Abstract
Background
Currently, scant data exists regarding ECMO support in neonates with herpes virus infection.
Objectives
We investigated outcomes among neonates with herpes virus infection reported to the Extracorporeal Life Support Organization (ELSO) registry and analyzed factors associated with death prior to hospital discharge with this virus.
Design
Retrospective analysis of ELSO registry dataset from 1985–2005.
Setting
114 ECMO centers contributing data to the ELSO registry.
Patients
Patients 0–31 days of age with herpes simplex virus infection supported with ECMO and reported to the ELSO registry.
Interventions
None
Methods
Clinical characteristics, outcomes, and factors associated with death prior to hospital discharge were investigated for patients in the virus group. Kaplan-Meier estimates of survival to hospital discharge according to virus type were investigated.
Measurements and Main Results
Newborns with HSV infection requiring ECMO support demonstrated much lower hospital survival rates (25%). Clinical presentation with septicemia/ shock was significantly associated with mortality for the HSV group on multivariate analysis. There was no difference in HSV mortality when comparing two era’s (≥ 2000 versus < 2000).
Conclusions
In this cohort of neonatal patients with overwhelming infections due to HSV who were supported with ECMO, survival was dismal. Patients with disseminated HSV infection presenting with septicemia/ shock are unlikely to survive, even with aggressive extracorporeal support.
Keywords: ECMO, neonates, HSV, CMV, outcomes
Introduction
Herpes simplex virus (HSV) is an enveloped, double-stranded, DNA virus which belong to the herpes virus family and causes significant morbidity and mortality in the neonatal period (1,2). HSV causes a wide spectrum of illness severity, ranging from an asymptomatic infection to organ-specific complications, to fulminant multi-system disease. The infection may be transmitted from the infected mother to the neonate either via intrauterine, perinatal or postnatal routes of transmission. The majority (85%) of neonatal HSV infections are acquired during delivery, although in-utero (5%) and postnatal (10%) infections do occur (1–3). Neonatal disseminated HSV infection is a devastating disease causing rapidly progressive multiple organ failure and death, often despite aggressive antiviral treatment and intensive care support.
Severe hemodynamic and respiratory instability associated primarily with the disseminated forms of HSV infection frequently requires the neonate to be cared for in the intensive care unit. A smaller sub-set with worsening cardio-respiratory compromise may need to be supported by extracorporeal techniques. Over a decade ago, Meyer, et al published a review of the existing experience with extracorporeal respiratory support for viral pneumonia in neonatal and pediatric patients reported to the ELSO Registry from 1988 – 1994, representing the early years of widespread extracorporeal membrane oxygenation (ECMO) use (5). In that study, 127 patients supported with ECMO for a documented viral infection were analyzed for survival, complications, and the impact of virus type on survival. The study reported on 13 patients with HSV infection. Meyer reported a 31% survival for herpes simplex virus. However, the impact of variables such as patient co-morbidities was not systematically assessed.
No recent study has sought to systematically evaluate the impact of patient co-morbidities and ECMO-related complications on the outcome of children with documented infection due to HSV supported with ECMO. The purpose of the present descriptive study was to analyze all neonates with HSV infection requiring ECMO reported to the ELSO Registry from 1985 – 2005 for such factors which may be associated with mortality prior to hospital discharge.
Material Methods
This study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences. Informed consent was not required for this analysis of retrospective registry data.
Subjects
The registry of the Extracorporeal Life Support Organization (ELSO) was queried for all patients in the neonatal period (0–31 days) with documented viral infection due to HSV from 1985 to 2005. The data source for the present study came from the ELSO registry (6–8). The ELSO registry was founded in 1982 and collects patient data from member institutions regarding extracorporeal support of cardio-respiratory functions using ECMO in children and adults. Data are collected and sent from the contributing centers with a standardized data sheet containing patient demographics, diagnosis/ procedure information, ECMO technique, complications during ECMO, and patient outcomes. Currently, 110 centers (including 14 outside of N. America) contribute data to the registry. Each individual member institution approves data reporting through its local institutional review board.
