Abstract
Background
Food preservation is basically done to preserve the natural characteristics and appearance of the food and to increase the shelf life of food. Food preservatives in use are natural, chemical and artificial. Keeping in mind the adverse effects of synthetic food preservatives, there is a need to identify natural food preservatives. The aims of this study were to evaluate in vitro antioxidant and antimicrobial activities of Dragon's blood resin obtained from Dracaena cinnabari Balf f., with a view to develop safer food preservatives.
Methods
In this study, three solvents of varying polarity were used to extract and separate the medium and high polarity compounds from the non-polar compounds of the Dragon's blood resin. The extracts were evaluated for their antimicrobial activity against the food borne pathogens. The antioxidant activities of the extracts were assessed using DPPH and ABTS radical scavenging, FRAP, metal chelating and reducing power assays. Total phenolics, flavonoids and flavonols of extracts were also estimated using the standard methods.
Results
Phytochemical analysis of extracts revealed high phenolic content in CH2Cl2 extract of resin. Free radical scavenging of CH2Cl2 extract was found to be highest which is in good correlation with its total phenolic content. All test microorganisms were also inhibited by CH2Cl2 extract.
Conclusions
Our result provide evidence that CH2Cl2 extract is a potential source of natural antioxidant compounds and exhibited good inhibitory activity against various food borne pathogens. Thus, CH2Cl2 extract of Dragon's blood resin could be considered as possible source of food preservative.
Background
There are many pathogenic organisms known to spoil refrigerated and ready to eat food products, often leading to food poisoning. Besides this all refrigerated and packed foods also undergo autooxidation during storage leading to formation of reactive oxygen species (ROS). These ROS cause oxidation of lipids producing secondary oxidants like heptanol and hexanal which lead to oxidative rancidity and deteriorate the flavor of the food [1,2]. Thus, preservatives may be antimicrobials, which inhibit the growth of bacteria or fungi, including mold, or antioxidants, which inhibit the oxidation of food constituents. Antioxidants also play an important role in preventing chronic diseases by reducing the oxidative damage to cellular components caused by ROS e.g. superoxide anions, hydrogen peroxide and hydroxyl, nitric oxide radicals. Hence, intervention of an antioxidant may have a therapeutic effect and also maintain the freshness of food products. A wide range of synthetic antimicrobial agents like sodium benzoate, sorbate and synthetic antioxidants like butylated hydroxy toluene (BHT), butylated hydroxyl anisole (BHA) are in use as food preservatives [3] but can cause liver damage, mutagenicity and neurotoxicity. Because of the lack of more safer and effective preservatives, lot of research is being directed towards searching the food preservatives of natural origin.
Dragon's blood is a name applied to many red resins described in the medical literature. Dracaena spp., Daemonorops spp., Croton spp. and Pterocarpus spp. are different sources of Dragon's blood [4,5]. Dragon's Blood resin in unani medicine is commonly known as Dammul-akhwain. It has wide medicinal uses: haemostatic, antidiarrhetic, antiulcer, antimicrobial, antiviral, wound healing, antitumor, anti-inflammatory and antioxidant [6,7]. There are few reports on antioxidative activity and chemoprotective potential of compounds isolated from resin [8,9]. The antimicrobial and antiviral potentials of resin extracts have previously been studied [10-13]. Previous studies have revealed that the resin is rich in flavanoids, homoisoflavanoids, chalcones, sterols and terpenoids [14-21]. Since it is the whole resin which is used in traditional medicinal systems, it is essential to have definite information on antioxidant potential of this resin. Being rich in phenolic compounds this resin holds possible use as food preservative, which has not been studied yet. This is the first ever report evaluating potential of dragon's blood resin as food preservative. The aim of this study was to evaluate in vitro antioxidant activity and antimicrobial activity of this resin to support its candidature as food preservative.
Methods
Chemicals
All chemicals and reagents used were of analytical grade and obtained mostly from Sigma. Folin-Ciocalteu phenol reagent, Gallic acid, Rutin, BHT, 6-Hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox), 2,20-Azinobis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS), 2,4,6-Tripyridyl-2-triazine (TPTZ), 1,1-Diphenyl-2-picrylhydrazyl (DPPH), [4,40-[3-(2-Pyridinyl-1,2,4-triazine-5,6-diyl] bisbenzenesulfonic acid] (Ferrozine), Nitro blue tetrazolium (NBT), Nicotineamide adenine dinucleotide (NADH), Phenazine methosulphate (PMS), Griess reagent, 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride (INT), 3-(4,5-dimethyiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Trichloroacetic acid (TCA) were purchased from Sigma chemical company. Tetracycline hydrochloride (Himedia), Cycloheximide (Himedia), Ascorbic acid, FeCl2.4H2O, anhydrous FeCl3, K3Fe(CN)6, anhydrous Na2CO3 and all other chemicals were of analytical grade.
Plant Materials
Dragon's blood resin was purchased from wholesale supplier of traditional unani medicine, Ballimaran, Delhi, India, in August 2008, and identified as Dracaena cinnabari Balf f. resin. A voucher specimen (NISCAIR/RHMD/Consult/2008-09/1069/100) has been deposited at NISCAIR, New Delhi, India.
Preparation of extracts
Finely ground samples (200 gm) were extracted sequentially with petroleum ether (PE), dichloromethane (CH2Cl2) and methanol (MeOH) using a soxhlet assembly for 12 h for each solvent. Extracts were filtered and concentrated under vacuum in a rotary evaporator and stored at 4°C.
Phytochemical screening of the extracts
Determination of the total phenolics content
Total phenolic content of extracts were estimated using a method based on Folin-Ciocalteu reagent [22]. The concentration of total phenolic compounds in different extracts was expressed as mg of gallic acid equivalents (GAE) per gm of dried extract, using a standard curve of gallic acid (concentration range, 0.002-0.01 mg/ml), described by the equation y = 0.0265x (R2 = 0.9977).
