Abstract
Given the increasing size of the older adult population in many countries, there is a pressing need to identify the nature of aging-related vision impairments, their underlying mechanisms, and how they impact older adults’ performance of everyday visual tasks. The results of this research can then be used to develop and evaluate interventions to slow or reverse aging-related declines in vision, thereby improving quality of life. Here we summarize salient developments in research on aging and vision over the past 25 years, focusing on spatial contrast sensitivity, vision under low luminance, temporal sensitivity and motion perception, and visual processing speed.
Keywords: aging, vision, vision impairment
1. Introduction
Fifty years ago there was little scientific information available on the effect of aging on vision except for that summarized in Robert Weale’s now classic book The Aging Eye (Weale, 1963). Twenty-five years ago scientists were just beginning to embark on comprehensive programs of research addressing how the aging process impacts various aspects of visual functioning. With the formation of the National Institute on Aging in 1974 as a separate institute within the National Institutes of Health in the U.S., the scientific spotlight was focused on vision and the aging process per sé, rather than solely on eye conditions and diseases prevalent in older adults (e.g., macular degeneration, glaucoma, cataract). By the late 1970s research increasingly addressed the role of lifespan changes during adulthood on visual function and task performance and the mechanisms underlying these aging-related changes. The initial years of this formative period were marked by a symposium entitled “Aging and Human Visual Function” in 1980 sponsored by the Committee on Vision of the National Research Council and convened at the National Academy of Sciences in Washington DC. The symposium, which helped to “jump-start” the field, was later published in an edited book (Sekuler, Kline & Dismukes, 1982) that served as one of the first reference volumes for the field. Many other overviews have appeared in the ensuing 25 years, and the reader is referred to these for additional perspectives on the field (e.g., Faubert, 2002; Kline & Schieber, 1985; Ordy, Wengenack & Dunlap, 1991; Owsley & Sloane, 1990; Sekuler & Sekuler, 2000a; Sekuler & Sekuler, 2000b; Spear, 1993; Weale, 1982a; Weale, 1986; Werner, Peterzell & Scheetz, 1990; Jackson, Owsley, & Curcio, 2002; Werner, Schefrin & Bradley, 2010).
Research on vision and aging is essential for several reasons. The percentage of the population in the U.S. and many other countries over age 60 is increasing, and thus eye conditions, diseases, and vision impairments associated with aging represent a larger segment of our societal health challenge on a population basis than in previous decades. Thus, there is a pressing need to identify the prevalence and incidence of various aging-related vision impairments in populations, the mechanisms underlying these impairments, and how they impact older adults’ performance of everyday visual tasks. The results of this research can then be used to develop and evaluate interventions to slow or reverse aging-related declines in vision, thereby improving quality of life. Research programs on how vision changes during the course of adulthood can also provide information about biomarkers or risk factors for the incident development of serious eye diseases and conditions common in late adulthood. For example, are there aging-related psychophysical deficits in certain aspects of visual function that are exacerbated in older adults who eventually develop serious eye diseases (e.g., age-related macular degeneration (AMD), glaucoma)? Vulnerabilities in visual function in late life can potentially reveal breakdowns in the visual system that are early markers or signs that disease development is likely. It is also important to know what biological (e.g., neural, optical) and environmental (e.g., smoking, dietary) characteristics differentiate between those older adults who “age well” (e.g., lose little to no visual sensitivity as they age) versus those who do not (e.g., experience substantial threshold elevations). Finally, our understanding of basic visual processes in general benefits from discoveries on older adults’ visual capabilities in that the most comprehensive theoretical frameworks and models of vision must account for visual changes over the lifespan.
A methodological challenge in defining normal aging needs to be mentioned at the outset. Many structural and physiological changes in the eye during aging often lie on the same continuum of those changes due to disease. For example, during adulthood the density of the crystalline lens increases (Xu, Pokorny & Smith, 1997); at what point is it considered cataract? Along similar lines, drusen appear in many older retinas (Vinding, 1990); what characteristics of drusen formation lead to a diagnosis of AMD? Because of the continuum of aging and disease it is important for studies purportedly studying aging to use, whenever possible, explicit inclusion and exclusion criteria that make reference to scales and/or clearly defined characteristics. Stating that the eye studied has been designated as “clinically normal” by an ophthalmologist or optometrist may not be adequate. This is because what one ophthalmologist or optometrist considers clinically normal can differ from one clinician to the next. Eligibility criteria based on retinal fundus grading systems (Age-Related Eye Disease Study Group, 2005) or lens grading systems (Chylack, Wolfe, Singer, Leske, Bullimore, Bailey, Friend, McCarthy, Wu & Group, 1993), or other more objective or standardized ways for measuring lens density and scatter are preferred in determining eligibility for recruitment into a study. Clear delineation of these criteria also facilitates comparison of results across studies. The good news is that an increasing number of investigators over the past 25 years are using more objective criteria in defining “normal aging” especially for retinal health (Dimitrov, Guymer, Zele, Anderson & Vingrys, 2008; Elliott & Werner, 2010; Owsley, McGwin, Jackson, Heimburger, Piyathilake, Klein, White & Kallies, 2006a), yet there are still many studies that rely on clinical judgment alone, self-report and/or a visual acuity cutpoint (e.g., 20/25) to define normal aging. We have already discussed the potential problem with relying on clinical judgment as a case definition of normal aging. Self-report is even more problematic since older adults are often unaware of whether they have early forms of conditions or diseases, which theoretically could be impacting their visual function. About half of older adults do not seek annual comprehensive eye care when conditions might be diagnosed (McGwin, Khoury, Cross & Owsley, 2010), so many older adults are not in the position to be made aware if they have eye conditions or diseases. The problem with relying on good visual acuity to define normal retinal aging is that good spatial resolution does not necessarily mean that the retina is free of disease; for example, good visual acuity can be retained even though AMD or primary open angle glaucoma is present. Yet these conditions can impact other aspects of retinal function, and thus impact higher order visual processing as well. Studies not using more objective and standardized criteria for normal retinal health must be viewed in light of the potential confounding effects of disease in interpreting thresholds.
