Abstract
BSI and acute GVHD (aGVHD) are serious complications of HSCT. We hypothesized that the two events were not independent of one another. We studied (1) associations between BSI and aGVHD; (2) the impact of BSI and/or aGVHD on death within 100 days after HSCT, employing a retrospective cohort analysis. Risk factor analysis was performed using multivariable Cox proportional hazards analyses. Of 211 subjects undergoing allogeneic HSCT from 1/00–12/05 (58% of whom underwent reduced intensity transplantation), 82 (39%) developed BSI. In 49 patients (23%), grades (gr) 2–4 aGVHD occurred. Early BSI was independently associated with an increased occurrence of subsequent aGVHD gr 2–4. Cytomegalovirus seropositivity was independently associated with decreased occurrence of aGVHD. Acute GVHD gr 2–4 independently predicted subsequent first BSI. Both BSI and aGVHD gr 2–4 were significant independent predictors of death within 100 days after HSCT. There is a strong, independent association between BSI and aGVHD. Potential explanations include the elaboration of cytokines during BSI favoring the development of aGVHD and/or the immunosuppressive treatment of aGVHD favoring the development of BSI. Future studies should be directed at mechanistic investigations of this association.
Keywords: hematopoietic stem cell transplantation, blood stream infection, acute graft-versus-host disease, mortality, multivariable model
INTRODUCTION
Blood stream infection (BSI), the most common infectious complication of hematopoietic stem cell transplantation (HSCT), and acute graft versus host disease (acute GVHD) are independent predictors of mortality after HSCT (1). The conditions favoring the development of acute GVHD are thought to occur in the earliest phases of stem cell transplantation (2–4). Intense chemotherapy and irradiation result in damage to the gastrointestinal tract, allowing bacterial constituents to enter the systemic circulation. This results in stimulation of the host immune response manifested, in part, by the production of inflammatory cytokines. Consequently, graft T cells are pushed along the Th-1 pathway and adopt an alloreactive phenotype, resulting in an immune response against the host. We felt that this sequence of events in the development of acute GVHD parallels the immunologic events that occur in infection.
Our primary hypothesis was that early BSI promotes the development of acute GVHD. A secondary hypothesis was that acute GVHD promotes the development of BSI, as a consequence of the immunosuppression used to treat GVHD, and skin and mucosal changes. Therefore, we studied (1) the temporal relationship of BSI and acute GVHD to determine if time-dependent relationships existed, and (2) the association of these complications of HSCT with all-cause mortality.
METHODS
Patient Population
We retrospectively studied consecutive allogeneic HSCT recipients cared for at Tufts Medical Center from January 1, 2000 through December 31, 2005. Most subjects undergoing full dose HSCT received a conditioning regimen of cyclophosphamide usually with total body irradiation. T-cell depletion was rarely performed. Subjects undergoing reduced intensity HSCT underwent extracorporeal photopheresis and reduced dose total body irradiation and were administered pentostatin (5). All subjects were cared for on the same unit by nurses specializing in HSCT, except for the extremely rare circumstance when they required intensive care. Supportive care for all subjects was identical and consisted of the following. Subjects received antimicrobial prophylaxis with a fluoroquinolone, fluconazole and acyclovir, all continuing until engraftment. After engraftment, patients received prophylaxis for Pneumocystis carinii pneumonitis with trimethoprim-sulfamethoxazole until one year after HSCT. Intravenous immunoglobulin was administered to patients undergoing allogeneic HSCT for the first seven months after HSCT. Initial acute GVHD prophylaxis in recipients of allogeneic HSCT consisted of cyclosporine and methotrexate, followed by cyclosporine alone. Cyclosporine was tapered off and replaced by mycophenolate mofetil that was continued for one year after HSCT unless GVHD developed. Central venous catheters were present in all patients for the HSCT and were generally removed after engraftment. Routine catheter care for all patients was identical and conformed to the Tufts Medical Center Infection Control standards. This study was approved by the Tufts Medical Center Institutional Review Board.
Definitions
The definition of BSI was adapted from the 2004 Center for Disease Control and Preventions definitions for nosocomial infections (6). BSI was defined as the isolation of bacteria not normally known to colonize the skin, such as Gram negative bacilli, or certain pathogens such as S. aureus or fungi, from at least one blood culture (7). For bacteria that typically colonize the skin, such as coagulase-negative Staphylococcus, Propionibacterium, viridans group of Streptococcus and non-JK strains of Corynebacterium, 2 consecutive positive blood cultures, 2 positive blood cultures within 72 hours, or one positive blood culture and one positive intravascular catheter tip culture within 72 hours constituted a BSI. All blood cultures were obtained in response to an indication of infection, usually fever (oral temperature ≥ 38°C). The criterion for engraftment was an absolute neutrophil count of 500/mm3 or greater for 3 consecutive days. Acute GVHD was graded according to standard criteria (8). Mucositis was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0 (9).
