Abstract
Obesity and diabetes are frequently associated with cardiovascular disease. When a normal heart is subjected to brief/sublethal repetitive ischemia and reperfusion (I/R), adaptive responses are activated to preserve cardiac structure and function. These responses include but are not limited to alterations in cardiac metabolism, reduced calcium responsiveness, and induction of antioxidant enzymes. In a model of ischemic cardiomyopathy inducible by brief repetitive I/R, we hypothesized that dysregulation of these adaptive responses in diet-induced obese (DIO) mice would contribute to enhanced myocardial injury. DIO C57BL/6J mice were subjected to 15 min of daily repetitive I/R while under short-acting anesthesia, a protocol that results in the development of fibrotic cardiomyopathy. Cardiac lipids and candidate gene expression were analyzed at 3 days, and histology at 5 days of repetitive I/R. Total free fatty acids (FFAs) in the cardiac extracts of DIO mice were significantly elevated, reflecting primarily the dietary fatty acid composition. Compared with lean controls, cardiac fatty acid oxidation capacity of DIO mice was significantly higher, concurrent with increased expression of fatty acid metabolism gene transcripts. Following 15 min of daily repetitive I/R for 3 or 5 days, DIO mice exhibited increased susceptibility to I/R and, in contrast to lean mice, developed microinfarction, which was associated with an exaggerated inflammatory response. Repetitive I/R in DIO mice was associated with more profound significant downregulation of fatty acid metabolism gene transcripts and elevated FFAs and triglycerides. Maladaptive metabolic changes of fatty acid metabolism contribute to enhanced myocardial injury in diet-induced obesity.
Keywords: fatty acids, hibernating, myocardium, metabolism, reperfusion
Introduction
Obesity is frequently accompanied by insulin resistance and is associated with metabolic abnormalities, namely hyperglycemia, increased plasma free fatty acid (FFA) concentrations, and ectopic accumulation of lipid metabolites in nonadipose tissues such as skeletal muscle, liver, and heart. There is growing evidence that excessive lipid accumulation in cardiomyocytes is toxic and contributes significantly to cardiac disorders commonly associated with obesity and/or diabetes (1).
Fatty acids (FAs) are the preferred substrate for ATP generation in a normal healthy heart (2), and the rate of FA uptake and oxidation is regulated at multiple levels. The adult heart has the metabolic capability to switch substrate preferences in various pathological conditions. When the heart is stressed either physiologically or pathologically, a series of adaptive responses are normally activated; metabolic remodeling is one of the foremost. In fact, we have proposed that metabolic remodeling precedes, triggers, and sustains subsequent functional and structural remodeling (3). A significant characteristic of the maladapted heart is loss of this metabolic flexibility. Increased reliance on any one metabolic pathway, along with reduced capacity for substrate switching, has been associated with accumulation of lipid species in the cardiomyocyte. For example, in diabetes, the heart almost exclusively relies on FA oxidation (FAO) (4, 5), whereas in hypoxic conditions (6) and hypertrophy (7), glucose utilization increases. Both conditions are associated with lipid accumulation within cardiomyocytes. Surplus accumulation of lipid species in cardiomyocytes has been shown to be associated with tissue dysfunction in genetic and transgenic animal models of obesity and diabetes (8, 9). Our recent observation in heart failure patients with nonischemic disease suggests that intramyocardial lipid deposition is a feature in diabetic patients with obesity (10). These findings tie in with the long suggested argument that development and progression of heart failure is related to abnormalities in myocardial substrate metabolism.
We have previously described a murine model of ischemic cardiomyopathy induced by brief (15 min) daily repetitive ischemia and reperfusion (I/R), which does not result in myocardial necrosis but is associated with development of interstitial fibrosis and ventricular dysfunction (11). Both ventricular dysfunction and cardiac fibrosis are reversible upon discontinuation of the ischemic protocol. The histological findings in the mouse closely resembled the pathology of human reversible ischemic cardiomyopathy that is associated with myocardial hibernation (11, 12). Induction of reactive oxygen species (ROS) is a critical early event that regulates processes such as the expression of the chemokine MCP-1 and subsequent inflammatory response, fibrosis, and ventricular dysfunction (11). In addition, ROS regulate mRNA expression of antioxidant enzymes, FA metabolism, and myosin isoform genes (13). Our previous findings in this model suggest that downregulation of peroxisome proliferator activated receptor–α (PPAR-α) expression and FAO are elements of an adaptive response that prevent lipotoxicity in repetitive I/R despite the fact that occlusion occurs only 15 min/day and is not accompanied by infarction (11, 13). Although it is clear that diet-induced obesity is associated with cardiac lipotoxicity, the role of accumulated lipid metabolites and metabolic adaptation in repetitive I/R injury has not been described. Obesity induced by a “Western” high-fat diet in C57BL/6J mice is associated with cardiac metabolic changes very similar to the diabetic heart, namely, increased expression of FA metabolism transcripts, increased capacity for FAO, and ectopic lipid accumulation. We hypothesized that dysregulation of adaptive responses in diet-induced obese (DIO) mice would enhance susceptibility to brief repetitive I/R.