This database was queried using ICD-9 diagnostic codes and a set of specific codes used by the ELSO registry for herpes simplex virus to identify the study cohort. Subjects who received ECMO support more than once or had more than one documented type of viral infection concomitantly and those more than one month of age were excluded from the study.
Outcomes
Death prior to hospital discharge was our defined primary outcome variable.
Variables
The following pre-ECMO variables (demographics, virus type, secondary diagnoses) and post-ECMO variables (complications on ECMO) were identified for patients with HSV infection. Secondary diagnoses considered for the study included the following: cardiac disease (congenital or acquired); septicemia/ shock; persistent pulmonary hypertension; congenital diaphragmatic hernia, and meconium aspiration syndrome. ECMO-related complications were defined as: central nervous system (CNS) hemorrhage/ infarction; gastrointestinal hemorrhage; disseminated intravascular coagulopathy; and need for renal replacement therapy.
Statistical Analysis
We summarized the independent variables, which were all defined as categorical, using frequencies and percentages. Both univariate and multivariate logistic regression analyses were used to estimate odds ratios for the association between the independent variables and the clinical outcome endpoints. Univariate analyses of associations between pre-ECMO variables (demographics, virus type, secondary diagnoses) and ECMO complications were carried using the Wald test.
Pre-ECLS variable’s with p-values < 0.2 in the univariate analyses were included in the multivariate logistic regression analysis. Serum bicarbonate, PaCO2 were not included in the model due to collinearity with pH, and only pH was included for the final model and analysis. When the final model was identified, factors that had not been retained in the model were re-evaluated for inclusion. The model’s goodness of- fit was evaluated using the Hosmer-Lemeshow chi square test and the discrimination of the model was assessed using the area under the receiver operating curve (ROC). Actuarial survival curves comparing death prior to hospital discharge in the HSV group were computed with the Kaplan-Meier analysis. HSV infection survival was compared to another comparative representative sample of patients from the ELSO Registry who had neonatal CMV infection. The comparison group is a heterogenous sample. A p value < 0.05 was considered to be statistically significant. All statistical calculations were performed using STATA version 10 (College Station, TX). Kaplan-Meier survival analysis was performed using the Medcalc version 10 (Mariakerke, Belgium) statistical package.
Results
Between 1985 and 2005, 40 patients with HSV infection (HSV group) were identified in the ELSO registry as having received ECMO support. Table 1 details the demographic variables, pre-ECMO gas exchange (in the 6 hours prior to going on ECMO) parameters, and complications on ECMO among survivors and non-survivors for the HSV virus group. Majority of the ECMO supported subjects were from North America, which reflects the composition of the contributing centers to the ELSO database. The median age at the time of hospitalization for the HSV group was 5 days. The median duration of ECMO support for HSV group was 163 hours.
Table 1.
Clinical and demographic variables for patients with HSV infections supported on ECMO
| Variables | HSV (n=40) |
|||
|---|---|---|---|---|
| N | Alive (n=10) |
Dead (n=30) |
||
| Demographics | ||||
| Admission age (days)* | 40 | 5(2–30) | 5(3–12) | |
| Race White | 40 | 6 | 20 | |
| Gender (Female) | 40 | 7 | 18 | |
| Veno-Arterial ECMO mode | 40 | 6 | 26 | |
| Weight (kg)* | 40 | 3(2.9–3.8) | 3(2–5) | |
| Gestational Age (wks)* | 37 | 39(34–42) | 38(34–42) | |
| Era (<2000/> = 2000) | 40 | 4/6 | 15/15 | |
| ECMO in North America | 40 | 10 | 27 | |
| Admit to time on ECMO (Hrs) * | 39 | 17(6–134) | 14(1–233) | |
| Hours of ECMO* | 40 | 207(89–482) | 117(4–463) | |
| Secondary Diagnosis | ||||
| Septicemia/ shock | 40 | 5 | 21 | |
| Pulmonary hypertension | 40 | 5 | 13 | |
| Pre-ECLS Variables | ||||
| pH | 38 | 7.38(7.15–7.61) | 7.22(7–7.46) | |
| PaCO2 (mm Hg) | 38 | 37(28–69) | 47(23–85) | |
| PaO2 (mm Hg) | 37 | 45(33–55) | 39(16–95) | |
| PaO2/FiO2 | 33 | 45(35–72) | 40(16–216) | |
| SaO2 (%) | 38 | 82(57–100) | 67(14–96) | |
| HCO3 (mEq/L) | 38 | 24(20–33) | 20(10–38) | |
| Complications on ECMO | ||||
| DIC | 40 | 0 | 11 | |
| CNS infarct/ hemorrhage | 40 | 2 | 10 | |
| Seizures | 40 | 1 | 3 | |
| Renal replacement therapy | 40 | 6 | 9 | |
Median/ (range) where applicable; N= total sample size; DIC: disseminated intravascular coagulation; CDH-congenital Diaphragmatic hernia; MAS-meconium aspiration syndrome;
The overall survival to hospital discharge for the HSV group was 25%. Prior to the year 2000, 79% of the HSV group died prior to hospital discharge. No significant differences in mortality were observed in the HSV group when comparing the era before and after the year 2000. Among the HSV infected cohort 65% of subjects presented with septicemia/shock. While receiving ECMO support, patients in the HSV group required renal replacement therapy.
