evolution. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Boyd R., Richerson P. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 3.Danchin E., Giraldeau L. A., Valone T. J., Wagner R. H. 2004. Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 10.1126/science.1098254 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- 4.Laland K. N., Odling-Smee J., Feldman M. W. 2000. Niche construction, biological evolution, and cultural change. Behav. Brain Sci. 23, 131–175 10.1017/S0140525X00002417 (doi:10.1017/S0140525X00002417) [DOI] [PubMed] [Google Scholar]
- 5.Heyes C. M., Galef B. G., Jr 1996. Social learning in animals: the roots of culture. San Diego, CA: Academic Press [Google Scholar]
- 6.Laland K. N., Kendal J. R., Kendal R. L. 2009. Animal culture: problems and solutions. In The question of animal culture (eds Laland K. N., Galef B. G.), pp. 174–197 Cambridge, MA: Harvard University Press [Google Scholar]
- 7.Whiten A., Hinde R. A., Laland K. N., Stringer C. B. 2011. Culture evolves. Phil. Trans. R. Soc. B 366, 938–948 10.1098/rstb.2010.0372 (doi:10.1098/rstb.2010.0372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiten A., Mesoudi A. 2008. Establishing an experimental science of culture: animal social diffusion experiments. Phil. Trans. R. Soc. B 363, 3477–3488 10.1098/rstb.2008.0134 (doi:10.1098/rstb.2008.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laland K. N., Janik V. M. 2006. The animal cultures debate. Trends Ecol. Evol. 21, 542–547 10.1016/j.tree.2006.06.005 (doi:10.1016/j.tree.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 10.Perry S. 2011. Social traditions and social learning in capuchin monkeys (Cebus). Phil. Trans. R. Soc. B 366, 988–996 10.1098/rstb.2010.0317 (doi:10.1098/rstb.2010.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiten A. 2011. The scope of culture in chimpanzees, humans and ancestral apes. Phil. Trans. R. Soc. B 366, 997–1007 10.1098/rstb.2010.0334 (doi:10.1098/rstb.2010.0334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slagsvold T., Wiebe K. L. 2011. Social learning in birds and its role in shaping a foraging niche. Phil. Trans. R. Soc. B. 366, 969–977 10.1098/rstb.2010.0343 (doi:10.1098/rstb.2010.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laland K. N., Atton N., Webster M. M. 2011. From fish to fashion: experimental and theoretical insights into the evolution of culture. Phil. Trans. R. Soc. B 366, 958–968 10.1098/rstb.2010.0328 (doi:10.1098/rstb.2010.0328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laland K. N., Kendal J. R. 2003. What the models say about social learning. In The biology of traditions: models and evidence (eds Fragaszy D. M., Perry S.), pp. 33–55 Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Griffin A. S. 2004. Social learning about predators: a review and prospectus. Learn. Behav. 32, 131–140 [DOI] [PubMed] [Google Scholar]
- 16.Davies N. B., Welbergen J. A. 2009. Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320 10.1126/science.1172227 (doi:10.1126/science.1172227) [DOI] [PubMed] [Google Scholar]
- 17.Reader S. M., Kendal J. R., Laland K. N. 2003. Social learning of foraging sites and escape routes in wild Trinidadian guppies. Anim. Behav. 66, 729–739 10.1006/anbe.2003.2252 (doi:10.1006/anbe.2003.2252) [DOI] [Google Scholar]
