Abstract
Context
c-Met is important in the pathogenesis, invasion and spread of several forms of lung cancer and multiple c-Met inhibitors are undergoing clinical trials. PAX5 has been shown to upregulate c-Met in small cell lung carcinoma (SCLC), and co-inhibiting PAX5 and c-Met had a synergic effect in killing tumor cells. Paxillin is a downstream target of activated c-Met, and its activation leads to enhanced cell motility and tumor spread. The expression patterns of these functionally related proteins had not been systemically studied in neuroendocrine tumors of lung.
Objective
Our aim was to investigate the expression patterns of PAX5, paxillin, c-Met and phosphorylated c-Met (p-c-Met) in four categories of pulmonary neuroendocrine tumor.
Design
Tissue microarrays of 38 typical carcinoids (TC), 6 atypical carcinoids (AC), 34 SCLC, and 11 large cell neuroendocrine carcinomas (LCNEC) were studied with immunohistochemistry.
Results
A vast majority of four tumor types expressed c-Met, p-c-Met and paxillin. PAX5 was frequently expressed in AC, SCLC and LCNEC, but tended to be negative in TC. Coexpression of PAX5 with c-Met or p-c-Met was present in a majority of AC, SCLC and LCNEC. Significant correlation between PAX5 and paxillin was detected in SCLC and LCNEC, but not in carcinoid tumors.
Conclusions
The frequent coexpression of PAX5 with c-Met or p-c-Met in intermediate and high grade neuroendocrine tumors supports the therapeutic strategy of co-inhibiting these proteins. The discrepancy between high and low grade neuroendocrine tumors in terms of PAX5/paxillin expression correlation may be due to different underlying molecular genetics of these tumors.
INTRODUCTION
Neuroendocrine tumors of the lung include diverse entities ranging from highly aggressive small cell lung carcinoma (SCLC) and large cell neuroendocrine carcinoma (LCNEC), to relatively indolent carcinoid tumors. SCLC accounts for 16% of lung cancers, while the other two are relatively rare, together comprising 2–3% of lung cancers.1 They are designated as neuroendocrine tumors because many have so-called “neuroendocrine” features in regards to histology, electron microscopy and immunohistochemistry, such as organoid, trabecular, palisading, or rosettes growth patterns, finely granular chromatin, dense-core neurosecretory granules, and expression of neuroendocrine markers.2, 3 However, there are many exceptions, and each type of tumor has its own distinct morphological features that allow histopathological diagnosis in most cases. Their biological behaviors are also different. While SCLC and LCNEC are characterized by aggressive course and poor prognosis, carcinoids are typically indolent and have favorable prognosis. An intermediate category, atypical carcinoid (AC), is used to designate tumors with features between those of typical carcinoids (TC) and high grade neuroendocrine carcinomas (SCLC and LCNEC).4
The tyrosine kinase receptor c-Met is normally activated by its ligand hepatocyte growth factor (HGF), and plays an important role in the tumorigenesis of various cancers including lung cancers. Activating mutations of c-Met in SCLC were first identified by Ma et al,5 and were subsequently documented in non-small cell lung cancer (NSCLC) as well.6 Expression of c-Met was detected in nearly all NSCLC and SCLC cases, and strong expression was present in more than half of the tumors. Amplification of MET gene has also been identified and appeared to be one of the mechanisms causing acquired resistance to gefitinib in NSCLC.7 These findings prompted studies on various c-Met inhibitors, including small interfering RNA and small molecules such as SU11274. These inhibitors were shown to decrease the growth rate of lung cancer cells, further supporting the role of c-Met in lung cancers and giving hopes that c-Met might be used as a therapeutic target.6, 8 Multiple clinical trials are currently underway to evaluate the therapeutic value of a number of c-Met inhibitors.8 The significance of c-Met in lung carcinoid tumors has not been well characterized, although its strong expression was reported in a large proportion of these tumors.6
In SCLC, the expression level of c-Met did not appear to correlate with the presence of activating mutations.5 The expression regulation of c-Met in the setting of lung cancers may provide further insights to understanding its role in tumorigenesis. PAX5, a transcription factor essential for B cell development, was strongly expressed in most SCLC cases and appeared to upregulate c-Met transcription. This may be unique for SCLC because PAX5 expression was not detected in NSCLC and several other cancers studied.9
Activated c-Met produces its biological effects through a number of downstream proteins in the HGF/c-Met pathway. One of them is paxillin, a key focal adhesion protein that is essential for cell-matrix adhesion, cell motility and migration. HGF/c-Met signaling can induce paxillin phosphorylation at its tyrosine residue, which in turn promotes tumor progression by enhancing tumor cell migration and spread.10 Activating c-Met mutations have been shown to increase paxillin phosphorylation in SCLC.5 In addition, paxillin has been shown to be highly expressed, and its gene sometimes amplified or mutated in NSCLC 11. The role of paxillin in LCNEC and carcinoid has not been well studied.
