Abstract
The majority (>95%) of thymocytes undergo apoptosis during selection in the thymus. Several mechanisms have been proposed to explain how apoptosis of thymocytes that are not positively selected occurs, however it is unknown whether thymocytes die purely by “neglect” or whether signaling through a cell surface receptor initiates an apoptotic pathway. We have previously demonstrated that on double positive (DP) thymocytes the ligation of CD8 in the absence of TCR engagement results in apoptosis and have postulated this is a mechanism to remove thymocytes that have failed positive selection. On mature single positive (SP) T cells CD8 acts as a co-receptor to augment signaling through the T cell receptor (TCR) that is dependent on the phosphorylation of the adaptor protein, Linker for Activation of T cells (LAT). Here we show that during CD8-mediated apoptosis of DP thymocytes there is an increase in the association of CD8 with LAT and an increase in LAT tyrosine phosphorylation. Decreasing LAT expression and mutation of tyrosine residues of LAT reduced apoptosis upon crosslinking of CD8. Our results identify novel functions for both CD8 and LAT that are independent of TCR signal transduction and suggest a mechanism for signal transduction leading to apoptosis upon CD8 crosslinking.
Keywords: Apoptosis, Thymus, CD8, LAT, Signal Transduction
Introduction
Thymocyte development involves meticulous screening processes in which thymocytes must successfully navigate through several developmental checkpoints to become fully mature. Only ~3% of all thymocytes meet the rigorous selection criteria to become functional T cells [1] while the remainder undergo programmed cell death (PCD) or apoptosis in the thymus. During positive selection approximately 90% of immature CD4+CD8+ double positive (DP) thymocytes undergo apoptosis due to the inability of the newly rearranged αβ-TCR to interact with self-peptide-MHC complexes (pMHC). It is widely believed that most thymocytes that fail positive selection simply die by “neglect” due to the failure to receive signals from cytokines. Apoptosis of thymocytes can also be triggered by corticosteroids or by ligation of CD24, CD45, or Thy-1, however the role of these events in thymic selection is yet to be established.[2–4].
We have previously described a novel mechanism for apoptosis of immature DP thymocytes through the ligation of CD8 [5]. CD8 is traditionally thought to function as a co-receptor for the TCR that stabilizes the interaction of the TCR with the pMHC class I complex and augments signals through the TCR by virtue of its association with both Lck and LAT [6, 7]. Interestingly, on immature DP thymocytes, CD8 exists in a form that is able to bind MHC class I molecules independent of the TCR interaction [8, 9]. This increase in the ability of CD8 to bind MHC class I molecules has been correlated with the absence of sialylation[8], however this is disputed by others [10]. The ligation of CD8, in the absence of TCR engagement, results in apoptosis of a proportion of DP thymocytes and we hypothesize that this maybe a mechanism to remove DP thymocytes that have failed positive selection. The apoptotic mechanism induced by the ligation of CD8 does not require new gene transcription and thymocytes can be “rescued” from CD8-mediated apoptosis by the simultaneous ligation of the CD3 complex.
On murine and human thymocytes, the majority of CD8 is expressed as a heterodimer consisting of an α and β chain, although thymocytes also express CD8αα homodimers [11]. The CD8α- and β-chains are structurally and functionally different molecules. The extracellular portion of the CD8α chain interacts primarily with residues in the α3 domain of MHC class I molecules [12]. The intracellular portion of the α-chain is responsible for association with the src-family kinase p56lck (Lck) and the adaptor molecule LAT [6, 7]. The intracellular region of CD8β stabilizes the association of CD8α with Lck and also enhances the kinase activity of Lck [13], while CD8β also promotes the association of LAT with the CD8α chain [7]. CD8β is palmitoylated on a cysteine residue in its cytoplasmic tail and partitions CD8 heterodimers into lipid rafts [14]. Interestingly, the association of Lck and LAT with CD8α is developmentally regulated. In DP thymocytes CD8 preferentially associates with LAT while Lck associates with CD4 [7]. As thymocytes mature to SP thymocytes and enter the periphery, the association of CD8 with Lck and LAT changes such that on mature T cells approximately 65% of both CD8 and CD4 associates with Lck while the remaining 35% associates with LAT [7]. The purpose of the preferential association of CD8α with LAT on DP thymocytes has not been elucidated.
LAT is a 36–38 kDa transmembrane adaptor protein that is expressed in T cells, mast cells, NK cells, and megakaryocytes. LAT is the predominant target of tyrosine phosphorylation during T cell activation. Human LAT contains ten tyrosine residues, nine of which are conserved in mice [15]. LAT is palmitoylated on two cysteine residues that influences its localization within the plasma membrane [16]. The only known function of LAT in T cells is that it is required for the transduction of signaling events following the engagement of the TCR that lead to T-cell proliferation and differentiation. As stated above, LAT associates with both CD4 and CD8 and is recruited to the TCR upon antigen recognition. LAT coordinates the assembly of multiprotein signaling complexes at the plasma membrane. Only the five C-terminal tyrosines (Y127, Y132, Y171, Y191 and Y226) undergo detectable phosphorylation upon TCR engagement [17]. The majority of the tyrosine phosphorylation of LAT is attributed to the tyrosine kinase ZAP-70 [15], although, Lck and ITK have also been shown to phosphorylate LAT [18, 19]. Phosphorylated LAT serves as a scaffolding molecule for the formation of signaling complexes that lead to downstream signaling pathways including Ca2+ mobilization, cytoskeletal rearrangements, and gene transcription resulting in complete activation of the T cell [20].
In this study we investigated the signaling events initiated by cross-linking of CD8 independent of the TCR and whether the preferential association of LAT with CD8 is involved in the induction of CD8-mediated apoptosis in DP thymocytes. We found that upon ligation of CD8 the association of LAT with CD8 was increased and LAT was phosphorylated on several regulatory tyrosine residues. In addition, the expression and phosphorylation of LAT was required for apoptosis to occur following the ligation of CD8 while Lck did not play a role. In this study we present a model in which ligation of CD8 initiates a novel biochemical signaling pathway that leads to apoptosis and provides an explanation for the preferential association of LAT with CD8 during thymocyte development.
Results
CD8 crosslinking induces apoptosis of DP thymocytes and 3H6 thymoma cells
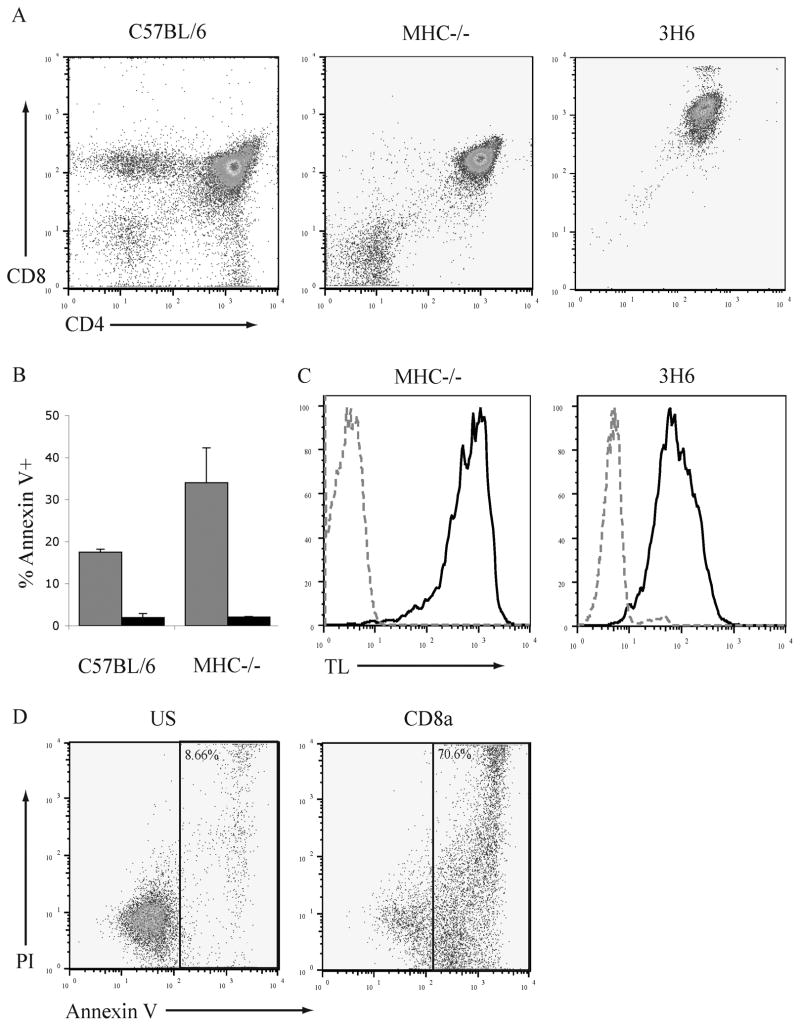
Within the thymus of wildtype C57BL/6 mice only a proportion (~25–35%) of DP thymocytes are susceptible to CD8-mediated apoptosis (5). Thymocytes from double knockout (B6.IAβb−/−β2M−/− referred to herein as MHC−/−) mice are developmentally arrested at the DP stage (Fig. 1A) and in these mice the proportion of DP thymocytes that is susceptible to apoptosis following ligation of CD8 is increased to approximately 40% whereas the ligation of CD4 does not induce apoptosis (Fig. 1B). The induction of apoptosis was analyzed by staining with annexin V that recognizes the exposure of phosphatidylserine and propidium iodide (PI). In our studies of the biochemical mechanism of CD8-mediated apoptosis we also used a cell line 3H6, that is a subclone of the ITT-1 75NS radiation-induced thymoma cell line and has a CD4+CD8+ phenotype (Fig. 1A right). The high affinity form of CD8 that can bind MHC class I molecules independent of the TCR can be detected by staining with tetrameric MHC class I molecules. To determine if the 3H6 thymoma cell line expresses the high affinity form of CD8 the binding of tetrameric TL molecules was examined (Fig. 1C). TL tetramers bind to the 3H6 thymoma cells indicating that 3H6 cells express the high affinity form of CD8.
