Abstract
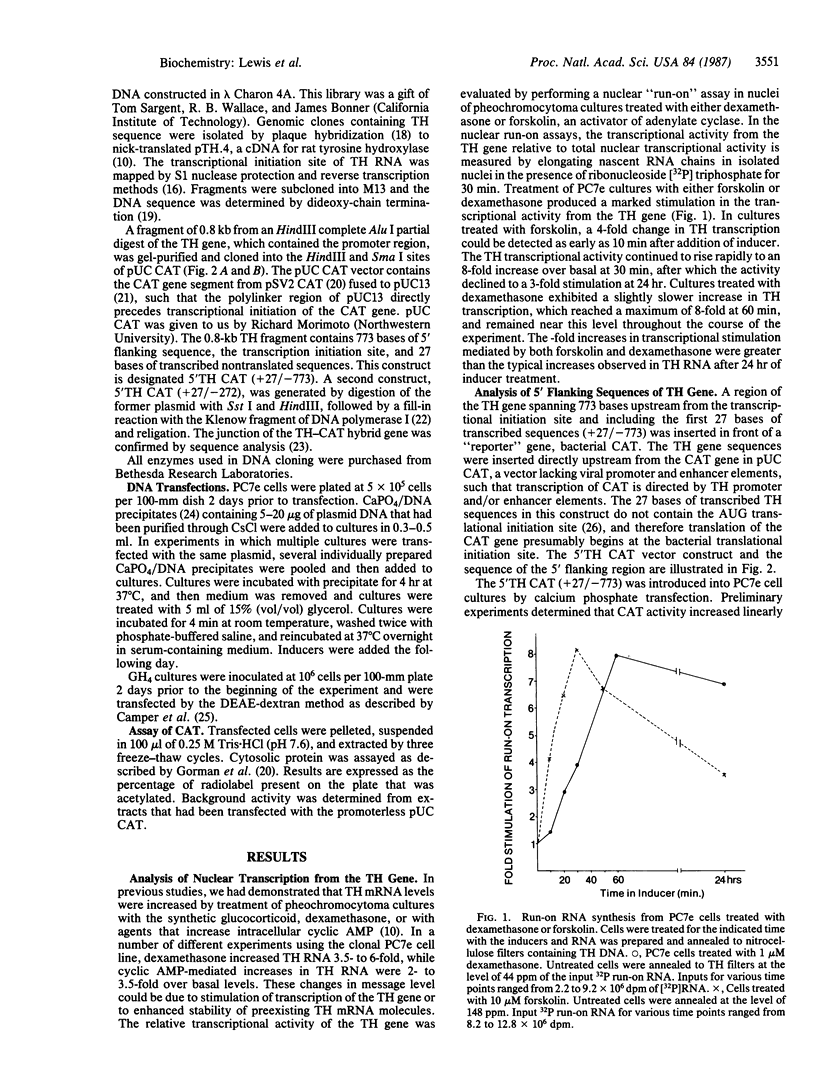
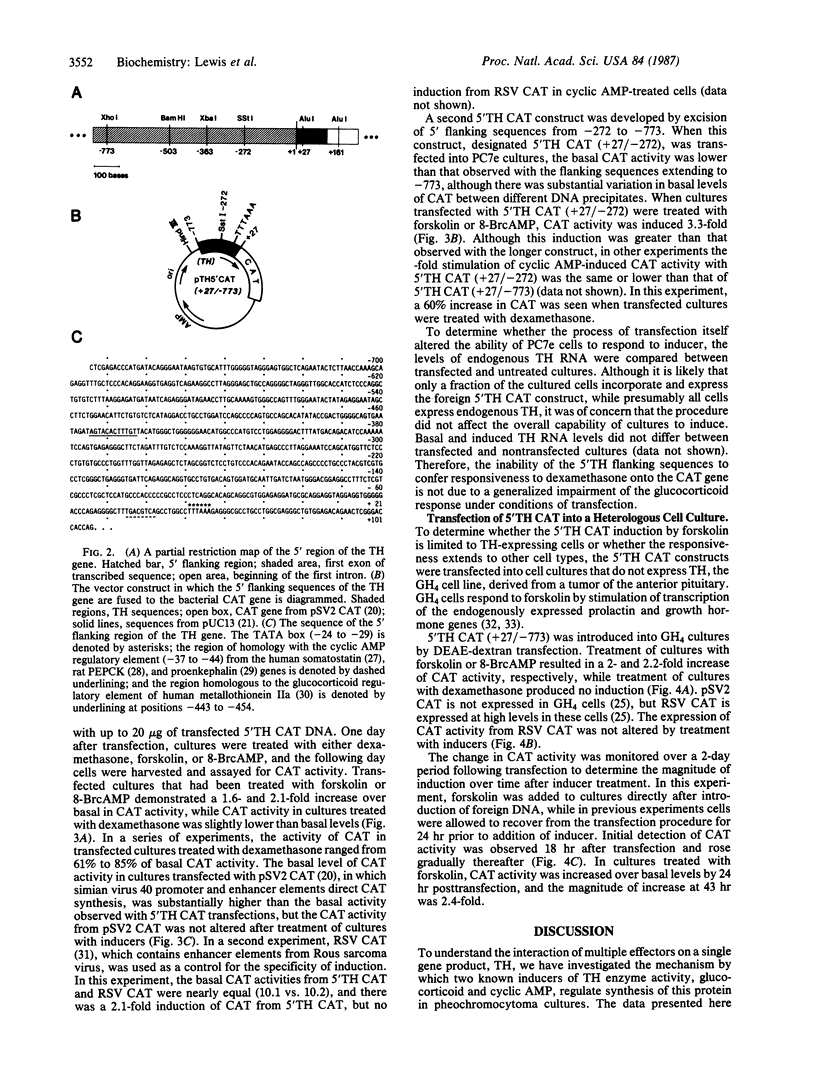
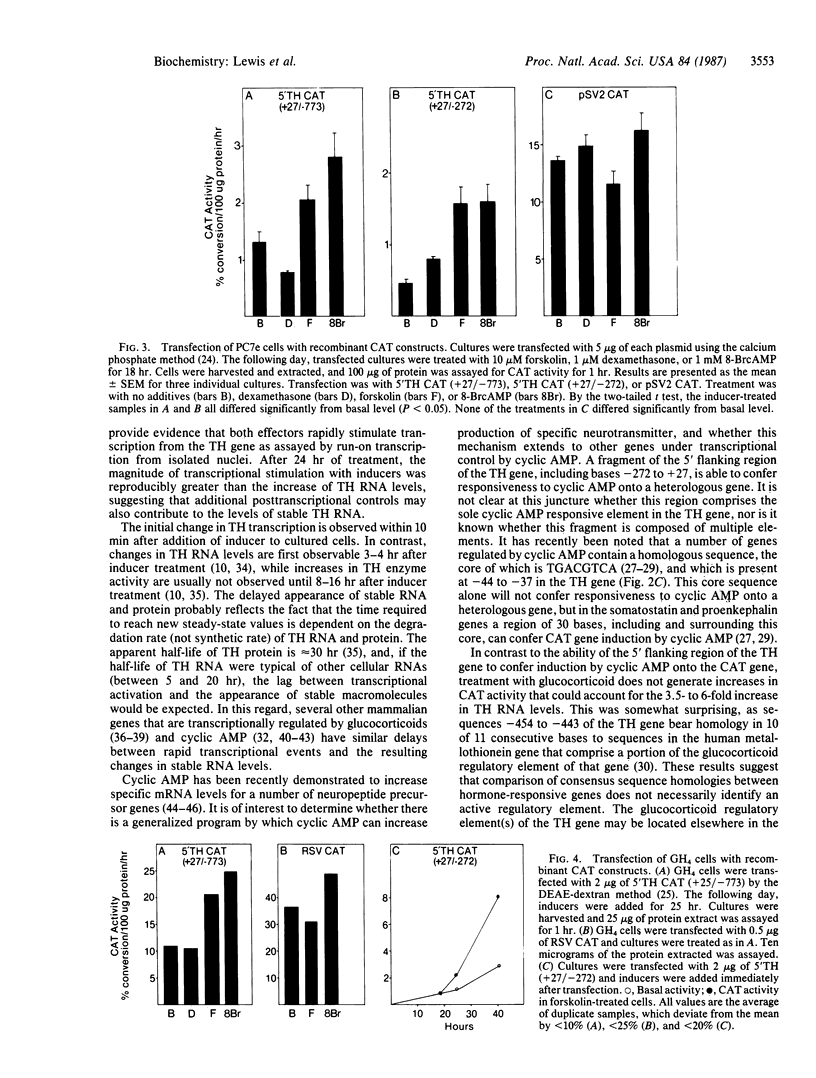
Glucocorticoid and cyclic AMP increase tyrosine hydroxylase (TH) activity and mRNA levels in pheochromocytoma cultures. The transcriptional activity of the TH gene, as measured by nuclear run-on assay, is also increased when cultures are treated with the synthetic glucocorticoid dexamethasone or agents that increase intracellular cyclic AMP, such as forskolin and 8-BrcAMP. Both inducers effect transcriptional changes within 10 min after treatment and are maximal after 30 min for forskolin and after 60 min for dexamethasone. The 5' flanking sequences of the TH gene were fused to the bacterial gene chloramphenicol acetyltransferase (CAT), and the hybrid gene was transfected into pheochromocytoma cultures and GH4 pituitary cells. In both cell lines, a region of the TH gene containing bases -272 to +27 conferred induction of CAT by cyclic AMP, but not by glucocorticoid. The same results were found when a region of the TH gene containing -773 to +27 was used. Thus, the sequences required for induction of TH by cyclic AMP are contained within 272 bases of 5' flanking sequence, but sequences sufficient for glucocorticoid regulation are not contained within 773 bases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson A. L., Naujoks K., Thoenen H. Nerve growth factor-mediated enzyme induction in primary cultures of bovine adrenal chromaffin cells: specificity and level of regulation. J Neurosci. 1984 Jul;4(7):1771–1780. doi: 10.1523/JNEUROSCI.04-07-01771.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter H. U., Reisine T. Corticotropin releasing factor increases proopiomelanocortin messenger RNA in mouse anterior pituitary tumor cells. J Biol Chem. 1985 Dec 15;260(29):15477–15481. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Black I. B., Chikaraishi D. M., Lewis E. J. Trans-synaptic increase in RNA coding for tyrosine hydroxylase in a rat sympathetic ganglion. Brain Res. 1985 Jul 22;339(1):151–153. doi: 10.1016/0006-8993(85)90635-3. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Yao Y. A., Rottman F. M. Hormonal regulation of the bovine prolactin promoter in rat pituitary tumor cells. J Biol Chem. 1985 Oct 5;260(22):12246–12251. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Danesch U., Hashimoto S., Renkawitz R., Schütz G. Transcriptional regulation of the tryptophan oxygenase gene in rat liver by glucocorticoids. J Biol Chem. 1983 Apr 25;258(8):4750–4753. [PubMed] [Google Scholar]
