Abstract
Impaired glucose regulation is a defining characteristic of type 2 diabetes mellitus (T2DM) pathology and has been linked to increased risk of cognitive impairment and dementia. Although the benefits of aerobic exercise for physical health are well-documented, exercise effects on cognition have not been examined for older adults with poor glucose regulation associated with prediabetes and early T2DM. Using a randomized controlled design, twenty-eight adults (57–83 y old) meeting 2-h tolerance test criteria for glucose intolerance completed 6 months of aerobic exercise or stretching, which served as the control. The primary cognitive outcomes included measures of executive function (Trails B, Task Switching, Stroop, Self-ordered Pointing Test, and Verbal Fluency). Other outcomes included memory performance (Story Recall, List Learning), measures of cardiorespiratory fitness obtained via maximal-graded exercise treadmill test, glucose disposal during hyperinsulinemic-euglycemic clamp, body fat, and fasting plasma levels of insulin, cortisol, brain-derived neurotrophic factor, insulin-like growth factor-1, amyloid-β (Aβ40 and Aβ42). Six months of aerobic exercise improved executive function (MANCOVA, p = 0.04), cardiorespiratory fitness (MANOVA, p = 0.03), and insulin sensitivity (p = 0.05). Across all subjects, 6-month changes in cardiorespiratory fitness and insulin sensitivity were positively correlated (p = 0.01). For Aβ42, plasma levels tended to decrease for the aerobic group relative to controls (p = 0.07). The results of our study using rigorous controlled methodology suggest a cognition-enhancing effect of aerobic exercise for older glucose intolerant adults. Although replication in a larger sample is needed, our findings potentially have important therapeutic implications for a growing number of adults at increased risk of cognitive decline.
Keywords: Aerobic exercise, Alzheimer’s disease, cognition, dementia, diabetes, executive function, glucose intolerance, prediabetes
INTRODUCTION
Abnormally high glucose levels in response to a glucose challenge suggest poor glucoregulation, a condition that has been linked to impaired cognition in older adults [1–3]. Type 2 diabetes mellitus (T2DM) is a condition defined by insulin resistance and inadequate compensatory insulin secretion that result in impaired glucose regulation. Poor glycemic control may be present for many years before T2DM is detected, and can have deleterious consequences for many target tissues without any noticeable symptoms. In the prodromal phase of the disease, abnormalities in glucose regulation can be detected by measuring glucose response to an oral glucose load. Impaired glucose tolerance (IGT) characteristic of prediabetes is identified when 2-h tolerance test glucose levels reach 140 mg/dL but fall below 200 mg/dL, while 2-h values that exceed this upper criterion indicate T2DM [4]. Abnormal glucose tolerance, a characteristic of prediabetes and T2DM, has been linked to an increased risk of cognitive impairment including prodromal and frank Alzheimer’s disease (AD) and vascular dementia [5–12].
Diet and exercise represent the first line of intervention in clinical practice to slow progression of metabolic disturbance associated with prediabetes and T2DM. Although increased physical activity has clear beneficial physiological effects for older adults with glucose intolerance [13], exercise effects on cognition have not been examined in this population. Physical activity has potent therapeutic effects on glucose regulation and cardiovascular health, both of which when compromised may threaten cognitive integrity [14–17]. Positive effects of aerobic exercise on cognition have been well documented in animal models and in aging clinical populations [18,19]. In one set of clinical studies, Colcombe and colleagues provide cross-sectional and prospective brain imaging data to suggest that aerobic exercise ameliorates age-related volume loss for older adults, changes that are most striking for brain regions that support executive control processes and memory [20–22] yet most vulnerable to the effects of aging [23]. Inhuman studies, enhanced executive function is the most frequently reported benefit attributable to exercise, and includes abilities such as selective attention, multi-tasking, cognitive flexibility, and working memory [19,20].
Although recent reports suggests that exercise can attenuate normal age-related cognitive changes and deficits associated with mild cognitive impairment [24, 25] and dementia [26–28], it has not been established whether aerobic exercise improves cognition for cognitively normal older adults with glucose intolerance who are at increased risk of cognitive decline. We hypothesized that a 6-month program of aerobic exercise relative to a stretching control would benefit cognition, particularly executive control processes in older glucose intolerant adults. We also examined intervention effects on insulin sensitivity, and on plasma levels of cortisol, brain-derived neurotrophic factor (BD-NF), insulin-like growth factor-1 (IGF-1) and amyloid-β 1–40 and 1–42 (Aβ40 and Aβ42), to explore putative mechanisms linking exercise with improved cognitive function for an at-risk group of older adults.
