Abstract
We used a new theory of the biological basis of the Big Five personality traits to generate hypotheses about the association of each trait with the volume of different brain regions. Controlling for age, sex, and whole-brain volume, results from structural magnetic resonance imaging of 116 healthy adults supported our hypotheses for four of the five traits: Extraversion, Neuroticism, Agreeableness, and Conscientiousness. Extraversion covaried with volume of medial orbitofrontal cortex, a brain region involved in processing reward information. Neuroticism covaried with volume of brain regions associated with threat, punishment, and negative affect. Agreeableness covaried with volume in regions that process information about the intentions and mental states of other individuals. Conscientiousness covaried with volume in lateral prefrontal cortex, a region involved in planning and the voluntary control of behavior. These findings support our biologically based, explanatory model of the Big Five and demonstrate the potential of personality neuroscience (i.e., the systematic study of individual differences in personality using neuroscience methods) as a discipline.
Keywords: personality, Big Five, neuroimaging, brain structure
Personality psychology promotes a systematic approach to understanding individual differences in behavior, emotion, motivation, and cognition, through the development of comprehensive taxonomies of personality traits. However, these descriptive taxonomies have not typically been linked to empirical data from neuroscience research. Individual differences are relevant to education and health, as well as basic science, and are therefore increasingly the focus of cognitive, affective, and social neuroscience research (Kosslyn et al., 2002). However, such efforts have not typically been systematic or comprehensive. Personality neuroscience is emerging as a subdiscipline focused on testing and refining neurobiological theories of personality (Canli, 2008; DeYoung & Gray, 2009). In the study reported by this article, we used magnetic resonance imaging (MRI) to test a biological theory of the Big Five personality traits (DeYoung & Gray, 2009): Extraversion, Neuroticism, Agreeableness, Conscientiousness, and Openness/Intellect.
The fact that many traits vary together (e.g., people who are talkative tend to experience more positive emotion than people who are quiet) implies that a limited number of underlying factors may account for much of the variation in personality traits. A large body of evidence has converged on a five-factor solution to the correlations among personality traits, leading to a taxonomy known as the Five-Factor Model, or Big Five (Costa & McCrae, 1992; John, Naumann, & Soto, 2008). However, the Big Five factors are descriptive rather than explanatory constructs and do not inherently provide a theory of the underlying forces that produce these five dimensions of individual differences in personality. A causal theory of the Big Five would be a significant advance in this field (cf. McCrae & Costa, 2008) and should include a biological component (i.e., it should specify not only the psychological mechanisms underlying each trait, but also the biological systems linked to these psychological mechanisms). Personality traits represent tendencies to manifest particular patterns of cognition, emotion, motivation, and behavior, in response to a variety of eliciting stimuli (Fleeson, 2001). These tendencies are posited to arise from regularities in the functioning of relevant brain systems (DeYoung & Gray, 2009).
Our aim in this study was to test whether individual variation in the volume of different brain regions relates to personality in a manner consistent with our theory of the psychological mechanisms and biological systems underlying each of the Big Five factors (DeYoung & Gray, 2009). We outline here the psychological processes associated with each factor and the neural systems linked to these processes. Our intent was not to provide an exhaustive review of the psychological mechanisms or neural systems involved in each of the Big Five, but rather to identify those likely to be most central to these traits. We therefore developed hypotheses regarding the structural covariation of specific brain regions with each of the Big Five. It is important to note that variation in function is not inevitably associated with variation in brain structure, so we did not expect to confirm every plausible association between traits and the volume of specific regions.
A Biological Model of the Big Five
Of the Big Five, Extraversion and Neuroticism are the best understood in terms of their underlying processes. There is considerable theoretical and experimental evidence that these two traits represent the primary manifestations in personality of sensitivity to reward and sensitivity to threat and punishment, respectively (Clark & Watson, 2008; Depue & Collins, 1999). Much is known about the biological systems governing reward and punishment, and findings from neuroimaging and psychopharmacological research on Extraversion and Neuroticism are consistent with the involvement of these biological systems in these traits (DeYoung & Gray, 2009).