Research Methods and Procedures
Animal Care
All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” (NIH publication 85–23, revised 1985) and the protocol was approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. C57BL/6J mice (The Jackson Laboratory, ME) were bred in-house. Mice (3–5 per cage) were allowed ad libitum access to water and a standard chow diet containing ~13.5% kcal from fat (Picolab Rodent Diet 5010, Purina Mills, Inc, St. Louis, MO) after weaning until 6–8 weeks of age. Obesity was induced by allowing ad libitum access to a high-fat diet containing ~42% kcal from fat (Dyet #112734, Research Diets, Inc, New Brunswick, NJ) starting at age 6–8 weeks for 24 weeks. The lean controls continued to feed on chow diet until the time of experiment.
Cardiac Fatty Acid Determination by Gas Chromatography–Mass Spectrometry
Methylation of plasma nonesterified FAs were analyzed implementing a method by Lepage et al (14). Briefly, an internal standard of heptadecanoic acid (17:0) dissolved in methanol-acetyl chloride 50:1 (v/v) and 150 μl of tissue extract was methylated at 24–29°C for 45 min on a stir plate. The methylation reaction was stopped with 6% K2CO3 solution and lipids were extracted with hexane. Following evaporation, the sample was dissolved in 100 μl of decane and transferred to a small 2-ml vial with a 250-μl insert. Vials were placed in a Hewlett-Packard 7683 automatic injector. FAs were chromatographed as methyl esters on a 30-m fused silica column with an internal diameter of 0.25 mm. The AT-255 column was wall-coated with 0.25 μm (50% cyanopropylphenyl)-dimethylpolysiloxan bonded phase (Alltech, Deerfield, IL). Analysis was performed on a Hewlett-Packard 6890GC coupled to a 5973 Mass Selective Detector. The machine was operated in the electron impact mode (70 eV) with full scan monitoring. Helium was used as carrier gas (1.0 ml/min) and was set to splitless mode. After an initial isothermal period of 2 min at 70°C, the temperature was programmed to 180°C, rising by 20°C/min, then a subsequent increase to 220°C at a rate of 3.0°C/min and maintained for 16 min. Injector and detector temperatures were set to 250°C, respectively. The gas chromatograph was calibrated using standard mixtures of different short- and long-chain FAs (Sigma, St. Louis, MO). For quantification, the FA calibration curves measuring peak area versus that of internal standard were used. Peak identification was confirmed by relative retention time and mass spectral comparison with authentic standards, as well as with the NIST standard reference database number 69.
Fatty Acid Oxidation Studies in vitro
The FAO studies were performed in heart homogenates from lean and obese mice by a protocol described by Sekiguchi et al (15) using [1-14C] palmitic acid. The FA β-oxidation capacity was expressed as cpm/mg heart tissue.
Lipid Extraction and Triglyceride Determination
Hearts were excised and processed for lipid extraction as described in detail by Thakker et al (16). Lipids were extracted with chloroform, methanol, and 0.9% sodium chloride according to the method of Folch (17). Triglycerides were measured with a commercially available kit (Sigma Diagnostics Inc.) using triolein as the standard.
Brief Repetitive Ischemia–Reperfusion
Lean and DIO mice were instrumented using a closed-chest model of ischemia and reperfusion as described by Dewald et al (11). Briefly, mice were anesthetized with sodium pentobarbital (10 μl/gm body weight, Nembutal, Abbott Laboratories, North Chicago, IL). Following a midline skin incision and intubation, a left parasternal thoracotomy was performed between the third and fifth rib. An 8–0 Prolene R (Ethicon, Sommerville, NJ) suture was placed around the left anterior descending artery, both ends of which were threaded through a 1-mm piece of PE-10 plastic tube (Beckton Dickinson), exteriorized through the thorax wall, and stored subcutaneously. The chest and skin were closed and mice were allowed to recover for 7–9 days. After recovery, mice were anesthetized, the skin was opened, and the ends of the suture were attached to heavy metal picks. Ischemia was initiated by pulling apart the picks until an ST elevation of 1 min or greater was observed on the electrocardiogram. Fifteen minutes of ischemia was followed by reperfusion (resolution of ST elevation). Mice underwent brief (15 min) episodes of repetitive ischemia for 3 or 5 consecutive days. Sham-operated animals underwent the initial surgery and waited for the same period of time as I/R groups. Five hours after the last ischemic episode, mice were anesthetized with an overdose of sodium pentobarbital; the heart was excised, stopped in diastole by rinsing in cardioplegic solution, perfusion fixed, and paraffin embedded for histological studies or immersed in TRIzol reagent for RNA extraction.
Histology and Immunohistochemistry
Paraffin-embedded hearts were sectioned at intervals of 200 μm from base to apex. Sections were stained with hematoxylin and eosin for initial evaluation and identification of the area below the suture. After the initial evaluation, serial sections were stained with picrosirius red to identify collagen fibers (18). The picrosirius-stained slides were scanned using a Zeiss Axioskop microscope, and quantitative evaluation was performed using ImagePro software as described before (19). Percent collagen staining was assessed in the anterior wall after 5 days of repetitive I/R.
Immunohistochemistry with specific anti-mouse antibody was performed to identify the following cell types: neutrophils (rat anti-mouse neutrophil antibody, Serotec, Oxford, UK), and myofibroblasts (monoclonal α–smooth muscle actin antibody, Sigma, St. Louis, MO). Staining was performed using a peroxidase-based technique with the Vectastain Elite kit (Vector laboratories, Burlingame, CA). Sections were counterstained with eosin, and quantitative analysis was performed by counting the density of labeled cells in the anterior wall after 5 days of repetitive I/R. Myofibroblasts were identified as spindle-shaped α–smooth muscle actin–positive cells located outside the vascular media and their density was expressed as cells/mm2.