Data Collection
Prespecified demographic and clinical variables were abstracted from the HSCT database and the medical record. The time period of interest was from 7 days prior to stem cell infusion, which marked the initiation of the conditioning regimen, until 100 days after the infusion. Variables included those fixed prior to the initiation of the transplantation process: age, gender, prior transplantation, the indication for HSCT, disease status at transplantation, type of HSCT, degree of HLA matching, and cytomegalovirus (CMV) serologies of the recipient and donor. Additional variables were potential events occurring at the start of the conditioning regimen through the end of the observation period. These included time to first BSI, time to early BSI, defined as occurring with 7 days prior until 10 days after HSCT, time to engraftment, time to first GVHD grade 2 or higher event, and time to death. We chose to study acute GVHD grade 2 or higher since these grades are associated with increased morbidity and mortality and require immunosuppressive treatment (10, 11).
Statistics
Cox proportional hazards regression reporting hazard ratios (HR) and 95% confidence intervals (CI) were used in univariate analyses and the multivariable analysis for time to death within 100 days after HSCT. Outcomes of interest were time to first acute GVHD grade 2 or higher event, time to first BSI and time to death. Variables analyzed included demographic factors (age, gender), underlying disease necessitating HSCT, number of chemotherapy cycles and classes of agents administered in the 6 months prior to HSCT, and type of HSCT. In addition, the following variables were expressed as time-dependent variables to examine their temporal relationship: time to engraftment, time to first BSI and time to first acute GVHD grade 2 or higher event. Observations were censored at death or day 100 if the patient survived without the outcome of interest. In addition, for certain analyses, patients were censored at day 42 after HSCT if they did not engraft by then. The assumption of proportional hazards was checked using Schoenfeld residuals, Pearson’s correlation coefficient and the %schoen and %daspline macros in SAS (12, 13).
For multivariable analyses, variables with parameter estimates with a p value ≤ 0.10 in the univariate analyses were included. Multivariable analyses of the outcomes of time to acute GVHD or BSI were performed with competing risks models that utilized cause-specific hazard ratios (14, 15). The competing risk was all-cause mortality. Differences between groups in Kaplan-Meier analyses were determined via the log-rank test. In all analyses, a p-value of ≤0.05 was considered statistically significant.
RESULTS
Patient Population
Two hundred and eighteen patients underwent consecutive allogeneic HSCT during the study period. Of these, 211 had data on the outcomes of acute GVHD, BSI and death. Their characteristics are displayed in Table 1. Data on mucositis was complete for 207 patients. Data on chemotherapy in the 6 months prior to HSCT was complete in 199 to 204 patients, depending upon the class of agent (Table 2).
Table 1.
Characteristics of the Study Population
| Characteristic | Total N=211 (%) | With BSI N=82 (%) | With GVHD grade ≥ 2 N=49 (%) |
|---|---|---|---|
| Age | 44 (34, 53)1 | 46 (37, 54)1 | 43 (34, 51)1 |
| Male gender | 115 (55) | 50 (61) | 26 (53) |
| Underlying disease | -- | -- | -- |
| Acute myelogenous leukemia | 63 (30) | 26 (32) | 11 (22) |
| Non-Hodgkins lymphoma2 | 28 (13) | 12 (15) | 5 (10) |
| Chronic myelogenous leukemia | 26 (12) | 9 (11) | 8 (16) |
| Acute lymphocytic leukemia | 25 (12) | 10 (12) | 8 (16) |
| Myelodysplastic syndrome | 19 (9) | 8 (10) | 7 (14) |
| Multiple myeloma | 13 (6) | 5 (6) | 0 (0) |
| Hodgkins lymphoma2 | 12 (6) | 5 (6) | 2 (4) |
| Chronic lymphocytic leukemia | 8 (4) | 2 (2) | 2 (4) |
| Myelofibrosis | 7 (3) | 3 (4) | 3 (6) |
| Aplastic anemia | 5 (2) | 1 (1) | 1 (2) |
| Renal cell carcinoma | 3 (1) | 1 (1) | 1 (2) |
| Paroxysmal nocturnal hemoglobinuria | 2 (1) | 0 (0) | 1 (2) |
| Prior HSCT | 44 (21) | 20 (24) | 5 (10) |
| Reduced intensity HSCT | 89 (42) | 53 (65) | 25 (51) |
| 6/6 HLA match | 196 (93) | 73 (89) | 46 (94) |
| Complete response at HSCT | 55 (26) | 19 (23) | 11 (22) |
| Donor/Recipient CMV serostatus3 | 102 (48) | 44 (54) | 16 (33) |
| D−/R− | 76 (36) | 26 (32) | 23 (47) |
| D+/R+ | 61 (29) | 26 (32) | 9 (18) |
| D+/R− | 33 (16) | 12 (15) | 10 (20) |
| D−/R+ | 41 (19) | 18 (22) | 7 (14) |
Abbreviations: BSI, blood stream infection; GVHD, acute graft versus host disease; HSCT, hematopoietic stem cell transplantation..
Median (interquartile range).
Lymphomas were further classified within the entire group as diffuse large B cell (4), mantle cell (4), follicular cleaved small cell (3), diffuse large cell (2), T cell lymphoblastic (2); and angioimmunoblastic, small B cell, B cell, anaplastic large cell, small noncleaved Burkitts lymphoma, low grade mucosa-associated lymphoid tissue with plasmacytoid features, follicular mixed small and large cell, marginal zone B cell, biphenotypic precursor B cel leukemia/lymphoma, peripheral T cell, immunoblastic, cutaneous T cell, low grade follicular (1 each).
D, donor; R, recipient; “+”, positive; “−”, negative.
Table 2.