Extraversion is linked to the tendency to experience positive emotions (Clark & Watson, 2008; Costa & McCrae, 1992), which typically stem from experiences of reward or the promise of reward. Extraversion encompasses an array of traits, such as assertiveness, sociability, and talkativeness, that appear to be linked to the approach tendencies that accompany sensitivity to reward. Although Extraversion is often manifested in social behavior, this is probably because many human rewards involve social affiliation or status; reward sensitivity thus remains at the core of Extraversion. We therefore hypothesized that Extraversion would be associated with structural variation in some or all of the brain systems responsible for sensitivity to reward, including the nucleus accumbens, amygdala, and orbitofrontal cortex (Depue & Collins, 1999).
Neuroticism is linked to the tendency to experience negative emotions (Clark & Watson, 2008; Costa & McCrae, 1992), and includes such traits as anxiety, self-consciousness, and irritability. We therefore hypothesized that Neuroticism would be associated with structural variation in some or all of the brain systems associated with sensitivity to threat and punishment, including the amygdala, anterior and mid-cingulate cortex, medial prefrontal cortex (PFC), and hippocampus (Eisenberger & Lieberman, 2004; J.A. Gray & McNaughton, 2000; Heatherton, Macrae, & Kelley, 2004). Although our previous presentation of our biological theory of the Big Five (DeYoung & Gray, 2009) did not review evidence for the involvement of medial PFC in Neuroticism, recent studies have shown an association between this trait and neural activity in this region (Haas, Constable, & Canli, 2008; Williams et al., 2006). In addition, involvement of this region in self-evaluation and emotion regulation is well established (Heatherton et al., 2004; Ochsner & Gross, 2005), and low self-esteem, rumination, and emotional dysregulation are all hallmarks of Neuroticism (Costa & McCrae, 1992; John et al., 2008).
Agreeableness appears to identify the collection of traits related to altruism: one's concern for the needs, desires, and rights of others (as opposed to one's enjoyment of others, which appears to be related primarily to Extraversion). The positive pole of Agreeableness describes prosocial traits, such as cooperation, compassion, and politeness, whereas its negative pole describes antisocial traits, such as callousness and aggression. Agreeableness has been linked to psychological mechanisms that allow the understanding of others’ emotions, intentions, and mental states, including empathy, theory of mind, and other forms of social information processing (e.g., Graziano, Habashi, Sheese, & Tobin, 2007; Nettle & Liddle, 2008). We therefore hypothesized that Agreeableness would be associated with brain structures involved in these mechanisms, including the superior temporal sulcus, temporoparietal junction, and posterior cingulate cortex (Pelphrey & Morris, 2006; Saxe & Powell, 2006).
Conscientiousness appears to reflect the ability and tendency of individuals to inhibit or constrain impulses in order to follow rules or pursue nonimmediate goals. This trait is linked to both academic and occupational success, as well as to behavior that promotes health and longevity (Ozer & Benet-Martinez, 2006). Conscientiousness is characterized by traits such as industriousness, orderliness, and self-discipline, as opposed to impulsivity, distractibility, and disorganization. Conscientiousness is likely to be associated with functions of PFC, which is thought to be responsible for much of the human ability to plan and follow complex rules (Bunge & Zelazo, 2006; Miller & Cohen, 2001). Functional neuroimaging studies have linked trait impulsivity to both dorsal and ventral regions of lateral PFC (Asahi, Okamato, Akado, Yamawaki, & Yokota, 2004; Brown, Manuck, Flory, & Hariri, 2006). We therefore hypothesized that Conscientiousness would be associated with structural variation in lateral PFC.
Openness/Intellect appears to reflect the tendency to process abstract and perceptual information flexibly and effectively, and includes traits such as imagination, intellectual engagement, and aesthetic interest (DeYoung, Peterson, & Higgins, 2005). A higher degree of Openness/Intellect suggests a larger bandwidth of information processing: an increase in the “breadth, depth, and permeability of consciousness” (McCrae & Costa, 1997, p. 826). This trait is likely to involve the PFC and functionally related regions, particularly those involved in working memory, abstract reasoning, and the control of attention (DeYoung et al., 2005). Openness/Intellect is the only Big Five trait to be consistently and positively associated with intelligence (DeYoung et al., 2005), a faculty that appears to be governed by brain systems that overlap substantially with the working memory network (J.R. Gray, Chabris, & Braver, 2003). We hypothesized that Openness/Intellect would be associated with structural variation in some or all of the brain systems involved in the regulation of working memory, attention, and reasoning, including dorsolateral PFC, anterior PFC (frontal pole), and anterior parietal cortex (DeYoung et al., 2005; DeYoung, Shamosh, Green, Braver, & Gray, 2009).