Table 2 depicts the risk factors associated with death prior to hospital discharge in the HSV group on univariate analysis. Those who died prior to hospital discharge were more likely to have a secondary diagnosis of septicemia/ shock (p = 0.044) and have a lower blood pH (p=0.014), serum bicarbonate (p =0.035) levels in the 6 hour prior to going on ECMO.
Table 2.
Univariate analysis comparing survivors and non-survivors with HSV infections supported on ECMO
| Variables | Odds Ratio |
P value |
95% confidence interval |
|
|---|---|---|---|---|
| Age (days) | 1.070 | 0.403 | 0.916 | 1.244 |
| Gestational Age | 1.190 | 0.305 | 0.851 | 1.673 |
| Female Gender | 0.629 | 0.573 | 0.138 | 2.990 |
| Weight (kg) | 1.249 | 0.696 | 0.409 | 3.810 |
| White race | 0.750 | 0.702 | 0.172 | 3.280 |
| ≥ 2000 Era | 0.667 | 0.585 | 0.156 | 2.852 |
| VA ECMO | 4.333 | 0.081 | 0.836 | 22.47 |
| Septicemia/ shock | 5.000 | 0.044 | 1.042 | 23.99 |
| Pulm hypertension | 0.765 | 0.714 | 0.182 | 3.210 |
| Dissem. Intravascular Coag. | 5.211 | 0.141 | 0.580 | 46.81 |
| pH | 0.401 | 0.014 | 0.194 | 0.830 |
| PaO2/ FiO2 ratio | 0.999 | 0.916 | 0.976 | 1.022 |
| PaCO2 | 0.963 | 0.183 | 0.982 | 1.098 |
| HCO3 | 0.853 | 0.035 | 0.735 | 0.989 |
| SaO2 | 0.941 | 0.062 | 0.883 | 1.003 |
| Renal replacement therapy | 0.286 | 0.099 | 0.064 | 1.264 |
On stepwise multivariate logistic regression analyses (Table 3) of variables impacting death prior to hospital discharge for the HSV group only a secondary diagnosis of septicemia/ shock (OR-10.2; 95% CI 1.069–96.33; p = 0.044) was associated with death prior to hospital discharge. For this model, the Hosmer and Lemeshow goodness of fit test demonstrated a X2 p value of 0.374 which suggests an adequate fit for the model. The area under the receiver operating curve was 0.845.
Table 3.
