Abstract
Reactive oxygen species (ROS) play an important role in the pathogenesis of hypertension, disease in which ROS levels and markers of oxidative stress are increased. Xanthine oxidase (XO) is a ROS-producing enzyme the activity of which may increase during hypertension. Studies on XO inhibition effects on BP have yielded controversial results. We hypothesized that XO inhibition would decrease BP or attenuate the development of DOCA-salt hypertension. We administered the XO inhibitor, allopurinol (50 mg/kg/day, orally) or its vehicle to rats during the established or development stages of DOCA-salt hypertension. We validated XO inhibition by HPLC measurements of XO metabolites in urine, serum and tissues demonstrating decrease in products, increase in substrates and detection of the active metabolite of allopurinol, oxypurinol. We monitored BP continuously via radiotelemetry and performed gross evaluations of target organs of hypertension. Allopurinol treatment did not impact the course of DOCA-salt hypertension, regardless of the timing of administration. Aside from a significant decrease in pulse pressure in allopurinol-treated rats, no positive differences were observed between the allopurinol and the vehicle-treated rats. We conclude that XO does not play an important role in the development or maintenance of hypertension in the rat DOCA-salt hypertension model.
Keywords: xanthine oxidase, hypertension, reactive oxygen species, allopurinol, blood pressure
Introduction
A large body of evidence supports the involvement of reactive oxygen species (ROS) and oxidative stress in the pathogenesis of hypertension as well as in the development of its complications 1. As one of several ROS sources, xanthine oxidase (XO) has therefore also been associated with hypertension. XO, a flavoprotein with a high degree of homology between mouse, rat and human 2, is encoded by the evolutionarily conserved housekeeping gene xanthine oxidoreductase (XOR). Both XO and its interconvertible dehydrogenase isoform (XDH), using different electron acceptors, catalyze the same steps in purine catabolism: the transformation of hypoxanthine and xanthine to uric acid, with superoxide/H2O2 generated as by-products 2.
In vitro, the activity of XO is increased in various tissues in several animal models of hypertension. The spontaneously hypertensive rat (SHR) displays increased renal XO activity during the development of hypertension 3. The same model exhibits higher mesenteric artery XO activity 4. Similarly, mesenteric artery XO activity is increased in the DOCA-salt model of hypertension 5.
Various human and animal studies have demonstrated beneficial effects of XO inhibition in hypertension, coronary artery disease or cardiomyopathies by lowering blood pressure (BP), decreasing end organ damage, improving endothelial function and heart function (for review, see 6). However, XO effects in pathophysiology may be mediated by at least one other mechanism that is not related to XO-mediated ROS production: the production of uric acid, a molecule with controversial cardiovascular effects that can act both as an antioxidant and as a deleterious product 7–10.
Pharmacological inhibition of XO by allopurinol or oxypurinol has lead to contradictory results. Allopurinol treatment improved vascular function in diabetic rats 11, and oxypurinol improved coronary and peripheral endothelial function in patients with coronary artery disease 12. Treatment with oxypurinol acutely decreased the BP in the SHR model, while it had no effect on the BP of normal counterparts 13. Treatment with allopurinol also decreased systolic blood pressure (SBP) in DOCA-salt 14 and in dexamethasone15 hypertensive rats. Although allopurinol is widely used to treat patients with hyperuricemia or gout, there have been few published results of clinical studies regarding allopurinol use in hypertensive patients. A unique clinical study seeking an association between allopurinol administration and BP decrease found a decrease in SBP with allopurinol in newly diagnosed hypertensive adolescents that have concomitant hyperuricemia. 16
Several other studies, however, have reported a lack of effects of XO inhibition on BP. Chronic treatment of SHR with allopurinol did not prevent the development of hypertension or decrease BP in this model 17,18. Allopurinol also did not have BP lowering effects in Dahl-salt sensitive19 or glucocorticoid 20, L-NAME 21, ACTH 22 or hypercholesterolemia 23 -induced hypertension. Thus, the BP effects of XO inhibition are overall mixed, appearing to be species and model-dependent.
In this study, we sought to investigate the effects of XO inhibition on BP in the DOCA-salt model of hypertension. This is a mineralocorticoid-induced, salt dependent model of hypertension, in which ROS levels are increased 24. We hypothesized that allopurinol administration would effectively block XO action, reduce ROS, and thus either ameliorate the already established hypertension and/or attenuate the development of hypertension in DOCA-salt rats. In an attempt to resolve some of the contradictory findings in the aforementioned published reports, we used an HPLC-based method to confirm the effects of allopurinol on overall XO metabolism in hypertensive rats.
Methods
Animal use
All animal procedures were approved and performed in accordance with regulations of the Institutional Animal Care and Use Committee at Michigan State University. Male Sprague-Dawley rats (225–250 g)(Charles River Laboratories, Indianapolis, IN) were used. Rats were kept in clear plastic boxes, with access to standard rat chow (Teklad®) and treatment water ad libitum. Rats were euthanized with pentobarbital (60–80 mk/kg i.p.).
DOCA-salt hypertension
Rats were uninephrectomized under isoflurane anesthesia (0.8 L/min, to effect), received a subcutaneous implant at the nape of the neck of a pellet of 200 mg/kg deoxycorticosterone acetate (DOCA) and were given drinking water supplemented with 1% NaCl and 0.2% KCl for a duration of 4 weeks. All animals received analgesic (carprofen 5 mg/kg s.c., at the time of surgery and 2 days postoperatively) and antibiotic (enrofloxacin 10 mg/kg i.m. at the time of surgery) treatment.
Radiotelemetric measurement of mean arterial pressure (MAP)
Under isoflurane anesthesia (0.8 L/min, to effect), radiotelemeter devices (TA11PA-C40, Data Sciences International, St. Paul, MN) with attached catheters with pressure-sensing tips were implanted into a subcutaneous pouch in the abdominal wall through a 1–1.5 cm incision in the left inguinal area. Catheters were introduced into the left femoral artery 3 to 5 mm distal to the level of the peritoneal wall, and the tip was advanced to the abdominal aorta. Animals received analgesic (carprofen 5 mg/kg s.c., at the time of surgery and 2 days postoperatively) and antibiotic (enrofloxacin 10 mg/kg i.m. at the time of surgery) treatment. Rats were allowed 3 days to recover postoperatively. Systolic pressure, diastolic pressure, mean arterial pressure, pulse pressure, heart rate and activity (movement) were then recorded throughout the duration of the study; at a sampling rate of 10 seconds each 10 minute interval (Dataquest ART 4.1, DSI). Data are presented as mean ± SEM of 24h–averaged parameters.
