Abstract
The advent of real‐time neurofeedback techniques has allowed us to begin to map the controllability of sensory and cognitive and, more recently, affective centers in the brain. The subgenual anterior cingulate cortex (sACC) is thought to be involved in generation of affective states and has been implicated in psychopathology. In this study, we examined whether individuals could use real‐time fMRI neurofeedback to modulate sACC activity. Following a localizer task used to identify an sACC region of interest, an experimental group of eight women participated in four scans: (1) a pretraining scan in which they were asked to decrease activity in the sACC without neurofeedback; (2) two training scans in which sACC neurofeedback was presented along with instructions to decrease sACC activity; and (3) a neurofeedback‐free post‐training scan. An additional nine women in a yoked feedback control group saw sACC activity from the participants in the experimental group. Activity in the sACC was significantly reduced during neurofeedback training in the experimental group, but not in the control group. This training effect in the experimental group, however, did not generalize to the neurofeedback‐free post‐training scan. A psychophysiological interaction analysis showed decreased correlation in the experimental group relative to the sham control group between activity in the sACC and the posterior cingulate cortex during neurofeedback training relative to neurofeedback‐free scans. The finding that individuals can down‐modulate the sACC shows that a primary emotion center in which functional abnormality has been strongly implicated in affective disorders can be controlled with the aid of neurofeedback. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: real‐time MRI, cingulate cortex, biofeedback, emotion regulation, psychopathology
INTRODUCTION
Real‐time neurofeedback training paradigms are designed to teach individuals volitional control over brain states by presenting them with continuously updated metaphors of brain activity and asking them to learn to modulate these representations, often through a process of trial and error. The first neurofeedback studies estimated brain activity with electroencephalography (EEG) and showed not only that humans are capable of gaining volitional control over regionally specific brain activity [e.g., Mulholland et al.,1976; Schwartz et al.,1976], but further, that controlling brain activation can be of therapeutic benefit [e.g., Lubar,1977; Mills and Solyom,1974; Rosenfeld et al.,1996; Sterman et al.,1975]. The advent of functional magnetic resonance imaging (fMRI)—with spatial resolution approximating the size of functional neural modules suggested both by cytoarchitectural and electrophysiological data—brought the promise of greater spatial specificity to investigations of the feasibility of neurofeedback training. Indeed, as fMRI data acquisition and analytic techniques developed to allow processing of multiple‐voxel fMRI datasets in real time, pioneering studies using real‐time fMRI (rtfMRI) documented the controllability of several brain regions; see DeCharms [2007] and Weiskopf et al. [2004b] for reviews.
rtfMRI neurofeedback training studies have demonstrated that individuals can learn to control various brain regions of interest (ROIs) with the aid of a neurofeedback signal. Through neurofeedback training, participants have learned to control activity in auditory cortex [Yoo et al.,2006], sensorimotor cortex [DeCharms et al.,2004; Yoo and Jolesz,2002], supplementary motor area [Weiskopf et al.,2004a], parahippocampal place area [Weiskopf et al.,2004a], and dorsal/rostral ACC [DeCharms et al.,2004; Weiskopf et al.,2003]. In addition to these areas, which have been implicated primarily in sensorimotor and cognitive processing, investigators have examined the ability of individuals to learn to control key areas involved in emotional experience and regulation, such as the amygdala [Johnston et al.,2010; Posse et al.,2003], rostral/ventral ACC [Weiskopf et al.,2003], and insula [Caria et al.,2007; Johnston et al.,2010]. This work is important both because it extends the scope of research examining control of regionally specific brain processes and because it provides insight about the neural mechanisms underlying emotional control.
Posse et al. [2003] asked participants to generate a sad mood over a 30‐s interval and then presented them with a single estimate of their amygdala activity to reinforce their mood induction strategy. While this study demonstrated that amygdala activity can be estimated and its response detected in real time, amygdala neurofeedback was not used to change activity in this structure, but instead, was presented to confirm the effectiveness of participants' mood regulatory strategy. In an rtfMRI neurofeedback study of the insula, Caria et al. [2007] presented participants with estimates of brain activity from this structure, updated on a moment‐by‐moment basis. These researchers demonstrated both that control of the insula increased in a linear manner with neurofeedback training, and that these training effects generalized to a neurofeedback‐free post‐training scan.
In the first rtfMRI neurofeedback study of a rostral/ventral ACC ROI, Weiskopf et al. [2003] presented continuously updated indices of activity from dorsal and rostral/ventral ACC ROIs simultaneously over several training runs. These investigators showed that an exemplary participant became increasingly proficient in increasing activity in both dorsal and rostral/ventral ACC ROIs. Subsequent analyses indicated that learned modulation of the rostral/ventral ACC ROI occurred in the rostral but not the ventral aspect of the ACC. In a subsequent study from our laboratory, however, we showed that, when presented with an rtfMRI training signal from the subgenual ACC (sACC) specifically, participants could effectively increase and decrease activity in this structure by using, respectively, negative and positive emotion regulatory strategies [Hamilton et al.,2007].