MATERIALS AND METHODS
Subjects
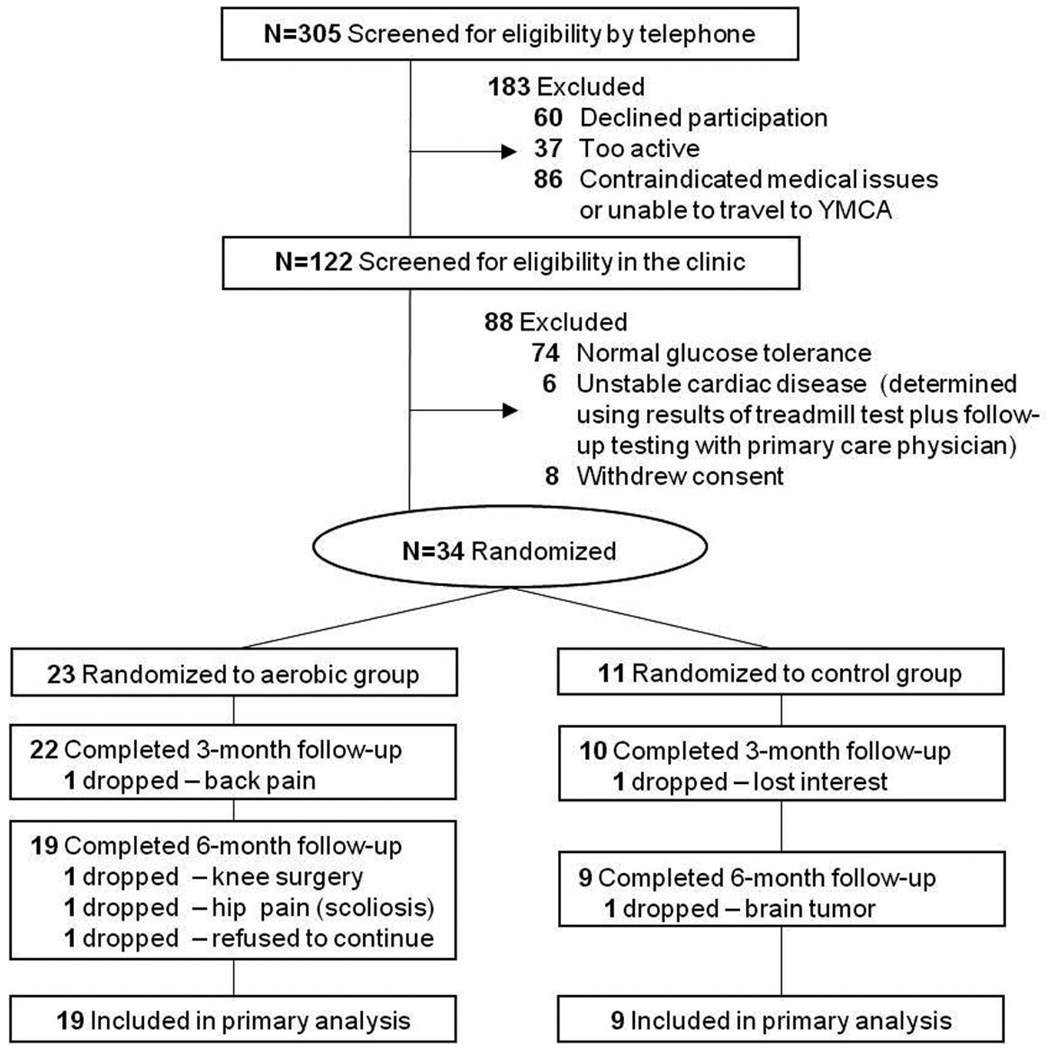
The study was approved by the University of Washington Institutional Review Board and the Research and Development Committee of the VA Puget Sound Health Care System. Thirty-four subjects with normal cognitive status as determined by neuropsychological assessment and meeting criteria for abnormal glucose tolerance via oral glucose tolerance test (2-h glucose ≥ 140 mg/dL [4]) provided written informed consent and were enrolled into the study. Subjects included those meeting glucose tolerance criteria for prediabetes (140 mg/dL ≥ 2-h glucose < 200 mg/dL) or newly diagnosed (at the time of study screening) T2DM (2-h glucose ≥ 200 mg/dL). Exclusion criteria included unstable cardiac disease, significant cerebrovascular disease, musculoskeletal impairment, or presence of other medical conditions with significant psychiatric, neurologic, or metabolic sequelae. Only sedentary adults (self-report of < 30 min of structured physical activity < 3 times/week in last 6 months) were enrolled. Use of statins or anti-hypertensives was permitted while current or prior use of diabetes medications was not. All subjects were newly diagnosed with glucose intolerance as a result of study participation. Twenty-eight participants successfully completed the trial and were comparable with respect to baseline general cognitive status, cardiorespiratory fitness, adiposity, glucose tolerance, and fasting plasma levels of insulin, glucose, and lipids (all ps > 0.22). Although all subjects met criteria for glucose intolerance during screening (2-h glucose ≥ 140 mg/dL), 6 of 28 subjects (21%) met the more stringent criteria for T2DM (2-h glucose ≥ 200 mg/dL), and 14 of 28 subjects (50%) also met criteria for impaired fasting glucose (IFG, ≥ 100 mg/dL [4]). T2DM and IFG were proportionately distributed across the two treatment groups, and for all cases, pharmacological treatment was deemed unwarranted by the participants’ primary care providers. Baseline characteristics for completers are provided in Table 1, and subject flow from initial contact through study completion is depicted in Fig. 1 using a CONSORT-style diagram. Relative to completers, the dropouts (n = 6 women) were younger (p = 0.04) and tended to have higher baseline fasting LDL levels (p = 0.09) but were otherwise comparable with respect to other measures at study entry.
Table 1.