- 18.Graw B., Manser M. B. 2007. The function of mobbing in cooperative meerkats. Anim. Behav. 74, 507–517 10.1016/j.anbehav.2006.11.021 (doi:10.1016/j.anbehav.2006.11.021) [DOI] [Google Scholar]
- 19.Manser M. B., Seyfarth R. M., Cheney D. L. 2002. Suricate alarm calls signal predator class and urgency. Trends Cogn. Sci. 6, 55–57 10.1016/S1364-6613(00)01840-4 (doi:10.1016/S1364-6613(00)01840-4) [DOI] [PubMed] [Google Scholar]
- 20.Hollén L. I., Manser M. B. 2006. Ontogeny of alarm call responses in meerkats, Suricata suricatta: the roles of age, sex and nearby conspecifics. Anim. Behav. 72, 1345–1353 10.1016/j.anbehav.2006.03.020 (doi:10.1016/j.anbehav.2006.03.020) [DOI] [Google Scholar]
- 21.Lonsdorf E. V., Eberly L. E., Pusey A. E. 2004. Sex differences in learning in chimpanzees. Nature 428, 715–716 10.1038/428715a (doi:10.1038/428715a) [DOI] [PubMed] [Google Scholar]
- 22.Cheney D., Seyfarth R. 1990. How monkeys see the world: inside the mind of another species. Chicago, IL: University of Chicago Press [Google Scholar]
- 23.Hollén L. I., Clutton-Brock T., Manser M. B. 2008. Ontogenetic changes in alarm-call production and usage in meerkats (Suricata suricatta): adaptations or constraints? Behav. Ecol. Sociobiol. 62, 821–829 10.1007/s00265-007-0508-x (doi:10.1007/s00265-007-0508-x) [DOI] [Google Scholar]
- 24.Mateo J. M., Holmes W. G. 1997. Development of alarm-call responses in Belding's ground squirrels: the role of dams. Anim. Behav. 54, 509–524 10.1006/anbe.1996.0446 (doi:10.1006/anbe.1996.0446) [DOI] [PubMed] [Google Scholar]
- 25.Mineka S., Cook M. 1988. Social learning and the acquisition of snake fear in monkeys. In Social learning: psychological and biological perspectives (eds Zentall T. R., Galef B. G.), pp. 51–73 Hillsdale, NJ: Erlbaum [Google Scholar]
- 26.Davies N. B., Madden J. R., Butchart S. H. M. 2004. Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc. R. Soc. Lond. B 271, 2297–2304 10.1098/rspb.2004.2835 (doi:10.1098/rspb.2004.2835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinsohn R. G. 1991. Slow learning of foraging skills and extended parental care in cooperatively breeding white-winged choughs. Am. Nat. 137, 864–881 10.1086/285198 (doi:10.1086/285198) [DOI] [Google Scholar]
- 28.Thornton A., Hodge S. J. 2009. The development of foraging microhabitat preferences in meerkats. Behav. Ecol. 20, 103–110 10.1093/beheco/arn120 (doi:10.1093/beheco/arn120) [DOI] [Google Scholar]
- 29.Giraldeau L. A. 1984. Group foraging: the skill pool effect and frequency-dependent learning. Am. Nat. 124, 72–79 10.1086/284252 (doi:10.1086/284252) [DOI] [Google Scholar]
- 30.Galef B. G., Giraldeau L. A. 2001. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 10.1006/anbe.2000.1557 (doi:10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
- 31.Galef B. G. 2003. ‘Traditional’ foraging behaviors of brown and black rats (Rattus norvegicus and Rattus rattus). In The biology of traditions: models and evidence (eds Fragaszy D. M., Perry S.), pp. 159–186 Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Hepper P. G. 1988. Adaptive fetal learning: prenatal exposure to garlic affects postnatal preferences. Anim. Behav. 36, 935–936 10.1016/S0003-3472(88)80177-5 (doi:10.1016/S0003-3472(88)80177-5) [DOI] [Google Scholar]