Gene Expression by Real-Time PCR
The methods for RNA isolation, cDNA synthesis, and quantitive RT-PCR have been described previously (20). The nucleotide sequence for primers and probes for PPAR-α, muscle-type carnitine palmitoyltransferase–1 (mCPT-1), and medium-chain acyl–coenzyme A dehydrogenase (MCAD) have also been published previously (20, 21). Taqman primers and probes for PPAR-β/δ and 18S were obtained from Applied Biosystems. Cyclophilin transcript levels were determined using SYBR Green chemistry using published primer sequences (22). Expression was normalized to either 18S or cyclophilin transcript levels. Internal standards were prepared using T7 RNA polymerase method.
Statistical Analysis
Data are expressed as mean±SEM. Statistical differences between groups were analyzed by the unpaired Student’s t-test. Data that did not follow normal Gaussian distribution were analyzed by a nonparametric Mann–Whitney test. A value of P≤0.05 was considered significant.
Results
C57BL/6J mice fed a “Western” high-fat diet for 24 weeks became obese and developed insulin resistance. Male obese mice were approximately 1.5 times heavier than lean male mice (47.5 gm vs 32.2 gm, P<0.001), while female obese mice were 2-fold heavier than lean female mice (51.4 gm vs 24.4 gm, P<0.001). Diet-induced obesity was associated with significantly elevated plasma insulin levels in female (0.88 ng/ml vs 0.33 ng/ml in lean controls, P<0.05), and male (3.9 ng/ml vs 0.28 ng/ml in lean controls, P<0.01) mice. Similarly, fasting plasma glucose levels were significantly (P<0.05) elevated in male obese mice (168 mg/dl) compared with male lean mice (126.2 mg/dL); in the female group no such difference was noted. As a consequence, HOMA-IR indicated that obese male mice were severely insulin resistant (16). Table 1 summarizes the FA composition of chow and “Western” high-fat diets. The “Western” high-fat diet contained markedly higher saturated FAs, 2 times more unsaturated FAs, and a greater n-3/n-6 ratio than chow diet.
Table 1.
Fatty acid composition of diets
| Fatty acid | Chow diet | High-fat diet |
|---|---|---|
| C4:0 | — | 0.71 |
| C6:0 | 0.01 | 0.42 |
| C8:0 | 0.01 | 0.25 |
| C10:0 | 0.01 | 0.57 |
| C12:0 | 0.01 | 0.63 |
| C14:0 | 0.09 | 2.25 |
| C14:1 (n-9) | 0.01 | 0.34 |
| C16:0 | 0.90 | 5.88 |
| C16:1 (n-9) | 0.13 | 0.53 |
| C18:0 | 0.26 | 2.73 |
| C18:1 (n-9) | 1.10 | 5.63 |
| C18:2 (n-6) | 1.88 | 0.53 |
| C18:3 (n-3) | 0.17 | 0.32 |
| C20:0 | 0.01 | 0.23 |
| C20:4 (n-6) | 0.01 | — |
| 20:1 (n-9) | 0.02 | — |
| 20:2 (n-6) | 0.01 | — |
| 20:5 (n-3) | 0.10 | — |
| 22:6 (n-3) | 0.11 | — |
| 22:5 (n-3) | 0.02 | — |
| saturated | 1.28 | 13.67 |
| unsaturated | 3.55 | 7.33 |
| n-6 | 1.90 | 0.53 |
| n-3 | 0.39 | 0.32 |
| n-3/n-6 ratio | 0.20 | 0.60 |
Values represent weight percent of total fatty acids in dietary lipids.
Cardiac Lipids and Fatty Acid Oxidation
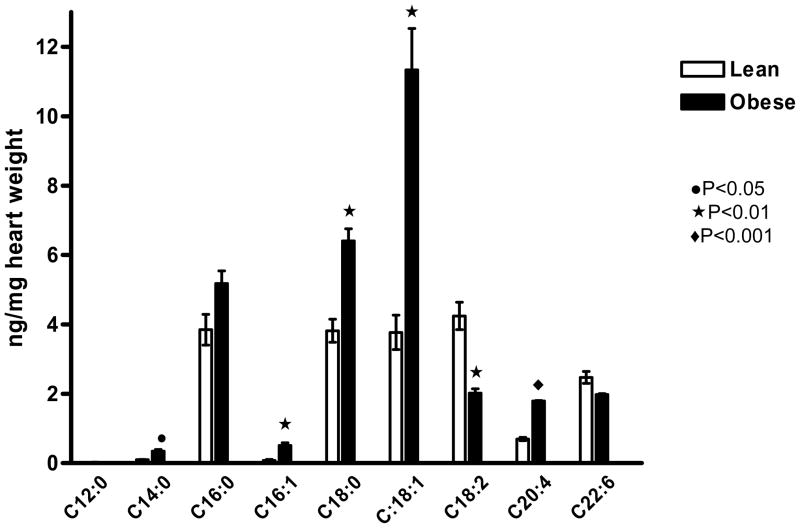
Altered cardiac lipid metabolism is frequently associated with lipid accumulation in the cardiomyocyte. We have previously reported that diet-induced obesity is associated with modest but significant triglyceride deposition in the heart (16). The total FFA concentration in the cardiac homogenates was 67% higher in obese (27.59±2.09 ng) than in lean (16.55±1.67 ng, P<0.01) mice. Analysis of individual FAs by gas chromatography–mass spectrometry (GC-MS) revealed that the concentrations of C14:0, C16:1, C18:0, C18:1, and C20:4 were significantly higher in cardiac extracts of obese than lean mice. However, C18:2 level was higher in the hearts of lean than obese mice (Figure 1). To estimate the cardiac FAO of lean and obese mice, we determined the oxidation of [1-14C] palmitate to its degradation products in cardiac homogenates. Mean FAO of obese mice was ~30% higher than lean mice (74.67±4.31 vs 57.84±3.48 cpm/mg heart weight, P<0.05).