In vivo allopurinol administration and biological sample collection
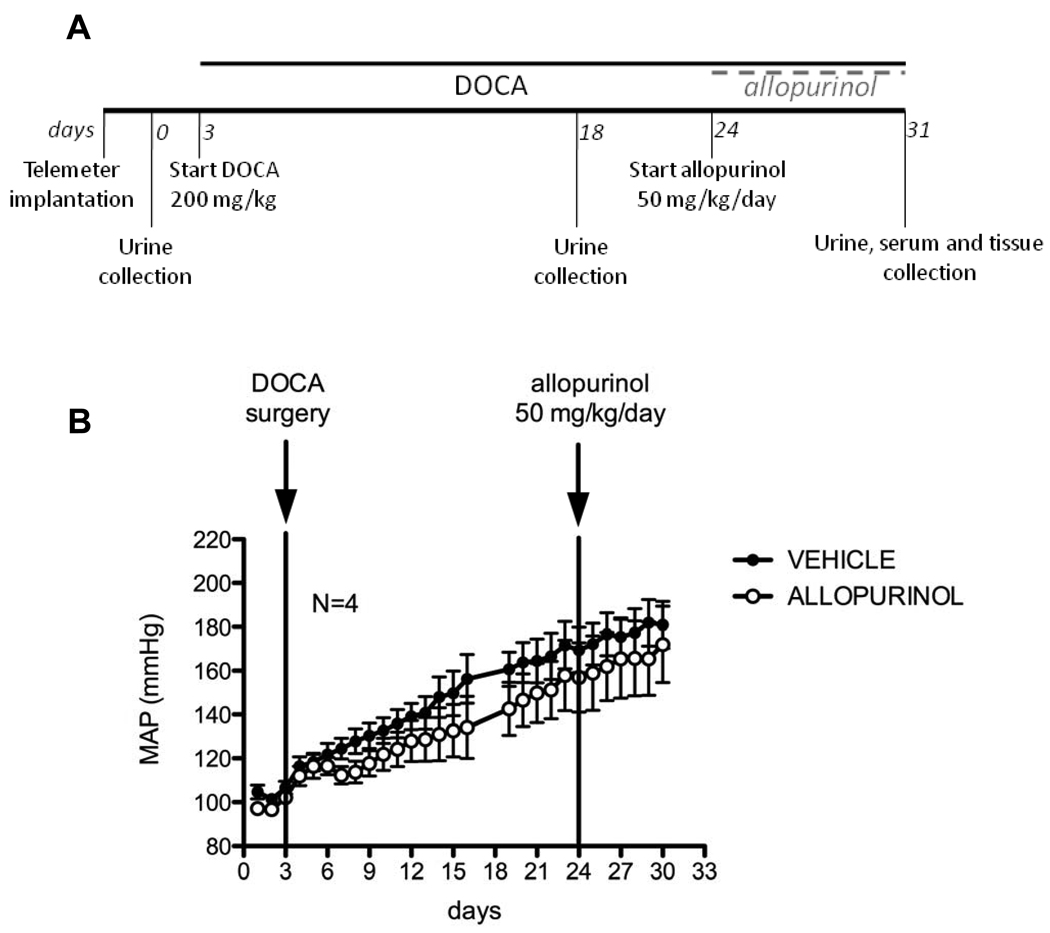
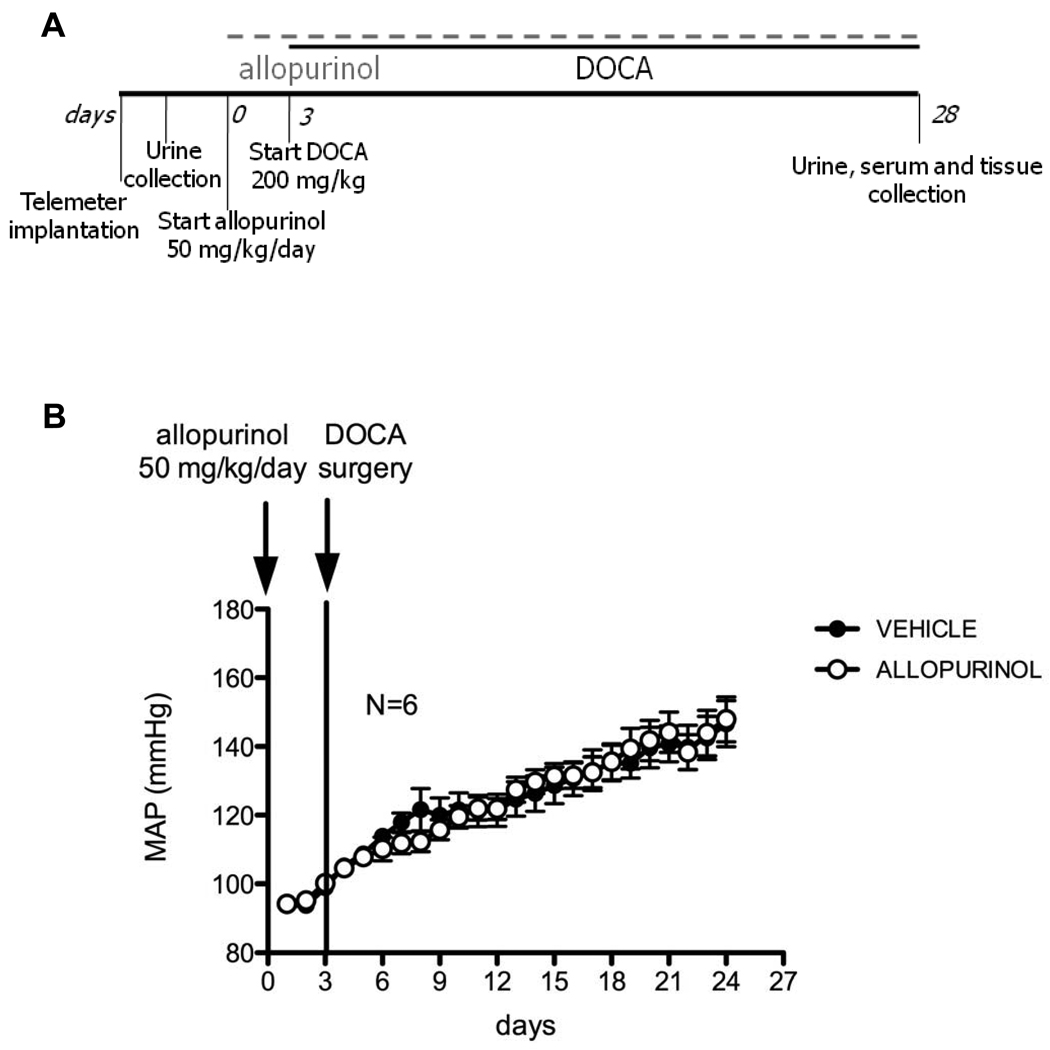
We performed two separate studies to investigate the effects of XO inhibition on BP at two different time points in the course of DOCA-salt hypertension. In the first study, to determine whether allopurinol would reduce BP of hypertensive animals, we administered allopurinol or vehicle for 7 days in the established phase of DOCA-salt hypertension, starting at 3 weeks after the DOCA-salt treatment was initiated (short-term - fig 1A). In the second study, to determine whether allopurinol would attenuate the development of DOCA-salt hypertension, we administered allopurinol or vehicle to rats 3 days before the start and throughout the course of DOCA-salt treatment (4 weeks) (long-term - fig 2A).