Note that we hypothesized both Conscientiousness and Openness/Intellect to be associated with lateral PFC. Individual brain regions can serve multiple functions, and Conscientiousness and Openness/Intellect may reflect complementary but potentially conflicting functions of lateral PFC: ensuring stable execution of plans and rules (associated with Conscientiousness) and manipulating abstract information in order to explore alternative possibilities (associated with Openness/Intellect; DeYoung & Gray, 2009). Nonetheless, the prediction of findings for two traits in the same general brain region raises the issue of discriminant validity, and some inferences can be made about which regions are not associated with particular traits. For example, Conscientiousness and Openness/Intellect are not likely to be associated with primarily affective regions, such as the amygdala and nucleus accumbens. Similarly, Extraversion and Neuroticism, which appear to reflect basic affective systems, are not likely to be associated with the areas of lateral cortex that are involved in top-down control and are hypothesized to be associated with Conscientiousness and Openness/Intellect. Finally, we would not expect any of the five traits to be associated with primary visual cortex.
In testing hypotheses about which brain regions are likely to be associated structurally with each of the Big Five factors, a preliminary question is how brain structure—specifically, the relative volume of different brain regions—relates to brain function. A greater-than-average volume of a specific brain structure may signify greater-than-average power to carry out specific functions associated with that structure, on the assumption that larger populations of neurons can produce larger outputs and can therefore be more influential than smaller populations of neurons. However, a smaller-than-average volume of a given structure might indicate increased efficiency, or that the structure is streamlined to perform a particular function or set of functions. Good evidence favors the larger-is-more-powerful position: Training on particular tasks has been shown to increase the volume of functionally relevant brain structures (Boyke, Driemeyer, Gaser, Buchel, & May, 2008). However, developmental studies on the association of cortical thickness with intelligence provide some evidence that efficiency may also be an important force, as individuals with above-average intelligence show greater reductions in cortical volume in late childhood in comparison with individuals of average intelligence (Shaw et al., 2006). Nonetheless, more intelligent children also show more cortical thickening prior to the period of thinning, and intelligence in both children and adults correlates positively with brain volume (McDaniel, 2005). Thus, it seems reasonable to generalize tentatively that volume tends to covary positively with function. It would be imprudent, however, to make strong predictions regarding the direction of effect for associations of personality traits with volume in relevant neural structures.
Although the direction of volume-function relations is not entirely certain, structural scans were more advantageous than functional scans for this study, as they provide data relevant to all brain systems simultaneously, rather than data relevant only to those systems engaged by the particular task being performed during a functional scan. Thus, we were able to test hypotheses for all of the Big Five traits using a single scan for each individual. Of course, a trait related to the function of a particular brain system is not necessarily associated with gross structural differences in that system. However, if the data indicate that the trait is related to structural differences in a given system, they provide evidence that the system is relevant to that trait. Thus, structural brain scans provide one important way to test theories in personality neuroscience. If a trait is hypothesized to be associated with the functions of a set of brain regions, the finding that the trait in question is associated with structural variation in any of those regions supports the hypothesis.
Method
Participants
Healthy, right-handed participants were recruited from Washington University in St. Louis and the surrounding community (N = 116: 58 females and 58 males; plus 1 female serving as a within-study reference subject; ages 18–40 years, mean age = 22.9, SD = 5.5). Participants gave informed consent and were prescreened to exclude anyone with a history of neurological or psychiatric disorders or use of psychoactive drugs. We utilized this sample in previous, unrelated studies (e.g., DeYoung et al., 2009), none of which reported on the relation of the Big Five to brain structure. The experimental protocol was approved by the Washington University Medical Center Human Subjects Committee.
Measures
We administered the self-report version of the Revised NEO Personality Inventory (NEO-PI-R; Costa & McCrae, 1992) to assess the Big Five personality factors. Cronbach's alphas for the Big Five scales were high—Neuroticism: .92; Extraversion: .87; Openness/Intellect: .89; Agreeableness: .91; Conscientiousness: .91. Participants returned individually on a different day for the MRI session.