- Eberwine J. H., Roberts J. L. Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J Biol Chem. 1984 Feb 25;259(4):2166–2170. [PubMed] [Google Scholar]
- Edgar D. H., Thoenen H. Selective enzyme induction in a nerve growth factor-responsive pheochromocytoma cell line (PC 12). Brain Res. 1978 Oct 6;154(1):186–190. doi: 10.1016/0006-8993(78)91070-3. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Affolter H. U., Herbert E., Hotchkiss A. J. Alternative modes of enkephalin biosynthesis regulation by reserpine and cyclic AMP in cultured chromaffin cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3949–3953. doi: 10.1073/pnas.81.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Birnberg N. C., Rosenfeld M. G. Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R., Herschman H. R. Nerve growth factor-mediated induction of tyrosine hydroxylase in a clonal pheochromocytoma cell line. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4587–4590. doi: 10.1073/pnas.75.9.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R., Slater E., Herschman H. R. Epidermal growth factor induces tyrosine hydroxylase in a clonal pheochromocytoma cell line, PC-G2. J Cell Biol. 1980 Mar;84(3):495–500. doi: 10.1083/jcb.84.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager L. J., Palmiter R. D. Transcriptional regulation of mouse liver metallothionein-I gene by glucocorticoids. Nature. 1981 May 28;291(5813):340–342. doi: 10.1038/291340a0. [DOI] [PubMed] [Google Scholar]
- Harrington C. A., Lewis E. J., Krzemien D., Chikaraishi D. M. Identification and cell type specificity of the tyrosine hydroxylase gene promoter. Nucleic Acids Res. 1987 Mar 11;15(5):2363–2384. doi: 10.1093/nar/15.5.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Schmid W., Schütz G. Transcriptional activation of the rat liver tyrosine aminotransferase gene by cAMP. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6637–6641. doi: 10.1073/pnas.81.21.