Figure 1.
Long-chain free fatty acids (FFAs) in cardiac extracts of diet-induced obese (DIO) mice were significantly elevated. Levels of C14:0, C16:1, C18:0, C18:1, and C20:4 as measured by GC-MS were significantly higher in obese than lean mice, whereas C18:2 and C22:6 levels were higher in lean compared with obese heart extracts n=4 each).
Cardiac Gene Expression after Repetitive I/R
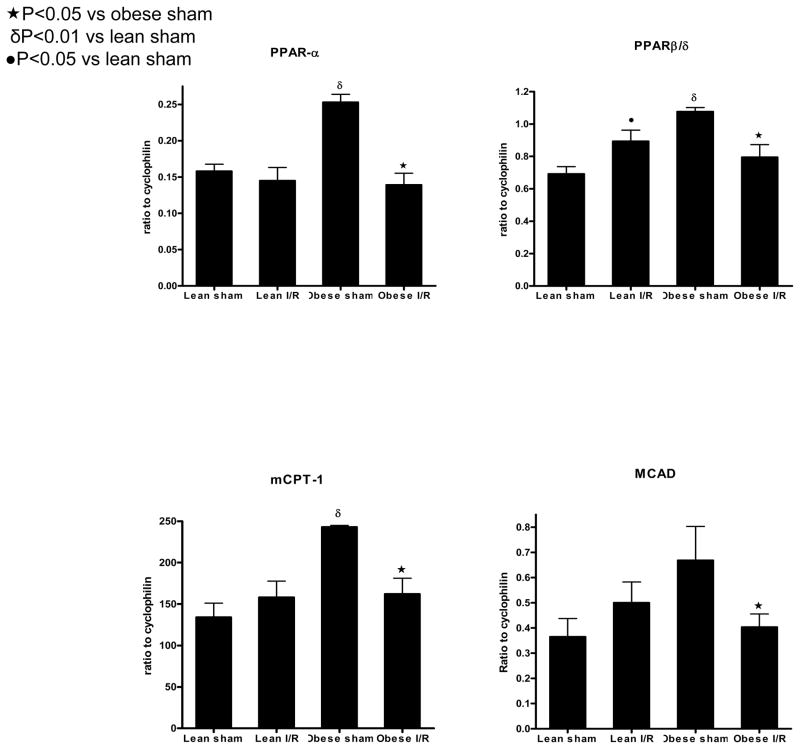
To evaluate the consequences of obesity, insulin resistance, and associated cardiac lipotoxicity in the development of fibrotic cardiomyopathy, gene expression and histopathology were examined in a model of brief repetitive myocardial I/R. Based on prior knowledge that ischemia profoundly affects cardiac FA metabolism, cardiac mRNA expression of PPAR-α and -β/δ, master regulators of FAO were measured after 3 days of repetitive I/R and compared with respective sham-operated animals (Figure 2). The transcript levels of PPAR-α and -β/δ in obese sham-operated mice were upregulated ~1.6-fold compared with lean sham-operated mice. Repetitive I/R protocol significantly decreased both PPAR-α and -β/δ in obese mice compared with obese sham animals. In lean animals subjected to repetitive I/R, expression of PPAR-α was not altered, but we did note a modest and significant increase in the expression of PPAR-β/δ. To determine whether elevated expression of PPAR-α and -β/δ correlated with increased expression of target genes involved in FAO, we examined the mRNA expression of two target genes, mCPT-1 and MCAD, in lean and obese animals after I/R. Obesity was associated with increased expression of mCPT-1, and there was a trend towards higher expression of MCAD (P=0.08). As noted above for PPAR-α and -β/δ, repetitive I/R significantly downregulated expression of mCPT-1 and MCAD in obese but not lean animals (Figure 2).
Figure 2.
Diet-induced obesity alters cardiac fatty acid metabolism gene expression. The transcript levels of PPAR-α, PPAR-β/δ, and their target gene mCPT-1 were significantly upregulated in obese (n=3) compared with lean sham animals (n=4). Transcript levels of MCAD tended (P=0.08) to be higher in obese compared with lean sham-operated animals. Repetitve I/R at 3 days was associated with significant downregulation of PPAR-α, mCPT-1, and MCAD transcripts in obese mice (n=8) but not in lean mice (n=9) compared with respective shams. PPAR-β/δ was significantly upregulated in lean repetitive I/R animals but was downregulated in obese repetitive I/R animals compared with respective shams..