Image acquisition and analysis
A 3-T Allegra System (Siemens, Erlangen, Germany) was used to acquire a high-resolution structural image—a whole-brain magnetization-prepared rapid gradient-echo (MPRAGE) image—for each subject. (See the Supplemental Material available online for additional methodological details.) Preprocessing steps for each subject involved extraction of brain data, coregistration of the resulting brain-only image to a reference subject using BioImage Suite (Papademetris et al., 2006), and image smoothing. The reference subject was selected from the sample of 117 participants on the basis of being near the group average for personality traits and having a good structural image scan and extraction. The reference subject's brain-only image was standardized by an affine transformation to a brain-only version of the Colin27 brain template (Holmes et al., 1998). All other subjects were then nonlinearly registered to the standardized reference subject, leaving each subject in standard space and coregistered to a within-study reference image. Registration quality was good for all subjects.
For any given subject's brain, the spatial transformation needed to achieve coregistration with the reference subject's brain contains the information of interest: the degree of local (voxel-level) expansion or contraction required for the subject image to match the reference image. The determinant of the Jacobian of the transformation is effectively a local scaling factor that, when applied, expands or contracts one brain locally so that it matches the other brain in structure. The nonlinear component of the transformation is of most interest because it does not include individual differences in whole-brain volume or other linear effects of no interest (such as translations) that apply uniformly across the brain (for that subject). For all subjects, a three-dimensional image of the nonlinear component of the determinant of the Jacobian across the brain was smoothed using an 8-mm full-width at half-maximum (FWHM) Gaussian kernel. The smoothed images were then used as the dependent measure in a voxel-based (mass-univariate) general linear model (GLM), with sex as a fixed effect, and age and the Big Five as continuous covariates.
To identify regions of interest (ROIs) in which local brain volume was significantly associated with each personality trait independently (controlling simultaneously for the other traits, plus age, sex, and whole-brain volume), we used a corrected threshold of p < .05, with a cluster-based correction for multiple comparisons based on effect size and spatial extent. An initial, uncorrected threshold of p < .01 at each voxel was chosen because it corresponded to an effect size (r) of .24 (with our sample size). Approximately half of all published significant effect sizes in psychology are equal to or greater than .24 (Hemphill, 2003), and we aimed to detect any effect larger than half of all published effects. With an uncorrected threshold of p < .01 at each voxel, we used Monte-Carlo simulation (AlphaSim, Ward, 2000; see the Supplemental Material) to identify the minimum cluster size needed to correct for multiple comparisons: 317 contiguous voxels. ROIs were then identified on the basis of meeting both the effect-size and the cluster-size criteria.
Results
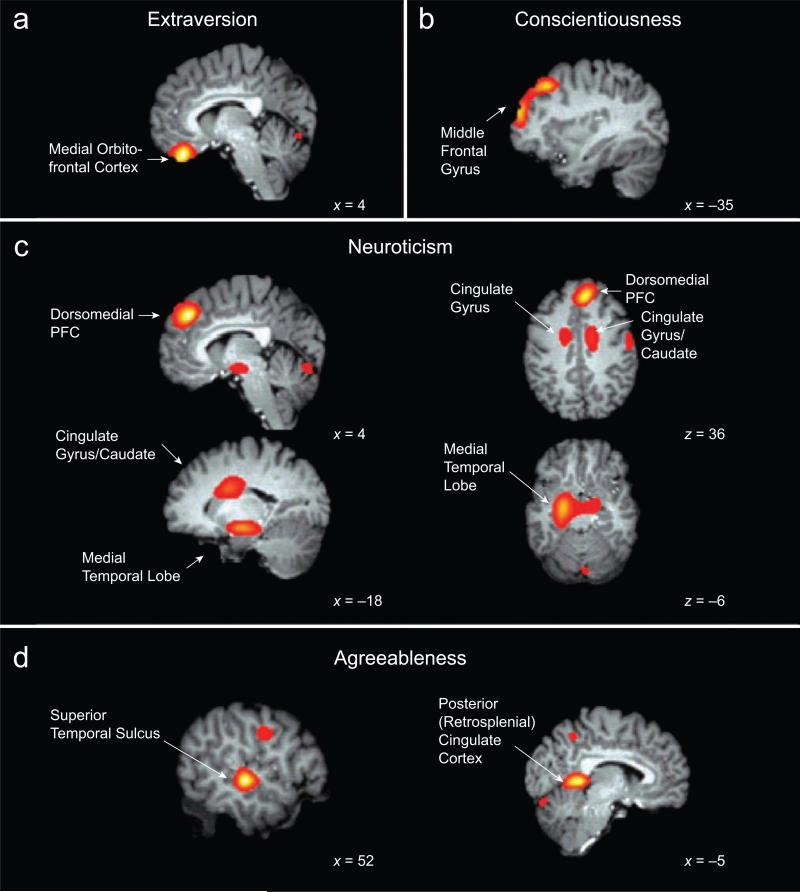
The results of our analysis are reported in Table 1, which lists all clusters related to each of the Big Five traits, controlling for age, sex, and the other traits. The table also indicates which of these clusters reached the cluster-size threshold for significance (p < .05, corrected), in order to be considered ROIs. (Controlling for indices of registration quality did not substantively change results; see the Supplemental Material.) We found evidence to support our hypotheses about four of the Big Five. Specifically, for Extraversion, Neuroticism, Agreeableness, and Conscientiousness, ROIs were in the set of regions hypothesized to be related to each trait (see Table 1 and Fig. 1).