Figure 1.
A. Study design for allopurinol effects on established DOCA-salt hypertension (short-term administration). B. Blood pressure of vehicle (closed symbols) and allopurinol (open symbols)-treated rats, measured by radiotelemetry. Timing of interventions is represented by vertical lines. Data are represented as means ± SEM of 24h average mean arterial pressure (MAP) for N=4.
Figure 2.
A. Study design for allopurinol effects on the development of DOCA-salt hypertension (long-term administration). B. Blood pressure of vehicle (closed symbols) and allopurinol (open symbols)-treated rats, measured by radiotelemetry. Timing of interventions is represented by vertical lines. Data are represented as means ± SEM of 24h average mean arterial pressure (MAP) for N=6.
Allopurinol was administered orally at 50 mg/kg/day mixed in the salt water. Water intake was monitored daily and allopurinol concentrations in the drinking water were varied according to this intake, in order to maintain the desired dosage.
Urine samples were collected at select time points by temporarily housing rats in metabolic cages that allow for separation of urine from feces and solid debris. In the short-term study, urine was collected at three time points: before the initiation of DOCA-salt, after 2 weeks of DOCA-salt treatment and at the end of the allopurinol administration. In the long-term study, urine was collected at two time points: before the initiation of allopurinol treatment and at the end of the 4 week DOCA-salt treatment. Urine samples were collected over 24h periods. The total volume of urine for 24 h was measured and used for normalization.
At the time of sacrifice, whole blood was collected into plastic tubes (non-heparin coated) by cardiac puncture from deeply anesthetized rats (pentobarbital 60–80 mg/kg i.p.). After 30 minutes at room temperature, clotted blood was spun (2500g, 10 minutes, twice, mechanically separating the clot from sides of tube if necessary between the two centrifugations), and the supernatant (serum) was collected.
Tissue samples (aorta, vena cava, carotid artery, jugular vein, liver, kidney, heart) were also collected at the time of sacrifice and cleaned in physiological salt solution containing (mM): NaCl, 130; KCl, 4.7; KH2PO4, 1.18; MgSO4·7H2O, 1.17; NaHCO3, 14.8; dextrose, 5.5; CaNa2EDTA, 0.03; CaCl2, 1.6. A segment of aorta was saved for histological analysis. The heart and the right kidney were wet-weighed.
High performance liquid chromatography (HPLC)
Serum and urine samples were diluted 1:10 in 0.2 M acetic acid and centrifuged (14,000g for 10 min at 4°C). All tissues were quick frozen in liquid nitrogen and pulverized with cooled mortar and pestle. Tissue homogenates were solubilized in 0.2 M acetic acid, sonicated and centrifuged (14,000g for 10 min at 4°C). Analysis of XO metabolites from supernatants of urine, serum and tissue samples was performed using Waters HPLC (Waters, Milford, MA) coupled with Photo Diode Array detection. Separation of analytes was achieved on a Phenomenex Luna, 5 µm, C-18, 250 × 4.6 mm column. Mobile phase was 0.02 sodium acetate (pH=4.5 with acetic acid). A gradient with methanol was used to clear the column after elution of analytes. The individual XO metabolites and compounds of interest were analyzed at their respective maximum wavelength intensities (uric acid at 284 nm, xanthine at 266 nm, hypoxanthine at 249 nm, allantoin, oxypurinol and allopurinol at 210 nm). Peak height values for each metabolite obtained in the same retention time window as standards were used as input into standard curves constructed with known analyte concentrations. Analytes were normalized to total protein content of HPLC samples determined by the Bradford method, with the exception of urine, for which they were normalized to the total urine volume for 24h.
Histology
Cross-sections of thoracic aorta segments collected at the time of sacrifice were paraffin-embedded and stained with hematoxylin and eosin. Morphometric assessment was performed using the MMI Cell Tools software (Molecular Machines and Industries, Glattbrugg, Switzerland) on a Nikon Eclipse microscope. Equally spaced (every 45°) measurements of lumen diameter (4 measurements) and wall thickness (8 measurements) were made. The averaged wall thickness was reported to the averaged lumen diameter for a final wall/lumen ratio.
Data analysis
Data are presented as mean ± SEM for the number of animals (N). Plotting and statistical analysis of data was accomplished using GraphPad Prism 5 (GraphPad, La Jolla, CA). When comparing groups, the appropriate Student’s t-test or ANOVA analysis was performed. For in vivo experiments, a 2 way repeated measures ANOVA with Bonferroni post-hoc analysis was performed. In all cases, a p value of ≤0.05 was considered statistically significant.
Results
Short-term XO inhibition did not decrease BP in the established DOCA-salt hypertension
The basic experimental design of the short-term study is depicted in figure 1A. The mean arterial pressure (MAP) observed over the course of the study is displayed in figure 1B. The missing data points on this figure correspond to the removal of rats from the close proximity of receivers and their placement in metabolic cages necessary for urine collection (second time point, day 18). Expectedly, MAP rose significantly following DOCA-salt treatment in all animals, reaching an average of 156 mmHg in the allopurinol group and 169 mmHg in the vehicle group after 3 weeks. At this time point, before initiation of allopurinol treatment, MAP was not significantly different between the two groups. Over the course of the 7-day allopurinol treatment that followed, MAP continued to rise in the allopurinol-treated group, following the same pattern observed in the vehicle group, with no statistically significant differences. Similarly, no changes were observed in the other hemodynamic parameters obtained in this study (data not shown).