The goal of this study was to evaluate the expression patterns of these three functionally related proteins, PAX5, c-Met and paxillin, in the setting of neuroendocrine tumors of the lung. We were particularly interested in possible correlation and coexpression between these markers.
MATERIALS AND METHODS
All tissues used in this study were under protocols approved by applicable Institutional Review Boards. Primary neuroendocrine tumors of the lung were selected from the archives of The Methodist Hospital, Houston, TX, including 38 TC, 6 AC, 34 SCLC and 11 LCNEC. Tissue microarrays (TMA) were assembled with 3 cores from each case, taken at representative foci and each measuring 1 mm in diameter. Monoclonal anti-PAX5 antibody was obtained from BD Biosciences (Franklin Lakes, NJ); monoclonal anti-c-Met antibody and polyclonal anti-phosphorylated c-Met (p-c-Met, pY1230/1234/1235) antibody were obtained from Biosource (Carlsbad, CA); monoclonal anti-paxillin antibody was obtained from Abcam (Cambridge, MA).
Immunohistochemical stains were performed with standard protocols. Briefly, 5 micron sections of TMA were first deparaffinized and rehydrated, followed by antigen retrieval by heating the sections in ethylenediaminetetraacetic acid (EDTA) buffer at pH 9 for 15 minutes. Endogenous peroxidase activity was removed by incubating the sections with 3% H2O2 in methanol for 5 minutes. Non-specific binding was minimized by incubation with Protein Block (DAKO, Carpinteria, CA) for 20 minutes. After that, the sections were incubated with the primary antibody for 1 hour, followed by the secondary antibody conjugated to a horseradish peroxidase-labeled polymer for 30 minutes. Slides were then developed with 3,3’-diaminobenzidine chromogen and counterstained with hematoxylin.
Scoring of the staining intensity in the cytoplasm and the nucleus was separately performed as follows: 0 if there was no stain; if there was any stain, a numeric score of 1, 2 or 3 was assigned semi-quantitatively corresponding to increasing intensity. Then, scores of the different cores of the same case were averaged, and the result was converted to a categorical score: negative (<0.5), weakly positive (0.5–2) and strong positive (≥2).
RESULTS
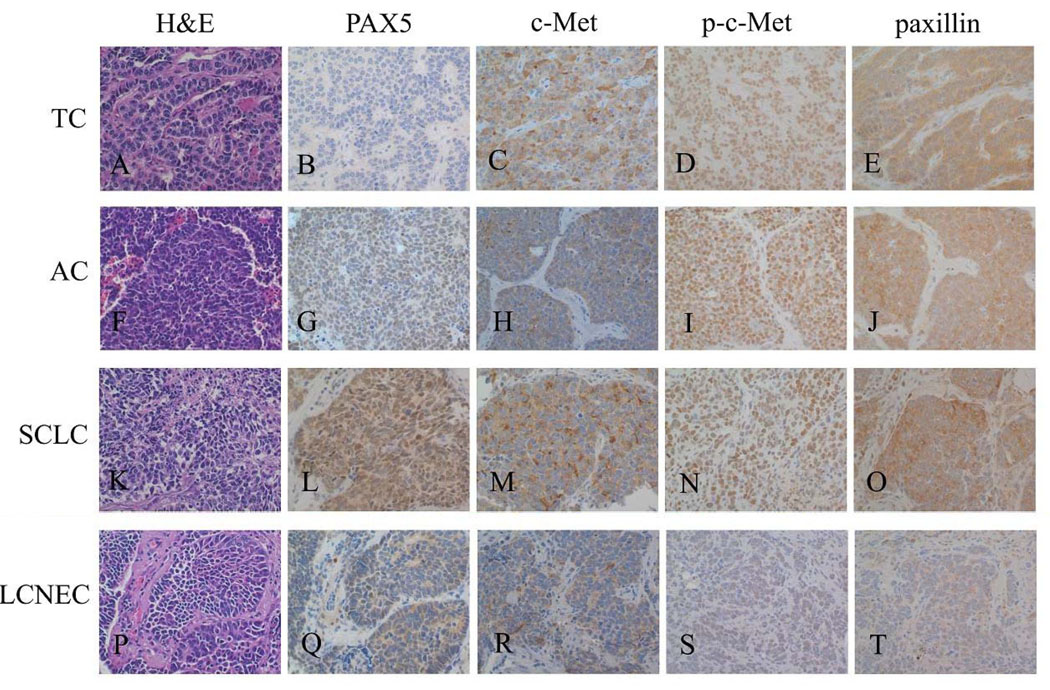