Table 1.
Brain Regions in Which Relative Local Volume Was Associated With a Big Five Trait
| Trait and brain region | Brodmann's area | Centroid (Talairach coordinates): x, y, z | Number of voxels | β |
|---|---|---|---|---|
| Extraversion | ||||
| Medial orbitofrontal cortex | 11 | 5, 33, –20 | 646* | 0.35 |
| Superior temporal sulcus | 22 | –60, –32, 4 | 57 | –0.27 |
| Cerebellum | — | 10, –74, –6 | 256 | –0.29 |
| Neuroticism | ||||
| Medial temporal lobe, basal ganglia | 28/34 | –12, –12, –7 | 1,822* | –0.41 |
| Medial frontal gyrus | 8/9 | 3, 38, 39 | 1,582* | –0.37 |
| Mid-cingulate gyrus | 24 | 15, –4, 31 | 693* | 0.31 |
| Mid-cingulate gyrus, caudate | 24 | –20, –1, 25 | 995* | 0.34 |
| Middle temporal gyrus | 37 | –52, –56, 2 | 654* | 0.36 |
| Precentral gyrus | 4 | 56, –10, 26 | 320* | –0.32 |
| Cerebellum | — | 22, –37, –42 | 697* | 0.39 |
| Cerebellum | — | –52, –46, –30 | 25 | 0.29 |
| Cerebellum | — | 3, –74, –8 | 124 | 0.28 |
| Agreeableness | ||||
| Superior temporal sulcus | 22/42 | 52, –20, 3 | 533* | –0.34 |
| Posterior cingulate | 29 | –5, –45, 6 | 813* | 0.34 |
| Fusiform gyrus | 18 | 36, –81, –7 | 1,059* | 0.37 |
| Middle temporal gyrus | 21/37 | –60, –54, 8 | 17 | 0.26 |
| Precentral gyrus | 6 | 55, –9, 31 | 105 | –0.28 |
| Precuneus | 7 | –2, –54, 43 | 144 | –0.28 |
| Middle frontal gyrus | 8/9 | –45, 25, 39 | 229 | –0.30 |
| Cerebellum | — | –6, –79, –13 | 64 | 0.26 |
| Conscientiousness | ||||
| Middle frontal gyrus | 8/9/10 | –35, 36, 32 | 629* | 0.43 |
| Inferior frontal gyrus | 44 | –54, 16, 12 | 35 | –0.27 |
| Paracentral lobule | 5 | –3, –34, 48 | 22 | –0.27 |
| Fusiform gyrus | 19 | 35, –80, –11 | 476* | –0.32 |
| Superior parietal lobule | 7 | 34, –60, 48 | 222 | 0.31 |
| Openness/Intellect | ||||
| Inferior parietal lobule | 40 | 47, –31, 43 | 188 | 0.31 |
| Lingual gyrus | 18 | 27, –78, –3 | 40 | –0.28 |
| Middle temporal gyrus | 37 | –60, –46, –10 | 19 | –0.28 |
Note: N = 116.
p < .05, corrected for cluster size.