Baseline subject characteristics and treatment effects on physical and physiological outcomes
| Stretching |
Aerobic |
|||
|---|---|---|---|---|
| Baseline | Month 6 | Baseline | Month 6 | |
| Total subjects, n (males) | 9 (1) | — | 19 (9) | — |
| IFG, n (%) | 5 (56) | — | 9 (47) | — |
| T2DM, n (%) | 2 (22) | — | 4 (21) | — |
| Taking a β-blocker, n (%) | 3 (33) | — | 6 (32) | — |
| Taking a statin, n (%) | 3 (33) | — | 5 (26) | — |
| Age, y* | 66 (6.0) | — | 71 (7.5) | — |
| MMSE | 28.8 (1.0) | — | 28.6 (1.2) | — |
| DRS | 140 (2.0) | — | 139 (3.2) | — |
| 2-h glu, mg/dL | 163 (31.8) | — | 184 (46.0) | — |
| VO2peak, L/min‡ | 1.69 (0.3) | 1.65 (0.3) | 1.79 (0.6) | 1.97 (0.7) |
| BMI, m2/kg† | 30.1 (7.2) | 29.6 (7.3) | 30.6 (3.9) | 29.7 (3.5) |
| Fat, %*† | 42.6 (7.1) | 41.0 (7.6) | 37.7 (6.6) | 36.1 (6.0) |
| FPI, mU/mL | 9.8 (3.9) | 8.4 (5.0) | 11.1 (9.0) | 9.0 (4.4) |
| FPG, mg/dL | 94 (11.4) | 91 (8.2) | 105 (30.0) | 100 (20.0) |
| GDR, mg/kgFFM/min‡ | 7.6 (2.5) | 6.7 (2.7) | 5.9 (3.3) | 6.5 (3.0) |
| TG, mg/dL† | 146 (77.3) | 108 (34.3) | 170 (114.3) | 137 (68.1) |
| HDL, mg/dL | 58 (16.2) | 60 (11.0) | 55 (11.9) | 56 (11.8) |
| LDL, mg/dL | 102 (16.8) | 100 (21.2) | 110 (29.5) | 114 (33.4) |
| Aβ42, pg/mL § | 58.9 (66.6) | 79.9 (43.8) | 87.1 (64.9) | 66.0 (42.7) |
Means (SD) are provided unless otherwise indicated. Abbreviations: IFG = number of subjects (% of group total) meeting criteria for impaired fasting glucose (≥ 100 mg/dL); T2DM = number of subjects (% of group total) meeting 2-h tolerance test glucose level criteria for type 2 diabetes mellitus (≥ 200 mg/dL); MMSE = Mini-Mental Status Exam (maximum score = 30); DRS = Dementia Rating Scale (maximum score = 44); 2-h glu = 2-hour plasma glucose concentration during oral glucose tolerance test performed at screening to assess study eligibility; VO2peak = peak oxygen uptake measured during maximal-graded exercise treadmill test; BMI = body mass index; Fat = Percent fat distributed across the body, excluding head, measured using dual energy X-ray absorptiometry; FPI = fasting plasma insulin; FPG = fasting plasma glucose; GDR = mean 30-min glucose disposal rate 120 min into the hyperinsulinemic-euglycemic clamp, adjusted for fat free mass in kgs (kgFFM); TG = fasting plasma triglyceride levels; HDL = fasting plasma high density lipoprotein concentration; LDL = fasting plasma low density lipoprotein concentration; Aβ42 = fasting plasma levels of amyloid-β 1–42.
Trend for baseline difference across treatment groups, p = 0.09; Decreased at month 6 vs. baseline for both groups, p < 0.05; Treatment-related improvement for aerobic group relative to controls, p = 0.05; Trended down at month 6 for aerobic group relative to controls, p = 0.07.
Fig. 1.
Subject flow from initial contact through study completion.
Procedure
Participants were randomized using a 2:1 ratio to an aerobic exercise or stretching control group. This schedule was used to offset an anticipated attrition imbalance across groups. Cognitive testing and 12-h fasting blood collection occurred between 8 am and 10 am at baseline and at months 3 and 6. Before and after the 6-month intervention, insulin sensitivity (via hyperinsulinemic-euglycemic clamp), peak cardiorespiratory capacity (via graded exercise treadmill test), and body fat (using dual energy X-ray absorptiometry) were assessed for all subjects. Study personnel involved in collection of outcome measures were blinded to randomization assignment.
Intervention protocols
Participants in both groups carried out their activity routines 4 d/wk for 45–60 min/session for 6 months. Participants were instructed to maintain constant diet and extracurricular activities for the duration of the study. The majority (90%) of exercise sessions were conducted at local YMCAs. All subjects in both groups received individualized supervision by a fitness trainer for the first 8 activity sessions. Thereafter, the trainer supervised 1 session/wk/participant. All subjects also received a weekly phone call to monitor compliance, and completed logs tracking exercise duration and heart rate (HR) monitor measurements. Exercise duration and intensity were titrated up over the first 6 weeks, until participants in the aerobic group were exercising at 75–85% of HR reserve [29] using a treadmill, stationary bicycle, or elliptical trainer. This intensity was maintained for the study duration. Participants in the control group carried out a prescribed routine of stretching and balance exercises, maintaining HR at or below 50% HR reserve. Compliance data included peak and mean HR measurements and exercise duration and frequency at the targeted HR intensity recorded by the trainer and by the subject. An exercise physiologist regularly reviewed these data to ensure that targeted goals were met, and assigned a weekly compliance rating (1 = failed to meet goals; 2 = met goals; 3 = exceeded goals) for each subject. When illness or travel prevented completion of 3 sessions/wk (n = 1 in stretching group, n = 2 in aerobic group), total study duration was increased by 1 week. Compliance outcomes are provided in Table 2, and were comparable across groups.
Table 2.
Compliance
| Stretching (n = 9) |
Aerobic (n = 19) |
|||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| Total weeks of exercise | 23 (0.5) | 22–25 | 23.9 (0.7) | 22–26 |
| Sessions/week* | 3.76 (0.7) | 3–5 | 3.74 (0.8) | 3–5 |
| Weekly compliance ratings* | 1.97 (0.07) | 1.8–2.0 | 1.87 (0.44) | 1–2.6 |
Tabled values for exercise sessions per week and weekly compliance ratings by the exercise physiologist (3-point scale: 1 = did not meet goals; 2 = met goals; 3 = exceeded goals) reflect data collected from week 6 (end of acclimation period) through the end of the study. Compliance outcomes did not differ between groups.
Cardiorespiratory fitness assessment
Pre- and post-intervention, participants performed a modified Balke maximal-graded exercise treadmill test [30], with HR and oxygen uptake monitored by an automated metabolic cart (MedGraphics, St. Paul, MN). Subjects began by walking on a treadmill at a slow speed and 0% grade. After 2 min, speed was increased to 3 mph at 0% grade. Thereafter, only the grade was increased by 2% every 2 min. Peak oxygen uptake (VO2peak) was measured at test termination triggered by the onset of symptoms or report of exhaustion.