Estimation of the flavonoids content
Total flavonoid content in the investigated extracts was determined spectrophotometrically [23]. The flavonoids content was expressed as mg of rutin equivalents (RE) per gm of dried extract, by using a standard graph of rutin, covering the concentration in between 0.02-0.2 mg/ml (y = 0.0025x, R2 = 0.9974).
Total flavonol content
Total flavonols of extracts were estimated as mg rutin equivalents (RE)/gm extract, from the rutin calibration curve in concentration range, 0.024-0.12 mg/ml (y = 0.0172x, R2 = 0.9979), using a previously reported method [24]. Here, y = absorbance and x = concentration. All experiments were done in triplicate.
In vitro Antioxidant Activities
DPPH free radical scavenging activity
Scavenging activity on DPPH was assessed according to the method reported by Blois [25] with a slight modification. Briefly, 100 μl of extracts (0.1-0.5 mg/ml) were mixed with 1 ml of methanolic solution of 0.1 mM DPPH. The mixture was shaken well and incubated at room temperature for 30 min and absorbance was measured at 517 nm in a spectrophotometer. Ascorbic acid, BHT and trolox were used as standards. Experiment was performed in triplicate and averaged. Percent inhibition was calculated from control using the following equation:
ABTS free radical scavenging activity
Trolox equivalent antioxidant capacity (TEAC) was estimated as ABTS radical scavenging activity according to the method of Re et al. [26]. Reagent solution consists of 7 mM ABTS and 2.45 mM potassium persulfate in 100 mM phosphate buffer solution (pH 7.4) and was left to stand for 12 h-16 h at laboratory temperature in the dark to form ABTS radical cation. A working solution was diluted to absorbance values 0.7 ± 0.02 at 734 nm with 100 mM phosphate buffer solution (pH 7.4). 10 μl of standards or resin extracts (2-10 μg/ml) were mixed with the working solution (990 μl) and absorbance was measured at 734 nm after 5 min. Trolox was used as a standard.
Nitric oxide radical scavenging assay
The nitric oxide radical scavenging activity of extracts was determined using the method of Sreejayan and Rao [27]. Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitrite ions determined by the Griess reagent. Two millilitre of 10 mM sodium nitroprusside dissolved in 0.5 ml phosphate buffer saline (pH 7.4) was mixed with 0.5 ml of extract at various concentrations (0.05-0.25 mg/ml). The mixture was incubated at 25°C. After 150 min, 0.5 ml of incubation solution was withdrawn and mixed with 0.5 ml of Griess reagent. The mixture was incubated at room temperature for 30 min. The absorbance was measured at 540 nm. The amount of nitric oxide radicals scavenged was calculated following this equation:
Superoxide radical scavenging activity
Superoxide anion scavenging activity was estimated by modified method of Robak and Gryglewski [28]. The reaction mixture consisting of 250 μl of NBT (150 μM), 250 μl of NADH (468 μM) and 250 μl of extract (0.1-0.5 mg/ml) was mixed in sodium phosphate buffer (100 mM, pH 7.4). The reaction was initiated by adding 250 μl of PMS (60 μM) to the mixture. The reaction mixture was incubated at 25°C for 5 min, and the absorbance was measured against the corresponding blank solution. L-Ascorbic acid and trolox were used as positive controls. The superoxide radical scavenging activity was calculated using the following formula:
Fe2+- chelating activity
The chelating activity of extract on Fe2+ was measured according to the method of Dinis et al. [29]. 1 ml of extracts (0.1-0.5 mg/ml) was incubated with 50 μl of 2 mM FeCl2. The reaction was started by the addition of 200 μl ferrozine (5 mM). After 10 min, the absorbance of ferrous ion-ferrozine complex at 562 nm was read. Na2EDTA served as positive control. Triplicate samples were run for each set and averaged. The ability of extracts to chelate ferrous ion was calculated using the following equation:
Ferric reducing antioxidant power (FRAP)
The assay was based upon the methodology of Benzie and Strain [30]. The FRAP reagent consisted of 10 mM TPTZ in 40 mM HCl, 20 mM FeCl3 and 250 mM sodium acetate buffer (pH 3.6). FRAP reagent was freshly prepared by mixing TPTZ solution, FeCl3 solution and acetate buffer in a ratio 1:1:10. A 100 μl of extract solution containing 0.1 mg extracts was mixed with 900 μl of FRAP reagent. After the mixture stood at 37°C for 4 min, the absorbance at 593 nm was determined against blank. Trolox was used as calibration standard in concentration range, 0.002-0.01 mg/ml (y = 0.160x, R2 = 0.981). FRAP values were calculated as mg of Trolox equivalents/gm extract from three determinations and are averaged.
Reducing power assay
The reducing power of extracts was determined as per the method of Oyaizu [31]. 1 ml of extracts (0.25-1 mg/ml) was mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml K3Fe(CN)6 (1%). After incubating the mixture at 50°C for 20 min., 2.5 ml of 10% TCA was added, and then mixture was centrifuged at 3000 rpm for 10 min. 2.5 ml of supernatant was mixed with 2.5 ml of distilled water and 0.5 ml FeCl3 (0.1%) and the absorbance was measured at 700 nm and compared with standard ascorbic acid.