Chemotherapy in the 6 months prior to HSCT
| # Cycles | N=201 |
|---|---|
| 0 | 46 (22.3) |
| 1–2 | 93 (45.2) |
| 3–4 | 49 (23.8) |
| ≥5 | 18 (8.7) |
| Classes1 | |
| Alkylating Agents | 49 (24.5) |
| Antimetabolites | 133 (65.2) |
| Anthracyclines | 89 (44.1) |
| Vinca Alkaloids | 48 (24.0) |
| Etoposide | 32 (16.1) |
| Antibodies | 20 (10.0) |
Numbers available for analysis per class: Alkylating agents, 200; antimetabolites, 204; anthracyclines, 202; vinca alkaloids, 200; etoposide, 199; steroids, 199; antibodies, 198.
Acute Graft versus Host Disease
Forty nine patients (23%) developed acute GVHD grade 2 or higher. Thirty four of these had acute GVHD grade 1 that then progressed to a more severe stage. There were 89 patients (42%) who developed acute GVHD grade 1 only and 73 (35%) who had no acute GVHD episodes. The median time to development of acute GVHD grade 1 was 16 days (interquartile range (25th and 75th percentiles, IQR) 12, 19 days) and of acute GVHD grades 2 or higher was 24 days (IQR 16, 36 days). Our preliminary analyses revealed that there was no relationship between the occurrence of BSI and subsequent acute GVHD grade 1 (data not shown). Because of this and the clinical significance of more severe grades of acute GVHD, we limited our remaining analyses to acute GVHD grades 2 or higher unless otherwise mentioned.
Univariate analysis of time to acute GVHD Grade 2 or higher
BSI occurring before acute GVHD was the primary predictor of interest. Thus, BSI that occurred after acute GVHD grade 2 or higher were censored, and only BSI that occurred before acute GVHD grade 2 or higher were assessed. In the univariate analysis, prior HSCT, recipient CMV seropositivity and receipt of etoposide within the 6 months prior to HSCT were predictive or trended toward being predictive of a reduced risk of acute GVHD grade 2 or higher. BSI that occurred at any time between the initiation of the conditioning regimen 7 days prior to stem cell infusion and acute GVHD grade 2 or higher, the censoring event of death or day 100, whichever came first, was not predictive of an increased risk of acute GVHD grade 2 or higher. However, early BSI occurring between the initiation of the conditioning regimen and 10 days after stem cell infusion (n=28) predicted an increased risk of acute GVHD grade 2 or higher. All early BSI occurred before any acute GVHD or censoring events. We graphically examined the relationship between early BSI and acute GVHD grade 2 or higher via Kaplan-Meier analysis (Figure 1). Again, it appeared that patients with early BSI were more likely to develop acute GVHD grade 2 or higher (p=0.05). Age, gender, HLA match between donor and recipient, underlying disease (categories collapsed to lymphoma, leukemia and other causes for the sake of analysis), reduced intensity HSCT, disease status at HSCT, donor CMV serology and time to engraftment did not predict subsequent acute GVHD grade 2 or higher. In addition, mucositis grades 3–4 within 14 days of HSCT, occurring in 21 (44%) of those with and 64 (41%) of those without acute GVHD grade 2 or higher, was not associated with acute GVHD when compared to mucositis grades 0–2. There was a trend toward reduced risk of developing acute GVHD grades 2 or higher in patients who received etoposide (HR 0.35, 95% CI 0.11, 1.11, p=0.08). When adjusting for underlying disease, this relationship did not change. There was no relationship between acute GVHD and the number of cycles of chemotherapy or classes of agents used in the 6 months prior to HSCT
Figure 1.
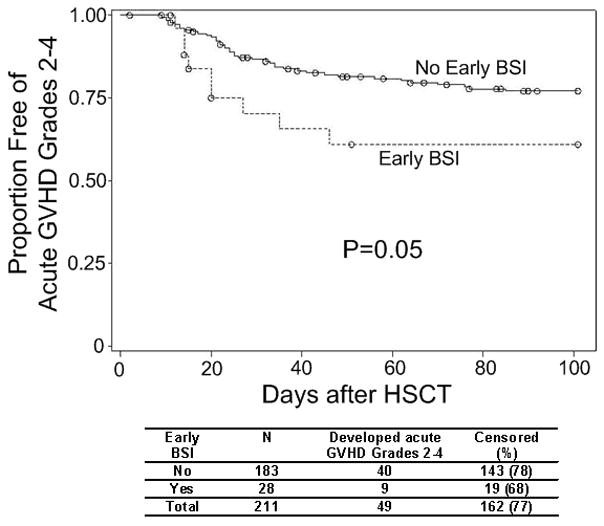
Time to acute GVHD grade 2 or higher with and without early BSI
Multivariable analysis of time to acute GVHD grade 2 or higher
We utilized cause-specific hazard ratios (CSHR) to assess risk factors for acute GVHD grade 2 or higher, along with the competing risk of all-cause mortality within 100 days of HSCT. Variables analyzed in this model were early BSI, prior HSCT, CMV seropositivity of the recipient and receipt of etoposide in the 6 months prior to HSCT (Table 3). The analysis revealed that those persons experiencing an early BSI had a more than two-fold increased risk of subsequent acute GVHD grade 2 or higher (CSHR 2.17, 95% CI 1.05, 4.49, p=0.04). CMV seropositivity of the recipient remained a significant predictor of reduced risk of acute GVHD grade 2 or higher whereas prior HSCT and receipt of etoposide in the 6 months prior to HSCT were not statistically significantly predictive. Since a predictor of BSI was engraftment (see below), to avoid confounding we repeated the analysis, censoring the 3 patients who did not engraft by day 42. The results of the analysis were not substantially different. Early BSI was still an independent risk factor for subsequent acute GVHD grade 2 or higher.