Long-term XO inhibition did not prevent or attenuate the development of DOCA-salt hypertension and had minimal effects on target organs
The basic experimental design of the long-term study is depicted in figure 2A. The MAP observed over the course of the long-term experiment is displayed in figure 2B. DOCA-salt treatment effectively raised the BP of rats in both treatment groups. However, there was no significant difference in the MAP of allopurinol-treated compared to vehicle treated rats. These data suggest that allopurinol did not prevent and did not attenuate the development of DOCA-salt hypertension.
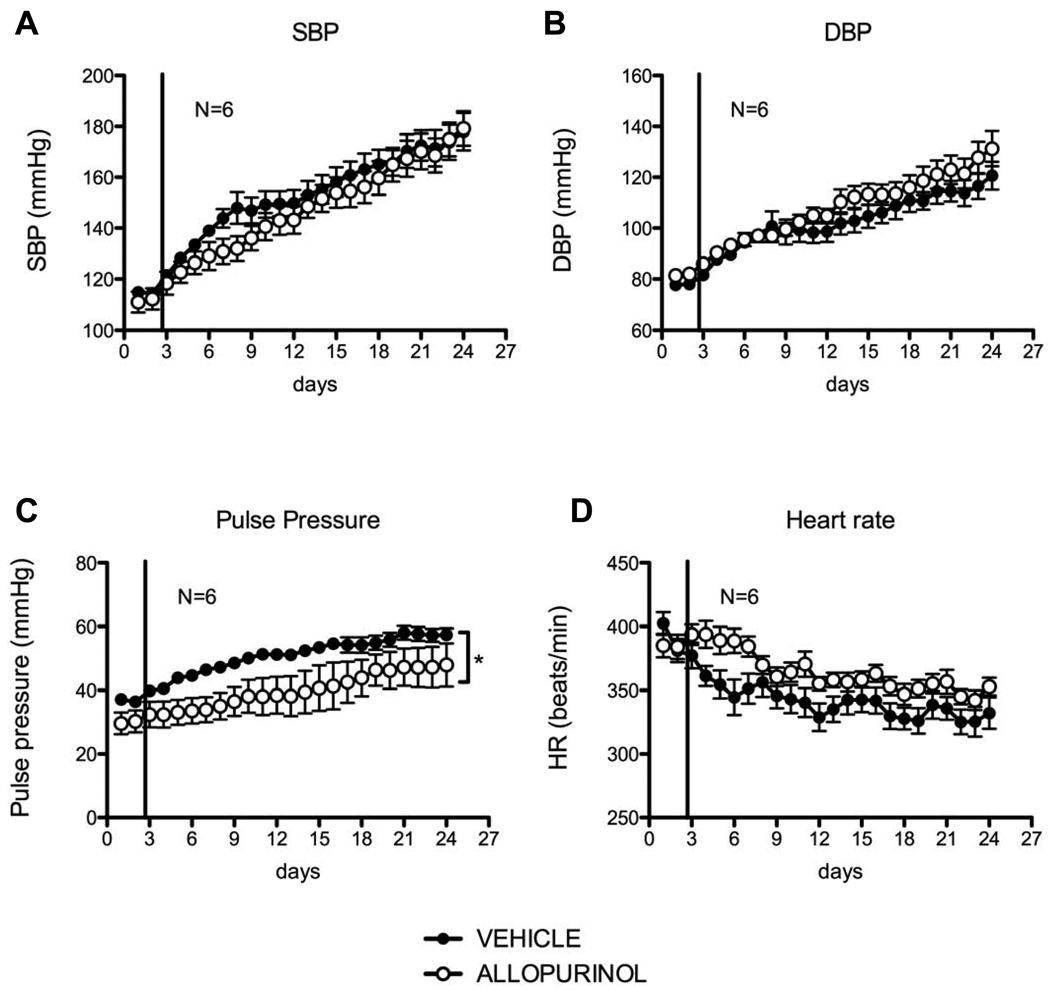
The other hemodynamic parameters obtained by radiotelemetry in the long-term study are displayed in figure 3. Despite an apparent initial decrease in systolic pressure in the allopurinol-treated group, this parameter was not significantly different over the course of allopurinol treatment as compared to the vehicle-treated rats (fig 3A). Diastolic pressure was increased by allopurinol treatment (fig 3B), and there was a statistically significant decrease in pulse pressure (Ppulse=Psys-Pdias) compared to the vehicle group (fig 3C). A trend for increased heart rate was observed in the allopurinol-treated group (fig 3D). No difference was observed in activity (movement) between the two groups (data not shown).
Figure 3.
Hemodynamic parameters: systolic blood pressure (A), diastolic blood pressure (B), pulse pressure (C), and heart rate (D) of DOCA-salt hypertensive rats under long-term treatment with vehicle (closed symbols) and allopurinol (open circles). The vertical line represents the initiation of vehicle or allopurinol treatment. Data are represented as means ± SEM of 24h averages (MAP) for N=6.
Despite the lack of effects of allopurinol on BP itself, chronic XO inhibition may have protective effects on hypertensive complications in target organs. Therefore in this study we also measured the heart and kidney weight at the end of the treatment (gross evaluation of left ventricular and renal hypertrophy) as well as performed histological morphometric analysis of hematoxylin-eosin stained aorta cross-sections (gross evaluation of arterial remodeling). We observed no difference in the heart weight (vehicle = 1.53 ± 0.04, allopurinol = 1.66 ± 0.05 g wet weight), but a surprising statistically significant increase in kidney weight in the allopurinol-treated group (vehicle = 2.99 ± 0.13, allopurinol = 3.46 ± 0.11 g wet weight, p=0.02). The histological characteristics of hematoxylin-eosin stained aorta sections did not appear qualitatively different and there was also no quantitative difference in the wall/lumen ratio between the two treatment groups (vehicle = 0.0813 ± 0.0029, allopurinol = 0.0813 ± 0.0033).
Allopurinol had measurable effects on XO metabolites
Urine, serum and tissue (aorta, vena cava, carotid artery, jugular vein, liver, kidney and heart) HPLC analysis of XO metabolites was used to verify XO inhibition in the two studies. Table 1 and Table 2 display the results for uric acid (the direct product of XO activity), hypoxathine (XO substrate) and oxypurinol (the active metabolite of allopurinol). Not shown are measurement results for allantoin (the product of uricase action on uric acid), which was variably detectable and displayed large in-group variations; xanthine (the other XO substrate), which followed the same pattern as hypoxanthine; and allopurinol (the administered drug), which followed the same pattern as oxypurinol. Oxypurinol was largely not detected or variable at the tissue level.
Table 1.