Antimicrobial assay
Test strains
The antimicrobial activity of the extracts was evaluated on various food borne pathogenic strains obtained from the IMTECH, Chandigarh, India. The antibacterial activity of extracts was screened on Gram-positive bacteria: Bacillus subtilis MTCC121 (Bs), Staphylococcus aureus MTCC96 (Sa), Micrococcus luteus MTCC106 (Ml) and Gram-negative bacteria: Shigella flexneri MTCC1457 (Sf), Salmonella enteritidis MTCC3219 (Se), Proteus mirabilis MTCC425 (Pm), Enterobacter aerogenes MTCC2822 (Ea), Escherichia coli MTCC739 (Ec), Pseudomonas aeruginosa MTCC2453 (Pa). The antifungal activity was tested on Candida albicans MTCC227 (Ca) and Aspergillus flavus MTCC277 (Af).
Agar disc diffusion method
Antimicrobial activity of extracts was evaluated using agar disc diffusion method [32] against test microorganisms. 100 μl of fresh culture suspension of the test microorganisms was spread on respective media agar plates. The concentration of cultures was 1 × 108 CFU/ml. For screening, sterile, 6 mm diameter filter paper disc were impregnated with 10 μl of extract equivalent to 0.1 mg of extract after being placed on the surface of the inoculated media agar plates. The plates were stood at 4°C for 2 h before being incubated under optimum conditions for 24 h. Clear inhibition zones around the discs indicated the presence of antimicrobial activity. The diameters of the inhibition zones were measured in millimeter, including the diameter of disc. The controls were set up with equivalent quantities of DMSO as control. Antibiotics tetracycline and cycloheximide were used as reference standards. An inhibition zone of 10 mm or more was considered as high antibacterial activity.
Determination of minimal inhibitory concentration (MIC)
Determination of the MIC of extracts against tested microbes was carried out by the broth microdilution method [33]. Extracts were dissolved in DMSO (10% of the final volume) and diluted with culture broth to a concentration of 5 mg/ml. Further 1:2 serial dilutions were performed by addition of culture medium to reach concentrations ranging from 5 to 0.078 mg/ml; 100 μl of each dilution were distributed in 96-well plates. Controls were set up as sterility control (containing culture medium only) and a growth control (containing culture medium plus DMSO). Each test and growth control well was inoculated with 5 μl of a microbial suspension (108 CFU/ml or 105 CFU/well). All experiments were performed in triplicate and the microdilution plates were incubated at 37°C for 24 h. For detecting growth, 20 μl of 70% alcoholic solution of INT (0.5 mg/ml) added to each well and incubated further for 30 min, and in those wells, where microbial growth occurred, INT changed from yellow to purple. MIC values were defined as the lowest concentration of each extract, which completely inhibited microbial growth. The results were expressed in milligrams per milliliters.
In vitro nehrotoxicity
Kidney cell culture
Porcine proximal tubule cells (LLC-PK1 cell line, National Centre for Cell Science, Pune) were grown until confluency in minimum essential medium (MEM) supplemented with nonessential amino acids and 10% fetal bovine serum without antibiotics, at 37° C in a humidified atmosphere containing 5% CO2. The medium was changed every 48 h and the cells were routinely subcultured every 4 d.
Cytotoxicity assay
Cells were seeded at a density of 3 × 105 cells/ml in 96-well plates and allowed to get confluent. Cytotoxicity of the extracts was measured by the MTT assay [34]. MTT (5 mg/ml) was added to the medium in each well, and plates were incubated for 3 h at 37°C. Medium was then removed, and dimethyl sulfoxide (200 μl) was added to each well to solubilize the purple formazan crystals created by mitochondrial dehydrogenase reduction of MTT. After 5 min of additional incubation, absorbance was read at 570 nm on a microplate spectrophotometer. The data were expressed as percent cell viability compared with control (dimethyl sulfoxide).
Statistical analysis
All experiments were conducted in triplicate (n = 3) and two-way ANOVA (using Graph pad prism 5 statistical software) was used to determine significance of each treatment among standards and extracts. Student's t-test was used to determine the significance between activities at different concentrations of the two extracts. P < 0.0001 vs 0 μg/ml was considered siginificant.
Results
Phytochemical screening
In this study, solvents of varying polarity were used to extract and separate the medium and high polarity compounds from the non-polar compounds of the resin. Resin was sequentially extracted with PE, CH2Cl2 and MeOH using a Soxhlet apparatus. The results of yield, total phenols, total flavonoids and total flavonols of extracts are presented in Table 1. Total phenolic, flavonoid and flavonol content of extracts were in following order: CH2Cl2 > MeOH > PE; and were significantly different (P < 0.05). Since, total phenolic, flavonoid and flavonol content of PE extract was very less, this extract was not further taken for antioxidant assays.
Table 1.
Yield and phytochemical content of the extracts from Dragon's blood resin
| Extracts | Yield (%) | Total phenols (mg GAE/g extract) | Total flavonoids (mg RE/g extract) | Total flavonols (mg RE/g extract) |
|---|---|---|---|---|
| PE | 0.556 ± 0.01 | 0.704 ± 0.246 | Tr | tr |
| CH2Cl2 | 2.605 ± 0.168 | 263.019 ± 3.717 | 229.333 ± 10.066 | 16.86 ± 0.581 |
| MeOH | 53.922 ± 0.468 | 177.358 ± 6.178 | 82.667 ± 8.327 | 56.395 ± 1.744 |
tr, trace (<0.05).
Antioxidant activity
The antioxidant effects of plant products must be evaluated by combining two or more different in vitro assays to get relevant data, because of the complex nature of phytochemicals. Each of these tests is based on one feature of the antioxidant activity, such as the ability to scavenge free radicals, or the metal ion chelation.