- 33.Galef B. G., Sherry D. F. 1973. Mothers milk: a medium for transmission of cues reflecting flavor of mother's diet. J. Comp. Physiol. Psychol. 83, 374–378 10.1037/h0034665 (doi:10.1037/h0034665) [DOI] [PubMed] [Google Scholar]
- 34.Galef B. G., Stein M. 1985. Demonstrator influence on observer diet preference: analyses of critical social interactions and olfactory signals. Anim. Learn. Behav. 13, 31–38 [Google Scholar]
- 35.Valsecchi P., Galef B. G. 1989. Social influences on the food preferences of house mice (Mus musculus). Int. J. Comp. Psychol. 2, 245–256 [Google Scholar]
- 36.Lupfer-Johnson G., Ross J. 2007. Dogs acquire food preferences from interacting with recently fed conspecifics. Behav. Processes 74, 104–106 10.1016/j.beproc.2006.09.006 (doi:10.1016/j.beproc.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 37.Box H. O., Gibson K. R. (eds) 1999. Mammalian social learning: comparative and ecological perspectives. Cambridge, UK: Cambridge University Press [Google Scholar]
- 38.Thornton A. 2008. Social learning about novel foods by young meerkats. Anim. Behav. 76, 1411–1421 10.1016/j.anbehav.2008.07.007 (doi:10.1016/j.anbehav.2008.07.007) [DOI] [Google Scholar]
- 39.Thornton A., Raihani N. J. 2008. The evolution of teaching. Anim. Behav. 75, 1823–1836 10.1016/j.anbehav.2007.12.014 (doi:10.1016/j.anbehav.2007.12.014) [DOI] [Google Scholar]
- 40.Sargeant B. L., Mann J. 2009. Developmental evidence for foraging traditions in wild bottlenose dolphins. Anim. Behav. 78, 715–721 10.1016/j.anbehav.2009.05.037 (doi:10.1016/j.anbehav.2009.05.037) [DOI] [Google Scholar]
- 41.Terkel J. 1996. Cultural transmission of feeding behavior in the black rat (Rattus rattus). In Social learning in animals: the roots of culture (eds Heyes C. M., Galef B. G. Jr), pp. 17–47 San Diego, CA: Academic Press [Google Scholar]
- 42.Premack D., Premack A. J. 1996. Why animals lack pedagogy and some cultures have more of it than others. In Handbook of education and human development: new models of learning, teaching and schooling (eds Olson D. R., Torrance N.), pp. 302–323 Oxford, UK: Blackwell [Google Scholar]
- 43.Strauss S., Ziv M., Stein A. 2002. Teaching as a natural cognition and its relations to preschoolers' developing theory of mind. Cogn. Dev. 17, 1473–1487 10.1016/S0885-2014(02)00128-4 (doi:10.1016/S0885-2014(02)00128-4) [DOI] [Google Scholar]
- 44.Tomasello M., Kruger A. C., Ratner H. H. 1993. Cultural learning. Behav. Brain Sci. 16, 495–552 10.1017/S0140525X0003123X (doi:10.1017/S0140525X0003123X) [DOI] [Google Scholar]
- 45.Caro T. M., Hauser M. D. 1992. Is there teaching in nonhuman animals? Q. Rev. Biol. 67, 151–174 10.1086/417553 (doi:10.1086/417553) [DOI] [PubMed] [Google Scholar]
- 46.Caro T. M. 1980. The effects of experience on the predatory patterns of cats. Behav. Neural Biol. 29, 1–28 10.1016/S0163-1047(80)92442-5 (doi:10.1016/S0163-1047(80)92442-5) [DOI] [PubMed] [Google Scholar]
- 47.Thornton A., McAuliffe K. 2006. Teaching in wild meerkats. Science 313, 227–229 10.1126/science.1128727 (doi:10.1126/science.1128727) [DOI] [PubMed] [Google Scholar]
- 48.Raihani N. J., Ridley A. R. 2008. Experimental evidence for teaching in wild pied babblers. Anim. Behav. 75, 3–11 10.1016/j.anbehav.2007.07.024 (doi:10.1016/j.anbehav.2007.07.024) [DOI] [Google Scholar]
- 49.Franks N. R., Richardson T. 2006. Teaching in tandem-running ants. Nature 439, 153. 10.1038/439153a (doi:10.1038/439153a) [DOI] [PubMed] [Google Scholar]