Multivariate analysis comparing survivors and non-survivors with HSV infections supported on ECMO
| Variables | Odds Ratio | P value | 95% Conf. Interval | |
|---|---|---|---|---|
| Septicemia/ shock | 10.2 | 0.044 | 1.069 | 96.33 |
| pH | 0.41 | 0.061 | 0.158 | 1.041 |
| VA ECMO | 5.47 | 0.067 | 0.848 | 96.11 |
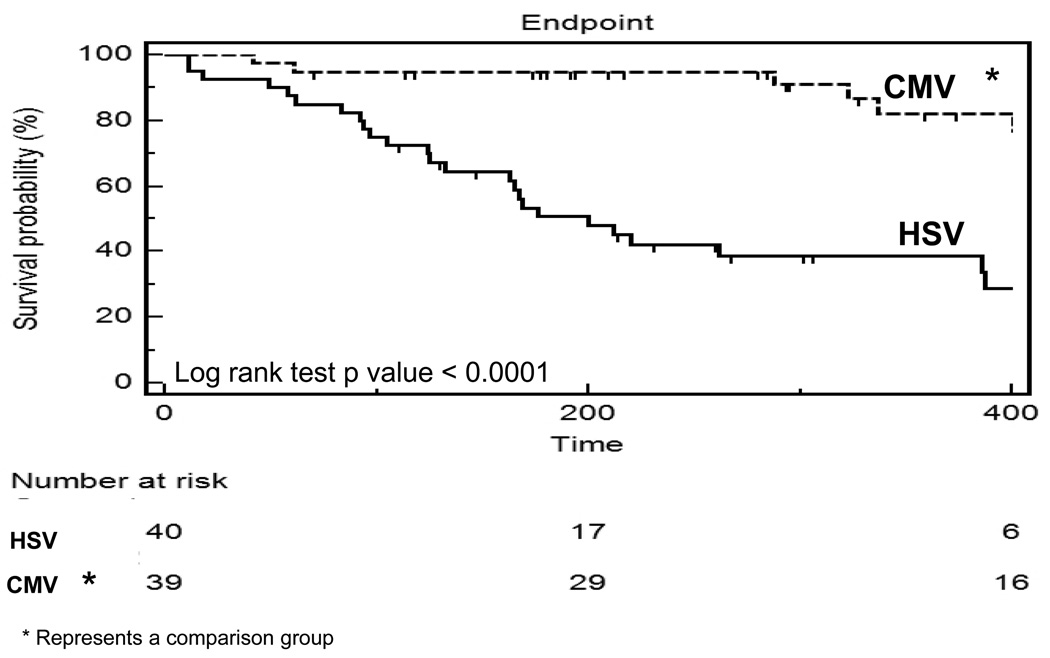
Figure 1 compares survival to hospital discharge between the HSV group and a representative group of neonates with CMV infection by the Kaplan-Meier survival curve in order to highlight the significantly increased mortality with HSV infection. This comparative group includes CMV positive neonates, but where the indication for ECMO support was most likely due to other primary diagnoses such as congenital diaphragmatic hernia. There was a significant difference between the HSV and comparison group (p =<0.0001). At 200 hours, greater than 90% of the comparison group survived while approximately 50% survived in the HSV group. Overall, infection with HSV requiring ECMO support conferred increased risk of death compared to the comparison group.
Figure 1.
Kaplan- Meier survival analysis highlighting survival from HSV virus type compared to a control group with neonatal CMV infection.
Discussion
This study is the largest descriptive cohort to date of neonates with HSV infection supported with ECMO. Critically ill newborns with HSV infection of such severity that extracorporeal support was utilized survived at a markedly lower rate (25%) than those with the comparative group with CMV infection (78%) (p = 0.0003).
These findings do not differ substantially from the survival rates reported for 13 patients with HSV by Meyer, et al in 1994 (5). There was no significant difference in HSV mortality based on the two era (≥ 2000 versus < 2000). Even though the dataset is limited in information related to specific therapy in this study we can speculate that the uniform mortality related to different era suggest the more fulminant nature of the disseminated HSV infection. It is possible that despite specific antiviral therapy for HSV they tend to be sicker prior to going on ECMO and significantly more ended up requiring veno-arterial ECMO support.
Presentation with septicemia/ shock was significantly associated with HSV infection mortality in multivariate analysis. Whitley and colleagues (1), in a cohort of 202 neonates with HSV infection, showed a significantly higher mortality rate in the 46 neonates with disseminated infection (57%) than in the 71 with encephalitis (15%). In addition, the risk of death was increased in neonates who were in or near coma at entry (relative risk, 5.2), had disseminated intravascular coagulopathy (relative risk, 3.8), or were premature (relative risk, 3.7). In babies with disseminated disease, HSV pneumonitis was also associated with greater mortality (relative risk, 3.6). However, in contrast to our study, none of the patients in that cohort were supported on ECMO.