Fig. 1. The 3H6 thymoma cell line is susceptible to apoptosis induced by the ligation of CD8.
A. Thymocytes from C57BL/6, MHC−/− mice, and 3H6 thymoma cells were stained with antibodies to CD4 and CD8α and analyzed by flow cytometry.
B. Thymocytes from C57BL/6 and MHC−/− mice were treated with antibody to CD8α and goat anti-rat Ig (grey bars), CD4 and goat anti-rat Ig (hatched bars), or goat anti-rat Ig alone (black bars) for 6hr. Cells were stained with FITC-conjugated annexin V and analyzed by flow cytometry. N=3; mean ± SEM.
C. Thymocytes from MHC−/− mice and 3H6 thymoma cells were stained with anti-CD4 (FITC), anti-CD8α (APC), and tetrameric TL molecules (PE) The binding of TL to DP cells is represented by the black line; unstained DP cells by the grey dotted line.
D. 3H6 thymoma cells were treated with antibody to CD8α and goat anti-rat Ig, CD4 and goat anti-rat Ig, or left unstimulated (US) for 6hr. Cells were stained with annexin V (FITC) and PI, the percentage of the cells in the indicated gate is given.
To determine if 3H6 is susceptible to CD8-mediated apoptosis cells were treated with antibodies to CD8α and goat anti-rat IgG. (Fig. 1D). Approximately 70% of 3H6 thymoma cells exhibit an apoptotic phenotype after 6 cross-linking of CD8. Consistent with data from ex vivo thymocytes crosslinking CD4 does not induce apoptosis. Therefore, a large population of 3H6 thymoma cells is susceptible to CD8-mediated apoptosis and 3H6 also provide a model to study the biochemical events that occur during CD8-mediated apoptosis in vitro.
The CD8β chain is involved in CD8-mediated apoptosis
DP thymocytes can express both CD8αβ heterodimers and CD8αα homodimers, therefore, we investigated the contribution of the CD8α chain versus the CD8β chain in the induction of apoptosis following ligation of CD8. To determine if separate crosslinking of CD8α or CD8β induced apoptosis 3H6 cells were treated with anti-CD8α or anti-CD8β antibodies and anti-rat IgG (Fig. 2A). Anti-CD8α and anti-CD8β both induced apoptosis in 70% of 3H6 cells following crosslinking as determined by the binding of annexin-V.
Figure 2. The CD8β chain is required for CD8-mediated apoptosis.
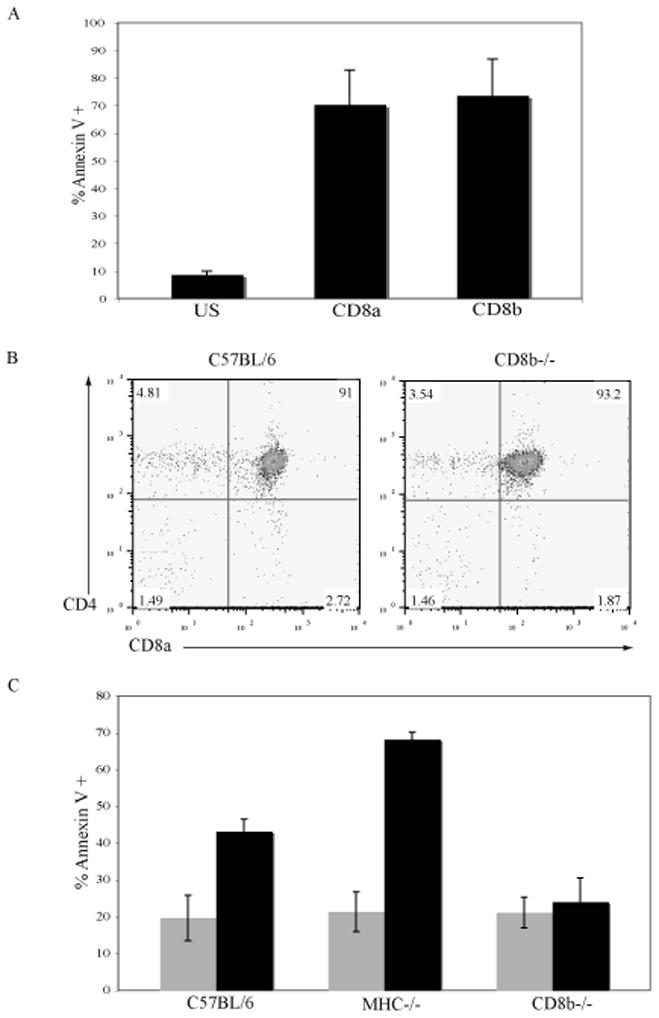
A. 3H6 thymoma cells were left unstimulated (US) or treated with anti-CD8α or anti-CD8β antibodies and goat anti-rat Ig to crosslink for 6 hours. Apoptosis was measured by the binding of annexin-V conjugated with FITC. N=4; mean ± SEM.
B. Thymocytes from C57BL/6 and CD8β−/− mice were stained with anti-CD4 and anti-CD8 antibodies and analyzed by flow cytometry.
C. Thymocytes from C57BL/6, MHC−/− and CD8β−/− mice were left US (grey bars) or crosslinked with anti-CD8α and goat anti-rat Ig (black bars) for 6 hours. Apoptosis was measured by the binding of annexin-V conjugated with FITC. N=5; mean ± SEM.
The CD8β-chain needs to form a heterodimer with the CD8α-chain to be expressed on the cell surface [21]. Therefore, in CD8β−/− mice the only form of CD8 that is expressed is CD8αα homodimers. CD8β−/− mice have a significant decrease in the number of peripheral CD8+ T cells compared to wildtype mice [22] however the number and frequency of DP thymocytes is similar to that in normal mice ([22] and Fig. 2B). The involvement of the CD8β-chain in CD8 mediated apoptosis was examined by analyzing thymocytes from CD8β−/− mice. After incubation with antibody to CD8α under crosslinking conditions the proportion of thymocytes that bound annexin V was greater (C57Bl/6 43%, MHC−/− 68%, Fig 2C) than in the untreated population. In contrast, the proportion of thymocytes from CD8β−/− mice that bound annexin V (24%) was not increased by ligation of CD8α. This observation indicates that CD8αβ heterodimers are the only form of CD8 that is involved in the induction of apoptosis.