In a recent neural model of emotional functioning, Critchley [2005] posits that the ACC is centrally involved in the generation of affective states. Critchley cites evidence of blunted autonomic response in individuals with dorsal ACC (dACC) lesions, and of dACC hyper‐responsivity following circumscribed denervation of the autonomic nervous system, in postulating that the dACC is integral in generating sympathetic autonomic arousal. More speculatively, but supported by a sizable body of research demonstrating that activity in the dorsal and subgenual ACC are negatively intercorrelated [e.g., Pochon et al.,2002], Critchley posits that the sACC plays a complementary role to the dACC in generating parasympathetic activity. Given this putative involvement of the sACC in emotion generation, it is important to investigate whether control of this structure—and, potentially, the primary emotion processes it subserves—can be learned with the aid of appropriate neurofeedback.
Examining whether individuals can learn to control sACC activity is also important given the consistent association of this structure with various forms of psychopathology. Functional anomalies in the sACC have been implicated in unipolar depression [e.g., Drevets et al.,1997; Gotlib et al.,2005; Mayberg et al.,1999], in bipolar disorder [e.g., Brooks et al.,2006; Krueger et al.,2000], and in obsessive‐compulsive disorder [e.g., Van Laere et al.,2006]. Moreover, Mayberg et al. [2005] demonstrated the antidepressant effect of exogenous down‐modulation of sACC activity with microelectrode stimulation, suggesting that endogenous sACC modulation via neurofeedback training may yield similar clinical benefit.
While findings of Hamilton et al. [2007] provided an important proof of concept regarding the controllability of sACC with emotion‐regulatory strategies, the rtfMRI technique used was applied to a small number of participants; thus, the generalizability of these results remains in question. Moreover, participants in that study were given generic seed strategies to regulate sACC activity over a single training run: participants upregulated negative emotion to increase sACC signal and upregulated positive emotion to decrease sACC signal. Thus, the findings from that study do not rule out the possibility that the sACC ROI signal was merely tracking the emotional state of the participant, and that sACC feedback itself did not play an important role in determining sACC activity. Finally, Hamilton et al. did not address whether learned control over the sACC could generalize in the absence of sACC neurofeedback. In addressing these concerns, the present rtfMRI study examines activity in a sACC ROI in an experimental group of participants before, during, and after sACC neurofeedback training, and in a control group run through an isomorphic paradigm using yoked, sham neurofeedback. Thus, this study allows us not only to assess whether sACC neurofeedback facilitates regulation of this structure beyond what may be associated with implementing an emotion‐regulatory strategy alone, but further, to determine whether sACC neurofeedback training effects generalize to a neurofeedback‐free context. We predicted that participants in the experimental group, but not in the sham neurofeedback control group, would be able to control sACC activity better when shown a sACC neurofeedback signal than when engaging in an emotional regulatory strategy before neurofeedback training. We also predicted that neurofeedback training effects in the experimental group would persist into a neurofeedback‐free post‐training scan.
MATERIALS AND METHODS
Participants
Seventeen adult females, recruited through community website postings, unselected for past or current psychopathology but who were not currently taking psychotropic medication, participated in this study. Inclusion criteria required that all participants: (1) were between the ages of 18 and 50; (2) had no reported history of brain injury; and (3) had no physical limitations that prohibited them from participating in an fMRI scan. Informed consent was obtained from all participants, who were paid $25 per hour. All aspects of this study complied with the ethical standards for treatment of human participants from the American Psychiatric Association.
rtfMRI Software Configuration
Processing and analysis of blood‐oxygen‐level dependent (BOLD) data in real time was conducted with software developed at the Lucas Center for Magnetic Resonance Imaging at Stanford University. At the end of each volume repetition, this software reconstructed spiral in‐out [Glover and Law,2001] data from k‐space to native brain space and then calculated a voxel‐wise weighted‐average of spiral‐in and spiral‐out acquisitions to optimize signal‐to‐noise ratio. Reconstructed data were then passed to a program that computed continuously updated BOLD levels over a user‐selected ROI and for the rest of the brain. Following low‐pass filtering at 0.25 Hz, these estimates were presented to participants as scrolling graphs. These graphs were centered at the mean signal value of the third, fourth, and fifth BOLD acquisitions—excluding the first two acquisitions to allow for equilibration of the longitudinal magnetization vector—of each scan. The y‐axis of the feedback plot was continuously auto‐scaled to fit the maximum and minimum BOLD value for a given block. A screenshot of our rtfMRI interface—scrolling graphs along with task instructions—is presented in Figure 1A. This program also calculated a continuously updated correlation coefficient for each voxel time course relative to a user‐defined reference function and overlaid these statistics on structural data. Data processing was completed within ∼750 ms of the conclusion of each BOLD data acquisition.