DPPH free radical scavenging
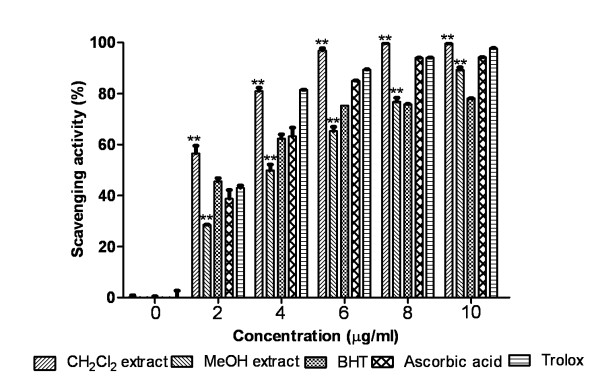
As depicted in Figure 1, CH2Cl2 extract and MeOH extract showed steady increase in percent inhibition of the DPPH radicals with concentration. MeOH extract showed higher DPPH scavenging activity than standard BHT but less than ascorbic acid and trolox. CH2Cl2 and MeOH extracts of resin were able to inhibit the formation of DPPH radicals with an IC50, 0.1357 ± 0.0069 mg/ml and 0.0942 ± 0.0024 mg/ml respectively. The extracts and standards were significantly different (P < 0.0001).
Figure 1.
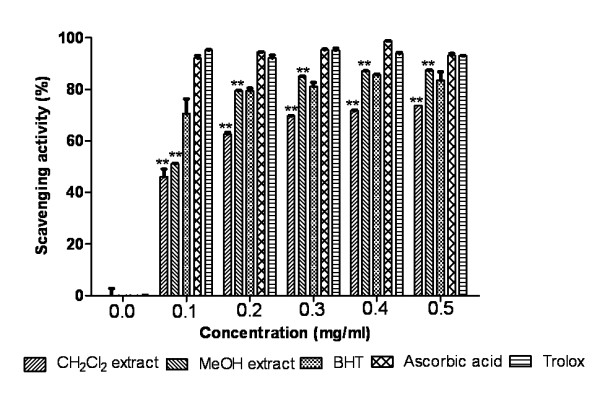
DPPH scavenging activity. Effect of Dragon's blood resin extracts and standards BHT, ascorbic acid, trolox on DPPH radical scavenging study. The data is expressed as% scavenging of DPPH radicals. **P < 0.0001 vs 0 μg/ml.
ABTS free radical scavenging
The results are demonstrated in Figure 2 and showed that CH2Cl2 extract (IC50= 0.0018 ± 0.0001 mg/ml) was more active than MeOH extract (IC50 = 0.0041 ± 0.0002 mg/ml) and standards BHT, trolox and ascorbic acid. The two extracts of the resin and standards showed significant difference (P < 0.0001) in ABTS scavenging activity.
Figure 2.
Total antioxidant activity. Effect of Dragon's blood resin extracts and standards BHT, ascorbic acid, trolox on ABTS radical cation decolorization assay. The percentage of inhibition was plotted against concentration of samples. **P < 0.0001 vs 0 μg/ml.
Nitric oxide radical scavenging assay
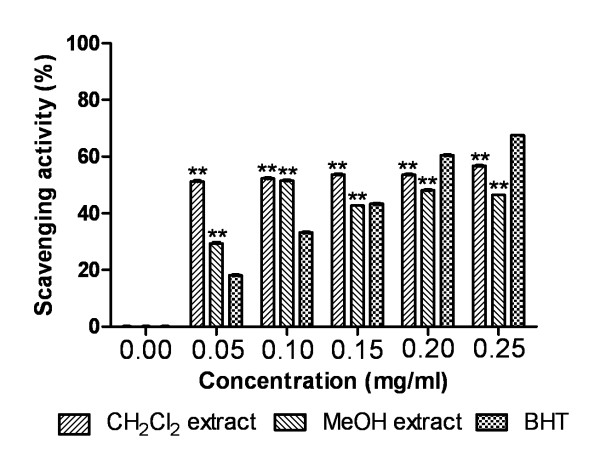
The percent inhibition of nitric oxide radicals was shown in Figure 3. The results show that CH2Cl2 extract had significantly higher scavenging activity (53.675 ± 0.111%) than standard BHT (43.341 ± 0.027%) at 0.15 mg/ml. MeOH extract was inconsistent in scavenging nitric oxide radicals with increase in concentration and found to show 51.51 ± 0.146% scavenging at 0.1 mg/ml. The extracts and standard were found to be significantly different (P < 0.0001).
Figure 3.
Nitric oxide radical scavenging activity. Effect of Dragon's blood resin extracts and standard BHT on nitric oxide scavenging assay. The percentage of scavenging was plotted against concentration of samples. **P < 0.0001 vs 0 μg/ml.
Superoxide radical scavenging assay
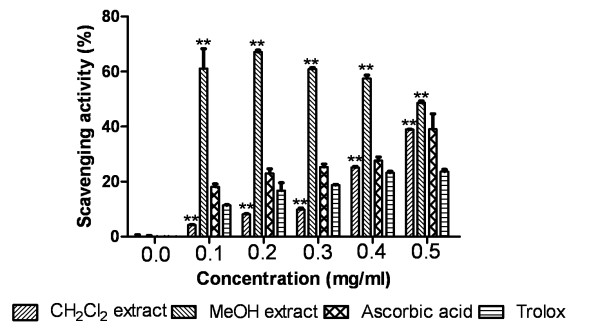
As demonstrated in Figure 4, MeOH extract showed significantly higher superoxide radical scavenging activity than CH2Cl2 extract, ascorbic acid and trolox. There was significant difference in superoxide radical scavenging activity of extracts and standards (P < 0.0001).
Figure 4.
Superoxide radical scavenging activity. Effect of Dragon's blood resin extracts and standards ascorbic acid, trolox on superoxide scavenging assay. The percentage of scavenging was plotted against concentration of samples. **P < 0.0001 vs 0 μg/ml.