The expression levels of the four markers are summarized in Table 1. Photomicrographs of representative cases, one from each tumor type, are shown in Figure 1. Both c-Met and p-c-Met were positive in a vast majority of all four tumor types, and were often strongly positive. In fact, all tumors (100%) included in this study expressed at least one of these two proteins, and more than 80% of them strongly expressed at least one of these two proteins (Table 2). Consistent with previous results, c-Met staining signal was mainly present in the cytoplasm, while p-c-Met showed a predominantly nuclear staining pattern (Figure 1).
Table 1.
Expression levels of PAX5, c-Met, p-c-Met and paxillin in neuroendocrine tumors of the lung
| Tumor Type |
PAX-5 | c-Met | p-c-Met | Paxillin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neg. | Weak | Strong | Neg. | Weak | Strong | Neg. | Weak | Strong | Neg. | Weak | Strong | |
| TC (n=38) |
27 (71%) |
11 (29%) |
0 (0%) |
3 (8%) |
10 (26%) |
25 (66%) |
2 (5%) |
17 (45%) |
19 (50%) |
0 (0%) |
8 (21%) |
30 (79%) |
| AC (n=6) |
2 (33%) |
3 (50%) |
1 (17%) |
0 (0%) |
2 (33%) |
4 (67%) |
0 (0%) |
2 (33%) |
4 (67%) |
0 (0%) |
2 (33%) |
4 (67%) |
| SCLC (n=34) |
6 (18%) |
13 (38%) |
15 (44%) |
3 (9%) |
14 (41%) |
17 (50%) |
0 (0%) |
11 (32%) |
23 (68%) |
1 (3%) |
17 (50%) |
16 (47%) |
| LCNEC (n=11) |
3 (27%) |
5 (46%) |
3 (27%) |
1 (9%) |
4 (36%) |
6 (55%) |
0 (0%) |
7 (64%) |
4 (36%) |
1 (9%) |
8 (73%) |
2 (18%) |
TC, typical carcinoid; AC, atypical carcinoid; SCLC, small cell lung carcinoma; LCNEC, large cell neuroendocrine carcinoma
Figure 1. Photomicrographs of representative cases.
Abbreviations: TC, typical carcinoid; AC, atypical carcinoid; SCLC, small cell lung carcinoma; LCNEC, large cell neuroendocrine carcinoma. Hematoxylin and eosin (H&E) stains and immunohistochemical stains of 4 representative cases are shown here. The magnification is 400× for all images. TC: 1A, H&E; 1B, negative PAX5; 1C, strong c-Met; 1D, strong p-c-Met; 1E, strong paxillin. AC: 1F, H&E; 1G, weak PAX5; 1H, weak c-Met; 1I, strong p-c-Met; 1J, strong paxillin. SCLC: 1K, H&E; 1L, strong PAX5; 1M, strong c-Met; 1N, strong p-c-Met; 1O, strong paxillin. LCNEC: 1P, H&E; 1Q, weak PAX5; 1R, weak c-Met; 1S, weak p-c-Met; 1T, weak paxillin.
Table 2.