Hyperinsulinemic-euglycemic clamp
Pre- and post-intervention, fasted participants underwent a hyperinsulinemic-euglycemic clamp [31] to assess insulin sensitivity. Before the clamp, one catheter was inserted into an antecubital vein for infusions, a second catheter was inserted into a brachial artery of the contralateral arm for blood sampling, and subjects rested with intravenous lines in place for a 30-min habituation period. Throughout the 2.5-h procedure, plasma insulin elevated using an insulin infusion dose of 1.0 mU/kg/min. Glucose levels were measured in duplicate at 5-min intervals using a whole blood glucose analyzer (HemoCue, Lake Forest, CA), at which time a variable rate infusion of 20% dextrose solution (D20) was adjusted according to a negative feedback algorithm described by Defronzo et al. [31] to maintain plasma glucose concentration at 95 mg/dL. Two hours into the procedure, the 30-min quantity of D20 infusate needed to maintain euglycemia under the condition of steady-state hyperinsulinemia was recorded. Plasma insulin levels were not measured during the clamp. Insulin sensitivity was estimated using mean quantity of dextrose infused per minute over this 30-min period adjusted for fat free body mass (kg).
Cognitive assessment
Three comparable versions of the cognitive protocol were randomly assigned in counterbalanced order to the three assessment visits. An additional version was administered prior to baseline to familiarize participants with procedures. The protocol included tests of executive function and short-term memory with documented sensitivity to the effects of aging or early neurodegenerative disease.
Tests of executive function
For the Trail-making Test [32], subjects drew lines to connect randomly placed alphanumeric stimuli in ascending order. In the more difficult condition (Trails B), subjects alternately tracked 2 different sets of stimuli (letters, numbers). Time to complete Trails B, adjusted for Trails A time, was subjected to analysis. Task Switching [33,34] measures the cost of switching between tasks. Pairs of stimuli including a letter and a number were presented clockwise around a 2 × 2 matrix displayed on a computer screen. Every 2 trials, the task alternated between having to make an odd-even decision or a consonant-vowel decision. Each new trial was triggered by the previous response. Mean reaction time, adjusted for accuracy, was subjected to analysis. Stroop Color-Word Interference [35,36], a test of selective attention and response inhibition, was administered via computer equipped with a voice key. Color names were presented on a computer screen, one at a time, in concordant or discordant font colors (e.g., the word “red” presented in red or green font). Subjects were instructed either to read the word or to name the color as quickly as possible, and voice onset latency and content were recorded. Each trial was preceded by a reminder regarding task instruction to minimize memory load. The Self-Ordered Pointing Test (SOPT) [37, 38] is a computer-administered test of working memory where subjects were instructed to touch each design of a multi-design array. After each touch, the designs were rearranged within the array. This procedure was repeated 10 times (trials) for the 10-design array, and 12 times for the 12-design array. Three consecutive trial blocks were completed for each of the 2 multidesign arrays, and number of errors was recorded. Verbal Fluency [39,40] was measured by the total number of words generated across four 60 s trials. Subjects listed words beginning with specified letters of the alphabet for the first 2 trials and that belonged to specified semantic categories for the remaining 2 trials.
Tests of memory
For Story Recall [41,42], a test of declarative short-term memory, subjects heard a brief narrative containing 44 informational bits, and were asked to recall as much as possible both immediately and after a 30-min delay. Credit was awarded for verbatim recall and accurate paraphrases, and delayed recall scores were subjected to analysis. For List Learning [43], subjects heard a list of 12 words and were asked to recall as many items as possible across 3 learning trials, and then again after a 20-min delay. Delayed recall scores were analyzed.
Assays
Plasma glucose was measured in duplicate using a HemoCue glucose analyzer (Hemocue, Lake Forest, CA). Radioimmunoassay was used to quantify plasma concentrations of insulin, total IGF-1 and IGF-binding protein 3 (IGFBP3) as previously described [24], and total cortisol (DSL Cortisol RIA kit, Diagnostics Systems Laboratories, Webster, TX) according to the manufacturer’s protocol. Plasma BDNF and platelet factor-4 (PF4) were quantified using BDNF Emax ELISA (Promega Co., Magison WI) and Zymutest PF4 ELISA (Aniara Co., Mason OH) according to the manufacturer’s instructions. Although BDNF is highly concentrated in the nervous system, it is also stored and released by activated platelets in the blood [44]. Thus we assayed PF4 as an estimate of platelet activity that could be used to adjust total BDNF levels in plasma for the contribution of activated platelets. Plasma Aβ40 and Aβ42 levels were determined using ELISA as previously described [45]. All assays were performed in duplicate, and pre- and post-intervention measurements were randomly distributed across plates when more than one plate was required.
Statistical analysis
Cognitive measures, reflected as difference scores (month 6 – baseline), were subjected to separate MAN-COVAs by domain (i.e., executive function, declarative memory), with treatment group serving as the independent variable. Age and gender were included as covariates in these analyses. Treatment effects on cardiorespiratory outcomes obtained during the treadmill test (VO2peak, treadmill grade, time to exhaustion) expressed as difference scores were examined using a similarly structured multivariate analysis. Significant findings from an omnibus test were examined using separate ANOVAs or ANCOVAs. Secondary analyses examined treatment effects on insulin sensitivity (glucose disposal during hyperinsulinemic-euglycemic clamp), cardiovascular outcomes (lipids, blood pressures), adiposity (%fat), and other AD biomarkers (plasma levels of cortisol, IGF-1, BDNF, Aβ) using one-way (treatment group assignment) ANCOVA. Age, fasting plasma insulin, and Dementia Rating Scale (DRS) score at baseline were initially included as covariates in the biomarker analyses, in light of scientific evidence to suggest a link between these outcomes, but dropped from the model if not contributory. For all analyses, pairwise comparisons were performed using t-tests when appropriate. Multiple regression and correlation procedures were used to examine exercise-induced associations between cognition, cardiorespiratory fitness, insulin sensitivity, adiposity, cortisol, BDNF, IGF-1, and Aβ. Positively skewed distributions were log-transformed prior to analysis. Adjustments for missing clamp data (unable to gain venous access for 5 individuals, n = 2 controls) were made using multiple imputation linear regression (STATA [46]). Imputations for missing data due to spoiled samples or testing error were not performed given the limited number of occurrences (< 5%).