Table 3.
Multivariable analysis of cause-specific hazard ratios for the acquisition of acute graft versus host disease grade 2 or higher
| Risk Factor | Possible Endpoints (Competing Risks) | |||||
|---|---|---|---|---|---|---|
| Acute GVHD Grade 2 or Higher | All-Cause Mortality | |||||
| CSHR | 95% CI | P value | CSHR | 95% CI | P value | |
| CMV seropositivity | 0.45 | 0.25, 0.81 | 0.008 | 1.18 | 0.59, 2.36 | 0.64 |
| Time to early BSI | 2.17 | 1.05, 4.49 | 0.04 | 2.80 | 1.12, 6.53 | 0.02 |
| Prior HSCT | 0.50 | 0.20, 1.27 | 0.15 | 2.22 | 1.08, 4.54 | 0.03 |
| etoposide | 0.39 | 0.12, 1.26 | 0.12 | 2.80 | 1.36, 5.76 | 0.005 |
Analysis represents 199 patients with complete data sets.
It was possible that acute GVHD grade 1 was increasing the risk for subsequent early BSI, and independently leading to progression to more severe grades of acute GVHD, without any influence of early BSI on the development of acute GVHD grade 2 or higher. However, in each patient with early BSI, any acute GVHD grade 1 events occurred after early BSI. Therefore, it would be unlikely that acute GVHD grade 1 increased the risk of early BSI. In addition, it was apparent that early BSI did not predict an increased risk of subsequent acute GVHD grade 1 (univariate analysis, HR= 1.05, 95% CI 0.61, 1.80, p=0.86). Therefore, there did not appear to be a confounding relationship between early BSI and acute GVHD grade 1.
Blood Stream Infection
Eighty two patients (39%) developed BSI during the study period at a median of 20 days (IQR 6, 46 days) after stem cell infusion. Twenty eight patients (13%) developed early BSI a median of 4 days (IQR -1, 7 days) after stem cell infusion. There were 96 blood isolates obtained from the first BSI episodes of the 82 individuals. Gram positive cocci were the most common type of isolate. Of these, 79% (or 49% of the total number of isolates) were coagulase negative staphylococci.
Univariate analysis of time to first BSI
We examined the outcome of time to first BSI, where the predictor variable of interest was first acute GVHD grade 2 or higher event. Events of acute GVHD grade 2 or higher that occurred after BSI were censored. In the univariate analysis, acute GVHD grade 2 or higher predicted a two-fold increase in subsequent BSI. Additionally, a 6 of 6 HLA locus match between donor and recipient, and engraftment as a categorical variable predicted a decrease in the likelihood of BSI. Underlying disease (collapsed to lymphoma, leukemia and other causes for the sake of analysis) and time to engraftment were not predictive of BSI. In addition, mucositis grades 3–4, occurring in 31 (40%) of those with and 54 (42%) of those without BSI, was not associated with BSI when compared to mucositis grades 0–2.
Agents used to treat hematologic diseases can have an effect on resident bacteria (16–18). Therefore, we sought to determine if the number of chemotherapy cycles or specific classes of chemotherapeutic agents used within the 6 months prior to HSCT were associated with BSI. Of the patients who developed BSI, 17 (22%), 33 (42%), 22 (29%) and 7 (9%) received 0, 1–2, 3–4 or at least 5 cycles, respectively, of chemotherapy 6 months prior to HSCT. This is in comparison to 29 (23%), 60 (47%), 27 (21%) and 11 (9%) of patients who did not develop BSI who received 0, 1–2, 3–4 or at least 5 cycles, respectively, prior to HSCT. Similarly, in those who did and did not develop BSI, there were no differences between the proportions of patients who received the following classes of agents: alkylating agents, antimetabolites, anthracyclines, vinca alkaloids, etoposide, or antibodies active against leukocytes (data not shown).
Multivariable analysis of time to first BSI
A competing risks model incorporating all cause mortality was constructed, using cause-specific hazard ratios. Variables incorporated into this model were degree of HLA matching, engraftment as a categorical variable, acute GVHD grade 2 or higher and prior HSCT. In this model, acute GVHD grade 2 or higher predicted an increased risk of subsequent BSI whereas HLA matching was associated with a reduction in the risk of BSI (Table 4). Prior HSCT and engraftment were not independent predictors of BSI.
Table 4.