Combined expression levels of c-Met and p-c-Met
| Tumor Type |
c-Met or p-c-Met | |
|---|---|---|
| Positive | Strong | |
| TC (n=38) |
38 (100%) |
30 (79%) |
| AC (n=6) |
6 (100%) |
6 (100%) |
| SCLC (n=34) |
34 (100%) |
28 (82.4%) |
| LCNEC (n=11) |
11 (100%) |
8 (72.7%) |
TC, typical carcinoid; AC, atypical carcinoid; SCLC, small cell lung carcinoma; LCNEC, large cell neuroendocrine carcinoma
The expression levels of c-Met and p-c-Met appeared similar among four tumor types, as Chi-square tests did not show significant difference (c-Met, P=.86; p-c-Met, P=.34). However, the expression of PAX5 varied significantly between different tumor types (P<.001, Chi-square), lower in TC than in AC, SCLC and LCNEC. Paxillin also showed significantly different expression levels (P=.008, Chi-square), highest in TC and lowest in LCNEC.
Because PAX5 has been shown to regulate the transcription of c-Met, we analyzed the coexpression pattern of these two proteins. There was frequent coexpression of PAX5 with c-Met or p-c-Met in AC, SCLC and LCNEC, and a significant proportion of cases had strong coexpression (Table 3). In contrast, coexpression was relatively rare in TC (Table 3).
Table 3.
Coexpression pattern of PAX5 with c-Met or p-c-Met
| Tumor Type |
PAX5 + c-Met | PAX5 + p-c-Met | PAX5 + c-Met or p-c-Met | |||
|---|---|---|---|---|---|---|
| Positive | Strong | Positive | Strong | Positive | Strong | |
| TC (n=38) |
11 (29%) |
0 (0%) |
10 (26%) |
0 (0%) |
11 (29%) |
0 (0%) |
| AC (n=6) |
4 (67%) |
1 (17%) |
4 (67%) |
1 (17%) |
4 (67%) |
1 (17%) |
| SCLC (n=34) |
26 (76%) |
9 (26%) |
28 (82%) |
9 (26%) |
28 (82%) |
11 (32%) |
| LCNEC (n=11) |
7 (64%) |
1 (9%) |
8 (73%) |
1 (9%) |
8 (73%) |
2 (18%) |
TC, typical carcinoid; AC, atypical carcinoid; SCLC, small cell lung carcinoma; LCNEC, large cell neuroendocrine carcinoma
The semi-quantitative staining intensities of the four markers were also compared with each other by Pearson’s correlation coefficient. The correlation between PAX5 and paxillin was moderate to strong in SCLC (r=0.5, P=.001) and LCNEC (r=0.81, P=.001), but very weak in TC (r=0.22, P=.11). Their correlation in AC (r=0.5) failed to show statistical significance (P=.15), possibly due to the small sample size of AC (n=6). Correlation between other markers was weak and did not show statistical significance.
DISCUSSION
All four types of neuroendocrine tumors of the lung showed frequent expression of c-Met and p-c-Met. A majority of these tumors had strong expression, supporting the role played by c-Met in tumor biology as well as the potential use of c-Met as a therapeutic target, especially in SCLC and LCNEC for which there are currently only limited and largely unsuccessful treatment options. Nuclear translocation of phosphorylated c-Met was observed, although its biological significance is not fully understood. We did not see any significant correlation between the expression levels of c-Met and p-c-Met, suggesting that independent mechanisms are in place to control the expression of c-Met and the activation/phosphorylation of c-Met in the setting of neuroendocrine tumors. This is in keeping with the previous observation that there was no correlation between c-Met mutations and its expression level in SCLC.5 It is known that immunohistochemistry has inherent limitations as a technique for measuring the level of protein, especially in formalin fixed paraffin embedded tissues. Therefore, it is possible that the results were biased.
PAX5 is a transcription factor essential for B cell development, and is widely used in hematopathology practice as a specific marker to recognize B cell lineage. It was shown recently that PAX5 was also expressed in neuroendocrine tumors of the lung, especially SCLC and LCNEC.9 More importantly, PAX5 appeared to directly promote the transcription of c-Met; and knocking down PAX5 had a synergizing effect with c-Met inhibitors in killing SCLC cells.9 This observation brought up the possibility of co-targeting both proteins for the treatment of lung cancers. Our results showed that coexpression of PAX5 and c-Met or p-c-Met was frequent in AC, SCLC and LCNEC, supporting that the co-targeting strategy may be useful.