RESULTS
Cardiorespiratory fitness
A 6-month trial of aerobic exercise versus stretching improved cardiorespiratory fitness (F3,23 = 3.63; p = 0.03) as measured by treadmill measures of VO2peak (L/min: +9% vs. −1.3%, p = 0.03, Table 1), treadmill grade (+70% vs. +8%, p = 0.002), and time to exhaustion (+59% vs. +5%, p = 0.001). These results were not altered when the statistical model was adjusted for β-blocker use.
Cognitive function
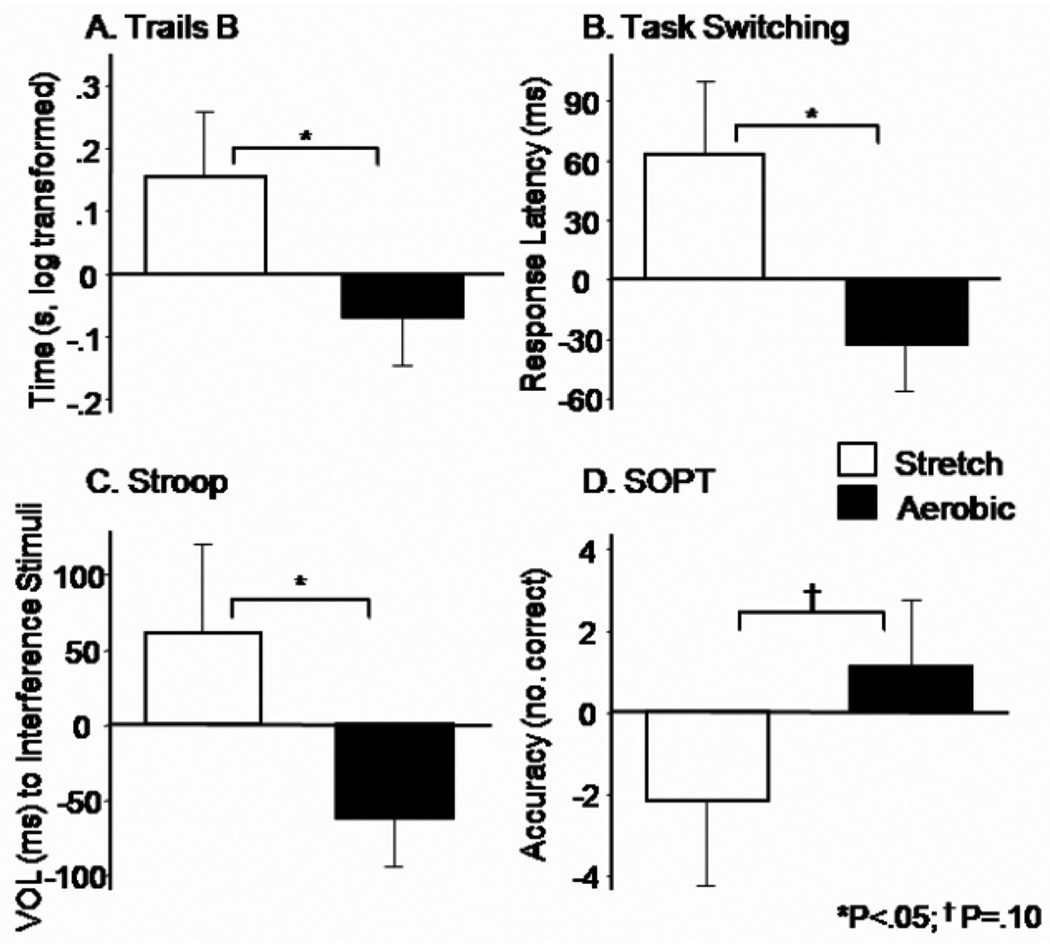
Six months of controlled aerobic exercise had a beneficial effect on executive function (F5,16 = 3.01; p = 0.04). The results of univariate analyses for the constituent cognitive tests and associated effect size estimates (Cohen’s f) are described below. Relative to controls, performance in the aerobic group improved on Trails B (f = 0.36, p = 0.04; Fig. 2A), Task Switching (f = 0.39, p = 0.03; Fig. 2B), and interference trials of the Stroop (f = 0.38, p = 0.04; Fig. 2C). Trends in the data suggested improved performance for the aerobic group vs. controls on SOPT (f = 0.29, p = 0.10; Fig. 2D) and Verbal Fluency (f = 0.25, p = 0.11; data not shown). Consistent with other reports in the literature [19,21,47], benefits were confined to executive control processes and did not impact memory (F2,22 = 0.56; p = 0.58). Analysis of cognitive outcomes collected 3 months into the study (6 weeks following titration to maximum intensity) failed to reach significance.
Fig. 2.
Treatment effects on cognitive outcomes of executive function. Means (standard error of measurement) represent 6-month change relative to baseline, expressed as difference scores. All means are adjusted for age and gender as well as other task-specific variables when indicated. A) Trails B time to complete the task (seconds, log transformed, adjusted for Trails A time) was faster for subjects in the aerobic group relative to controls, p = 0.04. B) Task Switching response latency for all trials, adjusted for accuracy, was faster for the aerobic group relative to controls, p = 0.03. C) Stroop voice onset latency to interference stimuli was faster for subjects in the aerobic group versus controls, p = 0.04. D) SOPT performance, indexed by number of correct responses across all trials, tended to improve for aerobic exercisers relative to controls, p = 0.10.