- 50.McComb K., Clutton-Brock T. 1994. Is mate choice copying or aggregation responsible for skewed distributions of females on leks? Proc. R. Soc. Lond. B 255, 13–19 10.1098/rspb.1994.0003 (doi:10.1098/rspb.1994.0003) [DOI] [PubMed] [Google Scholar]
- 51.Godin J. G. J., Herdman E. J. E., Dugatkin L. A. 2005. Social influences on female mate choice in the guppy, Poecilia reticulata: generalized and repeatable trait-copying behaviour. Anim. Behav. 69, 999–1005 10.1016/j.anbehav.2004.07.016 (doi:10.1016/j.anbehav.2004.07.016) [DOI] [Google Scholar]
- 52.White D. J., Galef B. G. 2000. ‘Culture’ in quail: social influences on mate choices of female Coturnix japonica. Anim. Behav. 59, 975–979 10.1006/anbe.1999.1402 (doi:10.1006/anbe.1999.1402) [DOI] [PubMed] [Google Scholar]
- 53.Janik V. M., Slater P. J. B. 2000. The different roles of social learning in vocal communication. Anim. Behav. 60, 1–11 10.1006/anbe.2000.1410 (doi:10.1006/anbe.2000.1410) [DOI] [PubMed] [Google Scholar]
- 54.Beltman J. B., Haccou P., Ten Cate C. 2004. Learning and colonization of new niches: a first step toward speciation. Evolution 58, 35–46 [DOI] [PubMed] [Google Scholar]
- 55.Laland K. N. 1994. On the evolutionary consequences of sexual imprinting. Evolution 48, 477–489 10.2307/2410106 (doi:10.2307/2410106) [DOI] [PubMed] [Google Scholar]
- 56.Clutton-Brock T., McAuliffe K. 2009. Female mate choice in mammals. Q. Rev. Biol. 84, 3–27 10.1086/596461 (doi:10.1086/596461) [DOI] [PubMed] [Google Scholar]
- 57.Davidson S. M., Wilkinson G. S. 2004. Function of male song in the greater white-lined bat, Saccopteryx bilineata. Anim. Behav. 67, 883–891 10.1016/j.anbehav.2003.06.016 (doi:10.1016/j.anbehav.2003.06.016) [DOI] [Google Scholar]
- 58.Rendell L., Whitehead H. 2001. Culture in whales and dolphins. Behav. Brain Sci. 24, 309–324 [DOI] [PubMed] [Google Scholar]
- 59.Fragaszy D. M., Perry S. 2003. Towards a biology of traditions. In The biology of traditions: models and evidence (eds Fragaszy D. M., Perry S.), pp. 1–32 Cambridge, UK: Cambridge University Press [Google Scholar]
- 60.McGrew W. C. 2009. Ten dispatches from the chimpanzee culture wars, plus postcript (revisiting the battlefronts). In The question of animal culture (eds Laland K. N., Galef B. G.). Cambridge, MA: Harvard University Press [Google Scholar]
- 61.Laland K. N., Hoppitt W. 2003. Do animals have culture? Evol. Anthropol. 12, 150–159 10.1002/evan.10111 (doi:10.1002/evan.10111) [DOI] [Google Scholar]
- 62.Whiten A., van Schaik C. P. 2007. The evolution of animal ‘cultures’ and social intelligence. Phil. Trans. R. Soc. B 362, 603–620 10.1098/rstb.2006.1998 (doi:10.1098/rstb.2006.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perry S. 2009. Are nonhuman primates likely to exhibit cultural capacities like those of humans? In The question of animal culture (eds Laland K. N., Galef B. G.). Cambridge, MA: Harvard University Press [Google Scholar]
- 64.Tomasello M. 1994. The question of chimpanzee culture. In Chimpanzee cultures (eds Wrangham R., McGrew W., de Waal F., Heltne P.), pp. 301–317 Cambridge, MA: Harvard University Press [Google Scholar]