Paxillin is one of the downstream molecules of the HGF/c-Met signaling pathway. It undergoes phosphorylation upon receiving the HGF/c-Met signal, and enhances tumor cell migration and spread. Strong expression of paxillin was observed in a large proportion of NSCLC, and seemed to correlate with higher stage and metastasis. Paxillin gene amplification and mutation were also identified in lung cancers.11 Interestingly, our results showed a moderate to strong correlation between the expression levels of paxillin and PAX5 in SCLC and LCNEC. We could not find any evidence in the literature that suggests an intrinsic linkage between the expression control mechanisms of these two proteins. Whether it is simply a coincidence or intrinsically associated with the biology of these tumors would be an interesting topic for future investigation. Unlike SCLC and LCNEC, no correlation between paxillin and PAX5 was detected in TC. In fact, TC had much scantier PAX5 expression than SCLC and LCNEC, despite having similar expression for the other three markers tested. This discrepancy may be due to different molecular genetics underlying these neuroendocrine tumors. SCLC and LCNEC have been regarded as closely-related, and some authors think they are actually similar entities within a spectrum. Clinically, tumors with overlapping features of SCLC and LCNEC exist that cannot be confidently diagnosed as one or the other by histopathology. Carcinoid, on the other hand, is quite distinct both clinically and biologically compared to SCLC and LCNEC.1 Our results provide another piece of evidence in this regard. In this study, the PAX5 expression level in AC appeared to be stronger than TC, but weaker than SCLC and LCNEC. There was no statistically significant correlation between PAX5 and paxillin in AC. However, the sample size of AC in this study was small (n=6).
In summary, we have shown that a vast majority of all four categories of neuroendocrine tumors of the lung express c-Met, p-c-Met and paxillin. In SCLC and LCNEC, PAX5 is frequently expressed and its expression level correlates with that of paxillin.
Acknowledgments
Supported by NIH/NCI Grants: 5RO1CA125541-03, 3RO1CA125541-03S109, 5RO1CA129501-02, 3RO1CA129501-02S109, 5R01CA100750-06, 3R01CA100750-06S109, Cancer Research Foundation and American Respiratory Health Association of America (RS)
REFERENCES
- 1.Jafri N, Salgia R. Biology and novel therapeutics for neuroendocrine tumors of the lung. J Biol Regul Homeost Agents. 2004;18(3–4):275–290. [PubMed] [Google Scholar]
- 2.Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma: an ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15(6):529–553. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Gal AA, Colby TV, Klimstra DS, Falk R, Koss MN. Reproducibility of neuroendocrine lung tumor classification. Hum Pathol. 1998;29(3):272–279. doi: 10.1016/s0046-8177(98)90047-8. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22(8):934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ma PC, Kijima T, Maulik G, et al. c-MET mutational analysis in small cell lung cancer: Novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63(19):6272–6281. [PubMed] [Google Scholar]
- 6.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65(4):1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 8.Kim ES, Salgia R. MET pathway as a therapeutic target. J Thorac Oncol. 2009;4(4):444–447. doi: 10.1097/JTO.0b013e31819d6f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanteti R, Nallasura V, Loganathan S, et al. PAX5 is expressed in small-cell lung cancer and positively regulates c-met transcription. Lab Invest. 2009;89(3):301–314. doi: 10.1038/labinvest.2008.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parr C, Davies G, Nakamura T, Matsumoto K, Mason MD, Jiang WG. The HGF/SF-induced phosphorylation of paxillin, matrix adhesion, and invasion of prostate cancer cells were suppressed by NK4, an HGF/SF variant. Biochem Biophys Res Commun. 2001;285(5):1330–1337. doi: 10.1006/bbrc.2001.5307. [DOI] [PubMed] [Google Scholar]
- 11.Jagadeeswaran R, Surawska H, Krishnaswamy S, et al. Paxillin is a target for somatic mutations in lung cancer: Implications for cell growth and invasion. Cancer Res. 2008;68(1):132–142. doi: 10.1158/0008-5472.CAN-07-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]