Figure 1.
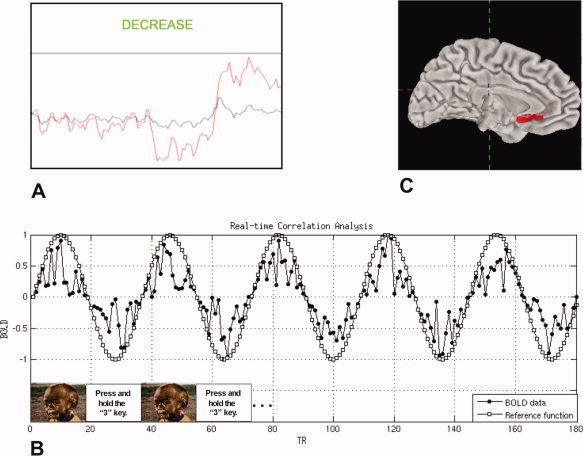
A: Real‐time neurofeedback interface with scrolling graphs of ROI (red line) and whole‐brain (black line) activity and task instruction window; B: schematic of functional localizer scan and real‐time correlational analysis used to locate sACC ROIs; C: visual rendering of sACC definition used to bound neurofeedback ROI selection.
rtfMRI Scanning Protocol
Overview
In this rtfMRI protocol, participants were first introduced to the rtfMRI interface and neuromodulation task outside of the scanner. After entering the scanner, they underwent anatomical and shimming scans followed by a functional localizer scan and, finally, four neuromodulation scans.
Scanner and pulse sequence
Scanning was conducted on a 1.5T General Electric Signa MR scanner with a single channel, whole‐head quadrature imaging coil. Anatomical underlay data [20 sagittal slices, 0.859 mm2 in‐plane, and 4 mm through‐plan resolution, echo time (TE) = 7 ms, flip angle = 90°, field of view (FOV) = 22 cm] were acquired first. FMRI data were then acquired [20 sagittal slices with 3.44 mm2 in‐plane and 4 mm through‐plane resolution, TE = 40 ms, flip angle = 80°, FOV = 22 cm, acquisition time (TR) = 2,000 ms per frame, number of frames = 90 per run for the localizer scan, 160 per run for neuromodulation scans] using a spiral‐in/out pulse sequence [Glover and Law,2001], which has been demonstrated to have superior recovery of activation signal in frontal orbital regions [Preston et al.,2004]. A high‐resolution structural scan (115 slices, 1 mm2 in‐plane, and 1.5 mm through‐plane resolution, TE = 7 ms, flip angle = 15°, FOV = 22 cm) was performed following BOLD scanning runs.
Prescan preparation
Before scanning, participants were introduced to the neurofeedback interface and the task design of the study. They were told that they would see a neurofeedback signal from a brain structure involved in emotion (red line) and a neurofeedback signal from the rest of the brain (black line, see Fig. 1A). Consistent with previous research [Caria et al.,2007; deCharms et al.,2005], the results of pretesting indicated that neuromodulation training with the region of the sACC identified with our localizer scan (see below) progressed slowly if participants were not given a basic seed strategy. Because results from a previous study suggested that participants fatigued quickly if they were asked to alternately increase and decrease sACC activity [Hamilton et al.,2007], in this study, they were asked only to down‐modulate sACC activity during “Decrease” blocks and to do so by upregulating positive mood in some way. We found that this “increase positive mood” strategy suggestion was sufficiently specific to allow participants to make progress in a single training session and sufficiently flexible to allow participants to make substantive changes in neuromodulatory strategies in response to neurofeedback. Additionally, participants were informed that it might take 4–6 s for the signal to change following execution of a particular Decrease strategy. Finally, participants were reminded that the neurofeedback signal was there to help teach them, and that if one strategy did not produce the desired change in the neurofeedback signal, they should try another.
Localizer scan
Given evidence that the sACC is spatially heterogeneous with respect to the type of affect it subserves [Damasio et al.,2000; Gotlib et al.,2005; Maddock et al.,2003], we developed a functional localizer scan designed to efficiently locate the specific area of the sACC in each participant that was most strongly involved in the processing of negative affective information. We defined the sACC ROI in this way and asked participants to downregulate activity in this ROI by increasing positive mood so that we could explore the feasibility of sACC neurofeedback training in this unselected control population as a novel neural‐level intervention for major depression, which has been found consistently to be associated with elevated sACC activity [e.g., Mayberg et al.,1999]. On the basis of findings that the sACC is involved in negative mood in both depressed and never‐depressed persons and in response to affectively valenced information [Maddock et al.,2003], we designed a scan in which blocks of passive viewing of negative pictures from the International Affective Picture System [IAPS; Lang and Greenwald,1993] were contrasted with a baseline task. In this 3‐min scan, participants engaged in five 18‐s blocks—each composed of three, six‐second trials—of passive viewing of negatively valenced (rating of three or less, on average) IAPS pictures. Interleaved with these picture‐viewing blocks were five blocks of a baseline task. During baseline blocks, participants were given a simple yet engaging task in which they were instructed to “Press and hold the N key,” where the value of N changed every 3 s, indicating that the participant should press and hold the 1, 2, 3, or 4 key on a four‐button fMRI‐compatible response box. Data from this scan were analyzed concurrently with their collection (see Analysis section, below). The content and structure of this scan are shown in Figure 1B.