Multivariable analysis of cause-specific hazard ratios for the acquisition of blood stream infection
| Risk Factor | Possible Endpoints (Competing Risks) | |||||
|---|---|---|---|---|---|---|
| Blood Stream Infection | All-Cause Mortality | |||||
| CSHR | 95% CI | P value | CSHR | 95% CI | P value | |
| HLA matching | 0.40 | 0.20, 0.81 | 0.01 | 0.13 | 0.04, 0.41 | 0.0004 |
| Engraftment | 0.74 | 0.31, 1.81 | 0.51 | 1.58 | 0.21, 12.0 | 0.66 |
| Time to acute GVHD grade ≥ 2 | 2.21 | 1.15, 4.22 | 0.02 | 1.89 | 0.67, 5.32 | 0.23 |
| Prior HSCT | 1.38 | 0.83, 2.29 | 0.22 | 1.58 | 0.57, 4.38 | 0.38 |
Analysis represents data on 211 patients with complete data records.
Mortality
Forty nine patients (23%) died by the end of the observation period of 100 days. The median time to death of those patients was 53 days (IQR 28, 78 days). We examined the factors that were associated with death, including BSI and acute GVHD grade 2 or higher.
Univariate analysis of time to death
BSI, expressed as either a categorical or a time-dependent variable, was associated with an increased risk of death within 100 days after HSCT. Acute GVHD grade 2 or higher when expressed as a time dependent variable, prior HSCT, receipt of etoposide in the 6 months prior to HSCT and of an alkylating agent during that time frame were also associated with an increased risk of death. When adjusted for underlying disease, receipt of these chemotherapeutic classes were still predictive of mortality. Engraftment, expressed as a categorical variable, and a 6/6 HLA match between the donor and recipient were associated with a decreased risk of death. Age, gender, underlying disease, type of HSCT (reduced intensity versus full dose HSCT), mucositis grades 3–4, time to engraftment and early BSI were not associated with an increased or decreased risk of death (data not shown).
Multivariable analysis of time to death
In the first multivariable analysis, the following variables were incorporated: BSI, acute GVHD grade 2 or higher, HLA match, engraftment as a categorical variable and prior HSCT. BSI, acute GVHD grade 2 or higher and prior HSCT were independently associated with death within 100 days after HSCT (Table 5). In the multivariable model, engraftment was highly associated with survival whereas there was a trend toward an association between degree of HLA matching and survival. We performed subsequent analyses identical to the one described above except that receipt of etoposide or receipt of an alkylating agent within the 6 months prior to HSCT was substituted for HLA matching. Receipt of either agent was associated with an increased risk of death (etoposide: n=199, HR 2.65, 95% CI 1.37, 5.13, p=0.004; alkylating agent: n=200, HR 2.04, 95% CI 1.10, 3.76, p=0.02).
Table 5.
Independent predictors of all-cause mortality
| HR | 95% CI | P value | |
|---|---|---|---|
| Time to acute GVHD grade ≥ 2 | 3.34 | 1.70, 6.56 | 0.0005 |
| Time to first BSI | 2.79 | 1.53, 5.09 | 0.0008 |
| Engraftment | 0.14 | 0.06, 0.29 | <0.0001 |
| Prior HSCT | 1.99 | 1.06, 3.74 | 0.03 |
| HLA Match | 0.49 | 0.22, 1.09 | 0.08 |
Analysis represents data on 211 patients with complete data records.
DISCUSSION
We found that early BSI occurring between the start of the conditioning regimen and 10 days after HSCT were independently associated with a two-fold increase of subsequent acute GVHD grade 2 or higher. CMV serology of the recipient but not of the donor was also independently associated with acute GVHD grade 2 or higher in that a positive serology was predictive of a substantial decrease in the risk. We also determined that the development of acute GVHD grade 2 or higher was associated with an increased risk of subsequent first BSI. Engraftment was not associated with BSI. Finally, both BSI and acute GVHD grade 2 or higher were independently associated with mortality.
Bacterial components, specifically lipopolysaccharide from gastrointestinal microorganisms, leak from the gastrointestinal tract after tissue damage induced by the conditioning regimen and enter the systemic circulation. There, the bacterial components contribute to the activation of donor T cells via antigen presenting cells, favoring the development of acute GVHD (3). As supporting evidence, patients with higher degrees of gastrointestinal injury and mucosal permeability during the HSCT process have an increased likelihood of developing acute GVHD (19). We reasoned that there would be similar consequences on acute GVHD in the course of an active blood stream infection. To our knowledge, this is the first demonstration of an association between BSI and subsequent acute GVHD. In our study, only early BSI was associated with an increased risk of acute GVHD. Acute GVHD appeared a median of 16 days after stem cell infusion, implying that the critical events that promote acute GVHD occur within days to weeks after stem cell infusion, consistent with the timing of early BSI.
The relationship between CMV and acute GVHD is complex as is reflected by a lack of consensus in the literature. Contributing to the complexity are differences in study populations with regard to stem cell source, donor/recipient matching status, and immunosuppressive and conditioning regimens. In addition, the studies differ in the approaches to CMV assessment such as by donor and/or recipient serostatus, CMV antigenemia, CMV viremia, the presence of CMV in tissue specimens and CMV disease.
Several studies focused on whether or not CMV increased the likelihood of acute GVHD. Recipient CMV seropositivity was associated with an increased likelihood of acute GVHD in some but not all studies (20–23). Similar disagreement has been found with regard to the association of donor CMV serostatus on acute GVHD (24, 25). CMV reactivation as detected by CMV DNA in peripheral blood leukocytes, positive blood culture or positive tissue biopsy was not associated with an increased risk of subsequent acute GVHD (26–28). Several investigators have demonstrated an increased risk of CMV reactivation or disease after the onset of acute GVHD although this finding was not universal (28–37). However, to our knowledge, no study has demonstrated an association between recipient CMV seropositivity and a reduction in acute GVHD as we have in the current study.