- 65.Kendal R. L., Kendal J. R., Hoppitt W., Laland K. N. 2009. Identifying social learning in animal populations: a new ‘option-bias' method. PLoS ONE 4, e6541. 10.1371/journal.pone.0006541 (doi:10.1371/journal.pone.0006541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Müller C. A., Cant M. A. 2010. Imitation and traditions in wild banded mongooses. Curr. Biol. 20, 1171–1175 10.1016/j.cub.2010.04.037 (doi:10.1016/j.cub.2010.04.037) [DOI] [PubMed] [Google Scholar]
- 67.Sargeant B. L., Mann J. 2009. From social learning to culture: intrapopulation variation in bottlenose dolphins. In The question of animal culture (eds Laland K. N., Galef B. G.). Cambridge, MA: Harvard University Press [Google Scholar]
- 68.Thornton A., Malapert A. 2009. Experimental evidence for social transmission of food acquisition techniques in wild meerkats. Anim. Behav. 78, 255–264 10.1016/j.anbehav.2009.04.021 (doi:10.1016/j.anbehav.2009.04.021) [DOI] [Google Scholar]
- 69.Helfman G. S., Schultz E. T. 1984. Social transmission of behavioral traditions in a coral reef fish. Anim. Behav. 32, 379–384 10.1016/S0003-3472(84)80272-9 (doi:10.1016/S0003-3472(84)80272-9) [DOI] [Google Scholar]
- 70.Warner R. R. 1988. Traditionality of mating site preferences in a coral reef fish. Nature 335, 719–721 10.1038/335719a0 (doi:10.1038/335719a0) [DOI] [Google Scholar]
- 71.van de Waal E., Renevey N., Favre C. M., Bshary R. 2010. Selective attention to philopatric models causes directed social learning in wild vervet monkeys. Proc. R. Soc. B 277, 2105–2111 10.1098/rspb.2009.2260 (doi:10.1098/rspb.2009.2260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Claidière N., Sperber D. 2010. Imitation explains the propagation, not the stability of animal culture. Proc. R. Soc. B 277, 651–659 10.1098/rspb.2009.1615 (doi:10.1098/rspb.2009.1615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galef B. G., Allen C. 1995. A new model system for studying behavioral traditions in animals. Anim. Behav. 50, 705–717 10.1016/0003-3472(95)80131-6 (doi:10.1016/0003-3472(95)80131-6) [DOI] [Google Scholar]
- 74.Giraldeau L. A., Lefebvre L. 1987. Scrounging prevents cultural transmission of food-finding behavior in pigeons. Anim. Behav. 35, 387–394 10.1016/S0003-3472(87)80262-2 (doi:10.1016/S0003-3472(87)80262-2) [DOI] [Google Scholar]
- 75.Rieucau G., Giraldeau L.-A. 2011. Exploring the costs and benefits of social information use: an appraisal of current experimental evidence. Phil. Trans. R. Soc. B 366, 949–957 10.1098/rstb.2010.0325 (doi:10.1098/rstb.2010.0325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thornton A., Malapert A. 2009. The rise and fall of an arbitrary tradition: an experiment with wild meerkats. Proc. R. Soc. B 276, 1269–1276 10.1098/rspb.2008.1794 (doi:10.1098/rspb.2008.1794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henrich J., et al. 2010. Markets, religion, community size, and the evolution of fairness and punishment. Science 327, 1480–1484 10.1126/science.1182238 (doi:10.1126/science.1182238) [DOI] [PubMed] [Google Scholar]
- 78.Thornton A., Samson J., Clutton-Brock T. 2010. Multi-generational persistence of traditions in neighbouring meerkat groups. Proc. R. Soc. B 277, 3623–3629 10.1098/rspb.2010.0611 (doi:10.1098/rspb.2010.0611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laland K. N., Odling-Smee J., Myles S. 2010. How culture shaped the human genome: bringing genetics and the human sciences together. Nat. Rev. Genet. 11, 137–148 10.1038/nrg2734 (doi:10.1038/nrg2734) [DOI] [PubMed] [Google Scholar]