Neuromodulation scans
Participants engaged in four neuromodulation scans, each lasting 5 min and 20 s. In the first scan (NoFB1) and the fourth scan (NoFB2), participants saw only instruction screens without neurofeedback; in the second and third scans (FB1 and FB2), participants saw instruction screens accompanied by neurofeedback. All four scans were composed of five Decrease blocks (each 32 s), interspersed with five baseline blocks (each 32 s) like those from the localizer scan. The first eight participants run through the protocol saw actual sACC neurofeedback (REAL); nine additional participants were run through a yoked sham neurofeedback protocol (SHAM). This protocol was equivalent to the real‐neurofeedback protocol with the exception that during FB1 and FB2 scans, participants saw neurofeedback that had been generated and seen by the participants in the REAL neurofeedback group.
For the NoFB1 scan, participants were asked to pick one strategy for increasing positive mood and to use it during Decrease blocks. For the Decrease blocks in the FB1 and FB2 scans, participants were instructed to cycle through positive‐mood strategies until they found one that was effective in making the sACC signal (red line) go below the whole‐brain‐minus‐sACC signal (black line). For the Decrease blocks in the NoFB2 scan, participants were instructed to use whichever strategy was most effective in reducing the sACC signal during the neurofeedback training scans.
Postscanning interview
Following scanning, participants engaged in a brief, informal interview wherein they reported the positive affect regulation strategies they used during the NoFB1 scan and during successful sACC regulation for the FB1 and FB2 scans.
Analysis
Functional localizer scan
The sACC voxel with the highest statistical value and the eight within‐plane voxels directly adjacent to it were selected as the sACC ROI for neurofeedback training for each participant. The sACC was defined as the region of the ACC ventral and posterior to the most anterior portion of the genu of the corpus callosum (see Fig. 1C for a graphical depiction of this definition). To find the sACC voxel that was maximally active for viewing negative material relative to baseline, we calculated the Pearson product‐moment correlation coefficient of each voxel time course with a sine wave with a period equal to the task cycle of the localizer scan (see Fig. 1B).
Neuromodulation training effects
Voxel time courses for the whole brain were slice‐time corrected relative to the tenth slice for each acquisition with the program 3dtshift from the Analysis of Functional Neuroimages [AFNI; Cox,1996] package. For each scan, these data were volume registered relative to the 80th acquisition with the AFNI program 3dvolreg and were then spatially smoothed with AFNI's 3dmerge [full width at half maximum (FWHM) = 4 mm]. These data were then band‐pass filtered with AFNI's 3dFourier (low pass criterion = 0.25 Hz; high pass criterion = 0.016 Hz) and were then converted to units of percent signal change relative to the time course mean.
For each run, each participant's average sACC time course was extracted from whole‐brain data by applying to the whole‐brain data the sACC mask identified with the localizer scan detailed earlier. Next, both to reduce noise and to correct for any changes in sACC BOLD that resulted from participants inadvertently learning to move to control the sACC BOLD signal, we subjected the sACC time course data to linear detrending against three translational and three rotational motion estimates. Similarly, to reduce noise and correct for the effects of neuromodulatory strategies that might have influenced BOLD signal at the whole‐brain level such as changing respiration rate [Birn et al.,2008], we detrended the motion‐detrended ROI data against whole‐brain (except for the sACC ROI) BOLD time series data. To perform these detrending procedures, we used the AFNI program, 3dDetrend, which uses a linear least squares algorithm to remove components from voxel time series. From the detrended sACC time courses, Decrease and baseline epochs from each of the four scans were averaged for each participant. Finally, average sACC BOLD for baseline blocks was subtracted from that for Decrease blocks to render four data points per participant corresponding to the difference in sACC data for Decrease versus baseline blocks for each run. To examine whether presentation of an sACC neurofeedback signal was effective in helping participants learn to down‐modulate activity in this structure, we conducted a two‐ (group) by‐four (scan) mixed‐model analysis of variance (ANOVA) on sACC activation. We analyzed significant main effects and interactions from the omnibus test with one‐ and two‐sample t‐tests.