There are potential biological mechanisms to explain the association between recipient CMV seropositivity and a reduction in subsequent acute GVHD. For example, CMV infection, but not latency, induces immunomodulatory changes including a decrease in expression of major histocompatibility antigens and inhibition of NK cell activation (reviewed in (38–40)). In addition, the CMV genome encodes for a protein that is a homolog of interleukin-10, a human cytokine thought through its inflammatory activity to diminish the acute GVHD response. In summary, it could be postulated that silent reactivation of CMV in the early post-HSCT time period could result in a dampening of the immune response at a time when donor cells are most susceptible to either pro- or anti-inflammatory influences, resulting in a decrease in acute GVHD.
CMV reactivation can occur early, even within one week of stem cell infusion although most patients reactivate later in their course (27, 29–32, 35). In these studies, the predominant methods of determining reactivation were by antigen detection, culture or PCR of the blood. It is possible that the blood compartment is not the most relevant site to detect CMV reactivation associated with the immunomodulation of donor cells. In addition, in some studies, CMV reactivation was not sought until after engraftment, after acute GVHD developed or until at least one week after stem cell infusion. Therefore, little detailed information if available regarding CMV reactivation in the blood or other compartments in the days prior to and just after stem cell infusion.
It seems intuitive that acute GVHD is associated with an increased risk for bacterial infection, either due to immunosuppressive medications used in its treatment (41), or by providing portals of entry through the skin and/or gastrointestinal tract. However, there is conflicting data. Several studies showed an increased risk of bacterial infection, particularly BSI, after the onset of acute GVHD as was shown in the present study whereas others have not (30, 41–44). Possible reasons for different findings among studies include differences in analytic approaches, such as assessing acute GVHD as a time-dependent variable, and heterogeneity in types of HSCT such as reduced intensity HSCT, use of T cell depletion, and the proportion of related versus unrelated donors.
There are several limitations to this study. For example, the association between early BSI and subsequent acute GVHD grade 2 or higher is at the borderline of statistical significance. This might be related to the small number of early BSI or to a weak relationship between the two events. Additionally, since this was a retrospective study, certain variables, such as the occurrence of CMV reactivation and disease, were not available.
The association between BSI or acute GVHD and mortality after HSCT has been observed by us and others (1, 11, 45–47). In addition, BSI and/or acute GVHD are associated with longer lengths of stay, readmission to the hospital, fungal infection resulting in death, and increased costs of care (1, 47–50). Therefore, it is important to further study the interrelationships between these two common and serious complications in the hopes of improving the outcomes of HSCT.
Acknowledgments
This study was supported in part by the National Institutes of Health/National Center for Research Resources (5K23 RR020042-04 to DDP), Tufts Clinical and Translational Science Institute (1UL1RR025752/1KL2RR025751, DDP), and the Harold William, MD Medical Student Research Fellowship, Tufts University School of Medicine (DM).
Footnotes
CONFLICTS OF INTEREST
There were no conflicts of interest for any authors.
References
- 1.Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40(1):63–70. doi: 10.1038/sj.bmt.1705690. [DOI] [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation [see comment] New Engl J Med. 2006;354(17):1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 3.Duran-Struuck R, Reddy P. Biological advances in acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transplantation. 2008;85(3):303–8. doi: 10.1097/TP.0b013e318162d357. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara JL, Levine JE, Reddy P, Holler E, Ferrara JLM, Levine JE, et al. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KB, Roberts TF, Chan G, Schenkein DP, Lawrence D, Sprague K, et al. A novel reduced intensity regimen for allogeneic hematopoietic stem cell transplantation associated with a reduced incidence of graft-versus-host disease. Bone Marrow Transplant. 2004;33(9):881–9. doi: 10.1038/sj.bmt.1704454. [DOI] [PubMed] [Google Scholar]
- 6.Horan T, Gaynes R. Hospital Epidemiology and Infection Control. 3. Lippincott, Williams & Wilkeins; Philadelphia: 2004. Surveillance of nosocomial infections; pp. 1659–1702. [Google Scholar]
- 7.DesJardin JA, Falagas ME, Ruthazer R, Griffith J, Wawrose D, Schenkein D, et al. Clinical utility of blood cultures drawn from indwelling central venous catheters in hospitalized patients with cancer. Ann Intern Med. 1999;131(9):641–7. doi: 10.7326/0003-4819-131-9-199911020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 9.National Cancer Institute. Common Terminology Criteria for Adverse Events. 2006. [Google Scholar]