HPLC measurements of uric acid, hypoxanthine and oxypurinol in urine, serum and tissues during the short-term administration of vehicle or allopurinol to DOCA-salt hypertensive rats.
| XO metabolite group (n) |
Uric acid vehicle (n=4) |
Uric acid allopurinol (n=4) |
Hypoxanthine vehicle (n=4) |
Hypoxanthine allopurinol (n=4) |
Oxypurinol vehicle (n=4) |
Oxypurinol allopurinol (n=4) |
|---|---|---|---|---|---|---|
| Sample (unit) | ||||||
| Urine day 0 (mg/day) | 2.508 ± 0.561 | 2.662 ± 0.383 | 0.017 ± 0.005 | 0.039 ± 0.005 | 0.017 ± 0.009 | 0.074 ± 0.026 |
| Urine day 18 (mg/day) | 1.687 ± 0.824 | 2.718 ± 0.165 | 0.029 ± 0.011 | 0.028 ± 0.006 | 0.255 ± 0.091 | 0.216 ± 0.118 |
| Urine day 31 (mg/day) | 1.776 ± 0.886 | 2.621 ± 0.597 | 0.016 ± 0.003 | 0.280 ± 0.096* | 0.170 ± 0.070 | 17.899 ± 4.357* |
| Serum (µg/g protein) | 154.0 ± 64.37 | 35.37 ± 6.903 | 0.0 | 48.54 ± 15.94* | 0.0 | 10.82 ± 8.724* |
| Aorta (µg/g protein) | 78.33 ± 26.34 | 15.24 ± 3.589* | 20.14 ± 10.16 | 67.75 ± 21.90* | ||
| Vena cava (µg/g protein) | 514.0 ± 173.7 | 100.8 ± 35.24* | 54.50 ± 17.06 | 155.2 ± 66.34* | ||
| Carotid artery (µg/g protein) | 91.68 ± 21.25 | 14.93 ± 2.556* | 30.06 ± 16.44 | 65.63 ± 16.70 | ||
| Jugular vein (µg/g protein) | 137.3 ± 10.11 | 27.78 ± 3.853* | 18.25 ± 4.369 | 51.16 ± 9.950* | ||
| Liver (µg/g protein) | 1039 ± 198.4 | 1064 ± 439.0 | 7.953 ± 6.760 | 37.52 ± 27.15 | ||
| Kidney (µg/g protein) | 3228 ± 131.7 | 3381 ± 1171 | 5.134 ± 2.800 | 514.9 ± 511.9* | ||
| Heart (µg/g protein) | 139.0 ± 32.80 | 71.28 ± 13.10 | 72.95 ± 6.872 | 368.2 ± 77.74* |
represents a statistically significant (p<0.05) difference between treatment groups.
Table 2.
HPLC measurements of uric acid, hypoxanthine and oxypurinol in urine, serum and tissues during the long-term administration of vehicle or allopurinol to DOCA-salt hypertensive rats.
| XO metabolite group (n) |
Uric acid vehicle (n=6) |
Uric acid allopurinol (n=6) |
Hypoxanthine vehicle (n=6) |
Hypoxanthine allopurinol (n=6) |
Oxypurinol vehicle (n=6) |
Oxypurinol allopurinol (n=6) |
|---|---|---|---|---|---|---|
| Sample (unit) | ||||||
| Urine basal (mg/day) | 4.813 ± 0.468 | 5.415 ± 0.526 | 0.056 ± 0.015 | 0.066 ± 0.018 | 0.0 | 0.087 ± 0.041 |
| Urine post-treat. (mg/day) | 1.925 ± 0.247 | 2.474 ± 0.296 | 0.052 ± 0.031 | 0.334 ± 0.164* | 0.0 | 17.34 ± 4.707* |
| Serum (µg/g protein) | 62.93 ± 8.086 | 42.02 ± 17.61 | 0.0 | 27.78 ± 7.437* | 0.0 | 18.69 ± 6.786* |
| Aorta (µg/g protein) | 85.33 ± 20.50 | 10.92 ± 6.205* | 48.10 ± 18.90 | 46.23 ± 17.11 | ||
| Vena cava (µg/g protein) | 460.9 ± 94.27 | 83.40 ± 43.66* | 33.75 ± 14.28 | 97.33 ± 28.82* | ||
| Carotid artery (µg/g protein) | 168.37 ± 94.94 | 28.80 ± 12.15 | 84.69 ± 42.94 | 67.34 ± 19.06 | ||
| Jugular vein (µg/g protein) | 199.3 ± 84.55 | 33.71 ± 15.18* | 24.20 ± 9.769 | 44.24 ± 17.56 | ||
| Liver (µg/g protein) | 358.1 ± 98.59 | 27.44 ± 22.51* | 66.51 ± 19.51 | 245.8 ± 55.95* | ||
| Kidney (µg/g protein) | 726.0 ± 276.4 | 381.1 ± 130.3* | 799.3 ± 212.8 | 1030 ± 165.7 | ||
| Heart (µg/g protein) | 17.37 ± 9.966 | 2.776 ± 0.836 | 201.4 ± 44.54 | 125.2 ± 18.15 |
represents a statistically significant (p<0.05) difference between treatment groups.
Urinary levels of uric acid were not changed by allopurinol treatment in both studies. Urinary levels of hypoxanthine increased substantially after allopurinol administration in both studies. Oxypurinol was detected in urine following allopurinol treatment in both studies. None of the XO metabolites were changed by the DOCA treatment per se (table 1, compare day 0 to day 18).
Uricemia levels displayed a trend for reduction following allopurinol treatment in both studies, although this was not statistically significant. Serum levels of hypoxanthine increased dramatically after allopurinol administration in both studies. Oxypurinol was detected in serum following allopurinol treatment in both studies .
Uric acid levels were decreased in the allopurinol treated rats in vascular tissues (aorta, vena cava, carotid artery, jugular vein) in both studies. In the liver and kidney, these levels were decreased only in the second study. Hypoxanthine levels were increased in the vascular tissues, the kidney and the heart in the short-term study and only in the vena cava and the liver in the long-term study. Oxypurinol was not detected in most tissues of allopurinol treated rats (data not shown).
Collectively, these studies validate that XO was inhibited in multiple tissues of the cardiovascular system, and dissociates XO inhibition from modification of blood pressure.
Discussion
In this study we tested the hypothesis that XO inhibition in vivo would impact the course of hypertension in the DOCA-salt rat model, by either decreasing BP when administered during the established phase and/or by attenuating the development of this hypertension when administered before the initiation of DOCA-salt treatment. We sought to achieve XO inhibition by allopurinol administration (50 mg/kg/day, orally). XO inhibition was verified through HPLC analysis of XO metabolites in urine, serum and tissues. Despite effective XO inhibition, allopurinol treatment did not lead to BP changes in either the established or the development phase of DOCA-salt hypertension.