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann R. A., Kelley D. C., Miles M. F., Milkowski D. M. Cyclic AMP regulation of lactate dehydrogenase. Isoproterenol and N6,O2-dibutyryl cyclic amp increase the rate of transcription and change the stability of lactate dehydrogenase a subunit messenger RNA in rat C6 glioma cells. J Biol Chem. 1983 Apr 25;258(8):5312–5318. [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Kumakura K., Guidotti A., Costa E. Primary cultures of chromaffin cells: molecular mechanisms for the induction of tyrosine hydroxylase mediated by 8-Br-cyclic AMP. Mol Pharmacol. 1979 Nov;16(3):865–876. [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. J., Tank A. W., Weiner N., Chikaraishi D. M. Regulation of tyrosine hydroxylase mRNA by glucocorticoid and cyclic AMP in a rat pheochromocytoma cell line. Isolation of a cDNA clone for tyrosine hydroxylase mRNA. J Biol Chem. 1983 Dec 10;258(23):14632–14637. [PubMed] [Google Scholar]
- Mallet J., Faucon Biguet N., Buda M., Lamouroux A., Samolyk D. Detection and regulation of the tyrosine hydroxylase mRNA levels in rat adrenal medulla and brain tissues. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):305–308. doi: 10.1101/sqb.1983.048.01.033. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Montminy M. R., Low M. J., Tapia-Arancibia L., Reichlin S., Mandel G., Goodman R. H. Cyclic AMP regulates somatostatin mRNA accumulation in primary diencephalic cultures and in transfected fibroblast cells. J Neurosci. 1986 Apr;6(4):1171–1176. doi: 10.1523/JNEUROSCI.06-04-01171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. D., Marks A. R., Buckley D. I., Kapler G., Payvar F., Goodman H. M. The first intron of the human growth hormone gene contains a binding site for glucocorticoid receptor. Proc Natl Acad Sci U S A. 1985 Feb;82(3):699–702. doi: 10.1073/pnas.82.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Increase in tyrosine hydroxylase activity after reserpine administration. J Pharmacol Exp Ther. 1969 Sep;169(1):74–79. [PubMed] [Google Scholar]
- Murdoch G. H., Rosenfeld M. G. Eukaryotic transcriptional regulation and chromatin-associated protein phosphorylation by cyclic AMP. Science. 1982 Dec 24;218(4579):1315–1317. doi: 10.1126/science.6293056. [DOI] [PubMed] [Google Scholar]
- Naujoks K. W., Korsching S., Rohrer H., Thoenen H. Nerve growth factor-mediated induction of tyrosine hydroxylase and of neurite outgrowth in cultures of bovine adrenal chromaffin cells: dependence on developmental stage. Dev Biol. 1982 Aug;92(2):365–379. doi: 10.1016/0012-1606(82)90182-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Wynshaw-Boris A., Short H. P., Hanson R. W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. II. Identification of cAMP and glucocorticoid regulatory domains. J Biol Chem. 1986 Jul 25;261(21):9721–9726. [PubMed] [Google Scholar]