- 10.Flowers ME, Traina F, Storer B, Maris M, Bethge WA, Carpenter P, et al. Serious graft-versus-host disease after hematopoietic cell transplantation following nonmyeloablative conditioning. Bone Marrow Transplant. 2005;35(3):277–82. doi: 10.1038/sj.bmt.1704767. [DOI] [PubMed] [Google Scholar]
- 11.Leisenring WM, Martin PJ, Petersdorf EW, Regan AE, Aboulhosn N, Stern JM, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108(2):749–55. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergstralh E, Therneau T. Mayo Foundations for Medical Education and Research. 2002. %Schoen.sas. Copyright 2006. [Google Scholar]
- 13.Harrell JFE. Mayo Foundations for Medical Education and Research. 1991. %daspline.sas. Copyright 2006. [Google Scholar]
- 14.Allison PD. Survival Analysis Using SAS(TM): A Practical GuideEstimating Cox regression models with PROC PHREG. SAS Institute, Inc; Cary, NC: 1995. [Google Scholar]
- 15.Wolkewitz M, Vonberg RP, Grundmann H, Beyersmann J, Gastmeier P, Barwolff S, et al. Risk factors for the development of nosocomial pneumonia and mortality on intensive care units: application of competing risks models [see comment] Critical Care (London, England) 2008;12(2):R44. doi: 10.1186/cc6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neher A, Arnitz R, Gstottner M, Schafer D, Kross EM, Nagl M, et al. Antimicrobial activity of dexamethasone and its combination with N-chlorotaurine. Archives of Otolaryngology -- Head & Neck Surgery. 2008;134(6):615–20. doi: 10.1001/archotol.134.6.615. [DOI] [PubMed] [Google Scholar]
- 17.Unsal H, Balkaya M, Unsal C, Biyik H, Basbulbul G, Poyrazoglu E, et al. The short-term effects of different doses of dexamethasone on the numbers of some bacteria in the ileum. Digestive Diseases & Sciences. 2008;53(7):1842–5. doi: 10.1007/s10620-007-0089-6. [DOI] [PubMed] [Google Scholar]
- 18.van Vliet MJ, Tissing WJ, Dun CA, Meessen NE, Kamps WA, de Bont ES, et al. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis. 2009;49(2):262–70. doi: 10.1086/599346. [DOI] [PubMed] [Google Scholar]
- 19.Johansson JE, Ekman T, Johansson J-E, Ekman T. Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Digestive Diseases & Sciences. 2007;52(9):2340–5. doi: 10.1007/s10620-006-9404-x. [DOI] [PubMed] [Google Scholar]
- 20.Bostrom L, Ringden O, Sundberg B, Linde A, Tollemar J, Nilsson B. Pretransplant herpesvirus serology and acute graft-versus-host disease. Transplantation. 1988;46(4):548–52. doi: 10.1097/00007890-198810000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Broers AE, van Der Holt R, van Esser JW, Gratama JW, Henzen-Logmans S, Kuenen-Boumeester V, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood. 2000;95(7):2240–5. [PubMed] [Google Scholar]
- 22.Meijer E, Dekker AW, Rozenberg-Arska M, Weersink AJ, Verdonck LF. Influence of cytomegalovirus seropositivity on outcome after T cell-depleted bone marrow transplantation: contrasting results between recipients of grafts from related and unrelated donors. Clin Infect Dis. 2002;35(6):703–12. doi: 10.1086/342332. [DOI] [PubMed] [Google Scholar]
- 23.Remberger M, Aschan J, Lonnqvist B, Carlens S, Gustafsson B, Hentschke P, et al. An ethnic role for chronic, but not acute, graft-versus-host disease after HLA-identical sibling stem cell transplantation. Eur J Haematol. 2001;66(1):50–6. doi: 10.1034/j.1600-0609.2001.00331.x. [DOI] [PubMed] [Google Scholar]
- 24.Gratama JW, Zwaan FE, Stijnen T, Weijers TF, Weiland HT, D’Amaro J, et al. Herpes-virus immunity and acute graft-versus-host disease. Lancet. 1987;1 (8531):471–4. doi: 10.1016/s0140-6736(87)92088-5. [DOI] [PubMed] [Google Scholar]
- 25.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102(13):4255–60. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 26.Appleton AL, Sviland L, Peiris JS, Taylor CE, Wilkes J, Green MA, et al. Role of target organ infection with cytomegalovirus in the pathogenesis of graft-versus-host disease. Bone Marrow Transplant. 1995;15(4):557–61. [PubMed] [Google Scholar]
- 27.Meyers JD, Ljungman P, Fisher LD. Cytomegalovirus excretion as a predictor of cytomegalovirus disease after marrow transplantation: importance of cytomegalovirus viremia. J Infect Dis. 1990;162(2):373–80. doi: 10.1093/infdis/162.2.373. [DOI] [PubMed] [Google Scholar]
- 28.Miller W, Flynn P, McCullough J, Balfour HH, Jr, Goldman A, Haake R, et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease. Blood. 1986;67(4):1162–7. [PubMed] [Google Scholar]
- 29.Asano-Mori Y, Oshima K, Sakata-Yanagimoto M, Nakagawa M, Kandabashi K, Izutsu K, et al. High-grade cytomegalovirus antigenemia after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36(9):813–9. doi: 10.1038/sj.bmt.1705134. [DOI] [PubMed] [Google Scholar]
- 30.Daly A, McAfee S, Dey B, Colby C, Schulte L, Yeap B, et al. Nonmyeloablative bone marrow transplantation: Infectious complications in 65 recipients of HLA-identical and mismatched transplants. Biol Blood Marrow Transplant. 2003;9(6):373–82. doi: 10.1016/s1083-8791(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 31.Frere P, Baron F, Bonnet C, Hafraoui K, Pereira M, Willems E, et al. Infections after allogeneic hematopoietic stem cell transplantation with a nonmyeloablative conditioning regimen. Bone Marrow Transplant. 2006;37(4):411–8. doi: 10.1038/sj.bmt.1705255. [DOI] [PubMed] [Google Scholar]