Iron (II) chelating activity
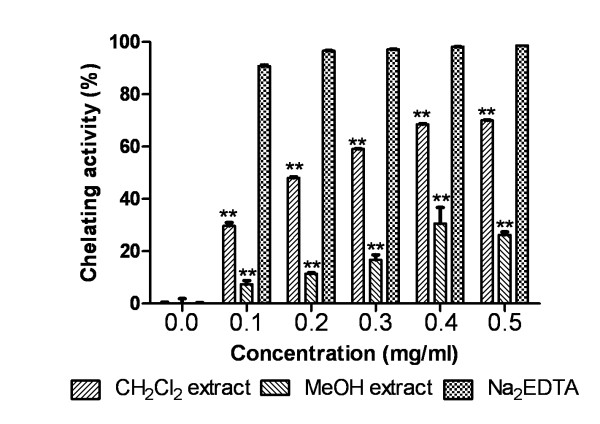
As demonstrated in Figure 5, CH2Cl2 extract showed metal chelating activity with an IC50 value of 0.2195 ± 0.0052 mg/ml and was less potent chelator than standard Na2EDTA. MeOH extract was found to be very less reactive showing a maximum of 25.96% inhibition at 0.5 mg/ml. The two extracts and standard were significantly different in metal chelation (P < 0.0001).
Figure 5.
Metal chelating activity. Effect of Dragon's blood resin extracts and standard Na2EDTA on metal chelating assay. The percentage of chelation was plotted against concentration of sample. **P < 0.0001 vs 0 μg/ml.
FRAP activity
In this assay, reduction of the ferric-tripyridyltriazine to the ferrous complex forms an intense blue color which can be measured at a wavelength of 593 nm. The intensity of the color is related to the amount of antioxidant reductants in the samples. The ferric reducing activity of CH2Cl2 and MeOH extracts of resin were found to be 2.831 ± 0.598 and 1.366 ± 0.017 mg trolox equivalents/gm extract respectively. There was significant difference in FRAP values of the two extracts with P < 0.05.
Reducing power assay
Figure 6 depicts the reducing power activity of extracts were comparable to that of standard ascorbic acid. Reducing ability increased well with amount of extracts. Extracts and standard were significantly different (P < 0.0001).
Figure 6.
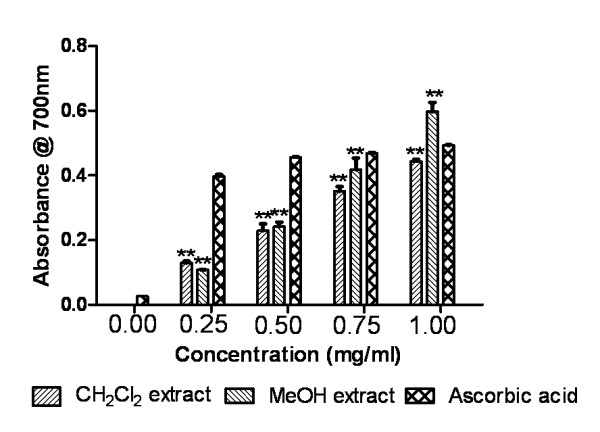
Reducing power assay. Reducing power activities of Dragon's blood resin extracts and standard ascorbic acid. The absorbance (A700) was plotted against concentration of sample. **P < 0.0001 vs 0 μg/ml.
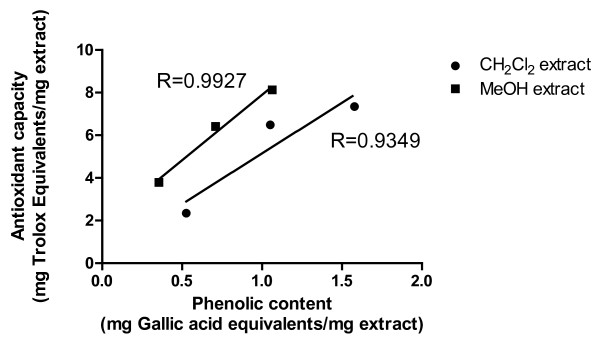
Correlation between the total phenolic contents with the antioxidant activity
As showed in Figure 7, the total phenolic content of MeOH extract significantly correlated with total antioxidant activity (R = 0.9927), whereas the correlation coefficients for CH2Cl2 extract was found to be greater than 0.9 (R = 0.9349) which proved that the phenolic contents of these extracts highly attributed their antioxidant activity.
Figure 7.
Correlation of antioxidant activity with phenolic contents. The relationship between total phenolic content in individual extract and their antioxidant capacity. The correlation analyses were described as linear correlation coefficient (R).
Antimicrobial activity
In this study, nine different bacterial and two fungal species were used to screen the possible antimicrobial activities of Dragon's blood resin extracts. Antimicrobial activity assay against these microbial strains showed that CH2Cl2 extract was most active amongst extracts, as shown in Table 2. PE extract exhibited weak antibacterial and antifungal activities (data not shown here). While, CH2Cl2 and MeOH extracts displayed activity against all test bacteria except S. enteritidis. It is remarkable to note that CH2Cl2 extract was also active against fungi C. albicans and A. flavus.
Table 2.