Psychophysiological interaction analysis
To examine the neural correlates of effective down‐regulation of the sACC, we conducted a psychophysiological interaction [Friston et al.,1997; PPI; also known as “functional connectivity”] analysis on each of the four scans. The first step in this analysis was to deconvolve the detrended sACC time courses with the AFNI program 3dTfitter, using a γ‐function model of the hemodynamic response function (HRF) and minimizing first and second derivatives in the deconvolution. Next, we multiplied the deconvolved sACC time course by a task vector in which “1”s was assigned to TRs during Decrease epochs and “−1”s was assigned to TRs during baseline epochs. This interaction vector was then convolved with a gamma function. The resulting regressor and the detrended sACC time course were entered as covariates of interest, and three translational and three rotational motion estimates and one whole‐brain‐minus‐sACC average BOLD time series were entered as covariates of no interest, into a full regression model against voxel time courses from the whole brain. To allow for subsequent statistical comparisons, the whole‐brain fit coefficients for the interaction (sACC‐by‐task) were warped to Talairach space [Talairach and Tournoux,1988]. These data were then analyzed with a voxel‐wise, mixed‐model ANOVA performed with the AFNI program 3dANOVA4. The statistical criterion for this exploratory analysis was P = 0.01 at the individual voxel level with a cluster threshold of k = 10 (nearest neighbor) voxels (effective P = 0.0001, uncorrected).
RESULTS
Participant Characteristics
Mean age and years of education as well as group composition by handedness are presented in Table I. The REAL and SHAM groups did not differ on any of these variables (all P > 0.10).
Table I.
Participent characteristics
| REAL | SHAM | |
|---|---|---|
| Average Age | 31.0 (3.99) | 28.8 (1.36) |
| Handedness (right:left) | 7:1 | 7:2 |
| Average yrs of education | 16.5 (0.50) | 16.4 (0.87) |
Standard error in parentheses.
Functional Localizer Scan
For each participant, an sACC region was identified that correlated significantly with an ideal reference function. Peak voxel statistics and Talairach coordinates for the sACC ROI identified for each participant by real‐time correlation are presented in Table II.
Table II.
Localizer‐selected ROI and statistics comparison values
| Participant | Region | Peak voxel t | P (one‐tailed, Uncorrected) | X | Y | Z |
|---|---|---|---|---|---|---|
| REAL1 | Left subgenual ACC | 3.29 | 0.0005 | −4 | 20 | −17 |
| REAL2 | Left subgenual ACC | 3.09 | 0.0010 | −3 | 24 | −5 |
| REAL3 | Right subgenual ACC | 2.19 | 0.0140 | 3 | 22 | −4 |
| REAL4 | Left subgenual ACC | 3.58 | 0.0001 | −2 | 14 | −15 |
| REAL5 | Right subgenual ACC | 2.39 | 0.0085 | 3 | 31 | 5 |
| REAL6 | Right subgenual ACC | 2.72 | 0.0035 | 5 | 1 | −5 |
| REAL7 | Left subgenual ACC | 2.22 | 0.0130 | −6 | 23 | −12 |
| REAL8 | Left subgenual ACC | 2.81 | 0.0025 | −5 | 31 | −4 |
| SHAM1 | Left subgenual ACC | 2.47 | 0.0070 | −5 | 23 | −10 |
| SHAM2 | Right subgenual ACC | 2.31 | 0.0150 | 1 | 16 | −10 |
| SHAM3 | Right subgenual ACC | 3.25 | 0.0006 | 4 | 19 | −3 |
| SHAM4 | Right subgenual ACC | 2.40 | 0.0082 | 8 | 27 | −9 |
| SHAM5 | Left subgenual ACC | 2.77 | 0.0028 | 3 | −4 | −10 |
| SHAM6 | Left subgenual ACC | 4.43 | <0.0001 | −8 | 13 | −8 |
| SHAM7 | Right subgenual ACC | 3.59 | 0.0002 | 4 | 22 | −3 |
| SHAM8 | Right subgenual ACC | 2.48 | 0.0065 | 5 | 1 | −5 |
| SHAM9 | Right subgenual ACC | 2.62 | 0.0044 | 5 | 2 | −2 |
Warping from native‐ to Talairach‐space caused artificial displacement along y‐ and z‐axes for some ROIs.
Neuromodulation Training
The two (group) by four (scan) mixed‐model ANOVA conducted on average sACC activity estimates for NoFB1, FB1, FB2, and NoFB2 scans for Decrease minus baseline blocks for REAL and SHAM groups yielded no main effect of group [F(1,15) = 1.06; P > 0.10], but a significant main effect of scan [F(3,45) = 5.15; P < 0.05], which was qualified by a significant interaction of group and scan [F(3,45) = 3.10; P < 0.05]. Follow‐up contrasts indicated that participants in the REAL group exhibited significantly lower sACC activity during FB1 and FB2 scans than during NoFB1 scan, ts(7) = 3.71 and 2.92, respectively, both P < 0.05. sACC activity during NoFB2 Decrease blocks was not significantly lower than that during NoFB1 [t(7) = 1.22, P > 0.10]. Decrease‐block sACC activity during FB1 was lower than activity during NoFB2 [t(7) = 2.36, P = 0.05]. Finally, FB2 sACC activity during Decrease blocks was marginally significantly lower than was NoFB2 sACC activity during Decrease blocks, t(7) = 1.76, P = 0.06. These same contrasts conducted on the data from the SHAM group showed no significant or marginally significant within‐group differences (all P > 0.10). Between‐groups comparisons of REAL and SHAM groups yielded no significant difference for NoFB1 and NoFB2 scans [t(15) = 0.68 and 0.01, respectively, both P > 0.10] but significantly reduced sACC activity during Decrease blocks in the REAL relative to the SHAM group during FB1 and FB2 scans [t(15) = 1.70 and 1.64, respectively, P = 0.05 and 0.06]. Cell‐mean data are presented in Figure 2. Average Decrease block sACC time courses for each group for FB1 and FB2 (FB) and NoFB1 and NoFB2 (NoFB) scans are presented in Figure 3.