Cardiac Lipids and Plasma FFAs after Repetitive I/R
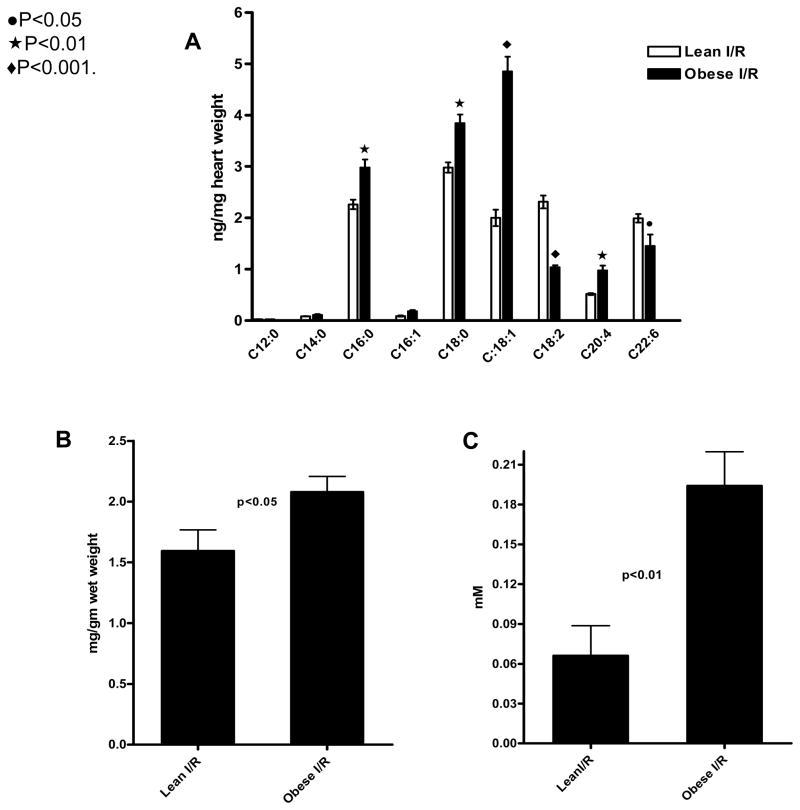
To address whether downregulation of FA metabolism genes in obese mice after repetitive I/R resulted in changes in cardiac lipid profile, we measured lipids after 3 days of repetitive I/R. The total FFAs remained significantly elevated in obese mice after repetitive I/R (P<0.001), with significantly higher levels of C16:0, C18:0, C18:1 and C20:4 in obese than lean mice. C18:2 and C22:6 levels were higher in lean mice (Figure 3a). In addition, cardiac triglyceride concentration was significantly elevated in obese mice after I/R (Figure 3b). Because high levels of FAs are commonly seen after myocardial infarction or following reperfusion after cardiac surgery in humans, we measured the total plasma FFAs after 3 days of repetitive I/R. As shown in Figure 3c, the plasma FFAs at 3 days of repetitive I/R were markedly higher in obese than lean animals.
Figure 3.
Cardiac FFAs, triglycerides, and circulating FFAs after 3 days of repetitive I/R. Cardiac levels of the FFAs C16:1, C18:0, C18:1, and C20:4 (A) and triglycerides (B) were significantly higher in obese than lean mice, whereas C18:2 and C22:6 (A) were higher in obese mice. Circulating FFAs (C) after 3 days of repetitive I/R were markedly higher in obese than lean mice.
Histopathology after Repetitive I/R
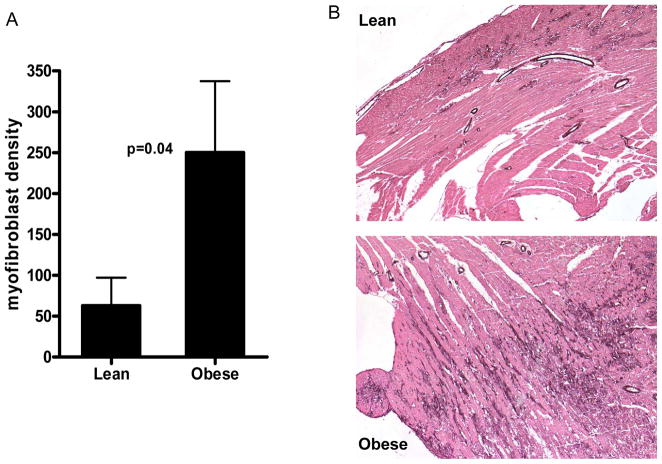
We have reported previously that short episodes of ischemia lead to reversible ischemic cardiomyopathy (11). Figures 4 and 5 summarize the histological evaluation in lean and obese mice after 5 days of repetitive I/R. In lean mice, we noted interstitial widening and marked fibrosis in the anterior wall with no infarction. However, obese mice reacted adversely to these brief episodes of I/R and developed microinfarcts. Microinfarction, as expected, was associated with an increased inflammatory infiltrate. Myofibroblast density and fibrosis were markedly increased in DIO mice compared with lean mice. Neutrophil density was also significantly increased in DIO mice (17.43±6.98 cells/mm2) compared with lean mice (3.67±0.75 cells/mm2; P=0.04).
Figure 4.
Increased myofibroblast density in obese mice after 5 days of repetitive I/R. Cardiac myofibroblasts were identified as spindle-shaped α–smooth muscle actin–positive cells located outside the media of vessels (arrows). α–smooth muscle actin immunohistochemistry also stained vascular smooth muscle cells (arrowheads). Myofibroblast density (A) was markedly higher in obese (n=7) than lean (n=9) mice. A nonparametric Mann–Whitney test was used to compare groups (P=0.04). A representative image of the anterior wall of lean and obese mice depicts myofibroblasts (B).