- 80.Durham W. H. 1991. Coevolution: genes, culture, and human diversity. Stanford, CA: Stanford University Press [Google Scholar]
- 81.Mesoudi A., Whiten A., Laland K. N. 2006. Towards a unified science of cultural evolution. Behav. Brain Sci. 29, 329–383 [DOI] [PubMed] [Google Scholar]
- 82.Boesch C. 1993. Towards a new image of culture in wild chimpanzees. Behav. Brain Sci. 16, 514–515 10.1017/S0140525X00031277 (doi:10.1017/S0140525X00031277) [DOI] [Google Scholar]
- 83.Hunt G. R., Gray R. D. 2003. Diversification and cumulative evolution in New Caledonian crow tool manufacture. Proc. R. Soc. Lond. B 270, 867–874 10.1098/rspb.2002.2302 (doi:10.1098/rspb.2002.2302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kendal J. R., Rendell L., Pike T. W., Laland K. N. 2009. Nine-spined sticklebacks deploy a hill-climbing social learning strategy. Behav. Ecol. 20, 238–244 10.1093/beheco/arp016 (doi:10.1093/beheco/arp016) [DOI] [Google Scholar]
- 85.Galef B. G. 1992. The question of animal culture. Hum. Nat. 3, 157–178 10.1007/BF02692251 (doi:10.1007/BF02692251) [DOI] [PubMed] [Google Scholar]
- 86.Huber L., Range F., Voelkl B., Szucsich A., Viranyi Z., Miklosi A. 2009. The evolution of imitation: what do the capacities of non-human animals tell us about the mechanisms of imitation? Phil. Trans. R. Soc. B 364, 2299–2309 10.1098/rstb.2009.0060 (doi:10.1098/rstb.2009.0060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caldwell C. A., Millen A. E. 2009. Social learning mechanisms and cumulative cultural evolution: is imitation necessary? Psychol. Sci. 20, 1478–1483 10.1111/j.1467-9280.2009.02469.x (doi:10.1111/j.1467-9280.2009.02469.x) [DOI] [PubMed] [Google Scholar]
- 88.Csibra G., Gergely G. 2011. Natural pedagogy as evolutionary adaptation. Phil. Trans. R. Soc. B 366, 1149–1157 10.1098/rstb.2010.0319 (doi:10.1098/rstb.2010.0319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lyons D. E., Damrosch D. H., Lin J. K., Macris D. M., Keil F. C. 2011. The scope and limits of overimitation in the transmission of artefact culture. Phil. Trans. R. Soc. B 366, 1158–1167 10.1098/rstb.2010.0335 (doi:10.1098/rstb.2010.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thornton A., Raihani N. J. 2010. Identifying teaching in wild animals. Learn. Behav. 38, 297–309 10.3758/LB.38.3.297 (doi:10.3758/LB.38.3.297) [DOI] [PubMed] [Google Scholar]
- 91.Hrdy S. B. 2009. Mothers and others: the evolutionary origins of mutual understanding. Cambridge, MA: Harvard University Press [Google Scholar]
- 92.Tomasello M., Carpenter M., Call J., Behne T., Moll H. 2005. Understanding and sharing intentions: the origins of shared cognition. Behav. Brain Sci. 28, 675–735 [DOI] [PubMed] [Google Scholar]
- 93.Hewlett B. S., Fouts H. N., Boyette A. H., Hewlett B. L. 2011. Social learning among Congo Basin hunter–gatherers. Phil. Trans. R. Soc. B 366, 1168–1178 10.1098/rstb.2010.0373 (doi:10.1098/rstb.2010.0373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McComb K., Moss C., Durant S. M., Baker L., Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science 292, 491–494 10.1126/science.1057895 (doi:10.1126/science.1057895) [DOI] [PubMed] [Google Scholar]
- 95.Whiten A. 2005. The second inheritance system of chimpanzees and humans. Nature 437, 52–55 10.1038/nature04023 (doi:10.1038/nature04023) [DOI] [PubMed] [Google Scholar]