Antimicrobial activity of the extracts
| Microorganisms | Zone of inhibition (mm) | MIC (mg/ml) | ||||
|---|---|---|---|---|---|---|
| CH2Cl2 extract | MeOH extract | Tet | CyX | CH2Cl2 extract | MeOH extract | |
| Bs | 11.33 ± 0.58 | 10.33 ± 0.58 | 11.0 ± 0.0 | ND | 0.156 | 0.625 |
| Ml | 17.67 ± 0.58 | 17.33 ± 0.58 | 35.0 ± 0.0 | ND | 0.156 | 1.25 |
| Sa | 9.0 ± 0.0 | 10.33 ± 0.58 | 16.0 ± 0.0 | ND | 1.25 | 1.25 |
| Sf | 14.0 ± 0.0 | 10.33 ± 0.58 | 15.0 ± 0.0 | ND | 0.156 | 1.25 |
| Pm | 11.33 ± 0.58 | 12.0 ± 1.0 | 16.0 ± 1.0 | ND | 0.3125 | 1.25 |
| Se | NA | NA | NA | ND | >5 | >5 |
| Ea | 8.67 ± 0.58 | 9.67 ± 0.58 | 10.0 ± 0.0 | ND | 0.625 | 1.25 |
| Ec | 9.0 ± 0.0 | 9.0 ± 0.0 | 11.67 ± 0.58 | ND | 0.3125 | 0.3125 |
| Pa | 7.67 ± 0.58 | 8.0 ± 0.0 | NA | ND | >5 | >5 |
| Ca | 8.0 ± 0.0 | NA | ND | NA | >5 | >5 |
| Af | 9.0 ± 0.0 | NA | ND | 14.0 ± 3.61 | >5 | >5 |
NA, No antimicrobial activity. ND, Not determined, Tet, Tetracycline, CyX, Cycloheximide, Inhibition zones including the disc diameter (6 mm); <8.0 mm, weak activity; 8.0-9.0 mm, slight low activity; 9.1-10.0 mm, middle activity; 10.1-11.0 mm, slight high activity; 11.1-12.0 mm, moderate high activity; over 12.0 mm, very high activity.
In vitro nephrotoxicity
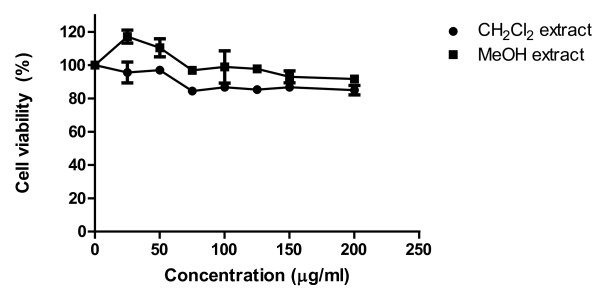
Cytotoxicity of resin extracts to LLC-PK1 cells was measured by MTT assay. As depicted in Figure 8, CH2Cl2 extract showed more than 80% viability of cells at concentrations ≤200 μg/ml. Cell viability was not also significantly altered by MeOH extract, at up to 200 μg/ml. These results suggest that concentrations of extracts were not toxic to LLC-PK1 cells below 200 μg/ml.
Figure 8.
Cytotoxicity of Dragon's blood resin extracts. LLC-PK1 cells were treated with various concentrations of extracts to determine percent cell viability through MTT assay.
Discussion
Food preservation is one of the oldest technologies used by human beings to avoid its spoilage. Food preservation by definition is a process by which certain foods like fruits and vegetables are prevented from getting spoilt for a long period of time. The color, taste and nutritive value of the food are also preserved. Besides damage to living cells, free radicals are the major cause of food deterioration through lipid oxidation, which ultimately affects the taste and smell of foods.
It is well known that phenolic compounds possess good antioxidant properties [35,36]. Phenolic compounds are secondary metabolites of plants and can act as antioxidants by many potential pathways such as free radical-scavenging, oxygen radical absorbance, and chelation of metal ions [37]. Flavonoids belong to phenolic compounds and are excellent antioxidant compounds because they are highly reactive as hydrogen and electron donor [38-41]. The mode of the actions of flavonoids is through scavenging or chelating processes [42,43]. CH2Cl2 and MeOH extracts of Dragon's blood resin were found to show high content of phenolic compounds and good antioxidant activity. DPPH assay demonstrated that both CH2Cl2 and MeOH extracts were potent in scavenging DPPH free radicals, suggesting the presence of compounds capable of donating hydrogen to the free radicals. In ABTS radical scavenging assay, CH2Cl2 extract showed significantly higher scavenging as compared to standards BHT, trolox and ascorbic acid; which implies that this extract could be a useful candidate for food preservation as well as for treatment of radical related pathological damage. Nitric oxide radicals play an important role in pathogenesis of inflammation and their toxicity multiplies when they form peroxynitrite after reacting with O2•- radicals, damaging biomolecules like proteins, lipids and nucleic acids [44]. The level of nitric oxide was significantly reduced in this study by the CH2Cl2 extract and was higher than BHT at 0.15 mg/ml. These results may explain the use of Dragon's blood resin for the treatment of inflammation and for wound healing. Superoxide anion radical is supposed to be one of the strongest reactive oxygen species among the free radicals. The scavenging of these radicals by the MeOH extract as compared to standards ascorbic acid and trolox, suggested that it is a potent scavenger of superoxide radicals.
The transition metal ion Fe2+ possesses the ability to move single electrons thus allowing the formation and propagation of many radical reactions, even starting with relatively nonreactive radicals [45]. The mechanism to avoid reactive oxygen species formation, associated with redox active metal catalysis is through chelation of the metal ions. In metal chelating assay, presence of chelating agents disrupts the formation of ferrozine-Fe+2 complex monitored by measuring the decrease in the red color of the complex at 562 nm and thus, estimating the chelating activity of the coexisting chelator. CH2Cl2 extract was found to be better metal chelator than MeOH extract.
The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. Fe+3 reduction, an indicator of electron donating activity, is considered to be an important mechanism of antioxidant activity of phenolics [46]. In the reducing power assay, the antioxidants in the samples reduce Fe3+ to Fe2+ by donating an electron. Amount of Fe2+ complex can then be measured spectrophotometrically at 700 nm. Increasing absorbance at 700 nm indicates an increase in the reductive ability. Both extracts displayed increasing reducing power in dose dependent manner. Since, MeOH extract showed higher reducing power than ascorbic acid at 1 mg/ml, this extract must be richer in compounds capable of donating hydrogen atom.