Fig. 1.
Brain regions in which local volume was significantly associated with (a) Extraversion, (b) Conscientiousness, (c) Neuroticism, and (d) Agreeableness, as hypothesized (see also Table 1). Coordinates indicate the locations of the brain slices. Color is related to effect size, with lighter color signifying a larger effect, and darker color signifying a smaller effect. PFC = prefrontal cortex.
For Extraversion, the only significant association with volume was a positive one in medial orbitofrontal cortex. For Neuroticism, the two largest regions of association were in right dorsomedial PFC and in portions of the left medial temporal lobe, including posterior hippocampus, as well as portions of basal ganglia and midbrain, including globus pallidus and bilateral subthalamic nuclei. Both of these associations were negative. Regions of positive association with Neuroticism were seen bilaterally in mid-cingulate cortex, extending into the white matter of the cingulate gyrus and, in the left hemisphere, into the caudate. In addition, three regions not hypothesized to be involved were found to be associated with Neuroticism: one region in the middle temporal gyrus and one in the cerebellum (both positive associations), and one region in right precentral gyrus (a negative association). Finding significant associations involving regions not included in our hypotheses in no way invalidates our theory but does suggest candidate brain regions and systems that might fruitfully be incorporated into a theory of the Big Five, given replication.
For Agreeableness, there was a significant positive association in the retrosplenial region of posterior cingulate cortex and a significant negative association in superior temporal sulcus and adjacent superior temporal gyrus. An additional, unpredicted, positive association with Agreeableness was found in fusiform gyrus.
Conscientiousness was associated positively with volume in a region of lateral PFC extending across most of the left middle frontal gyrus. An unpredicted negative association with Conscientiousness was found in posterior fusiform gyrus.
Openness/Intellect did not show any significant associations with relative local brain volume. This trait was associated with one cluster in right parietal cortex (p < .01, uncorrected), but this cluster was not large enough to reach the cluster-size threshold (p < .05, corrected). Although this association is consistent with our hypotheses, it could also be due to chance.
Discussion
To test our hypotheses on the biological substrates of the Big Five personality traits, we examined the association between the Big Five and relative local volume throughout the brain. We based specific hypotheses about individual traits on the premise that each trait would be associated with structure in one or more of the brain regions known to be involved in functions central to the trait in question. We also formulated more general hypotheses about regions we did not expect to be related to some or all traits. Our data clearly supported our hypotheses for four of the Big Five factors—Extraversion, Neuroticism, Agreeableness, and Conscientiousness—but were not significant for Openness/Intellect, despite appearing to be consistent with our hypothesis. These findings provide support for a model that posits biological systems and psychological mechanisms underlying the Big Five (DeYoung & Gray, 2009).
Extraversion was associated with the volume of medial orbitofrontal cortex. This region is involved in coding the reward values of stimuli, and has therefore been hypothesized to be a substrate of Extraversion (Depue & Collins, 1999), which appears to reflect sensitivity to reward. Increased volume of orbitofrontal cortex has been associated with Extraversion in two other studies (Omura, Constable, & Canli, 2005; Rauch et al., 2005), and our study provides further evidence for this association.
Neuroticism was associated with reduced volume in dorsomedial PFC and a segment of left medial temporal lobe including posterior hippocampus, and with increased volume in the mid-cingulate gyrus, including both gray and white matter. These associations are consistent with the theory that Neuroticism represents the primary manifestation in personality of sensitivity to threat and punishment, encompassing traits that involve negative emotion and emotional dysregulation. J.A.
These associations are consistent with the theory that Neuroticism represents the primary manifestation in personality of sensitivity to threat and punishment, encompassing traits that involve negative emotion and emotional dysregulation. J.A. Gray and McNaughton (2000) have implicated the hippocampus in the detection of uncertainty and goal conflict and in the control of rumination and anxiety, functions they linked to Neuroticism. In addition, reduced hippocampal volume has been associated with stress and depression (Bremner et al., 2000). The mid-cingulate cortex has been associated with detection of error and response to pain, both physical and emotional (Carter et al., 1998; Eisenberger & Lieberman, 2004). A larger volume in this region in individuals who score higher in Neuroticism may reflect higher sensitivity both to the possibility of error and to pain following punishment. Finally, dorsomedial PFC has been implicated in evaluation of the self and in emotion regulation (Heatherton et al., 2004; Ochsner & Gross, 2005). A smaller volume in this region may be related to the emotional dysregulation associated with Neuroticism and to the related tendency to evaluate the self negatively. Taken together, these associations present clear evidence that Neuroticism is broadly related to variation in brain systems governing sensitivity to threat and punishment.