Figure 2.

Average sACC Decrease versus baseline BOLD (± standard error) during NoFB1, FB1, FB2, and NoFB2 scans in REAL and SHAM groups.
Figure 3.
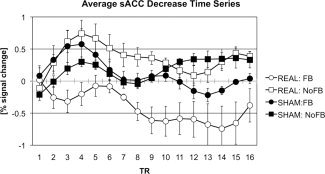
Group‐averaged BOLD time courses for Decrease blocks during NoFB and FB scans in REAL and SHAM groups.
Psychophysiological Interaction
Given that sACC activity decreased in the REAL feedback group during FB relative to NoFB scans but did not change in the SHAM group across FB and NoFB scans, we examined where activity interacted with sACC activity as a function of both group (REAL versus SHAM) and type of scan [NoFB (mean of NoFB1 and NoFB2) versus FB (mean of FB1 and FB2)]. These results are presented in Figure 4. FMRI time courses in left posterior cingulate (pCing) cortex and left cuneus were found to have decreased correlation with sACC ROI time courses during FB scans in the REAL relative to the SHAM group [pCing: t(15) = 3.96; cuneus: t(15) = 3.44; both P < 0.05]; no between‐group differences for NoFB scans in sACC functional connectivity were observed in these structures [pCing: t(15) = 0.31; cuneus: t(15) = 1.37; both P > 0.10]. Importantly, within‐group comparisons showed decreased sACC functional connectivity for FB relative to NoFB scans in the REAL group [pCing: t(7) = 3.50; cuneus: t(7) = 2.54; both P < 0.05], and increased sACC functional connectivity for FB relative to NoFB scans in the SHAM group [pCing: t(8) = 2.17; cuneus: t(8) = 3.09; both P < 0.05].
Figure 4.
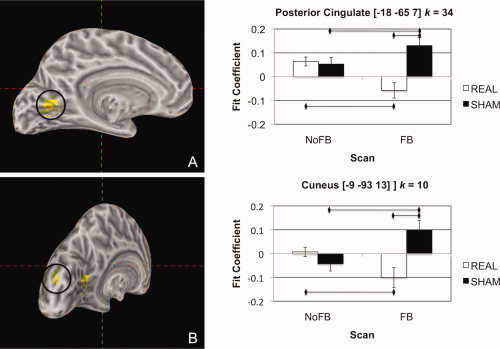
Regions of differential context‐dependent correlation with the sACC ROI across NoFB and FB scans in REAL and SHAM groups in the posterior cingulate cortex (A) and cuneus (B). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Postscanning Interview
While all participants indicated that they used positive affective strategies during neurofeedback scans, all but four of the REAL neurofeedback participants were unable to recall specific details of the strategies they used. We report the four recalled strategies in Supporting Information Table I.
DISCUSSION
This study examined the ability of adults to use neurofeedback to modulate activity in sACC. We examined activity in a sACC ROI before, during, and after real and sham sACC neurofeedback training. The results of this study indicated that participants were able to down‐modulate sACC activity significantly better when they were presented with a veridical sACC neurofeedback training signal than when they were not presented with such a signal; in contrast, participants who saw a sham neurofeedback signal were not able to modulate their sACC activity. The sACC down‐modulation observed in the REAL neurofeedback group during neurofeedback training, however, did not generalize to a post‐training, neurofeedback‐free scan. Finally, a PPI analysis used to identify the neural correlates of successful sACC ROI down‐modulation showed decreased correlation in the REAL neurofeedback group between sACC and posterior cingulate cortex during neurofeedback relative to neurofeedback‐free scans; SHAM control participants showed the opposite pattern, exhibiting an increased correlation between sACC and pCing during neurofeedback relative to neurofeedback‐free scans.