- 32.Lin TS, Zahrieh D, Weller E, Alyea EP, Antin JH, Soiffer RJ. Risk factors for cytomegalovirus reactivation after CD6+ T-cell-depleted allogeneic bone marrow transplantation. Transplantation. 2002;74(1):49–54. doi: 10.1097/00007890-200207150-00009. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura T, Narimatsu H, Kami M, Yuji K, Kusumi E, Hori A, et al. Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biology of Blood & Marrow Transplantation. 2007;13(5):577–83. doi: 10.1016/j.bbmt.2006.12.454. [DOI] [PubMed] [Google Scholar]
- 34.Meyers J, Flournoy N, Thomas E. Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis. 1986;153:478–488. doi: 10.1093/infdis/153.3.478. [DOI] [PubMed] [Google Scholar]
- 35.Mohty M, Jacot W, Faucher C, Bay JO, Zandotti C, Collet L, et al. Infectious complications following allogeneic HLA-identical sibling transplantation with antithymocyte globulin-based reduced intensity preparative regimen. Leukemia. 2003;17(11):2168–77. doi: 10.1038/sj.leu.2403105. [DOI] [PubMed] [Google Scholar]
- 36.Reddy V, Meier-Kriesche HU, Greene S, Schold JD, Wingard JR. Increased levels of tumor necrosis factor alpha are associated with an increased risk of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Biology of Blood & Marrow Transplantation. 2005;11(9):698–705. doi: 10.1016/j.bbmt.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 37.van Burik JA, Carter SL, Freifeld AG, High KP, Godder KT, Papanicolaou GA, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biology of Blood & Marrow Transplantation. 2007;13(12):1487–98. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends in Microbiology. 2002;10(7):332–9. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- 39.Mocarski ES., Jr Immune escape and exploitation strategies of cytomegaloviruses: impact on and imitation of the major histocompatibility system. Cellular Microbiology. 2004;6(8):707–17. doi: 10.1111/j.1462-5822.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 40.Peggs KS, Mackinnon S. Cytomegalovirus: the role of CMV post-haematopoietic stem cell transplantation. International Journal of Biochemistry & Cell Biology. 2004;36(4):695–701. doi: 10.1016/j.biocel.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Sayer HG, Longton G, Bowden R, Pepe M, Storb R. Increased risk of infection in marrow transplant patients receiving methylprednisolone for graft-versus-host disease prevention. Blood. 1994;84(4):1328–32. [PubMed] [Google Scholar]
- 42.Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN, et al. Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7(1):11–7. doi: 10.1111/j.1399-3062.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 43.Cappellano P, Viscoli C, Bruzzi P, Van Lint MT, Pereira CA, Bacigalupo A. Epidemiology and risk factors for bloodstream infections after allogeneic hematopoietic stem cell transplantion. New Microbiologica. 2007;30(2):89–99. [PubMed] [Google Scholar]
- 44.Ninin E, Milpied N, Moreau P, Andre-Richet B, Morineau N, Mahe B, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis. 2001;33(1):41–7. doi: 10.1086/320871. [DOI] [PubMed] [Google Scholar]
- 45.Kruger W, Russmann B, Kroger N, Salomon C, Ekopf N, Elsner HA, et al. Early infections in patients undergoing bone marrow or blood stem cell transplantation--a 7 year single centre investigation of 409 cases. Bone Marrow Transplant. 1999;23 (6):589–97. doi: 10.1038/sj.bmt.1701614. [DOI] [PubMed] [Google Scholar]
- 46.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biology of Blood & Marrow Transplantation. 2002;8(7):387–94. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 47.Sparrelid E, Hagglund H, Remberger M, Ringden O, Lonnqvist B, Ljungman P, et al. Bacteraemia during the aplastic phase after allogeneic bone marrow transplantation is associated with early death from invasive fungal infection. Bone Marrow Transplant. 1998;22(8):795–800. doi: 10.1038/sj.bmt.1701404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Lissovoy G, Hurd D, Carter S, Beatty P, Ewell M, Henslee-Downey J, et al. Economic analysis of unrelated allogeneic bone marrow transplantation: results from the randomized clinical trial of T-cell depletion vs unmanipulated grafts for the prevention of graft-versus-host disease. Bone Marrow Transplant. 2005;36 (6):539–46. doi: 10.1038/sj.bmt.1705078. [DOI] [PubMed] [Google Scholar]
- 49.Esperou H, Brunot A, Roudot-Thoraval F, Buzyn A, Dhedin N, Rio B, et al. Predicting the costs of allogeneic sibling stem-cell transplantation: results from a prospective, multicenter, French study. Transplantation. 2004;77(12):1854–8. doi: 10.1097/01.tp.0000129409.84087.62. [DOI] [PubMed] [Google Scholar]
- 50.Moya R, Espigado I, Parody R, Carmona M, Marquez F, De Blas JM. Evaluation of readmissions in hematopoietic stem cell transplant recipients. Transplantation Proceedings. 2006;38(8):2591–2. doi: 10.1016/j.transproceed.2006.08.057. [DOI] [PubMed] [Google Scholar]