Lck is not required for the induction of CD8-mediated apoptosis
The CD8α chain can associate with two intracellular molecules involved in signal transduction: the Src-family kinase Lck [6]and the adaptor molecule LAT[7]. Lck is the prominent tyrosine kinase that is active during the upstream signaling events during T cell activation. To determine if the association of Lck with CD8α is altered during the induction of CD8-mediated apoptosis CD8, immunoprecipitates of CD8 were analyzed (Fig. 3A). Thymocytes from MHC−/− mice were crosslinked with antibody to CD8β and CD8α was immunoprecipitated. The association of Lck was examined by probing western blots. The ligation of CD8 results in a slight increase in the association of CD8α with Lck. This result suggests that a small pool of Lck is recruited to CD8α upon crosslinking of CD8 and may be involved in coupling the apoptotic signals from CD8 to downstream pathways.
Figure 3. Lck is not required for CD8-mediated apoptosis of DP thymocytes.
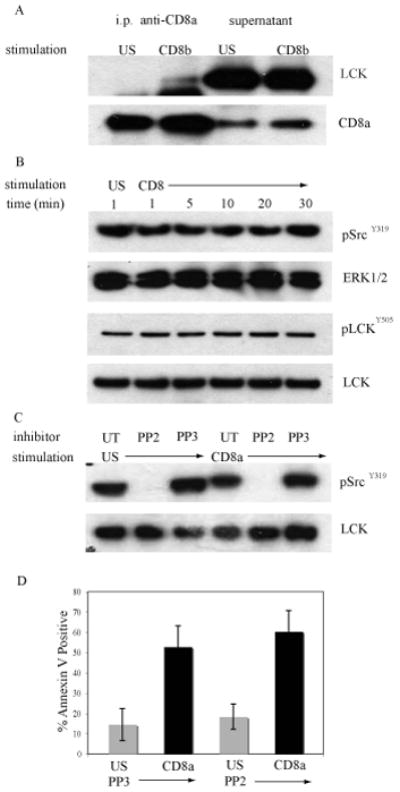
A. MHC−/− thymocytes were treated with antibody to CD8β and crosslinked with goat anti-rat Ig or left unstimulated (US) at 37°C for 30min. CD8α was immunoprecipitated and western blots of the immunoprecipitates and supernatants were probed with antibody to Lck and then reprobed with antibody to the intracellular tail of CD8α as a loading control.
B. MHC−/− thymocytes were treated with antibody to CD8α and crosslinked with goat anti-rat Ig for the indicated times or left unstimulated (US). Western blots of whole cell lysates were analyzed for expression of pSrcY416 or pLckY505 and reprobed with antibodies to either ERK1/2 or Lck to monitor equivalent loading in each lane.
C. MHC−/− thymocytes were treated with 5mM PP2, 5mM PP3, or left untreated (UT) for 30 minutes at 37°C. The thymocytes were then treated with anti-CD8α antibody and crosslinked with anti-rat Ig for 30 min at 37°C or left unstimulated. Western blots of whole cell lysates were analyzed with pSrcY416 antibody and reprobed with an anti-Lck antibody as a loading control.
D. MHC−/− thymocytes that were treated with PP2 or PP3 were cross-linked with antibody to CD8α (black bars) or left unstimulated (grey bars). At 6 hours apoptosis was measured by the binding annexin-V conjugated with FITC. N=3; mean ± SEM.
Lck is regulated by reversible phosphorylation of 2 tyrosine residues Y394 and Y505 [23]. Lck autophosphorylates Y394 leading to a conformational change in the activation loop of Lck. An increase in phosphorylation of Y394 is indicative of an increase in Lck kinase activity. Lck is also negatively regulated by phosphorylation of a conserved C-terminal tyrosine residue, Y505. When phosphorylated, Y505 binds to the SH2 domain in the same Lck molecule forcing Lck into an inactive conformation. MHC−/− thymocytes were crosslinked with CD8α antibody and goat anti-rat Ig and whole cell lysates were prepared. The western blots were probed with antibodies to phosphoSrc Y416 (pSrcY416) that recognizes the phosphorylated tyrosine in the active site of all src-family kinase members, and to phosphoLck Y505 (pLckY505) that binds to an epitope dependent on phosphorylation of the tyrosine residue in the inhibitory site (Fig. 3B). The ligation of CD8 with antibody did not induce either an increase or a decrease in the phosphorylation of Lck measured by pSrcY416 or pLckY505. These observations indicate that Lck is neither activated nor inactivated upon crosslinking of CD8-mediated on DP thymocytes.
The above findings suggest that Lck is not activated during CD8-mediated apoptosis but does not prove that Lck is not required for apoptosis to occur. The development of thymocytes in Lck−/− mice is arrested at the DN2 stage [24]. Therefore, we inhibited Lck kinase activity using PP2, a pharmacological inhibitor specific for the src-family tyrosine kinases [25]. PP2 does not inhibit other tyrosine kinase families such as Syk and Tec and it is a potent inhibitor of TCR signaling. To verify that PP2 inhibits the activity of src-family kinases in our system, MHC−/− thymocytes were treated with either PP2, or PP3 (a pharmacologically inactive control for PP2), or were left untreated. Thymocytes were then incubated with antibody to CD8α under crosslinking conditions or were left unstimulated and analyzed for the expression of pSrcY416 (Fig. 3C). Thymocytes that were treated with PP2 did not express pSrcY416 confirming that PP2 did inhibit src-family kinase activity.
The effect of inhibition of Lck on apoptosis following CD8 ligation was examined. Thymocytes from MHC−/− mice were pretreated with PP2 or PP3 and then incubated with antibody to CD8 under crosslinking conditions. Apoptosis was measured by the binding of annexin-V after ligation of CD8 (Fig. 3D). Treatment of thymocytes with PP2 did not affect the percentage of cells that bound annexin-V demonstrating that Lck is not involved in the initiation of CD8-mediated apoptosis in DP thymocytes.
LAT is required for apoptosis following CD8 crosslinking on DP thymocytes
In DP thymocytes CD8α preferentially associates with LAT whereas CD4 preferentially associates with Lck (7). To determine if the association of LAT with CD8α is altered during the induction of CD8-mediated apoptosis, the amount of LAT that co-precipitated with CD8 was determined (Fig. 4A). Thymocytes from MHC−/− mice were treated with antibody to CD8β under crosslinking conditions and CD8α was immunoprecipitated. In resting thymocytes a proportion of LAT is associated with CD8α. Upon the ligation of CD8 there is a dramatic increase in the amount of LAT that is associated with CD8α. This result suggests that during the induction of apoptosis LAT is recruited to CD8 and may be the link to downstream signaling pathways.
Fig. 4. LAT is required for CD8-mediated apoptosis of thymocytes.
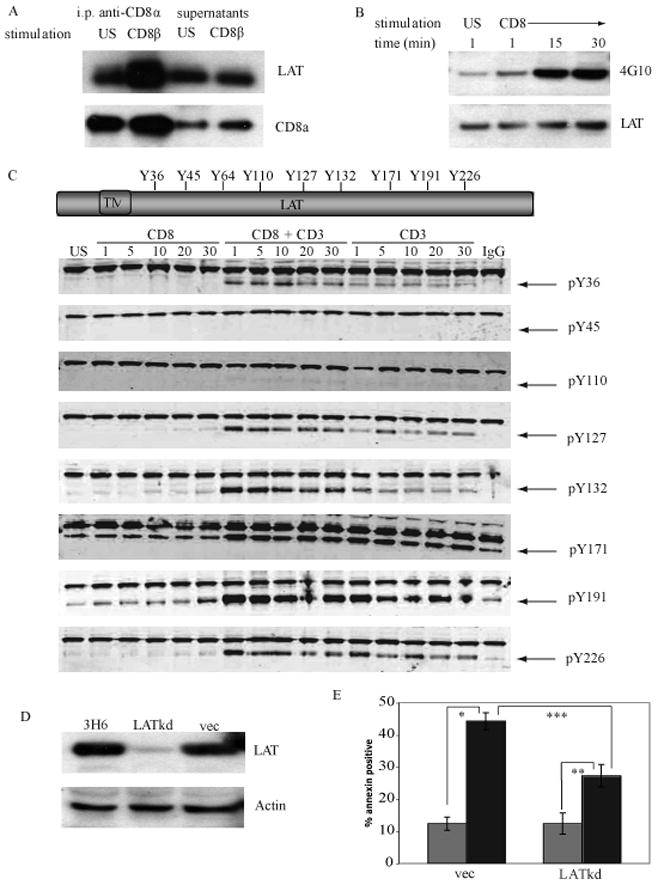
A. MHC−/− thymocytes were left unstimulated (US) or treated with antibody to CD8β and goat anti-rat Ig for 30 min. CD8 was immunoprecipitated with biotinylated antibody to CD8α coupled to Neutravidin beads for 90 min. Western blots of the immunoprecipitates and supernatants were probed with antibody to LAT and then reprobed with antibody to the CD8α intracellular region as a loading control.