This study of the ELSO Registry demonstrates 75% mortality among neonates with disseminated HSV requiring ECMO support. Our own institutional experience indicates a 100% mortality with disseminated HSV supported on ECMO. We believe that these results should steer the reader towards exercising great caution when considering ECMO for a patient with disseminated HSV. Septicemia/ shock, liver failure, and severe coagulopathy are such intrinsic parts of the disseminated HSV disease process that we in our institution consider ECMO support almost futile in this population. Additionally, due to the viral infection itself and to its complications such as shock and DIC, the few survivors are at high risk of substantial neurologic injury. The current study was unable to assess the neurologic status of survivors due to the limitations of the registry data.
Our study has certain additional limitations. As a retrospective study of registry data, it is subject to considerable bias, both in reporting and in selection. Perhaps most importantly, this study is without a means of assessing the accuracy of the information provided by each ECMO center to the ELSO registry. However, the Extracorporeal Life Support Organization has maintained an international registry since the 1980’s with great success, and the registry has been used to help answer a number of research questions. No other mechanism currently exists to glean information regarding such a large number of patients suffering from what is essentially an unusual, but critical, clinical problem. Additionally the ELSO registry is limited in the dataset collected on the reported cases. In the CMV study cohort (comparative group) which has been used for comparing HSV infection survival, it is highly likely that in cases with additional diagnoses, such as congenital diaphragmatic hernia (CDH), the other diagnoses are most likely the reason for ECMO support. Unfortunately, the ELSO Registry database is limited in its ability to distinguish this point. The ELSO Registry does not collect complete blood count parameters or any assessment of neurologic function in survivors which limits us from providing this data in this study.
Conclusions
In this cohort of neonatal patients with overwhelming infection with HSV who were supported with ECMO, survival outcome was dismal. Patients with disseminated HSV infection presenting with septicemia/ shock are unlikely to survive, even with aggressive extracorporeal support. As a result of high mortality even despite maximal cardio-pulmonary support, we recommend exercising great caution when considering ECMO for a patient with disseminated HSV.
Acknowledgments
Research support funding:
In part by NICHD grant support -5 U10 HD050009
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Name of Institution Where Work Performed:
Arkansas Children’s Hospital; College of Medicine-University of Arkansas for Medical Sciences, Little Rock, AR
Data obtained from the ELSO Registry, Ann Arbor, MI
The authors have no potential conflicts of interest to disclose.
References
- 1.Whitley R, Arvin A, Prober C, Corey L, Burchett S, Plotkin S, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991;324(7):450–454. doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- 2.Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Semin Pediatr Infect Dis. 2005 Oct;16(4):271–281. doi: 10.1053/j.spid.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez KM, Falkovitz Halpern MS, Maldonado Y, Arvin AM. The epidemiology of neonatal herpes simplex virus infections in California from 1985 to 1995. J Infect Dis. 1999;180(1):199–202. doi: 10.1086/314848. [DOI] [PubMed] [Google Scholar]
- 4.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Frenkel LM, Gruber WC, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223–229. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 5.Meyer TA, Warner BW. Extracorporeal life support for the treatment of viral pneumonia: collective experience from the ELSO registry. J Pediatr Surg. 1997;32:232–236. doi: 10.1016/s0022-3468(97)90185-8. [DOI] [PubMed] [Google Scholar]
- 6.ECLS Registry Report - International Summary. Ann Arbor, MI: Extracorporeal Life Support Organization; 2008. Jul, [Google Scholar]
- 7.Dalton HJ, Rycus PT, Conrad SA. Update on extracorporeal life support 2004. Semin Perinatol. 2005;29(1):24–33. doi: 10.1053/j.semperi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett RH, Roloff DW, Custer JR, et al. Extracorporeal life support: The University of Michigan experience. JAMA. 2000;283(7):904–908. [PubMed] [Google Scholar]