The finding that sACC BOLD signal was decreased during presentation of sACC neurofeedback in the REAL but not in the SHAM group represents strong evidence that sACC activity can be downregulated with the aid of a neurofeedback signal. We also found, however, that the ability to decrease sACC activity during veridical neurofeedback did not generalize to a neurofeedback‐free, post‐training scan. One reason for this lack of generalization involves the role of the sACC as a “visceromotor” region [Drevets et al.,2008; Johansen‐Berg et al.,2008]. More specifically, it is possible that effective sACC neuromodulatory strategies acquired during training could not be verbalized sufficiently to be easily recalled and implemented during the post‐training scan.
One limitation of this study is the lack of sensitivity and structure of the post‐scan, free‐recall probe used to explore effective neuromodulatory strategies; indeed, half of the participants in the experimental group could not easily verbalize the strategy they used in downregulating sACC activity. Including more sensitive strategy probes will benefit future work by highlighting the mechanisms underlying sACC modulation and better elucidating the function of this neural region. Moreover, given that future sACC neurofeedback research may, like this study, localize different regions of the sACC, more detailed descriptions of sACC neurofeedback strategies may help elucidate functional specialization within this region that is indicated by distinct patterns of white‐matter connectivity [Johansen‐Berg et al.,2008] within sACC.
Results from the PPI analysis showed decreased sACC‐pCing functional connectivity during FB scans in the REAL neurofeedback group relative both to NoFB scans in this group and to FB scans in the SHAM group. Posterior cingulate cortex is recruited reliably as part of the default mode network [Raichle et al.,2001]; indeed, functional connectivity approaches to defining the default mode network most often incorporate pCing as a seed region [Fox et al.,2005]. Data implicating this region in self‐reflective thought [Johnson et al.,2002], particularly in the retrieval of autobiographical memories [Maddock et al.,2001], help to delineate the role of pCing in default mode functioning. During self‐generation of emotion, this region and the sACC become coactive [Damasio et al.,2000], a finding replicated in this study in both the REAL and SHAM groups during NoFB scans and in the SHAM group during FB scans. The finding that this pattern of connectivity reverses during real neurofeedback training represents preliminary evidence that a neurofeedback signal may provide a means for conscious override of normal network dynamics. This pattern, combined with the present finding that only two regions showed a significant group by task relation with sACC activation despite a relatively sensitive statistical criterion (P = 0.01; k = 10), suggests that down‐modulation of the sACC during real neurofeedback is a neurally selective effect.
The central finding from this study—that a sample of adults can use neurofeedback to decrease activity in the sACC—is also clinically significant. This finding indicates that individuals who are experiencing forms of psychopathology in which anomalous sACC function has been implicated, such as clinical depression, may be able to use neurofeedback to modulate activation in this structure and, further, that this control may reduce emotional symptoms of their disorder. Moreover, given that the sACC has been shown to be over‐recruited in default mode network functioning in depression [Greicius et al.,2007], the current results, consistent with the formulation that sACC activity becomes decoupled from activity in pCing—a primary node in the default mode network—during real neurofeedback training bodes well for the potential clinical efficacy of sACC neurofeedback training in major depression. These data, considered together with the finding of therapeutic benefits in depression associated with exogenous down‐modulation of the sACC through direct electrode stimulation [Mayberg et al.,2005], indicate that sACC neurofeedback training may be effective in the treatment of depression. While this study is critical in demonstrating that individuals can modulate activation in the sACC with the aid of a veridical neurofeedback signal, it remains for future research to examine more directly the effects of this modulation in clinically depressed individuals.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Table 1
Acknowledgements
The authors thank Hannah Kang, Jennifer Cribbs, and Didem Bilensoy for their help with collecting the data presented in this manuscript. They also thank Helen Mayberg for her insightful comments that helped shape the experimental paradigm used in this study. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- Birn RM, Smith MA, Jones TB, Bandettini PA ( 2008): The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 40: 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JO, Wang PW, Strong C, Sachs N, Hoblyn JC, Fenn R, Ketter TA ( 2006): Preliminary evidence of differential relations between prefrontal cortex metabolism and sustained attention in depressed adults with bipolar disorder and healthy controls. Bipolar Disorder 8: 248–254. [DOI] [PubMed] [Google Scholar]
- Caria A, Veit R, Sitaram R, Lotze M, Welskopf N, Grodd W, Birbaumer N ( 2007): Regulation of anterior insular cortex activity using real‐time fMRI. Neuroimage 35: 1238–1246. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Critchley HD ( 2005): Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493: 154–166. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Decharms RC ( 2007): Reading and controlling human brain activation using real‐time functional magnetic resonance imaging. Trends Cogn Sci 11: 473–481. [DOI] [PubMed] [Google Scholar]
- DeCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JDE ( 2004): Learned regulation of spatially localized brain activation using real‐time fMRI. Neuroimage 21: 436–443. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JDE, Mackey SC ( 2005): Control over brain activation and pain learned by using real‐time functional MRI. Proc Natl Acad Sci USA 102: 18626–18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME ( 1997): Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386: 824–827. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M ( 2008): The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13: 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS ( 2001): Spiral‐in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med 46: 515–522. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JDE, Whitfield‐Gabrieli S, Goldin P, Minor KL, Canli T ( 2005): Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport 16: 1731–1734. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF ( 2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Gotlib IH ( 2007): Healthy individuals can use real‐time fMRI neurofeedback to modulate activity in the subgenual anterior cingulate cortex. Biol Psychiatry 61: 30S–30S. [Google Scholar]
- Johansen‐Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, Lozano AM, Mayberg HS ( 2008): Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment‐resistant depression. Cereb Cortex 18: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125: 1808–1814. [DOI] [PubMed] [Google Scholar]
- Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DEJ ( 2010): Neurofeedback: A promising tool for the self‐regulation of emotion networks. Neuroimage 49: 1066–1072. [DOI] [PubMed] [Google Scholar]
- Krueger S, Goldapple K, Liotti M, Mayberg HS. ( 2000): Regional changes in cerebral blood flow following transient sadness in bipolar affective disorder. Abstr Soc Neurosci 26; abstract no. 866.2. [Google Scholar]
- Lang PJ, Greenwald MK ( 1993): International affective picture system standardization procedure and results for affective judgments: Technical reports 1A‐1C. Gainesville: Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Lubar JF ( 1977): Electroencephalographic biofeedback methodology and management of epilepsy. Pavlov J Biol Sci 12: 147–185. [DOI] [PubMed] [Google Scholar]
- Maddock RF, Garrett AS, Buonocore MH ( 2001): Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104: 667–676. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH ( 2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT ( 1999): Reciprocal limbic‐cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH ( 2005): Deep brain stimulation for treatment‐resistant depression. Neuron 45: 651–660. [DOI] [PubMed] [Google Scholar]
- Mills GK, Solyom L ( 1974): Biofeedback of Eeg alpha in treatment of obsessive ruminations—Exploration. J Behav Ther Exp Psychiatry 5: 37–41. [Google Scholar]
- Mulholland T, McLaughlin T, Benson F ( 1976): Feedback‐control and quantification of response of Eeg alpha to visual stimulation. Biofeedback Self Regul 1: 411–422. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B ( 2002): The neural system that bridges reward and cognition in humans: An fMRI study. Proc Natl Acad Sci USA 99: 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao KX, Habel U, Rosenberg D, Moore GJ, Schneider F ( 2003): Real‐time fMRI of temporolimbic regions detects amygdala activation during single‐trial self‐induced sadness. Neuroimage 18: 760–768. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH ( 2004): Comparison of spiral‐in/out and spiral‐out BOLD fMRI at 1.5 and 3T. Neuroimage 21: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JP, Baehr E, Baehr R, Gotlib IH, Ranganath C ( 1996): Preliminary evidence that daily changes in frontal alpha asymmetry correlate with changes in affect in therapy sessions. Int J Psychophysiol 23: 137–141. [DOI] [PubMed] [Google Scholar]
- Schwartz GE, Davidson RJ, Pugash E ( 1976): Voluntary control of patterns of Eeg parietal asymmetry—Cognitive concomitants. Psychophysiology 13: 498–504. [DOI] [PubMed] [Google Scholar]
- Sterman M, Macdonald L, Stone R ( 1975): Biofeedback training of sensorimotor Eeg rhythm in man—Effects on epilepsy. Electroencephalogr Clin Neurophysiol 38: 104–104. [Google Scholar]
- Talairach J, Tournoux P. ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme. [Google Scholar]
- Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, Cosyns P ( 2006): Metabolic imaging of anterior capsular stimulation in refractory obsessive‐compulsive disorder: A key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med 47: 740–747. [PubMed] [Google Scholar]
- Weiskopf N, Veit R, Erb M, Mathiak K, Grodd W, Goebel R, Birbaumer N ( 2003): Physiological self‐regulation of regional brain activity using real‐time functional magnetic resonance imaging (fMRI): Methodology and exemplary data. Neuroimage 19: 577–586. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Mathiak K, Bock SW, Scharnowski F, Veit R, Grodd W, Goebel R, Birbaumer N ( 2004a) Principles of a brain‐computer interface (BCI) based on real‐time functional magnetic resonance imaging (fMRI). IEEE Trans Biomed Eng 51: 966–970. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Scharnowski F, Veit R, Goebel R, Birbaumer N, Mathiak K ( 2004b) Self‐regulation of local brain activity using real‐time functional magnetic resonance imaging (fMRI). J Physiol (Paris) 98: 357–373. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Jolesz FA ( 2002): Functional MRI for neurofeedback: Feasibility study on a hand motor task. Neuroreport 13: 1377–1381. [DOI] [PubMed] [Google Scholar]
- Yoo SS, O'Leary HM, Fairneny T, Chen NK, Panych LP, Park H, Jolesz FA ( 2006): Increasing cortical activity in auditory areas through neurofeedback functional magnetic resonance imaging. Neuroreport 17: 1273–1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Table 1


