Abstract
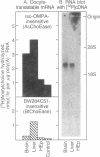
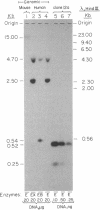
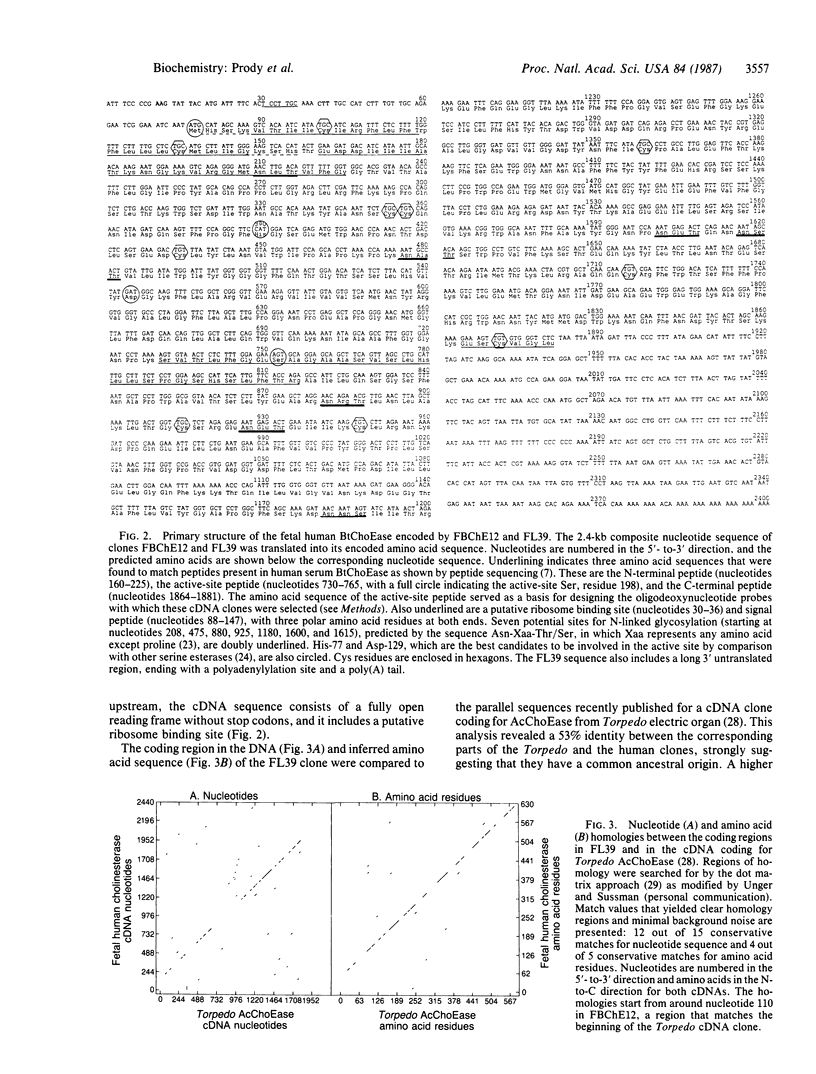
To study the primary structure and regulation of human cholinesterases, oligodeoxynucleotide probes were prepared according to a consensus peptide sequence present in the active site of both human serum pseudocholinesterase (BtChoEase; EC 3.1.1.8) and Torpedo electric organ "true" acetylcholinesterase (AcChoEase; EC 3.1.1.7). Using these probes, we isolated several cDNA clones from lambda gt10 libraries of fetal brain and liver origins. These include 2.4-kilobase cDNA clones that code for a polypeptide containing a putative signal peptide and the N-terminal, active site, and C-terminal peptides of human BtChoEase, suggesting that they code either for BtChoEase itself or for a very similar but distinct fetal form of cholinesterase. In RNA blots of poly(A)+ RNA from the cholinesterase-producing fetal brain and liver, these cDNAs hybridized with a single 2.5-kilobase band. Blot hybridization to human genomic DNA revealed that these fetal BtChoEase cDNA clones hybridize with DNA fragments of the total length of 17.5 kilobases, and signal intensities indicated that these sequences are not present in many copies. Both the cDNA-encoded protein and its nucleotide sequence display striking homology to parallel sequences published for Torpedo AcChoEase. These findings demonstrate extensive homologies between the fetal BtChoEase encoded by these clones and other cholinesterases of various forms and species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983 Feb 1;209(2):331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M., Avivi A., Schlessinger J., Soreq H. Production of EGF-containing polypeptides in Xenopus oocytes microinjected with submaxillary gland mRNA. EMBO J. 1984 Jul;3(7):1499–1505. doi: 10.1002/j.1460-2075.1984.tb02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dutta-Choudhury T. A., Rosenberry T. L. Human erythrocyte acetylcholinesterase is an amphipathic protein whose short membrane-binding domain is removed by papain digestion. J Biol Chem. 1984 May 10;259(9):5653–5660. [PubMed] [Google Scholar]
- Dziegielewska K. M., Saunders N. R., Schejter E. J., Zakut H., Zevin-Sonkin D., Zisling R., Soreq H. Synthesis of plasma proteins in fetal, adult, and neoplastic human brain tissue. Dev Biol. 1986 May;115(1):93–104. doi: 10.1016/0012-1606(86)90231-9. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Engel A. G., Rosenberry T. L. Acetylcholinesterase of human erythrocytes and neuromuscular junctions: homologies revealed by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1078–1082. doi: 10.1073/pnas.79.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Rosenberry T. L. Quantitative identification of N-terminal amino acids in proteins by radiolabeled reductive methylation and amino acid analysis: application to human erythrocyte acetylcholinesterase. Anal Biochem. 1985 Jul;148(1):154–162. doi: 10.1016/0003-2697(85)90640-2. [DOI] [PubMed] [Google Scholar]