Figure 5.
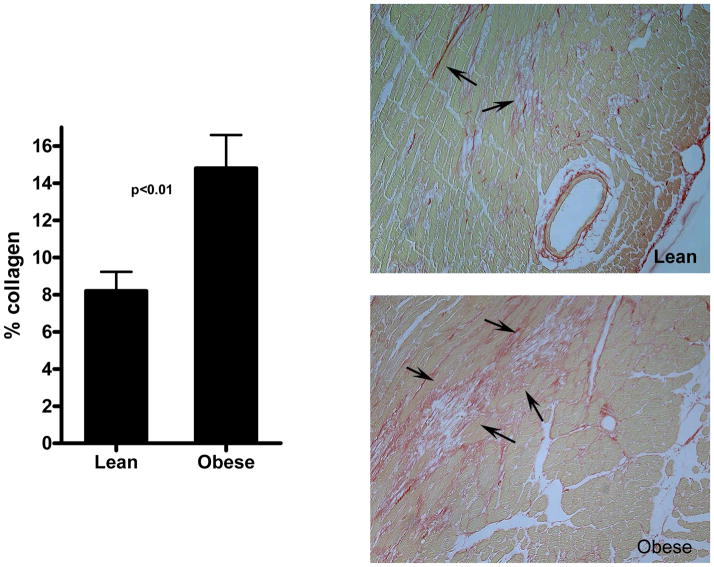
Obese mice had increased fibrosis associated with microinfarction after 5 days of repetitive I/R. Collagen was detected by picrosirius staining and quantitated in the anterior wall of lean (n=9) and obese (n=7) mice. Lean mice exhibited extensive interstitial fibrosis (arrows) in the absence of significant cardiomyocyte loss. In contrast, obese mice had microinfarction accompanied by replacement fibrosis (arrows) and markedly enhanced collagen deposition (p<0.01).
Discussion
In the present study, we investigated whether cardiac lipotoxicity and metabolic maladaptation in obesity induced by a high-fat “Western” diet contribute to increased myocardial susceptibility in a mouse model of ischemic cardiomyopathy, a model exhibiting pathological features similar to human hibernating myocardium (11, 12). The main findings of our study are [1] insulin-resistant diet-induced obese mice display elevated levels of lipid species in the heart along with altered mRNA expression of genes regulating myocardial FAO, [2] obesity is associated with increased myocardial susceptibility to repetitive I/R manifested as microinfarction with enhanced fibrosis, and [3] repetitive I/R in obese mice is associated with early and more profound downregulation of myocardial FAO-regulating genes and elevated lipids in the heart. The mouse model of diet-induced obesity used in this study develops hyperinsulinemia and insulin resistance with significantly elevated levels of FFAs in the plasma compared with lean mice (16). Although blood pressure and cardiac indices are similar in lean and obese mice, diet-induced obesity is associated with development of cardiac hypertrophy. Moreover, cardiac collagen content as measured by picrosirius staining was similar between lean and obese mice (16).
Cardiac Lipotoxicity in DIO Mice
A significant marker indicative of cardiac lipotoxicity is ectopic accumulation of triglyceride within cardiomyocytes (8). In pathological conditions, this is generally thought to result from a mismatch between uptake and utilization of FAs, which, in the cardiomyocyte, is regulated by PPAR-α. It should be noted that triglycerides themselves are considered relatively inert, and toxicity is mainly imparted by surplus long-chain nonesterified FFAs and their derivatives, ceramides and diacylglycerol. In cell culture studies with normal rat β-cells, oleate and palmitate induced apoptosis in a dose-dependent manner, while the cellular triglyceride content was inversely correlated with the percentage of dead cells (23). Similarly, Listerberger et al. have shown in CHO cells that oleic acid supplementation led to triglyceride accumulation and was well tolerated, whereas palmitic acid was poorly incorporated and led to apoptosis (24). Nonetheless, cellular triglycerides do represent a pool that can be potentially hydrolyzed to liberate toxic free fatty acyl moieties. We have demonstrated that diet-induced obesity is associated with ~30% increased cardiac triglyceride accumulation (16). Given that the rate of FA uptake by the heart is primarily determined by levels of FFAs in the plasma (25, 26), and our observation that DIO mice had elevated FFAs in the plasma compared with lean mice, we measured nonesterified FAs in the cardiac extracts of DIO and lean mice. In support of the above paradigm, the concentration of FFAs in heart extracts of DIO mice was 67% higher than those of lean mice. Also, the FFA profile in heart extracts of lean and obese mice largely reflected the FA composition of the diet. In particular, levels of arachidonic acid (n-6), a proinflammatory FA, were significantly higher, whereas levels of DHA, which is known to exert cardioprotective effects (27), were lower in heart extracts of obese mice. Palmitic acid levels, a saturated FA that induces apoptosis in cardiomyocytes (28), were ~7-fold higher in cardiac extracts of obese mice compared with lean mice. From a nutritional perspective, the FA composition of the high-fat diet used in our study has been associated with adverse cardiovascular outcomes in humans.