B. MHC−/− thymocytes were crosslinked with antibody to CD8α as in (A). LAT was immunoprecipitated using antibody to LAT coupled to rabbit Trublot beads for 90 min. Western blots of the immunoprecipitates were probed with the pan anti-phosphotyrosine antibody 4G10 and then reprobed with antibody to LAT as a loading control.
C. LAT contains 9 intracellular tyrosine residues. Phospho-site specific antibodies were generated in rabbits to all tyrosine residues of LAT except Y64. DP thymocytes from MHC−/− mice were either left unstimulated (US) or were crosslinked with antibody to CD8α, antibodies to both CD8α and CD3 (to mimic TCR signaling), or CD3 alone. Western blots were probed with the rabbit phospho-site specific antibodies indicated (bottom bands with arrows) and murine anti-ERK1 antibody (upper bands) to monitor for equivalent loading in each lane.
D. 3H6 thymoma cells were transduced with recombinant lentivirus encoding LAT shRNA (LATkd), empty vector (vec) or were untreated. Whole cell lysates were prepared and analyzed for expression of LAT by western blot. Actin serves as a loading control.
E. 3H6 thymoma cells that were stably transduced with LAT shRNA or vector alone were treated with antibody to CD8a and crosslinked with goat anti-rat IgG for 6 hours (black bars) or were unstimulated (grey bars). The induction of apoptosis was analyzed by the binding of annexin-V conjugated with FITC. N=5; mean ± SEM. * p< 0.001, ** p< 0.004, *** p< 0.005, Student’s t-test.
LAT is an adaptor molecule and therefore has no intrinsic enzymatic activity. During T cell activation LAT becomes rapidly phosphorylated on several tyrosine residues creating binding sites for proteins that contain Src homology 2 (SH2) domains, thus linking signals from the TCR to downstream signaling pathways [15]. To determine if the induction of CD8-mediated apoptosis induced the phosphorylation of tyrosine residues in LAT, we immunoprecipitated LAT from MHC−/− thymocytes that were either unstimulated or were treated with antibody to CD8α under crosslinking conditions and analyzed by western blot with the pan phosphotyrosine antibody 4G10 (Fig. 4B). Unstimulated thymocytes have low levels of LAT phosphorylation. Ligation of CD8 increases tyrosine phosphorylation of LAT within 1 minute with a sustained increase in phosphorylation until 30 minutes. Murine LAT contains 9 tyrosine residues that have the potential to become phosphorylated (Fig. 4C), however only the 4 c-terminal tyrosine residues are required for TCR-signaling [17]. Western blots were performed to determine which tyrosine residues were phosphorylated during the induction of CD8-mediated apoptosis of murine DP thymocytes compared to signals that mimic TCR signaling (CD3+CD8 and CD3 alone, Fig. 4C). 3 N-terminal tyrosine residues Y37, Y45, and Y110 did not become phosphorylated following the ligation of CD8. 3 C-terminal tyrosine residues; Y127 Y 132 and Y 191became phosphorylated following ligation of CD8 but with varying kinetics and intensity. There was a slow, gradual increase in phosphorylation of Y127 and Y 132. Phosphorylation of Y110Y127 and Y226 results in the formation of binding sites for the small adaptor molecule Grb2 while phosphorylation of Y132 creates a binding site for PLCγ1 [15]. There was a gradual increase in phosphorylation of Y191 that attained a greater intensity than Y127 or Y 132. The final residue, Y171, appears to have a high level of phosphorylation in unstimulated cells that is maintained following the ligation of CD8. Phosphorylation of either Y171 or Y191 enables the binding of Gads. These data demonstrates that the ligation of CD8 on DP thymocytes induces the phosphorylation on specific tyrosine residues of LAT and that these residues are within the binding sites for Grb2, Gads, and PLCγ1. Overall, these data clearly show that the intensity and kinetics of LAT phosphorylation induced during CD8-mediated apoptosis are distinct from TCR-mediated events which suggests that CD8 and LAT may have functions independent of TCR signaling.
LAT−/− mice also have a developmental block in thymocyte development at the DN2 stage and like the Lck knockout mice cannot be used to study the mechanism of CD8-mediated apoptosis in DP thymocytes. To determine whether LAT is required for apoptosis after crosslinking of CD8, we used a recombinant lentivirus to deliver a shRNA that “knocked down” the expression of LAT in the 3H6 thymoma cell line. Expression of the LAT shRNA reduced the expression of LAT by 90% compared to infection with the empty lentiviral vector (vec; Fig. 4D) The effect of the decrease in LAT expression on the induction of apoptosis following the ligation of CD8 was determined. 3H6 cells that were either uninfected, infected with the recombinant lentivirus containing either the shRNA that knocked down expression of LAT (LATkd-3H6), or empty vector (vec-3H6) were crosslinked with antibody to CD8α and apoptosis was measured by the binding of annexin-V (Fig. 4E) Approximately 45% of vec-3H6 cells but only 26% of LATkd-3H6 cells underwent apoptosis. The inability of the LAT shRNA to completely knock-down LAT expression in the LATkd-3H6 cells may account for the incomplete inhibition of apoptosis. Overall, these experiments demonstrated that LAT, but not Lck, is required for CD8-mediated apoptosis.
Tyrosine phosphorylation of LAT is required for CD8-mediated apoptosis
The experiments described above demonstrated that the expression of LAT is required for apoptosis following ligation of CD8 and that LAT exhibits an increase in tyrosine phosphorylation upon crosslinking of CD8. We investigated whether the phosphorylation of LAT is required for the induction of apoptosis and if so, which tyrosine residues are involved. A study by Zhu et al [17] examined the effect of phenyalanine substitutions for the tyrosine residues of LAT on TCR signaling in a Jurkat T cell line that did not express LAT. They established that the human equivalents of the 4 c-terminal tyrosine residues (Y132 Y171 Y191 and Y226) are important for TCR signaling and thymocyte development. We sought to determine if the apoptotic signal induced by the ligation of CD8 utilized a similar pattern of LAT phosphorylation. We created a panel of stable cell lines that express tyrosine to phenylalanine mutated forms of human LAT (mut-hLAT) expressed in the LATkd-3H6 cell line. To avoid confusion with the data from figure 4 we have labeled the human tyrosine residues as their murine equivalents (Fig. 5A). The LAT shRNA targets the 3′ UTR of the endogenous mLAT protein and did not degrade the mRNA encoding the mut-hLAT proteins. The LATkd-3H6 cells have a ~90% decrease in the amount of murine LAT (mLAT) protein that is expressed, therefore, the majority of the LAT protein that is expressed is the mut-hLAT. The human LAT construct also encoded a myc tag that enabled us to monitor the expression of the mut-hLAT proteins by probing western blots with antibody to the myc tag. Probing western blots using an antibody that reacts with both mLAT and mut-hLAT proteins demonstrated that expression of mut-hLAT protein restored the total LAT protein level to approximately 4 fold the level of endogenous murine LAT expressed in 3H6 cells (Fig 5B). Probing western blots with an antibody to the cytoplasmic tail of CD8α demonstrated that all of the cell lines expressed similar amounts of full length CD8α.