Correlation studies of extracts revealed strong correlation between total phenolic content and antioxidant activity of the extracts. Antioxidant activity of the two extracts of resin can be the result of their high phenolic content, as phenolics have been reported to act as reducing agents, hydrogen donors, free radical quenchers and metal chelators.
Apart from oxidative damage, contamination by microbes is another common problem during food preservation and also possesses serious health threats to those consuming it. Antimicrobial activity assay against different food borne pathogens showed that CH2Cl2 extract was most active amongst extracts, as shown in Table 2. Of the species used, S. aureus is the most common cause for food poisoning. B. subtilis is a common cause of food spoilage, mainly in canned foods. M. luteus has also been reported to be a food contaminant. In developing countries, S. flexneri is most predominant cause for acute bloody diarrhea known as bacillary dysentery. P. mirabilis has also been associated with food spoilage in fresh meat, poultry and seafood. S. enteritidis has become single most common cause of food poisoning causing gastroenteritis. E. coli, E. aerogenes and P. aeruginosa also causes food spoilage. CH2Cl2 extract showed strong antimicrobial activity with MIC values ranging from 0.156-1.25 mg/ml against test microorganisms and could be attributed to its high phenolic content. MeOH extract also exhibited good inhibition to test pathogens as MIC values were in range of 0.3125-1.25 mg/ml. Two extracts did not demonstrate inhibition to P. aeruginosa and S. enteritidis, while M. luteus was most sensitive to the extracts. Two extracts were also tested against fungal food pathogens C. albicans and A. flavus; only CH2Cl2 extract was able to inhibit fungal pathogens. Thus, the two extracts possess significant inhibitory activity to be used as antibacterial agent.
Exposure to drugs and chemicals often results in toxicity to living organisms and are not equally toxic to all parts of a living system because the toxic actions of many compounds are manifested in specific organs. Many organs, including the kidney, are capable of metabolizing chemicals to toxic reactive intermediates. The increasing use of in vitro techniques using isolated renal cells, nephron fragments, or cell cultures derived from specific renal cell types has helped in studying nephrotoxicity. Kidney derived cells are cultured in presence of test compounds whose cytotoxicity is then determined and serves as an indicator of potential nephrotoxicity. LLC-PK1 is an established cell line derived from normal porcine kidneys that has been widely used to study nephrotoxicity of a number of compounds [47,48]. Cytotoxicity studies showed that CH2Cl2 extract and MeOH extract were not toxic to LLC-PK1 cells upto the concentration of 200 μg/ml. Thus, resin extracts can be used as a source of food preservative at a concentration below 200 μg/ml without showing any nephrotoxicity.
Conclusions
Present study revealed that Dragon's blood resin extracts demonstrated high phenolic content and potent antioxidant activity, achieved by free radical scavenging, FRAP, metal chelating and reducing power assays. The CH2Cl2 extract had the highest radical-scavenging activity, metal chelating activity and highest total phenolic content among all extracts. CH2Cl2 extract also exhibited good inhibitory activity against bacterial and fungal food pathogens. Thus, CH2Cl2 extract of Dragon's blood resin could be considered as possible source of food preservative. The wide use of this resin in traditional medicinal systems as anti-inflammatory, wound healing agent and against diarrhea, dysentery may be in part due to its antioxidant and antimicrobial properties. Further activity guided isolation, characterization and toxicity of the extract is in progress to identify the full composition of the extract and the exact compound(s) responsible for its bioactivity, to be developed as food preservative agents.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DG carried out the study design, specimen collection, experimental work, data collection and interpretation, literature search and manuscript preparation. RKG supervised the work, evaluated the data and corrected the manuscript for publication. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Deepika Gupta, Email: deep_dr5@yahoo.co.in.
Rajinder K Gupta, Email: rkg67ap@yahoo.com.
Acknowledgements
We acknowledge the financial support from All India Council for Technical Education (8023/BOR/RID/RPS-27/2008-09).
References
- St Angelo AJ, Vercellotti JR, Vinenett CH, Kuan JW, James C Jr, Duputy HP. Chemical and instrumental analyses of warmed over flavor in beef. J Food Sci. 1987;52:1163–1168. doi: 10.1111/j.1365-2621.1987.tb14034.x. [DOI] [Google Scholar]
- Ladikos D, Lougovois V. Lipid oxidation of muscle foods: a review. Food Chem. 1990;35:295–314. doi: 10.1016/0308-8146(90)90019-Z. [DOI] [Google Scholar]
- Sherwin FR. In: In Food Additives. Branen R, editor. New York: Marcel Dekker; 1990. Antioxidants; pp. 139–193. [Google Scholar]
- Mabberley DJ. The Plant Book. Cambridge Univ. Press (rev. edn); 1998. [Google Scholar]
- Zheng QA, Chen JT, Zhang YJ, Yang CR. Origin and diffusion of Dragons Blood, a famous folk herb. Acta Bot Yunnan. 2003;Suppl:102–107. [Google Scholar]
- Milburn M. Dragon's Blood in East & West Africa, Arabia and the Canary Islands. Africa. 1984;39:486–493. [Google Scholar]
- Badib AS. Medicinal plants of the Yemen. Maktabah Alirsaid: Sanaa; 1991. p. 15. [Google Scholar]