Validation of XO inhibition by allopurinol
Pharmacological inhibition of XO classically involves use of purine analogues, such as allopurinol. Allopurinol serves both as an inhibitor and as a substrate (suicide inhibitor) for XO, which catalyzes its transformation to oxypurinol, an active and longer-lived metabolite. Despite questions raised on the specificity and potential ROS scavenging 25 activity of allopurinol, it is currently the most, and until recently also the only XO inhibitor used in humans.
In humans, allopurinol administration to patients with hyperuricemia and gout is monitored by measuring serum uric acid. Successful treatment is represented by a decrease in serum uric acid levels below the solubility limit of urate (6 mg/dl or 360 µM). This is variably achieved in ∼20 to 80% patients with a typical dose of allopurinol (300 mg/day). There is a high percentage of non-compliance to treatment 26. The dose of allopurinol used in humans is low in comparison with the one used in rodents (typically 1.25 – 3.75 to as high as 10 mg/kg/day in humans compared to 10–200 mg/kg/day for rodents), especially considering the lack of uricase activity in humans 27. This is partly due to adverse reactions observed in humans at higher allopurinol doses 28.
In rats, several of the published reports on allopurinol administration do not contain any type of validation for XO inhibition, while most show either a decrease in serum urate levels or in the XO activity of liver 14,20,29. A few studies reported analysis of urine or serum XO metabolites by HPLC 30–32, however to our knowledge no such analysis was performed for tissues from allopurinol-treated animals or for hypertensive animals.
As noted in the Introduction, disparate effects of XO inhibition on BP have been reported previously (table 3). In addition to differences in rat models and allopurinol doses used, the inconsistent results could be explained by varying degrees of XO inhibition achieved in different studies. Therefore, rather than only performing serum urate measurements or performing XO activity assays ex vivo, we chose a more comprehensive approach and investigated the alterations in XO metabolites caused by XO inhibition in biological fluids (urine, serum) and several cardiovascular tissues (artery-vein pairs and the heart) and other organs (liver and kidney). This approach however precluded simultaneous measurements of ROS levels in the same tissues.
Table 3.
Published reports of allopurinol effects on blood pressure in various rat hypertension models.
| Study first author (ref. no) | Rat hypertension model | Allopurinol administration | BP measurement | BP effect |
|---|---|---|---|---|
| Nakazono et al. (13) | SHR | Acute - iv - 17 mg/kg | Tail cuff - one timepoint | lower SBP |
| Yamamoto et al. (18) | SHR | Chronic - oral - 30 mg/kg/day | Tail cuff - two timepoints |
No effect |
| Laakso et al. (17) | SHR on high-salt diet | Chronic - oral - 10 mg/kg/day | Tail cuff - one timepoint | No effect |
| Wallwork et al. (15) | Dexamethasone | Chronic - oral - 20–25 mg/day | Direct arterial - one timepoint |
Lower SBP and MAP |
| Ong et al. (20) | Dexamethasone | Chronic - oral - 200 mg/kg/day | Tail cuff - timecourse | No effect |
| Viel et al. (14) | DOCA-salt | Chronic - oral - 100 mg/kg/day | Tail cuff - one timepoint | Lower SBP |
| Tian et al. (19) | Dahl-salt sensitive | Chronic - oral - 10 mg/kg/day | Direct arterial - timecourse |
No effect |
| Kasal et al. (21) | L-NAME | Chronic -oral - 40 mg/kg/day | Tail cuff - three timepoints |
No effect |
| Zhang et al. (22) | ACTH | Chronic -oral - 200 mg/kg/day | Tail cuff - timecourse | No effect |
| Minami et al. (23) | Hypercholesterolemia | Chronic - oral - 10 mg/kg/day | Tail cuff - timecourse | No effect |
We chose a dose of allopurinol previously proven to be effective in inhibiting XO activity in rats in vivo 29 and the oral route of administration. Following allopurinol administration, we expected decreases in urate levels and accumulations in xanthine/hypoxanthine, paralleling the findings during human allopurinol administration. We observed no significant changes of urate in urine; a trend for decrease in serum urate; and a significant decrease in tissue urate, accompanied by highly significant increases in hypoxanthine in urine, serum and tissues. We attribute these increases to the effective blockade of XO function, leading to accumulation of substrates. Effectiveness of the oral route of drug administration was further validated by detection of not only allopurinol, but also oxypurinol in both urine and serum of treated rats.
Blood pressure effects of XO inhibition
We observed no effects on MAP during treatment with 50 mg/kg/day allopurinol, regardless of the timing of XO inhibition. Aside from a significant decrease in pulse pressure observed in the long-term inhibition study (discussed in the speculation), other secondary parameters either were not changed by allopurinol or the changes were opposite to the ones expected if allopurinol would be antihypertensive. More specifically, there was no change in heart weight, no changes in aortic remodeling, and an increase in kidney weight. We measured kidney weight as a gross measure of hypertensive nephropathy, but this may also have changed due to adverse renal effects of allopurinol 33–35. However, absolute kidney weight is a limited measure of kidney hypertrophy and additional functional or histological studies would be required to characterize the renal changes in allopurinol-treated DOCA-salt hypertensive rats.
We concluded that XO is an unessential player in the pathogenesis of DOCA-salt hypertension. This would certainly be in agreement with several other negative reports using allopurinol in the SHR 17,18, Dahl salt-sensitive 19, L-NAME 21, glucocorticoid 20, ACTH 22 and hypercholesterolemia 23 -induced hypertension models. However, a few reports 13–15 contradict ours and there are alternative explanations on the lack of effects of allopurinol treatment on MAP.
We excluded the possibility of lack of blockade of XO with the chosen dose of allopurinol, based on the results of our HPLC analysis of XO metabolites. However, because we did not observe BP effects with 50 mg/kg/day, as opposed to another study using DOCA-salt hypertensive rats 14 (in which decreases in SBP were achieved with 100 mg/kg/day allopurinol), the question of insufficient XO blockade arises. Before repeating full BP studies in the DOCA-salt model with a higher allopurinol dose, we compared the effects of the two allopurinol doses (50 and 100 mg/kg/day) on XO metabolites only, in normal rats (data not shown). Overall, these experiments demonstrated that the increases observed in hypoxanthine, xanthine, allopurinol and oxypurinol in urine and serum were dose-dependent. Serum urate decreased significantly with the higher allopurinol dose; however this decrease was not significantly different from the one obtained with the lower dose. Additionally, the 100 mg/kg/day dose was previously reported as renal toxic in rats 34,35, and this nephrotoxicity appeared enhanced by hypertension 36. Therefore we did not repeat the BP studies with a higher dose of allopurinol, as little additional effect on BP could be expected with similar levels of XO inhibition.