- Slater E. P., Rabenau O., Karin M., Baxter J. D., Beato M. Glucocorticoid receptor binding and activation of a heterologous promoter by dexamethasone by the first intron of the human growth hormone gene. Mol Cell Biol. 1985 Nov;5(11):2984–2992. doi: 10.1128/mcb.5.11.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak M., Sebbane R., Stricker E. M., Zigmond M. J., Kaplan B. B. Effect of chronic cold exposure on tyrosine hydroxylase mRNA in rat adrenal gland. Brain Res. 1985 Dec 16;359(1-2):356–359. doi: 10.1016/0006-8993(85)91450-7. [DOI] [PubMed] [Google Scholar]
- Tank A. W., Curella P., Ham L. Induction of mRNA for tyrosine hydroxylase by cyclic AMP and glucocorticoids in a rat pheochromocytoma cell line: evidence for the regulation of tyrosine hydroxylase synthesis by multiple mechanisms in cells exposed to elevated levels of both inducing agents. Mol Pharmacol. 1986 Nov;30(5):497–503. [PubMed] [Google Scholar]
- Tank A. W., Ham L., Curella P. Induction of tyrosine hydroxylase by cyclic AMP and glucocorticoids in a rat pheochromocytoma cell line: effect of the inducing agents alone or in combination on the enzyme levels and rate of synthesis of tyrosine hydroxylase. Mol Pharmacol. 1986 Nov;30(5):486–496. [PubMed] [Google Scholar]
- Tank A. W., Lewis E. J., Chikaraishi D. M., Weiner N. Elevation of RNA coding for tyrosine hydroxylase in rat adrenal gland by reserpine treatment and exposure to cold. J Neurochem. 1985 Oct;45(4):1030–1033. doi: 10.1111/j.1471-4159.1985.tb05519.x. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970 Nov 28;228(5274):861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Reznikoff W. S. Cloning DNA restriction endonuclease fragments with protruding single-stranded ends. Gene. 1980 May;9(3-4):307–319. doi: 10.1016/0378-1119(90)90329-p. [DOI] [PubMed] [Google Scholar]
- Waterman M., Murdoch G. H., Evans R. M., Rosenfeld M. G. Cyclic AMP regulation of eukaryotic gene transcription by two discrete molecular mechanisms. Science. 1985 Jul 19;229(4710):267–269. doi: 10.1126/science.2990047. [DOI] [PubMed] [Google Scholar]