- Juránek I, Suchý V, Stará D, Mašterová I, Grančaiová Z. Antioxidative activity of homoisoflavonoids from Muscari racemosum and Dracaena cinnabari. Pharmazie. 1993;48:310–311. [PubMed] [Google Scholar]
- Machala M, Kubínová R, Hořavová P, Suchý V. Chemoprotective potentials of Homoisoflavonoids and Chalcones of Dracaena cinnabari: Modulations of drug-metabolizing enzymes and antioxidant activity. Phytother Res. 2001;15:114–118. doi: 10.1002/ptr.697. [DOI] [PubMed] [Google Scholar]
- Mothana RAA, Lindequist U. Antimicrobial activity of some medicinal plants of the island Soqotra. J Ethnopharmacol. 2005;96:177–181. doi: 10.1016/j.jep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Mothana RAA, Mentel R, Reiss C, Lindequist U. Phytochemical screening and antiviral activity of some medicinal plants from the Island Soqotra. Phytother Res. 2006;20:298–302. doi: 10.1002/ptr.1858. [DOI] [PubMed] [Google Scholar]
- Kumar VP, Chauhan NS, Padh H, Rajani M. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J Ethnopharmacol. 2006;107:182–8. doi: 10.1016/j.jep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Deepika G, Bleakley B, Gupta RK. Bioassay guided isolation of antibacterial homoisoflavone from Dragon's blood resin (Dammul-akhwain) Nat Prod Rad. 2009;8(5):494–497. [Google Scholar]
- Suchý V, Bobovnický B, Trojánek J, Buděsínšký M, Ubik K. In: Progress on Terrestrial and Marine Natural Products of Medicinal and Biological Interest: American Botanical Council: Austin. Pezzuto JM, editor. 1991. Homoisoflavans and other constituents of Dragon's blood from Dracaena cinnabari; pp. 110–118. [Google Scholar]
- Masaoud M, Himmelreich H, Ripperger H, Adam G. New bioflavonoids from Dracaena cinnabari. Planta Med. 1995;61:341–344. doi: 10.1055/s-2006-958096. [DOI] [PubMed] [Google Scholar]
- Masaoud M, Ripperger H, Porzel A, Adam G. Cinnabarone, a bioflavonoid from Dragon's blood of Dracaena cinnabari. Phytochemistry. 1995;38:751–753. doi: 10.1016/0031-9422(94)00718-9. [DOI] [Google Scholar]
- Masaoud M, Ripperger H, Porzel A, Adam G. Flavonoids of Dragon's blood from Dracaena cinnabari. Phytochemistry. 1995;38:745–749. doi: 10.1016/0031-9422(94)00738-F. [DOI] [Google Scholar]
- Masaoud M, Schmidt J, Adam G. Sterols and Triterpenoids from Dracaena cinnabari. Phytochemistry. 1995;38:795–796. doi: 10.1016/0031-9422(94)00743-D. [DOI] [Google Scholar]
- Himmelreich U, Masaoud M, Adam G, Ripperger H. Damalachawin, a triflavonoid of a new structural type from Dragon's blood of Dracaena cinnabari. Phytochemistry. 1995;39:949–951. doi: 10.1016/0031-9422(95)00044-8. [DOI] [Google Scholar]
- Veselá D, Marek R, Ubik K, Lunerová K, Sklenář V, Suchý V. Dracophane, a metacyclophane derivative from the resin of Dracaena cinnabari Balf. Phytochemistry. 2002;61:967–970. doi: 10.1016/s0031-9422(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Deepika G, Bleakley B, Gupta RK. Dragon's blood: Botany, chemistry and therapeutic uses. J Ethnopharmacol. 2008;115:361–380. doi: 10.1016/j.jep.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Miliauskas PR, Venskutonis TA, Van Beek. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans CA. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sreejayan N, Rao MNA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxides anions. Biochem Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetoaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a measure of ''Antioxidant Power'': The FRAP Assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- Bauer AW, Kirby WMM, Sheriss JC, Turck M. Antibiotic susceptibility testing by standardised single method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- Valgas C, de Souza SM, Smânia EFA, Smânia A Jr. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38:369–380. doi: 10.1590/S1517-83822007000200034. [DOI] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Hollman PCH, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–942. doi: 10.1016/S0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Halliwell B, Aeschbach R, Loliger J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–617. doi: 10.1016/0278-6915(95)00024-V. [DOI] [PubMed] [Google Scholar]
- Cotelle N. Role of flavonoids in oxidative stress. Curr Top Med Chem. 2001;1:569–590. doi: 10.2174/1568026013394750. [DOI] [PubMed] [Google Scholar]
- Pannala AS, Chan TS, O'Brien PJ, Rice-Evans CA. Flavonoid B-ring chemistry and antioxidant activity: fast reaction kinetics. Biochem Biophys Res Commun. 2001;282:1161–1168. doi: 10.1006/bbrc.2001.4705. [DOI] [PubMed] [Google Scholar]
- Yang B, Kotani A, Arai K, Kusu F. Estimation of the antioxidant activities of flavonoids from their oxidation potentials. Anal Sci. 2001;17:599–604. doi: 10.2116/analsci.17.599. [DOI] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NC, Samman S. Flavonoids- chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7:66–76. doi: 10.1016/0955-2863(95)00168-9. [DOI] [Google Scholar]
- Kessler M, Ubeaud G, Jung L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol. 2003;55:131–142. doi: 10.1211/002235702559. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- Yildirim A, Mavi A, Kara A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- Scoble JE, Senior JC, Chan P, Varghese Z, Sweny P, Moorhead JF. In vitro cyclosporine toxicity: the effect of verapamil. Transplantation. 1989;47:647–650. doi: 10.1097/00007890-198904000-00016. [DOI] [PubMed] [Google Scholar]
- Okuda M, Masaki K, Fukatsu S, Hashimoto Y, Inui K. Role of apoptosis in cisplatin-induced toxicity in the renal epithelial cell line LLC-PK1: implication of the functions of apical membranes. Biochem Pharmacol. 2000;59:195–201. doi: 10.1016/S0006-2952(99)00303-2. [DOI] [PubMed] [Google Scholar]