Insulin sensitivity, lipids, and adiposity
Glucose disposal during the 30 min steady-state period of the hyperinsulinemic-euglycemic clamp improved for the aerobic group relative to controls (F1,26 = 4.09; P = 0.05, Table 1). Across all subjects, treatment-related changes in glucose disposal and cardiorespiratory capacity were positively correlated (treadmill grade: r = 0.52, p = 0.005; time to exhaustion: r = 0.49, p = 0.01). At the end of the trial, adiposity and plasma triglyceride levels decreased for both groups relative to baseline (Table 1). Plasma levels of LDL trended down over the 6-month trial for all statin users (p = 0.08) but did not interact with treatment group.
Aβ, cortisol, BDNF, and IGF
Exploratory analyses were conducted to examine intervention effects on aging- and AD-related biomarkers. Plasma levels of Aβ42 were highly variable but nonetheless trended down for the aerobic group relative to controls (p = 0.07, Table 1). For this analysis, age, fasting plasma insulin, and DRS score were included as covariates. Mean plasma concentration of cortisol and BDNF increased for the stretching group and decreased for the aerobic group, consistent with our earlier findings [24], but this difference failed to reach statistical significance in the present study. Mean plasma levels of bioactive IGF-1 (total IGF-1 adjusted for IGFBP3) were higher for men than women at baseline (118 pg/mL vs. 89 pg/mL), but were not affected by treatment manipulation.
DISCUSSION
Six months of aerobic exercise improved cognitive performance on tasks of executive function including selective and divided attention, cognitive flexibility, and working memory in older adults with glucose intolerance. In addition, circulating levels of the AD biomarker Aβ42 tended to decrease for subjects in the aerobic group relative to controls. Although similar effects of aerobic exercise on executive control processes have been previously reported in normal adults [19], this is the first study to demonstrate that aerobic exercise can improve cognition in older adults with glucose intolerance who are at increased risk of cognitive decline associated with progression of T2DM pathology and AD.
Our results suggest that aerobic exercise has favorable effects on cognitive processes of executive function compromised by T2DM [48–50] and AD pathology [51,52]. Diabetes has numerous harmful consequences for peripheral systems but is also characterized by deleterious neurophysiologic and structural changes in the brain that adversely affect cognition [49] and ultimately increase risk of dementia [53–55]. Such neuropathological changes are believed to begin in the early stages of diabetes, conferring increased risk of cognitive decline for adults with prediabetes as well [17, 56,57].
In the present study, executive function and insulin sensitivity improved with aerobic exercise, a finding that implicates a potential benefit of improved glucose metabolism on cognitive processes. Although it is not possible to specify the mechanisms underlying these effects in our study, in animal models, exercise-induced cognitive benefits have been linked to improved energy metabolism and insulin signaling in the brain [58]. Executive control processes are supported in large part by frontal brain regions [21,22] that are particularly vulnerable not only to the effects of aging [23], but also to the effects of T2DM pathophysiology [59]. In epidemiological studies, glucose intolerance is linked to cognitive impairment in non-diabetic older adults [55], while in controlled studies pharmacological treatment of dysglycemia with consequences for insulin sensitivity is associated with improved cognitive function [60]. In light of the potent insulin sensitizing effects of aerobic exercise, it is conceivable that exercise-induced enhancements in cognition may be supported in part by improvements in glucoregulation.
We and others have demonstrated that the effects of exercise in humans are greatest for tasks of executive function mediated by frontal brain regions. These regions, and the cognitive processes they support, are particularly susceptible to deleterious neurophysiological and structural changes associated with aging [21–23]. Consistent with the idea that T2DM pathology represents a model of accelerated aging [6,61], these areas are likely affected to an even greater extent for prediabetic and diabetic adults. Adults with poor glucose regulation and reduced insulin sensitivity have impairments in executive function [2,62–64], and metabolic and structural disturbances in frontal cortex relative to controls [48,49,59]. Impairments in executive function have been linked to reduced vasodilation with pronounced effects on frontal-subcortical circuits that are particularly susceptible to microvascular dysfunction [65]. Thus, aerobic exercise may have its greatest remediating effect on frontal brain regions that are most vulnerable to aging, and for glucose intolerant adults with an increased risk of cognitive decline and AD, to deleterious consequences of diabetes- and AD-related vascular dysfunction [48,66,67].
The failure to observe consistent beneficial effects of exercise on memory in this and other studies is somewhat surprising given that a number of animal studies show benefits for spatial memory, a task supported by the hippocampus [68]. In human trials, the absence of exercise-induced memory benefits may relate to the type of tests administered (not tests of spatial memory) or the specific task demands that rely more heavily on brain regions other than the medial-temporal lobe and surrounding structures. Alternatively, as noted above, positive effects of aerobic exercise may be most noticeable for brain regions that are most compromised by age.
Impaired glucoregulation is implicated in a number of AD-related pathophysiological processes, including altered Aβ metabolism. Plasma Aβ42 levels are elevated for adults at high risk of AD [69], and increase in response to acute alterations in glucose load, particularly in the context of AD pathology [70,71]. In animal models of AD, exercise reduces Aβ burden in brain [72–74]. The results of our study suggest that aerobic exercise may also have an impact on circulating Aβ42 given that plasma levels tended to decrease for subjects in the aerobic exercise group relative to controls. Although we also reported a similar change in Aβ42 levels in response to a 6-month trial of aerobic exercise for older adults with mild cognitive impairment [24], the significance of this finding remains to be determined.