Figure 5. The phosphorylation of the 4 C-terminal tyrosines of LAT is required for the initiation of CD8-mediated apoptosis.
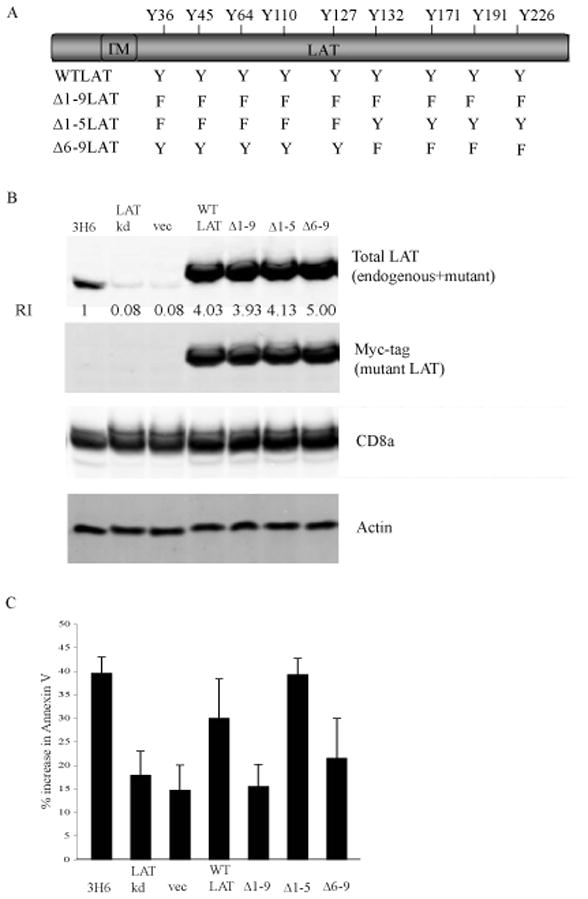
A. Schematic of the tyrosine to phenylalanine mutants in LAT generated by site-directed mutagenesis.
B. Whole cell lysates were prepared from untreated 3H6 cells, 3H6 cells transduced with vector alone or WT-LAT, LAT-Kd or mut-hLAT. The expression of total LAT (murine plus mut-hLAT, rabbit anti-LAT polyclonal antibody), mut-hLAT (myc antibody), CD8α, and actin were determined by probing western blots of whole cell extracts. The concentration of total LAT was quantified and normalized to the concentration of protein in wildtype 3H6 cells (RI = 1).
C. 3H6 cells, 3H6 cells transduced with vector alone or WT-LAT, LAT-Kd or mut-hLAT were treated with antibody to CD8α and crosslinked with goat anti-rat Ig for 6 hours at 37°C. Apoptosis was measured by the binding of annexin-V. N=4; mean ± SEM. * p< 0.001, ** p< 0.004, *** p< 0.005, Student’s t-test.
The effect of the expression of mut-hLAT on apoptosis following ligation of CD8 in LATkd-3H6 cells was examined. 3H6, LATkd-3H6 and LATkd-3H6 cells that expressed either wildtype (WT) or mutant (mut) human LAT were treated with CD8α antibody under crosslinking conditions and apoptosis was measured by the binding of annexin-V (Figure 5C). Crosslinking of CD8 resulted in the induction of apoptosis in 40% of 3H6 cells and18% of LATkd-3H6 cells. The expression of the empty vector alone (vec) had no effect on the number of apoptotic cells while the expression of WT human LAT restored the sensitivity of LATkd-3H6 cells to CD8 mediated apoptosis to 30%. These findings confirm that the shRNA does not target the exogenous human LAT and that the expression of LAT is required for the initiation of apoptosis following the ligation of CD8. The expression of the Δ1–9LAT mut-hLAT did not restore the susceptibility to CD8 mediated apoptosis indicating that phosphorylation of at least some of the tyrosine residues of LAT is necessary for apoptosis. The expression of the Δ1–5LAT mut-hLAT but not the expression of the Δ6–9LAT mut-hLAT restored susceptibility to apoptosis. Overall, this data demonstrates that the expression of LAT protein and the phosphorylation of the 4 C-terminal tyrosine residues of LAT are required for the initiation of CD8-mediated apoptosis.
ZAP-70, ITK, and RLK are not required for the CD8-mediated apoptosis
Several tyrosine kinases including ZAP-70 [26], Lck [15], and ITK [19] have been implicated in the phosphorylation of LAT in response to various stimuli. In addition, the phosphorylation of LAT is reduced in ZAP-70 or Syk deficient Jurkat cells implying that LAT is a substrate for the Syk family kinases [27]. However, some experiments suggest that other tyrosine kinases can phosphorylate LAT. Lck has been found to directly associate with, and phosphorylate LAT when co-transfected into HEK293 cells and LAT phosphorylation is absent in Lck-deficient Jurkat cells [28]. In addition, the TEC-family kinase ITK can phosphorylate LAT when co-transfected into COS cells. The data presented in figure 3C demonstrated that Lck is not required for CD8-mediated apoptosis so we examined whether ZAP-70, or the Tec kinases ITK and/or RLK, are involved in CD8-mediated apoptosis using ZAP-70−/−, ITK−/−, and ITK−/−RLK−/− mice.
Mice deficient in ZAP-70 have normal thymic development through the pre-TCR/β-selection stage but are unable to progress through positive and negative selection and are arrested at the DP stage [29]. Analysis of CD4 and CD8α expression confirms that ZAP-70−/− thymocytes are arrested at the DP stage (Fig. 6A). Thymocytes from MHC−/−, C57BL/6, and ZAP-70−/− mice were treated with antibody to CD8α under crosslinking conditions. Apoptosis was measured by the binding of annexin-V (Fig. 6B). Following crosslinking of CD8, the proportion of apoptotic cells in ZAP-70−/− thymocytes was similar to the levels obtained when MHC−/− or C57BL/6 thymocytes were treated in a similar manner. Although the above data suggests that Zap-70 is not required for apoptosis to occur, we also investigated if Zap-70 was responsible for the phosphorylation of LAT so that we would gain further insight into which tyrosine residues of LAT were involved in the propagation of apoptotic signals. Zap-70 has been shown to phosphorylate the 5 c-terminal tyrosine of LAT including Y132, Y171 and Y191 [26]. The ligation of CD8 induced an increase in the phosphorylation of Y191 (data not shown) in a similar pattern to MHC−/− thymocytes. In contrast, thymocytes from Zap-70−/− mice did not have an increase in phosphorylation on Y132 in response to ligation of CD8 compared to MHC−/− thymocytes (Fig 6C). This data demonstrates that Zap-70 is responsible for the increase in phosphorylation of LAT on Y132. Since Zap-70 is not required for apoptosis, this implies that phosphorylation of Y132 of LAT is also not involved in CD8-mediated apoptosis.
Figure 6. The tyrosine kinase ZAP-70, ITK, and RLK are not required for CD8-mediated apoptosis of DP thymocytes.
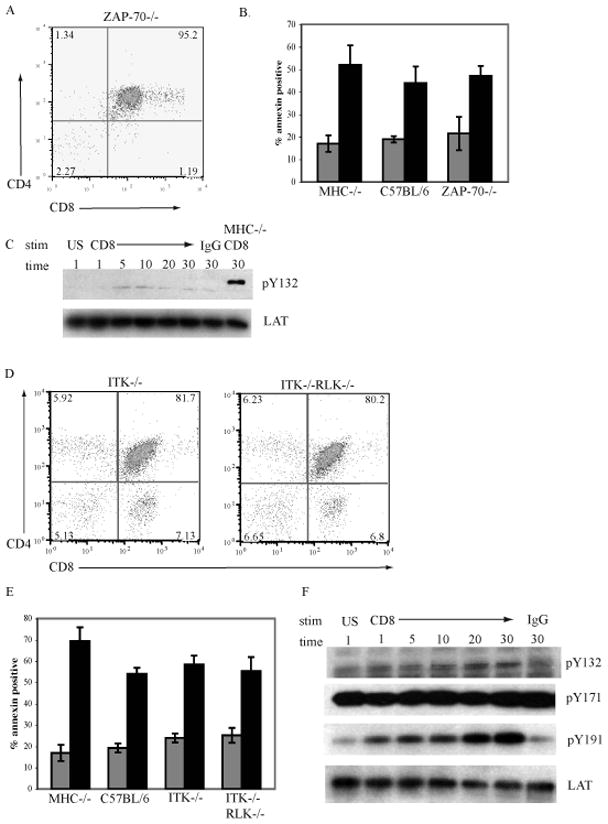
A. Thymocytes from ZAP-70−/− mice were stained with antibodies to CD8α and CD4 and analysed by flow cytometry.
B. Thymocytes from MHC−/− C57BL/6, and ZAP-70−/− mice were left unstimulated (grey bars) or treated with antibody to CD8α and crosslinked with goat anti-rat Ig for 6hrs (black bars). The induction of apoptosis was measured by the binding of annexin-V. N=3; mean ± SEM.