Moreover, other factors may account for the discrepancy between our results and the aforementioned study 14, such as differences in the DOCA-salt model (DOCA dose was 250 mg/kg as opposed to 200 mg/kg in our case), or in the BP measurement method (single timepoint tail-cuff measurement as opposed to continuous long-term radiotelemetric measurement in our case). We could only fully exclude inadequate inhibition by inhibiting XO via different means: another pharmacological inhibitor, such as the newer, more specific, non-purine based febuxostat 37; or another method, such as in vivo siRNA inhibition of XO.
Another possible explanation for the lack of BP changes with XO inhibition is that the reduction in total ROS thus obtained may not be sufficient to impact mineralocorticoid hypertension pathogenesis. This idea would be addressed directly in DOCA-salt hypertension by ROS measurements during XO inhibition. However, in a study attempting to determine the relative contribution of ROS sources to total ROS in human vascular tissues, XO was a non-essential ROS producer compared to NADPH oxidase 38. Consistent with this idea are positive studies demonstrating a reduction in BP following administration of apocynin in the DOCA-salt model of hypertension 39.
Speculation
We have previously shown that vascular XO is locally-produced, and that XO expression, activity and impact on vascular function is higher in veins than in arteries in the normal rat 40. Venous XO activity increases during DOCA-salt hypertension (unpublished observation). Additionally, mean circulatory filling pressure (gross measure of venomotor tone) was increased in DOCA rats 41, raising the question of venous contributions to this model of hypertension. Therefore, we originally speculated that should our hypothesis be correct and a BP decrease be observed with allopurinol, this might be explained by a vascular (specifically, venous) XO-dependent mechanism.
However, the single positive hemodynamic finding in this study was a statistically significant decrease in pulse pressure during long-term allopurinol administration. This hemodynamic parameter is independently associated with cardiovascular risk in humans 42. Decreased pulse pressure therefore may be a beneficial effect of allopurinol treatment, highlighting a potentially important role of XO aside from its impact on MAP. The decreased pulse pressure in allopurinol treated rats could be due to reduced stroke volume caused by diminished venous smooth muscle tone. Alternatively, reduced pulse pressure could reflect an effect of allopurinol on aortic stiffness, since increased XO activity has been linked to aortic stiffening in both humans 43 and rats 44.
Conclusion
In this study, we investigated the BP effects of XO inhibition in the DOCA-salt rat hypertension model. No MAP changes were observed following allopurinol administration in this model, regardless of the timing of the intervention. We conclude that there is minimal, if any, contribution of XO activity to the pathogenesis and the maintenance of hypertension in the rat DOCA-salt model. Our study does not however exclude the role of ROS in mineralocorticoid-induced hypertension or hypertension in general, nor the role of XO in other cardiovascular diseases. Further studies are needed to elucidate the role played by XO in cardiovascular pathology through both its ROS and uric acid-producing activities. Furthermore, XO inhibition in hypertensive patients is currently only considered in the case of hyperuricemia. In this case, allopurinol might act synergistically with the antihypertensive medication, and BP or protective target organ effects may be thus masked. However, a better understanding of XO function is required for establishing the benefit/risk ratio of potential XO inhibition treatments in hypertensive patients without concomitant hyperuricemia.
Acknowledgments
Funding: This work was supported by NHLBI PO1 HL70687 (GDF, SWW) and AHA Predoctoral Fellowship 0715679Z (TS).
Abbreviations
- BP
blood pressure
- DOCA
deoxycorticosterone acetate
- HPLC
high performance liquid chromatography
- L-NAME
L-NG-Nitroarginine methyl ester
- ROS
reactive oxygen species
- XO
xanthine oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 2.Borges F, Fernandes E, Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem. 2002;9:195–217. doi: 10.2174/0929867023371229. [DOI] [PubMed] [Google Scholar]
- 3.Laakso JT, Teravainen TL, Martelin E, Vaskonen T, Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hypertensive rats. J Hypertens. 2004;22:1333–1340. doi: 10.1097/01.hjh.0000125441.28861.9f. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, DeLano FA, Parks DA, Jamshidi N, Granger DN, Ishii H, Suematsu M, Zweifach BW, Schmid-Schonbein GW. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc Natl Acad Sci U S A. 1998;95:4754–4759. doi: 10.1073/pnas.95.8.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 2006;110:243–253. doi: 10.1042/CS20050307. [DOI] [PubMed] [Google Scholar]
- 6.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 9.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 10.Waring WS, McKnight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55:3127–3132. doi: 10.2337/db06-0283. [DOI] [PubMed] [Google Scholar]
- 11.Inkster ME, Cotter MA, Cameron NE. Treatment with the xanthine oxidase inhibitor, allopurinol, improves nerve and vascular function in diabetic rats. Eur J Pharmacol. 2007;561:63–71. doi: 10.1016/j.ejphar.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Baldus S, Koster R, Chumley P, Heitzer T, Rudolph V, Ostad MA, Warnholtz A, Staude HJ, Thuneke F, Koss K, Berger J, Meinertz T, Freeman BA, Munzel T. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radic Biol Med. 2005;39:1184–1190. doi: 10.1016/j.freeradbiomed.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viel EC, Benkirane K, Javeshghani D, Touyz RM, Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H281–H288. doi: 10.1152/ajpheart.00304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallwork CJ, Parks DA, Schmid-Schonbein GW. Xanthine oxidase activity in the dexamethasone-induced hypertensive rat. Microvasc Res. 2003;66:30–37. doi: 10.1016/s0026-2862(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 16.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. Jama. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laakso J, Mervaala E, Himberg JJ, Teravainen TL, Karppanen H, Vapaatalo H, Lapatto R. Increased kidney xanthine oxidoreductase activity in salt-induced experimental hypertension. Hypertension. 1998;32:902–906. doi: 10.1161/01.hyp.32.5.902. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Ogino K, Igawa G, Matsuura T, Kaetsu Y, Sugihara S, Matsubara K, Miake J, Hamada T, Yoshida A, Igawa O, Yamamoto T, Shigemasa C, Hisatome I. Allopurinol reduces neointimal hyperplasia in the carotid artery ligation model in spontaneously hypertensive rats. Hypertens Res. 2006;29:915–921. doi: 10.1291/hypres.29.915. [DOI] [PubMed] [Google Scholar]