Agreeableness was associated with reduced volume in posterior left superior temporal sulcus and with increased volume in posterior cingulate cortex. The superior temporal sulcus is involved in the interpretation of other individuals’ actions and intentions on the basis of biological motion (Pelphrey & Morris, 2006), a process that may be more efficient in individuals who score higher in Agreeableness. The posterior cingulate has been implicated in the process of understanding other individuals’ beliefs, a sophisticated, late-emerging component of theory of mind (Saxe & Powell, 2006). These associations are consistent with the hypothesis that Agreeableness is associated with the social information processing that enables and motivates altruistic behavior. We also found an association between Agreeableness and volume in the fusiform gyrus, which we did not predict, but which is nonetheless consistent with a social information processing function, given the area's proximity to the fusiform region specialized for perceiving faces (Kanwisher, McDermott, & Chun, 1997).
Conscientiousness was associated positively with volume of the middle frontal gyrus in left lateral PFC. The region of association was large, stretching from close to the frontal pole to the posterior region of lateral PFC. The middle frontal gyrus is crucially involved in maintaining information in working memory and in the execution of planned action. In terms of brain function, moving from posterior to anterior regions of lateral PFC appears to entail an increasing hierarchy of abstraction and complexity, in terms of rules that are maintained and selected to guide behavior (Bunge & Zelazo, 2006). Our results may therefore reflect the association of Conscientiousness with effective self-regulation at multiple levels of complexity, which would be in keeping with this trait's importance as a predictor of academic and occupational performance, health, and longevity.
We found no associations with Openness/Intellect in regions large enough to be significant at p < .05, corrected. However, we did find that Openness/Intellect was associated—at p < .01, uncorrected—with one region consistent with our hypotheses: an area of parietal cortex involved in working memory and the control of attention. A previous study found that a nearly identical region (Talairach coordinates: 46, −33, 45) showed the strongest correlation between neural activity (during a difficult working memory task) and intelligence (J.R. Gray et al., 2003). This finding is significant because Openness/Intellect is the only Big Five trait that has been consistently and positively associated with intelligence (DeYoung et al., 2005). Our current finding for Openness/Intellect is not conclusive, but it does indicate a specific ROI for testing in future studies.
The associations of personality traits with volume in predicted brain regions were generally consistent with the hypothesis that larger brain tissue volume is associated with increased function (with the exception of the negative association of Agreeableness with volume in superior temporal sulcus). For example, Neuroticism was positively associated with volume in a region of the cingulate linked to the detection of error and response to pain, both of which increase with Neuroticism. Also, Neuroticism was negatively associated with volume in a region of PFC associated with emotional regulation, which decreases with Neuroticism. However, our findings do not provide definitive evidence to allow generalizations about the relation of volume to function, and further research should target this question directly.
Conclusion
Personality neuroscience is a rapidly expanding area of research, incorporating not just structural and functional neuroimaging, but also molecular genetics, psychophysiology, and psychopharmacological research. If results in this field are to accumulate systematically, broad theoretical frameworks are needed to organize findings and generate predictions. The Big Five model offers a promising taxonomy of traits around which to build such a framework. The Big Five factors are important predictors of outcomes in mental and physical health, well-being, education, work, and relationships (Ozer & Benet-Martinez, 2006), and a theory of their biological roots is an important step toward the integration of individual differences research in psychology and neuroscience. The results of this study support such a theory, demonstrating the feasibility of using personality neuroscience to advance understanding of human psychology.