C. ZAP-70−/− thymocytes were treated with antibody to CD8α and crosslinked with goat anti-rat Ig for the indicated times, treated only with goat anti-rat Ig or left unstimulated (US). Western blots of whole cell lysates were analyzed for expression of pLATY132 and reprobed with antibodies to LAT as a loading control. MHC−/− cells treated with antibody to CD8α and crosslinked with goat anti-rat Ig served as a positive control.
D. Thymocytes from ITK−/− and ITK−/−RLK−/− mice were stained with antibodies to CD8α and CD4 and analysed by flow cytometry.
E. Thymocytes from MHC−/−, C57BL/6, ITK−/− and ITK−/−RLK−/− mice were left unstimulated (grey bars) or treated with antibody to CD8α and crosslinked with goat anti-rat Ig for 6hrs (black bars). The induction of apoptosis was measured by the binding of annexin-V. N=3; mean ± SEM.
F. ITK−/−RLK−/− thymocytes were treated with antibody to CD8α and crosslinked with goat anti-rat Ig for the indicated times, treated only with goat anti-rat Ig or left unstimulated (US). Western blots of whole cell lysates were analyzed for expression of pLATY132, pLATY171. pLATY191 and reprobed with antibodies to LAT as a loading control.
The Tec-family kinase ITK has been shown to phosphorylate the c-terminal tyrosines of LAT when both LAT and ITK are transfected into COS cells. In addition, the expression of a dominant negative form of ITK in Jurkat cells significantly decreased the phosphorylation of tyrosine residues in LAT in response to TCR signaling [27]. Similar to the Syk-family kinases, there are multiple members of the Tec-family kinases and they may have redundant functions. In T cells the primary Tec kinases are ITK and RLK, whereas Tec is expressed at much lower levels [30]. ITK-deficient mice have relatively normal thymocyte development with only a decrease in mature CD4+ T cells [31], suggesting that there is functional redundancy between the Tec kinases. RLK−/−ITK−/− double knockout mice have normal numbers of T cells, however, these T cells fail to respond to TCR activation [32]. Staining for expression of CD8 and CD4 (Fig. 6D) reveals that ITK−/− and RLK−/−ITK−/− mice have a higher percentage of CD8 SP thymocytes (7.3% and 6.8% respectively) compared to C57BL/6 mice (1.5%). The percentage of CD4+CD8+ DP thymocytes is slightly lower in ITK−/− (81.7) and RLK−/−ITK−/− (80.2%) mice compared to C57BL/6 mice (85%). The effect of ITK or RLK and ITK deficiency on apoptosis following the ligation of CD8 was examined. Thymocytes from MHC−/−, C57BL/6, ITK−/−, and RLK−/−ITK−/− mice were crosslinked with CD8α antibody and apoptosis was measured by binding of annexin-V (Fig. 6E). The proportion of thymocytes from ITK−/− and RLK−/− ITK−/− mice that were apoptotic following ligation of CD8 was comparable to those obtained with C57BL/6 and MHC−/− thymocytes. The ligation of CD8 induced the phosphorylation of LAT on Y132 and Y191 indicating that ITK and RLK are not involved in the phosphorylation of LAT during apoptosis (Fig 6F). Overall these findings demonstrate that the expression of ZAP-70, ITK, and RLK tyrosine kinases are not required for the induction of CD8-mediated apoptosis.
Discussion
In this study we investigated the initial events of the biochemical signaling pathway in DP thymocytes that links the ligation of CD8 on the cell surface to apoptosis. Our previous report demonstrated that this apoptotic pathway is characterized by the exposure of phoshophatidylserine on the cell surface, the loss of mitochondrial membrane potential, and the activation of Caspase-3. In this report we focused on the phosphorylation events initiated by CD8 ligation.
The ligation of CD8 on DP thymocytes with antibody to either CD8α or CD8β resulted in apoptosis, however there was a significant reduction in the proportion of apoptotic cells from CD8β−/− mice compared to C57Bl/6 and MHC−/− mice. The inhibition of apoptosis in CD8β−/− thymocytes was complete and this suggests that the primary form of CD8 that induces apoptosis is CD8αβ heterodimers. The role of CD8β chain is generally considered to be to enhance the co-receptor function of CD8 [33]. Other investigators have suggested that expression of CD8αα and CD8αβ represent alternate differentiation [34]. The palmitoylation of a cysteine residue in the cytoplasmic tail of CD8β partitions CD8αβ into lipid rafts [14]. This localization facilitates the association of CD8 with CD3 as well as with the signaling molecules Lck and LAT. In contrast CD8αα does not localize to lipid rafts and there is a significant decrease in the amount of Lck and LAT that associates with CD8 in thymocytes from CD8β−/− mice [7].
Lck and LAT associate with CD8α and the binding sites for these molecules in the cytoplasmic domain of CD8α overlap. In DP thymocytes CD8α preferentially associates with LAT rather than with Lck [7]. In our experiments the ligation of CD8 resulted in a large increase in the amount of CD8α associated with LAT while the association of CD8α with Lck was only slightly increased (Figure 3, 4). It has been reported that in thymocytes, only 2% of CD8 is associated with Lck [35]. The amount of CD8 that is associated with LAT has not been quantified but speculated to be significantly higher, possibly 40–50% based on the preferential association of CD8 with LAT [7]. If these estimates are relatively correct, a significant portion of CD8 is not associated with either Lck or LAT. Overall our observations are consistent with a signaling model in which the ligation of the CD8 that is associated with LAT and/or the CD8 that is not associated with either Lck or LAT, results in the increased association of LAT with CD8α and the induction of the apoptotic signaling cascade. The ligation of Lck-associated CD8 is not required for apoptosis but is involved in TCR signaling. This proposed model might explain why the ligation of CD8 activated several signaling molecules, including ZAP-70, MAP kinases, AKT, and ROS, (data not shown) even though these molecules are not required for the apoptosis initiated by the ligation of CD8.
The comparison of the expression of the LAT tyrosine mutants revealed that the phosphorylation of the C-terminal tyrosine residues plays a role in the induction of CD8-mediated apoptosis. This result is intriguing because analysis of knockin mice in which the same 4 C-terminal tyrosine residues were mutated to phenylalanine (LAT4YF/4YF) demonstrated that these tyrosine residues are required for thymocyte development and T cell signaling [36]. This adds an additional layer of complexity to the outcome of the phosphorylation of LAT if the engagement of CD8 results in the phosphorylation of these same 4 tyrosine residues during positive selection. How does the thymocyte distinguish between the pro-survival signal induced by the TCR, and the pro-apoptotic phosphorylation of LAT induced by CD8? This may be due to the intensity and kinetics of the phosphorylation events. In Figure 4 we examined the induction of LAT phosphorylation using a panel of phospho-LAT antibodies and compared the ligation of CD8 to the ligation of CD3 plus CD8 or CD3 alone. 3 of the 5 c-terminal tyrosine residues exhibit an increase in phosphorylation following the induction of apoptosis. However, the intensity of phosphorylation of all 3 tyrosine residues in response to CD8 and CD3 was approximately 3 times greater than the phosphorylation induced by the crosslinking of CD8. In addition, the kinetics of the phosphorylation response differs between the 2 stimuli. In general, the ligation of CD8 induced a slow increase in phosphorylation that peaked at 30 minutes after crosslinking. In contrast, the ligation of CD3 and CD8 induces a rapid phosphorylation response that peaked at 1 minute after crosslinking and then gradually declined. These differences could have implications on the recruitment and binding of signaling complexes to LAT in response TCR signaling compared with signaling during CD8-mediated apoptosis. Further experiments analyzing the proteins that immunoprecipitated with LAT following the ligation of CD8 versus CD8 and CD3 will reveal if the difference in activating pro-survival or pro-apoptotic signals is due to the recruitment of the same signaling complexes with different kinetics or if the signaling complexes are comprised of different proteins.