PPAR-α and -β/δ are the principal regulators of long-chain FA metabolism in the heart and regulate genes involved in virtually every step of cardiac long-chain FA utilization (29). We analyzed cardiac expression of PPAR-α and -β/δ, and found their expression to be significantly upregulated in DIO mice compared with lean mice. Moreover, mRNA expression of mCPT-1 and MCAD, enzymes whose expression is inducible by PPAR-α and -β/δ, were upregulated in DIO mice. We also found that in DIO mice, increased expression of FA metabolism gene transcripts was accompanied by increased capacity for mitochondrial FAO. Despite the increased expression of FA metabolism genes and capacity for metabolism, obesity was associated with triglyceride accumulation in the heart, suggesting that the tightly regulated process of FA uptake and utilization was perturbed in DIO mice.
Myocardial Susceptibility to Repetitive I/R
In the ischemic cardiomyopathy model used in this study, we have shown previously that daily episodes of brief I/R resulted in ROS-dependent upregulation of MCP-1 that was followed by infiltration of phenotypically modified α–smooth muscle actin–positive myofibroblasts and macrophages. Brief repetitive I/R episodes led to reversible interstitial fibrosis and ventricular dysfunction in the absence of myocardial infarction and necrosis (11, 30). . Furthermore, expression of metabolic and myosin isoform genes was altered, again in a ROS-dependent pattern. The pathology characteristic of repetitive I/R was prevented in transgenic mice overexpressing superoxide dismutase (11).. The infarction model, in contrast, is attended by acute chemokine and cytokine induction, a robust inflammatory response, and clearing of dead necrotic tissue that is eventually replaced with a collagen-based scar. . In the present study, the histology in lean mice after 5 days of repetitive I/R was similar to what we reported earlier (11): interstitial fibrosis was associated with infiltrating myofibroblasts and macrophages in the absence of cardiomyocyte death and necrosis. In contrast, after 5 days of repetitive I/R in obese mice, we observed microinfarcts in the anterior wall, suggesting enhanced myocardial susceptibility to brief episodes of I/R. Not surprisingly, microinfarction in obese mice was associated with significantly increased fibrosis in the anterior wall. The density of myofibroblasts, cells critical for progression of fibrosis because of their ability to secrete procollagen, was also significantly increased in the anterior wall of obese compared with lean mice.
Metabolic Maladaptation to Repetitive I/R in DIO Mice
Studies in animal models have shown that the adult heart responds to stress triggers such as pressure overload, hypertension, and myocardial infarction by structural remodeling that is accompanied by a switch to the fetal gene program (31). Most notably, to improve cardiac efficiency, the heart switches from fatty acid metabolism to oxidation of more efficient substrates such as glycogen, lactate, and glucose (32, 33) . In the present study, after 3 days of repetitive I/R, expression of transcripts regulating cardiac FA metabolism was significantly different in lean and obese groups. When studied at 3 days, PPAR-α and its target genes mCPT-1 and MCAD were significantly downregulated in obese animals exposed to repetitive I/R compared with sham animals, whereas in the lean group expression of these genes was not significantly altered. Expression of PPAR-β/δ, an equally important regulator of cardiac FA metabolism, was significantly increased in lean mice subjected to repetitive I/R compared with sham animals. In contrast, PPAR-β/δ expression was downregulated after repetitive I/R in obese mice. We previously demonstrated in a time course study of wild-type lean C57BL/6J mice that expression of PPAR-α and its target genes was significantly altered only at 7 days of repetitive I/R in a ROS-dependent manner (13). Reactivation of PPAR-α in these lean mice was associated with increased cellular injury. Thus, downregulation of PPAR-α in repetitive I/R may be an adaptive mechanism to prevent lipotoxicity in the ischemic myocardium. In the current study, downregulation of PPAR-α and -β/δ and their target genes at 3 days of repetitive I/R indicates that the metabolism switches from FAO much earlier in the obese mice. At the same time, obese mice had significantly elevated plasma FFAs compared with lean mice, likely because they continue to feed on their respective diets. Furthermore, cardiac FFAs and triglycerides were higher in obese compared with lean mice exposed to repetitive I/R. We propose that the adaptive response of downregulating FAO machinery to prevent lipotoxic injury caused by repetitive I/R becomes maladaptive in obese insulin-resistant mice when cardiac supply of FFAs exceeds FAO. Unmetabolized FFAs are capable of inflicting cellular damage through mechanisms such as induction of apoptosis by palmitic acid (34), and activation of cytokines and inflammatory pathways via activation of NF-κB–regulated genes (35). At the cellular level, excess saturated FFAs may be directed to nonoxidative pathways that promote synthesis of ceramide (36), a sphingolipid that can activate JNK, p38, and IκB kinase (37), thereby activating pathways that lead to both inflammation and development of tissue-specific insulin resistance (38).
In summary, we show that obesity induced by a “Western” high-fat diet is associated with cardiac lipid accumulation and alterations in FA metabolism in the heart, indicative of cardiac lipotoxicity. Activation of adaptive mechanisms in response to stress is an inherent feature of the heart and preserves structure and function. Our studies indicate that repetitive I/R–induced adaptive mechanisms are severely impaired in DIO mice and contribute to increased myocardial injury. Because of the experimental design of these studies, we are not able to determine how much of the observed increase in myocardial susceptibility to repetitive ischemia was due to the effects of high–saturated fat “Western” diet versus obesity. However, future studies can target selected interventions on diet and obesity to determine the optimal strategy to reduce myocardial susceptibility to repetitive ischemia.