The limitations of our study include small sample size and disproportionate representation by gender across groups. Despite a high risk of type II error, an inherent disadvantage of trials with small n, we detected exercise-related improvements across several tasks of executive function. In larger trials, it is conceivable that subtle effects involving memory could be detected. In addition, we chose to exercise adults at a high level of intensity to maximize our ability to detect a true effect. Consequently, we were conservative regarding inclusion criteria to ensure patient safety and minimize liability, and this selection process potentially limits our ability to extrapolate the results to larger, older adult populations.
Increased physical activity is a potent non-pharmacological intervention for physiological symptoms associated with impaired glucose metabolism and T2DM, conditions that confer increased risk of AD. Our results suggest that exercise also has a positive effect on cognitive function for older adults with glucose intolerance, without the cost and adverse side effects associated with most medication therapies. The cognition-enhancing effects of aerobic exercise were confined to executive control processes and did not include declarative memory. Exercise-induced improvements in insulin sensitivity, cerebral blood flow, and other metabolic parameters may contribute to the observed cognitive benefits. The results of this study also suggest that 6 months of moderate to high intensity aerobic exercise may influence circulating levels of Aβ42, a finding with potential implications for AD pathology. Future controlled trials of aerobic exercise that include brain imaging measures of glucose metabolism and blood flow will likely help to identify specific mechanisms to account for cognition-enhancing effects.
ACKNOWLEDGMENTS
This work was supported by the Office of Research and Development Medical Research Service and the Geriatric Research, Education and Clinical Center of the Department of Veterans Affairs, and the American Diabetes Association (Clinical Research Award: 7-04-CR-02). Neither funding source provided scientific input to the study. The principal investigator, Dr. Baker, had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis which was conducted without input from the funding agencies. Throughout the study, the YMCA of Greater Seattle, the YMCA of Tacoma-Pierce County, and the South Sound YMCA worked closely with us to provide exercise facilities for participants. We are grateful to the members of our laboratory who contributed many hours to this project including Karen Enstrom, RN, Darla Chapman, RN, Donna Davis, RN, Laura Fisher, Lauren Smith, Jaime Tidwell, Tracia Clark, Amy Morgan, Brenna Renn, and Meg Wojtowicz, and to Elizabeth Colasurdo for her assistance with the cortisol assays.
Footnotes
The authors have no conflict of interest to disclose.
Clinical Trials Registration: NCT00220441, ClinicalTrials.gov
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=527).
REFERENCES
- 1.Messier C, Tsiakas M, Gagnon M, Desrochers A, Awad N. Effect of age and glucoregulation on cognitive performance. Neurobiol Aging. 2003;24:985–1003. doi: 10.1016/s0197-4580(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 2.Messier C, Tsiakas M, Gagnon M, Desrochers A. Effect of age and glucoregulation on cognitive performance. J Clin Exp Neuropsychol. 2010;5:1–13. doi: 10.1080/13803390903540323. [DOI] [PubMed] [Google Scholar]
- 3.Vanhanen M, Koivisto K, Kuusisto J, Mykkanen L, Helkala E-L, Hanninen T, Riekkinen P, Soininen H, Laakso M. Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care. 1998;21:398–402. doi: 10.2337/diacare.21.3.398. [DOI] [PubMed] [Google Scholar]
- 4.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 5.Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- 6.Baquer NZ, Taha A, Kumar P, McLean P, Cowsik SM, Kale RK, Singh R, Sharma D. A metabolic and functional overview of brain aging linked to neurological disorders. Biogerontology. 2009;10:377–413. doi: 10.1007/s10522-009-9226-2. [DOI] [PubMed] [Google Scholar]
- 7.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 8.Craft S. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26 Suppl 1:65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 10.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 11.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 12.Luchsinger JA. Type 2 diabetes and related conditions in relation to dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20 doi: 10.3233/JAD-2010-091687. 723-723. [DOI] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program Research Group. The Diabetes Prevention Program: reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEJM. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 15.Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends Neurosci. 2003;26:404–406. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 16.Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuusisto J, Koivisto K, Mykkanen L, Helkala E-L, Vanhanen M, Hanninen T, Kervinen K, Kesaniemi YA, Riekkinen PJ, Laakso M. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 20.Colcombe S, Erickson K, Raz N, Webb A, Cohen N, McAuley E, Kramer A. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol Med Sci. 2003;58A:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 21.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 23.Daniels K, Toth J, Jacoby L. The aging of executive functions. In: Bialystok E, Craik F, editors. Lifespan Cognition: Mechanisms of Change. New York, New York: Oxford University Press; 2006. pp. 96–111. [Google Scholar]
- 24.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol. 2010;67:1–9. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 26.Broe GA, Henderson AS, Creasey H, McCusker E, Korten AE, Jorm AF, Longley W, Anthony JC. A case-control study of Alzheimer’s disease in Australia. Neurology. 1990;40:1698–1707. doi: 10.1212/wnl.40.11.1698. [DOI] [PubMed] [Google Scholar]
- 27.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Shen YC, Chen CH, Zhau YW, Li SR, Lu M. An epidemiological survey of age-related dementia in an urban area of Beijing. Acta Psychiatr Scand. 1989;79:557–563. doi: 10.1111/j.1600-0447.1989.tb10303.x. [DOI] [PubMed] [Google Scholar]
- 29.Pate R, Blair S, Durstine J, Eddy D, Hanson P, Painter P, Smith L, Wolfe L. In: Pate R, editor. Philadelphia, PA: Lea and Febiger; 1991. [Google Scholar]