We were unable to identify the tyrosine kinase that is responsible for the phosphorylation of LAT following engagement of CD8 as ZAP-70, ITK, RLK, and Src-family kinase activity was not required for apoptosis. There are a plethora of other tyrosine kinases present in DP thymocytes that may phosphorylate LAT following the ligation of CD8. Syk, functions in a similar manner to ZAP-70 and although it is expressed at low levels in DP thymocytes, it could possibly phosphorylate LAT. ZAP-70−/−Syk−/− double-deficient mice have been generated but thymocyte development is arrested at an early DN stage so we were unable to examine the effects of CD8 engagement on DP thymocytes from these mice. We have tested several different pharmacological inhibitors of Syk and did not observe any effect on apoptosis. There are other members of the TEC-family kinases expressed in DP thymocytes including Etk that could possibly phosphorylate LAT. There is so much redundancy between family members of all of the known tyrosine kinases that it may be impossible to determine which individual kinase is primarily responsible for the phosphorylation of LAT during CD8-mediated apoptosis using genetically deficient mice. The use of the lentiviral shRNA system in the 3H6 cell line will be an invaluable tool to systematically knockdown the expression of individual or selected combinations of tyrosine kinases to determine what kinases are required for CD8-mediated apoptosis.
A major hurdle in understanding the mechanisms of thymic selection is to determine how specific signal transduction pathways influence the deletion of immature thymocytes in vivo. Various models of apoptosis have been documented during thymocyte development including cytokine withdrawal, steroids, death receptors, and stimulation through the TCR/CD3 complex. The majority of these mechanisms are extremely important during negative selection however, none of these mechanisms can accurately account for the removal of the 90% thymocytes that have failed positive selection. Thymocytes are also susceptible to the induction of apoptosis by the ligation of molecules such as Thy-1, CD24 and CD45. Our studies indicate that ligation of CD8 can also induce apoptosis in a proportion of DP thymocytes. One question that arises is “Why are there so many different ways to initiate apoptosis in thymocytes and do they have a role in the deletion of thymocytes during positive and negative selection?” It has been proposed that the induction of apoptosis through these molecules may constitute mechanisms that delete thymocytes that fail positive selection and whose fate is usually referred to as “Death by Neglect. A cellular process as important as positive selection may need to have redundancy to ensure that thymocytes that are incapable of antigen recognition are deleted. However, the numerous mechanisms of apoptosis of thymocytes that are not positively selected that have been described in the literature are so diverse that it is difficult to propose a pattern to link them all. The crosslinking of CD45, CD24, and Thy-1 with antibodies induces apoptosis of thymocytes and the characterization of the pathways initiated by cross-linking of these molecules has suggested that there are both similar and distinct events.
Not only is the mechanism of CD8-mediated apoptosis unique compared to apoptosis induced by the ligation of other cell surface receptors such as CD45, but it is also distinctive from the models of cell death implicated in β-selection and in negative selection. The comparison of CD8-mediated apoptosis to apoptosis induced by the TCR/CD3 complex is the most intriguing because these two pathways have access to a similar pool of signaling molecules such as Lck and LAT, but may induce apoptosis at completely different stages of thymocyte development. We postulate that CD8-mediated apoptosis occurs during positive selection whereas TCR/CD3 induced apoptosis occurs during negative selection. The role of LAT in TCR/CD3 induced apoptosis during negative selection has not been examined and it would be interesting to determine if the ligation of TCR/CD3 in “post-positive selection” DP thymocytes induced a similar pattern of LAT phosphorylation and other downstream signaling events as the ligation of CD8 in “pre-positive selection” DP thymocytes.
Up until now the primary functions ascribed to both CD8 and LAT providing activation signals upon the engagement of the TCR. The data in this study demonstrates that CD8 can induce a signaling pathway independent of the TCR that induces apoptosis of DP thymocytes. The ligation of CD8 induces a unique pattern of phosphorylation on LAT that utilizes the same tyrosine residues as TCR signaling, but with different kinetics and intensities. It would be interesting to examine the phosphorylation pattern of LAT during β-selection of DN thymocytes to see how it compares to LAT phosphorylation during CD8-mediated apoptosis and TCR signaling. This data suggests that “tweaking” the phosphorylation pattern of LAT may lead to either cellular activation and proliferation or cell death and demonstrates that LAT is a key regulator of thymocyte development.
Materials and Methods
Mice and Cell Lines
C57BL/6 and B6.MHC−/− (Aβb−/− and β2M−/−) were purchased from Taconic Farms (Germantown, NY). B6.129.TCRα−/− mice were obtained from Dr. Philippa Marrack (National Jewish Medical Center, Denver CO). B6.CD8β−/− mice were obtained from Dr. Alfred Singer (National Institutes of Health, Bethesda MD); B6.ZAP-70−/− mice were obtained from Dr. Arthur Weiss (University of California, San Francisco, San Francisco CA); and B6.ITK−/− and B6. ITK−/−RLK−/− mice were obtained from Dr. Pamela Schwartzberg (National Human Genome Research Institute, National Cancer Institute, Bethesda, MD). The thymoma cell line ITT-1 75NS was derived from B6.PL (75NS) mice by Dr. Mark Hogarth (Austin Research Institute, Melbourne, Australia) and was subcloned by limiting dilution and is referred to herein as 3H6.
Cell culture medium and preparation of ex vivo thymocytes
The cell line 3H6 as well as ex vivo thymocytes were cultured at 37°C in 7.5% CO2 in DMEM (GIBCO) supplemented with non-essential amino acids, essential amino acids, dextrose, glutamine, gentamycin, penicillin, streptomycin, 2-mercaptoethanol, antibiotic/antimycotic and 10% fetal calf serum (FCS; Atlanta Biologicals). 4–6 week old mice were sacrificed by CO2 asphyxiation. The thymus was removed and passed through a 0.2μM cell strainer washed 2 times and resuspended in DMEM/FCS at 10×106/mL.
Induction of Apoptosis by Crosslinking of CD8 with antibodies
Thymocytes or 3H6 thymoma cells at 5×106/mL were incubated with antibodies to CD8α (clone YTS169), or CD8β (clone 53–5.8) at a final concentration of 10μg/mL for 10 minutes at room temperature. To crosslink the bound antibody 50μg/mL of anti-rat IgG (Jackson Immunolabs) was added to the cultures and incubated at 37°C for the indicated time periods. 15 min prior to each timepoint samples were washed with PBS, and stained with annexin V-Alexa 647 and 5μg/ml propidium iodide.
Western blotting
Thymocytes and 3H6 cells were harvested and stimulated as above. At indicated timepoints cells were lysed in ice-cold OβG lysis buffer (60mM Octyl-β-glucopyranoside [Calbiochem], 0.44% tetrasodium pyrophosphate (Sigma), 150mM NaCl (Fisher), 10mM Tris pH 7.3, and a cocktail of inhibitors consisting of 5mM EDTA, 1mM Na3VO4, 100mM NaF, 10mM aprotinin) for 15 min on ice. Lysates were prepared and loaded into Bis-Tris NuPage ready cast gels (Invitrogen). Separated proteins were transferred to a Polyvinylidene fluoride (PVDF) membrane (Millipore) and after incubation with antibodies, were processed on the Odyssey infrared imaging system (LiCor). All western blots are representative of a minimum of 3 independent experiments.