- Lockridge O., Bartels C. F., Vaughan T. A., Wong C. K., Norton S. E., Johnson L. L. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987 Jan 15;262(2):549–557. [PubMed] [Google Scholar]
- MacPhee-Quigley K., Taylor P., Taylor S. Primary structures of the catalytic subunits from two molecular forms of acetylcholinesterase. A comparison of NH2-terminal and active center sequences. J Biol Chem. 1985 Oct 5;260(22):12185–12189. [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ott P. Membrane acetylcholinesterases: purification, molecular properties and interactions with amphiphilic environments. Biochim Biophys Acta. 1985 Dec 9;822(3-4):375–392. doi: 10.1016/0304-4157(85)90016-4. [DOI] [PubMed] [Google Scholar]
- Prody C., Zevin-Sonkin D., Gnatt A., Koch R., Zisling R., Goldberg O., Soreq H. Use of synthetic oligodeoxynucleotide probes for the isolation of a human cholinesterase cDNA clone. J Neurosci Res. 1986;16(1):25–35. doi: 10.1002/jnr.490160105. [DOI] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Rakonczay Z., Brimijoin S. Monoclonal antibodies to rat brain acetylcholinesterase: comparative affinity for soluble and membrane-associated enzyme and for enzyme from different vertebrate species. J Neurochem. 1986 Jan;46(1):280–287. doi: 10.1111/j.1471-4159.1986.tb12959.x. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L. Asymmetric acetylcholinesterase is assembled in the Golgi apparatus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):479–483. doi: 10.1073/pnas.81.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Silman I., di Giamberardino L., Lyles L., Couraud J. Y., Barnard E. A. Parallel regulation of acetylcholinesterase and pseudocholinesterase in normal, denervated and dystrophic chicken skeletal muscle. Nature. 1979 Jul 12;280(5718):160–162. doi: 10.1038/280160a0. [DOI] [PubMed] [Google Scholar]
- Soreq H., Zevin-Sonkin D., Avni A., Hall L. M., Spierer P. A human acetylcholinesterase gene identified by homology to the Ace region of Drosophila. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1827–1831. doi: 10.1073/pnas.82.6.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Zevin-Sonkin D., Razon N. Expression of cholinesterase gene(s) in human brain tissues: translational evidence for multiple mRNA species. EMBO J. 1984 Jun;3(6):1371–1375. doi: 10.1002/j.1460-2075.1984.tb01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz M., Bjerrum O. J., Brodbeck U. Characterization of an active hydrophilic erythrocyte membrane acetylcholinesterase obtained by limited proteolysis of the purified enzyme. Biochim Biophys Acta. 1984 Sep 19;776(1):65–74. doi: 10.1016/0005-2736(84)90251-7. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut H., Matzkel A., Schejter E., Avni A., Soreq H. Polymorphism of acetylcholinesterase in discrete regions of the developing human fetal brain. J Neurochem. 1985 Aug;45(2):382–389. doi: 10.1111/j.1471-4159.1985.tb03999.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]