Acknowledgments
We are thankful to Jennifer Pocius, Roy Hendley, Ugochi Uzoka, and Christa Unsinn for performing the animal surgeries. Editorial assistance from Kerrie Jara is acknowledged. Current affiliation for GDT: Takeda Pharmaceuticals North America, Inc., Deerfield, IL.
Grants
This work was supported by National Institutes of Health (NIH) grants P01 HL-42550 (CMB, MLE and NGF), R01 HL-76246 (NGF), and R01 HL-61483 (HT) and American Diabetes Association grant 1-04-RA-03 (CMB). GDT was supported by NIH training grant T32 HL-007812, and SS was supported by NIH training grant T32 HL-07591.
References
- 1.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–24. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 2.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 3.Taegtmeyer H, Wilson CR, Razeghi P, Sharma S. Metabolic energetics and genetics in the heart. Ann N Y Acad Sci. 2005;1047:208–18. doi: 10.1196/annals.1341.019. [DOI] [PubMed] [Google Scholar]
- 4.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues B, Cam MC, McNeill JH. Myocardial substrate metabolism: implications for diabetic cardiomyopathy. J Mol Cell Cardiol. 1995;27:169–79. doi: 10.1016/s0022-2828(08)80016-8. [DOI] [PubMed] [Google Scholar]
- 6.Razeghi P, Young ME, Abbasi S, Taegtmeyer H. Hypoxia in vivo decreases peroxisome proliferator-activated receptor α-regulated gene expression in rat heart. Biochem Biophys Res Commun. 2001;287:5–10. doi: 10.1006/bbrc.2001.5541. [DOI] [PubMed] [Google Scholar]
- 7.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-α during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–30. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu HC, Kovacs A, Ford DA, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J. 2004;18:1692–700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 11.Dewald O, Frangogiannis NG, Zoerlein M, et al. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100:2700–5. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangogiannis NG. The pathological basis of myocardial hibernation. Histol Histopathol. 2003;18:647–55. doi: 10.14670/HH-18.647. [DOI] [PubMed] [Google Scholar]
- 13.Dewald O, Sharma S, Adrogue J, et al. Downregulation of peroxisome proliferator-activated receptor-α gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation. 2005;112:407–15. doi: 10.1161/CIRCULATIONAHA.105.536318. [DOI] [PubMed] [Google Scholar]
- 14.Lepage G, Roy CC. Specific methylation of plasma nonesterified fatty acids in a one-step reaction. J Lipid Res. 1988;29:227–35. [PubMed] [Google Scholar]
- 15.Sekiguchi K, Tian Q, Ishiyama M, et al. Inhibition of PPAR-α activity in mice with cardiac-restricted expression of tumor necrosis factor: potential role of TGF-β/Smad3. Am J Physiol Heart Circ Physiol. 2007;292:H1443–51. doi: 10.1152/ajpheart.01056.2006. [DOI] [PubMed] [Google Scholar]
- 16.Thakker GD, Frangogiannis NG, Bujak M, et al. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H2504–14. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Frangogiannis NG, Perrard JL, Mendoza LH, et al. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687–98. doi: 10.1161/01.cir.98.7.687. [DOI] [PubMed] [Google Scholar]
- 19.Frangogiannis NG, Ren G, Dewald O, et al. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–42. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 20.Depre C, Shipley GL, Chen W, et al. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–75. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 21.Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 2001;89:1199–208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 22.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50:1771–7. doi: 10.2337/diabetes.50.8.1771. [DOI] [PubMed] [Google Scholar]
- 24.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–82. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med. 1954;16:504–15. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- 26.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta. 1994;1213:263–76. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 27.Nasa Y, Hayashi M, Sasaki H, Hayashi J, Takeo S. Long-term supplementation with eicosapentaenoic acid salvages cardiomyocytes from hypoxia/reoxygenation-induced injury in rats fed with fish-oil-deprived diet. Jpn J Pharmacol. 1998;77:137–46. doi: 10.1254/jjp.77.137. [DOI] [PubMed] [Google Scholar]
- 28.de Vries JE, Vork MM, Roemen TH, et al. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res. 1997;38:1384–94. [PubMed] [Google Scholar]
- 29.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med. 2000;10:238–45. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 30.Dewald O, Frangogiannis NG, Zoerlein MP, et al. A murine model of ischemic cardiomyopathy induced by repetitive ischemia and reperfusion. Thorac Cardiovasc Surg. 2004;52:305–11. doi: 10.1055/s-2004-821153. [DOI] [PubMed] [Google Scholar]
- 31.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–43. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 32.Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1345–51. doi: 10.1152/ajpheart.2000.278.4.H1345. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–9. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 34.Kong JY, Rabkin SW. Palmitate-induced cardiac apoptosis is mediated through CPT-1 but not influenced by glucose and insulin. Am J Physiol Heart Circ Physiol. 2002;282:H717–25. doi: 10.1152/ajpheart.00257.2001. [DOI] [PubMed] [Google Scholar]
- 35.Ajuwon KM, Spurlock ME. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNFα expression in 3T3-L1 adipocytes. J Nutr. 2005;135:1841–6. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- 36.Unger RH. Hyperleptinemia: protecting the heart from lipid overload. Hypertension. 2005;45:1031–4. doi: 10.1161/01.HYP.0000165683.09053.02. [DOI] [PubMed] [Google Scholar]
- 37.Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res. 2003;47:383–92. doi: 10.1016/s1043-6618(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 38.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]