Production of LAT shRNA lentivirus
shRNA targeting the 3′ UTR LAT were selected based on the 8 criterion algorithm designed by Reynolds et al [37]. Duplexes with a Reynolds score of 8 were selected for validation. The following oligonucleotides were synthesized: LAT sense 3: 5′-TGC AGC TCT CTG TCC TGA AAT TCA AGA GAT TTC AGG ACA GAC AGC TGC TTT TTT C-3′ LAT antisense 3: 5′-TCG AGA AAA AAG CAG CTG TCT GTC CTG AAA TCT CTT GAA TTT CAG GAC AGA CAG CTG CA-3′. Annealed oligonucleotides were cloned into the pLL3.7 vector and plasmid DNA was transfected into 293FT fibroblasts using the Effectene transfection reagent (Qiagen) according to the manufacturers protocol using the following reagent quantities, 20μg pLL3.7-LAT, 10μg pLL3.7MOG, 10μg pLL3.7GAGPol, 10μg pLL3.7VSV-G. The supernatants containing the recombinant lentivirus were filtered and then used to infect 3H6 cells and after 48 hours GFP+ cells were isolated by flow cytometry.
Production of LAT tyrosine mutations
Plasmids encoding myc-tagged human LAT either wildtype (WTLAT) or mutants Δ1–9LAT, Δ1–5LAT, and Δ6–9LAT in pMSCVGFP were obtained from Dr. Weiguo Zhang (Duke University) and cloned into the pMSCVpuro retroviral vector. Purified and sequenced plasmid DNA was transfected into Phoenix fibroblasts using the Effectene transfection reagent (Qiagen) according to the manufacturers protocol using the following quantities of reagent: 1μg pMSCVpuro mutantLAT, 1μg pCLEco helper plasmid. 3H6 cells were transduced with retrovirus and selected by culture in DMEM/FCS containing 10μg puromycin (Sigma) for 7–14 days.
Statistical Analyisis
All data are expressed as the mean ± SEM. Statistical analysis was performed by using the Student’s t-test (* p< 0.001, ** p< 0.004, *** p< 0.005).
Acknowledgments
We would like to thank Dr. Alfred Singer, Dr. Pamela Schwartzberg, and Dr. Arthur Weiss for providing the knockout mice used in this study and Dr. Weiguo Zhang for providing the human LAT cDNA. This work was supported by by National Institutes of Health (AI 055701).
Footnotes
Conflict of Interest
The authors declare no commercial or financial conflict of interest.
References
- 1.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 2.Jung KC, Park WS, Kim HJ, Choi EY, Kook MC, Lee HW, Bae Y. TCR-independent and caspase-independent apoptosis of murine thymocytes by CD24 cross-linking. J Immunol. 2004;172:795–802. doi: 10.4049/jimmunol.172.2.795. [DOI] [PubMed] [Google Scholar]
- 3.Lesage S, Steff AM, Philippoussis F, Page M, Trop S, Mateo V, Hugo P. CD4+ CD8+ thymocytes are preferentially induced to die following CD45 cross-linking, through a novel apoptotic pathway. J Immunol. 1997;159:4762–4771. [PubMed] [Google Scholar]
- 4.Hueber AO, Raposo G, Pierres M, He HT. Thy-1 triggers mouse thymocyte apoptosis through a bcl-2-resistant mechanism. J Exp Med. 1994;179:785–796. doi: 10.1084/jem.179.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grebe KM, Clarke RL, Potter TA. Ligation of CD8 leads to apoptosis of thymocytes that have not undergone positive selection. Proc Natl Acad Sci U S A. 2004;101:10410–10415. doi: 10.1073/pnas.0402079101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 7.Bosselut R, Zhang W, Ashe JM, Kopacz JL, Samelson LE, Singer A. Association of the adaptor molecule LAT with CD4 and CD8 coreceptors identifies a new coreceptor function in T cell receptor signal transduction. J Exp Med. 1999;190:1517–1526. doi: 10.1084/jem.190.10.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moody AM, Chui D, Reche PA, Priatel JJ, Marth JD, Reinherz EL. Developmentally regulated glycosylation of the CD8alphabeta coreceptor stalk modulates ligand binding. Cell. 2001;107:501–512. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- 9.Daniels MA, Devine L, Miller JD, Moser JM, Lukacher AE, Altman JD, Kavathas P, Hogquist KA, Jameson SC. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15:1051–1061. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 10.Kao C, Sandau MM, Daniels MA, Jameson SC. The sialyltransferase ST3Gal-I is not required for regulation of CD8-class I MHC binding during T cell development. J Immunol. 2006;176:7421–7430. doi: 10.4049/jimmunol.176.12.7421. [DOI] [PubMed] [Google Scholar]
- 11.Moebius U, Kober G, Griscelli AL, Hercend T, Meuer SC. Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol. 1991;21:1793–1800. doi: 10.1002/eji.1830210803. [DOI] [PubMed] [Google Scholar]
- 12.Potter TA, Rajan TV, Dick RF, 2nd, Bluestone JA. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989;337:73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- 13.Irie HY, Ravichandran KS, Burakoff SJ. CD8 beta chain influences CD8 alpha chain-associated Lck kinase activity. J Exp Med. 1995;181:1267–1273. doi: 10.1084/jem.181.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arcaro A, Gregoire C, Boucheron N, Stotz S, Palmer E, Malissen B, Luescher IF. Essential role of CD8 palmitoylation in CD8 coreceptor function. J Immunol. 2000;165:2068–2076. doi: 10.4049/jimmunol.165.4.2068. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhu M, Janssen E, Zhang W. Minimal requirement of tyrosine residues of linker for activation of T cells in TCR signaling and thymocyte development. J Immunol. 2003;170:325–333. doi: 10.4049/jimmunol.170.1.325. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Cheng H. Evidence of LAT as a dual substrate for Lck and Syk in T lymphocytes. Leuk Res. 2006 doi: 10.1016/j.leukres.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Villar JJ, Whitney GS, Sitnick MT, Dunn RJ, Venkatesan S, O’Day K, Schieven GL, Lin TA, Kanner SB. Phosphorylation of the linker for activation of T-cells by Itk promotes recruitment of Vav. Biochemistry. 2002;41:10732–10740. doi: 10.1021/bi025554o. [DOI] [PubMed] [Google Scholar]
- 20.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–116. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 21.Terry LA, DiSanto JP, Small TN, Flomenberg N. Differential expression and regulation of the human CD8 alpha and CD8 beta chains. Tissue Antigens. 1990;35:82–91. doi: 10.1111/j.1399-0039.1990.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 22.Fung-Leung WP, Kundig TM, Ngo K, Panakos J, De Sousa-Hitzler J, Wang E, Ohashi PS, Mak TW, Lau CY. Reduced thymic maturation but normal effector function of CD8+ T cells in CD8 beta gene-targeted mice. J Exp Med. 1994;180:959–967. doi: 10.1084/jem.180.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 24.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 25.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 26.Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem J. 2001;356:461–471. doi: 10.1042/0264-6021:3560461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan X, Wange RL. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J Biol Chem. 1999;274:29323–29330. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Cheng H. Evidence of LAT as a dual substrate for Lck and Syk in T lymphocytes. Leuk Res. 2007;31:541–545. doi: 10.1016/j.leukres.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Kadlecek TA, van Oers NS, Lefrancois L, Olson S, Finlay D, Chu DH, Connolly K, Killeen N, Weiss A. Differential requirements for ZAP-70 in TCR signaling and T cell development. J Immunol. 1998;161:4688–4694. [PubMed] [Google Scholar]
- 30.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 31.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 32.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 33.Karaki S, Tanabe M, Nakauchi H, Takiguchi M. Beta-chain broadens range of CD8 recognition for MHC class I molecule. J Immunol. 1992;149:1613–1618. [PubMed] [Google Scholar]
- 34.Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity. 2008;28:149–159. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Wiest DL, Yuan L, Jefferson J, Benveniste P, Tsokos M, Klausner RD, Glimcher LH, Samelson LE, Singer A. Regulation of T cell receptor expression in immature CD4+CD8+ thymocytes by p56lck tyrosine kinase: basis for differential signaling by CD4 and CD8 in immature thymocytes expressing both coreceptor molecules. J Exp Med. 1993;178:1701–1712. doi: 10.1084/jem.178.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sommers CL, Menon RK, Grinberg A, Zhang W, Samelson LE, Love PE. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. J Exp Med. 2001;194:135–142. doi: 10.1084/jem.194.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]