- 19.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45:934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 20.Ong SL, Vickers JJ, Zhang Y, McKenzie KU, Walsh CE, Whitworth JA. Role of xanthine oxidase in dexamethasone-induced hypertension in rats. Clin Exp Pharmacol Physiol. 2007;34:517–519. doi: 10.1111/j.1440-1681.2007.04605.x. [DOI] [PubMed] [Google Scholar]
- 21.Kasal DA, Neves MF, Oigman W, Mandarim-de-Lacerda CA. Allopurinol attenuates L-NAME induced cardiomyopathy comparable to blockade of angiotensin receptor. Histol Histopathol. 2008;23:1241–1248. doi: 10.14670/HH-23.1241. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Chan MM, Andrews MC, Mori TA, Croft KD, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not allopurinol prevents and reverses adrenocorticotropic hormone-induced hypertension in the rat. Am J Hypertens. 2005;18:910–916. doi: 10.1016/j.amjhyper.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Minami M, Ishiyama A, Takagi M, Omata M, Atarashi K. Effects of allopurinol, a xanthine oxidase inhibitor, on renal injury in hypercholesterolemia-induced hypertensive rats. Blood Press. 2005;14:120–125. doi: 10.1080/08037050510008878. [DOI] [PubMed] [Google Scholar]
- 24.Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- 25.Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun. 1987;148:314–319. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]
- 26.Wortmann RL. Recent advances in the management of gout and hyperuricemia. Curr Opin Rheumatol. 2005;17:319–324. doi: 10.1097/01.bor.0000162060.25895.a5. [DOI] [PubMed] [Google Scholar]
- 27.Yeldandi AV, Yeldandi V, Kumar S, Murthy CV, Wang XD, Alvares K, Rao MS, Reddy JK. Molecular evolution of the urate oxidase-encoding gene in hominoid primates: nonsense mutations. Gene. 1991;109:281–284. doi: 10.1016/0378-1119(91)90622-i. [DOI] [PubMed] [Google Scholar]
- 28.So A. Developments in the scientific and clinical understanding of gout. Arthritis Res Ther. 2008;10:221. doi: 10.1186/ar2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fields M, Lewis CG, Lure MD. Allopurinol, an inhibitor of xanthine oxidase, reduces uric acid levels and modifies the signs associated with copper deficiency in rats fed fructose. Free Radic Biol Med. 1996;20:595–600. doi: 10.1016/0891-5849(95)02056-x. [DOI] [PubMed] [Google Scholar]
- 30.Turnheim K, Krivanek P, Oberbauer R. Pharmacokinetics and pharmacodynamics of allopurinol in elderly and young subjects. Br J Clin Pharmacol. 1999;48:501–509. doi: 10.1046/j.1365-2125.1999.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Putterman GJ, Shaikh B, Hallmark MR, Sawyer CG, Hixson CV, Perini F. Simultaneous analysis of substrates, products, and inhibitors of xanthine oxidase by high-pressure liquid chromatography and gas chromatography. Anal Biochem. 1979;98:18–26. doi: 10.1016/0003-2697(79)90700-0. [DOI] [PubMed] [Google Scholar]
- 32.Cooper N, Khosravan R, Erdmann C, Fiene J, Lee JW. Quantification of uric acid, xanthine and hypoxanthine in human serum by HPLC for pharmacodynamic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;837:1–10. doi: 10.1016/j.jchromb.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 33.Horiuchi H, Ota M, Nishimura S, Kaneko H, Kasahara Y, Ohta T, Komoriya K. Allopurinol induces renal toxicity by impairing pyrimidine metabolism in mice. Life Sci. 2000;66:2051–2070. doi: 10.1016/s0024-3205(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki Y, Sato Y, Sudo J, Tanabe T. Renal lipid peroxidation induced by allopurinol-administration in rats. Dev Toxicol Environ Sci. 1986;14:65–72. [PubMed] [Google Scholar]
- 35.Suzuki Y, Sudo J, Tanabe T. Allopurinol toxicity: its toxic organ-specificity between the liver and the kidney in the rat. J Toxicol Sci. 1984;9:343–351. doi: 10.2131/jts.9.343. [DOI] [PubMed] [Google Scholar]
- 36.Trachtman H, Valderrama E, Futterweit S. Nephrotoxicity of allopurinol is enhanced in experimental hypertension. Hypertension. 1991;17:194–202. doi: 10.1161/01.hyp.17.2.194. [DOI] [PubMed] [Google Scholar]
- 37.Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;76:1835–1847. doi: 10.1016/j.lfs.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, Korbut R, Channon KM. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol. 2004;24:1614–1620. doi: 10.1161/01.ATV.0000139011.94634.9d. [DOI] [PubMed] [Google Scholar]
- 39.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- 40.Szasz T, Thompson JM, Watts SW. A comparison of reactive oxygen species metabolism in the rat aorta and vena cava: focus on xanthine oxidase. Am J Physiol Heart Circ Physiol. 2008;295:H1341–H1350. doi: 10.1152/ajpheart.00569.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fink GD, Johnson RJ, Galligan JJ. Mechanisms of increased venous smooth muscle tone in desoxycorticosterone acetate-salt hypertension. Hypertension. 2000;35:464–469. doi: 10.1161/01.hyp.35.1.464. [DOI] [PubMed] [Google Scholar]
- 42.Thomas F, Blacher J, Benetos A, Safar ME, Pannier B. Cardiovascular risk as defined in the 2003 European blood pressure classification: the assessment of an additional predictive value of pulse pressure on mortality. J Hypertens. 2008;26:1072–1077. doi: 10.1097/HJH.0b013e3282fcc22b. [DOI] [PubMed] [Google Scholar]
- 43.Delles C, Zimmerli LU, McGrane DJ, Koh-Tan CH, Pathi VL, McKay AJ, Steedman T, Dargie HJ, Hamilton CA, Dominiczak AF. Vascular stiffness is related to superoxide generation in the vessel wall. J Hypertens. 2008;26:946–955. doi: 10.1097/HJH.0b013e3282f7677c. [DOI] [PubMed] [Google Scholar]
- 44.Soucy KG, Lim HK, Benjo A, Santhanam L, Ryoo S, Shoukas AA, Vazquez ME, Berkowitz DE. Single exposure gamma-irradiation amplifies xanthine oxidase activity and induces endothelial dysfunction in rat aorta. Radiat Environ Biophys. 2007;46:179–186. doi: 10.1007/s00411-006-0090-z. [DOI] [PubMed] [Google Scholar]