- 30.Hagberg J. Exercise assessment of arthritic and elderly individuals. Baillieres Clin Rheumatol. 1994;8:29–52. doi: 10.1016/s0950-3579(05)80223-7. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 32.Shibuya-Tayoshi S, Sumitani S, Kikuchi K, Tanaka T, Tayoshi S, Ueno S, Ohmori T. Activation of the prefrontal cortex during the Trail-Making Test detected with multichannel near-infrared spectroscopy. Psychiatry Clin Neurosci. 2007;61:616–621. doi: 10.1111/j.1440-1819.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 33.Kramer AF, Hahn S, McAuley E, Cohen NJ, Banich MT, Harrison C, Chason J, Boileau RA, Bardell L, Colcombe A, Vakil E. Exercise, Aging and Cognition: Healthy Body, Healthy Mind? In: Fisk AD, Rogers W, editors. Human Factors Interventions for the Health Care of Older Adults. Hillsdale, N.J.: Erlbaum; 2001. [Google Scholar]
- 34.Rogers R, Sahakian B, Hodges J, Polkey C, Kennard C, Robbins T. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121:815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 35.Golden CJ. Stroop Color and Word Test. Chicago: Stoelting; 1978. [Google Scholar]
- 36.Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. J Exp Psychol Hum Percept Perform. 1996;22:461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- 37.Daigneault S, Braun CM. Working memory and the self-ordered pointing task: Further evidence of early prefrontal decline in normal aging. J Clin Exp Neuropsychol. 1993;16:881–895. doi: 10.1080/01688639308402605. [DOI] [PubMed] [Google Scholar]
- 38.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 39.Lonie JA, Herrmann LL, Tierney KM, Donaghey C, O’Carroll R, Lee A, Ebmeier KP. Lexical and semantic fluency discrepancy scores in aMCI and early Alzheimer’s disease. J Neuropsychol. 2009;3:79–92. doi: 10.1348/174866408X289935. [DOI] [PubMed] [Google Scholar]
- 40.Nutter-Upham KE, Saykin AJ, Rabin LA, Roth RM, Wishart HA, Pare N, Flashman LA. Verbal fluency performance in amnestic MCI and older adults with cognitive complaints. Arch Clin Neuropsychol. 2008;23:229–241. doi: 10.1016/j.acn.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chodosh J, Reuben D, Albert M, Seeman T. Predicting cognitive impairment in high-functioning community-dwelling older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2002;50:1051–1060. doi: 10.1046/j.1532-5415.2002.50260.x. [DOI] [PubMed] [Google Scholar]
- 42.Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 43.Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Moss M, Albert M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64:862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 44.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- 45.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 46.StataCorp. College Station, TX: StataCorp; 2007. [Google Scholar]
- 47.Colcombe S, Kramer A. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 48.Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 49.Manschot SM, Biessels GJ, de Valk H, Algra A, Rutten GE, van der Grond J, Kappelle LJ. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50:2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thabit H, Kennelly SM, Bhagarva A, Ogunlewe M, McCormack PM, McDermott JH, Sreenan S. Utilization of Frontal Assessment Battery and Executive Interview 25 in assessing for dysexecutive syndrome and its association with diabetes self-care in elderly patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;86:208–212. doi: 10.1016/j.diabres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Chang YL, Jacobson MW, Fennema-Notestine C, Hagler DJ, Jr, Jennings RG, Dale AM, McEvoy LK. Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cereb Cortex. 2009;20:1305–1313. doi: 10.1093/cercor/bhp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen TF, Chen YF, Cheng TW, Hua MS, Liu HM, Chiu MJ. Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp. 2009;30:3826–3836. doi: 10.1002/hbm.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messier C, Gagnon M. Cognitive decline associated with dementia and type 2 diabetes: the interplay of risk factors. Diabetologia. 2009;52:2471–2474. doi: 10.1007/s00125-009-1533-2. [DOI] [PubMed] [Google Scholar]
- 54.Ott A, Stolk RP, vanHarskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 55.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 56.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 57.Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology. 2004;63:228–233. doi: 10.1212/01.wnl.0000129989.28404.9b. [DOI] [PubMed] [Google Scholar]
- 58.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–194. doi: 10.1016/j.brainres.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29:345–351. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
- 61.Messier C, Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast. 2005;12:311–328. doi: 10.1155/NP.2005.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbatecola AM, Paolisso G, Lamponi M, Bandinelli S, Lauretani F, Launer L, Ferrucci L. Insulin resistance and executive dysfunction in older persons. J Am Geriatr Soc. 2004;52:1713–1718. doi: 10.1111/j.1532-5415.2004.52466.x. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr. 2000;72:825–836. doi: 10.1093/ajcn/72.3.825. [DOI] [PubMed] [Google Scholar]
- 64.Vanhanen M, Koivisto K, Karjalainen L, Helkala EL, Laakso M, Soininen H, Riekkinen P., Sr Risk for non-insulin-dependent diabetes in the normoglycaemic elderly is associated with impaired cognitive function. Neuroreport. 1997;8:1527–1530. doi: 10.1097/00001756-199704140-00041. [DOI] [PubMed] [Google Scholar]
- 65.Campbell JJ, 3rd, Coffey CE. Neuropsychiatric significance of subcortical hyperintensity. J Neuropsychiatry Clin Neurosci. 2001;13:261–288. doi: 10.1176/jnp.13.2.261. [DOI] [PubMed] [Google Scholar]
- 66.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 67.Stopa EG, Butala P, Salloway S, Johanson CE, Gonzalez L, Tavares R, Hovanesian V, Hulette CM, Vitek MP, Cohen RA. Cerebral cortical arteriolar angiopathy, vascular beta-amyloid, smooth muscle actin, Braak stage, and APOE genotype. Stroke. 2008;39:814–821. doi: 10.1161/STROKEAHA.107.493429. [DOI] [PubMed] [Google Scholar]
- 68.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, Small SA, Stern Y, Wisniewski HM, Mehta PD. Plasma amyloid beta-peptide 1–42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46:412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 70.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 71.Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Elevation of plasma beta-amyloid level by glucose loading in Alzheimer mouse models. Biochem Biophys Res Commun. 2009;385:193–197. doi: 10.1016/j.bbrc.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 72.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, Hwang DY, Cho JY. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–539. [PubMed] [Google Scholar]