Supplementary Material
Funding
This research was supported by grants from the National Institute of Mental Health (NIMH) to J.R.G. (MH R01 66088) and C.G.D. (F32 MH077382). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIMH or the National Institutes of Health (NIH).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Supplemental Material
Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Asahi S, Okamato Y, Akado G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. Journal of Neuroscience. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. American Journal of Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: Contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Zelazo PD. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15:118–121. [Google Scholar]
- Canli T. Toward a “molecular psychology” of personality. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. Guilford Press; New York: 2008. pp. 311–327. [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Temperament: An organizing paradigm for trait psychology. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. Guilford Press; New York: 2008. pp. 265–286. [Google Scholar]
- Costa PT, Jr., McCrae RR. NEO PI-R professional manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Gray JR. Personality neuroscience: Explaining individual differences in affect, behavior, and cognition. In: Corr PJ, Matthews G, editors. The Cambridge handbook of personality psychology. Cambridge University Press; New York: 2009. pp. 323–346. [Google Scholar]
- DeYoung CG, Peterson JB, Higgins DM. Sources of Openness/Intellect: Cognitive and neuropsychological correlates of the fifth factor of personality. Journal of Personality. 2005;73:825–858. doi: 10.1111/j.1467-6494.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Shamosh NA, Green EA, Braver TS, Gray JR. Intellect as distinct from openness: Differences revealed through fMRI of working memory. Journal of Personality and Social Psychology. 2009;97:883–892. doi: 10.1037/a0016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Fleeson W. Towards a structure- and process-integrated view of personality: Traits as density distributions of states. Journal of Personality and Social Psychology. 2001;80:1011–1027. [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford University Press; New York: 2000. [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Graziano WG, Habashi MM, Sheese BE, Tobin RM. Agreeableness, empathy, and helping: A person × situation perspective. Journal of Personality and Social Psychology. 2007;93:583–599. doi: 10.1037/0022-3514.93.4.583. [DOI] [PubMed] [Google Scholar]
- Haas BW, Constable RT, Canli T. Stop the sadness: Neuroticism is associated with sustained medial prefrontal cortex response to emotional facial expressions. NeuroImage. 2008;42:385–392. doi: 10.1016/j.neuroimage.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Macrae CN, Kelley WM. What the social brain sciences can tell us about the self. Current Directions in Psychological Science. 2004;13:190–193. [Google Scholar]
- Hemphill JF. Interpreting the magnitudes of correlation coefficients. American Psychologist. 2003;58:78–80. doi: 10.1037/0003-066x.58.1.78. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins DL, Woods R, Toga A, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big Five trait taxonomy: History, measurement, and conceptual issues. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. Guilford Press; New York: 2008. pp. 114–158. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Cacioppo JT, Davidson RJ, Hugdahl K, Lovallo WR, Spiegel D, Rose R. Bridging psychology and biology: The analysis of individuals in groups. American Psychologist. 2002;57:341–351. [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr. Conceptions and correlates of openness to experience. In: Hogan R, Johnson J, Briggs S, editors. Handbook of personality psychology. Academic Press; Boston: 1997. pp. 825–847. [Google Scholar]
- McCrae RR, Costa PT., Jr. The five factor theory of personality. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. Guilford Press; New York: 2008. pp. 159–181. [Google Scholar]
- McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nettle D, Liddle B. Agreeableness is related to social-cognitive, but not social-perceptual, theory of mind. European Journal of Personality. 2008;22:323–335. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Omura K, Constable RT, Canli T. Amygdala gray matter concentration is associated with extraversion and neuroticism. NeuroReport. 2005;16:1905–1908. doi: 10.1097/01.wnr.0000186596.64458.76. [DOI] [PubMed] [Google Scholar]
- Ozer DJ, Benet-Martinez V. Personality and the prediction of consequential outcomes. Annual Review of Psychology. 2006;57:201–221. doi: 10.1146/annurev.psych.57.102904.190127. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski M, Rajeevan N, Okuda H, Constable RT, Staib LH. BioImage Suite: An integrated medical image analysis suite: An update. [Computer software] 2006 Available from http://www.bioimagesuite.org. [PMC free article] [PubMed]
- Pelphrey KA, Morris JP. Brain mechanisms for interpreting the actions of others from biological-motion cues. Current Directions in Psychological Science. 2006;15:136–140. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. NeuroReport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It's the thought that counts: Specific brain regions for one component of theory of mind. Psychological Science. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Ward BD. [March 16, 2010];Simultaneous inference for fMRI data. 2000 from http://homepage.usask.ca/~ges125/fMRI/AFNIdoc/AlphaSim.pdf.
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years?: Neural basis of improving emotional stability over age. Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.