The purpose of this article is not to provide a comprehensive up-to-date overview of the broad field of vision and aging; the topic is simply too broad for an article of this type. As mentioned above, there are several summaries of the literature that when taken together provide a comprehensive overview of our current understanding of vision phenomena in later adulthood (e.g., Werner, Schefrin & Bradley, 2010; Jackson, Owsley, & Curcio, 2002; Sekuler & Sekuler, 2000b). Rather, our purpose here is to identify areas rather intensively studied since 1985, summarize and critically review the current state of knowledge, and discuss the unresolved or new questions emerging from this body of work. Our focus is on studies of visual function as psychophysically measured. The reader will no doubt note that there are many aging and vision phenomena not addressed here, and their omission by no means signifies that the author believes they are unimportant. The topics below were selected because from this author’s perspective they seemed the most salient.
2. Spatial Contrast Sensitivity
The processing of spatial contrast is a fundamental building block of pattern vision. Therefore it is not surprising that one of the first questions tackled by vision researchers interested in the aging process was how aging impacts spatial contrast sensitivity. As we will see below, research on this issue has continued into the present. In all the research discussed here, refractive error (including that from presbyopia) is removed as a potential contributor to contrast sensitivity loss by ensuring that observers are refracted for the target distance.
By the end of the 1980s several studies established that older adults have impaired contrast sensitivity under photopic conditions at intermediate and high spatial frequencies, with the magnitude of deficit increasing with increasing spatial frequency (Derefeldt, Lennerstrand & Lundh, 1979; Elliott, Whitaker & Mac Veigh, 1990; Kline, Schieber, Abusamra & Coyne, 1983; Owsley, Sekuler & Siemsen, 1983; Tulunay-Keesey, Ver Hoeve & Terkla-McGrane, 1988). Studies differ in the reported magnitude of this deficit undoubtedly stemming from the variations in lens density and retinal health in the samples used across studies. As mentioned earlier, cataract and AMD themselves can cause contrast sensitivity deficits even in their early forms (Elliott, Gilchrist & Whitaker, 1989; Kleiner, Enger, Alexander & Fine, 1988; Rubin, Adamsons & Stark, 1993); the first studies on aging and contrast sensitivity did not use more objective and standardized criteria for lens and retina in determining participant eligibility. Thus, for example, some study samples undoubtedly had older adults with more lens opacity than other studies, which could lead to differences among studies in the magnitude of psychophysical impairments reported. Thus, efforts to resolve why one study found a larger young-old difference than the other are bound to be difficult if not futile.
Older adults’ spatial contrast sensitivity deficits are present even when criterion-free methods of threshold estimation are used (Elliott et al., 1990; Higgins, Jaffe, Caruso & deMonasterio, 1988). Impairment in lower spatial frequency sensitivity seems to be spared under photopic conditions. However, if low spatial frequency targets (e.g., 0.5 cycles/degree) are presented with rapid temporal modulation, the sensitivity enhancement characteristic of younger adults is attenuated in older adults (Elliott et al., 1990; Habak & Faubert, 2000; Kline, 1987; Owsley et al., 1983; Sloane, Owsley & Jackson, 1988b). This finding is consistent with the literature on aging and temporal sensitivity showing that older adults exhibit sensitivity loss for flicker that is accentuated as temporal frequency increases (Kim & Mayer, 1994; Tyler, 1989).
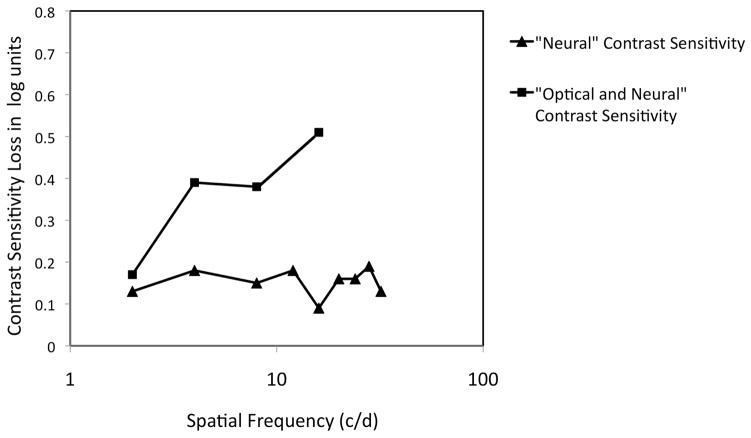
Retinal illuminance in older eyes is reduced due to pupillary miosis (Loewenfeld, 1979) and the increased density of the crystalline lens (Pokorny, Smith & Lutze, 1987; Said & Weale, 1959; Weale, 1961). There is also increased intraocular light scatter and increased optical aberrations in the aging eye (Artal, Guirao, Berrio, Piers & Norrby, 2003; Glasser & Campbell, 1998) that can reduce image contrast. Research has indicated that optical characteristics of older eyes are largely responsible for older adults’ spatial contrast sensitivity deficits at photopic light levels. When the optical performance of the human eye was compared for younger and older adults using a double-pass apparatus to measure the modulation transfer function, there were lower values of modulation in the older eyes (Artal, Ferro, Miranda & Navarro, 1993; Guirao, Gonzalez, Redondo, Geraghty, Norrby & Artal, 1999). This lower modulation difference was similar to the psychophysically measured loss in spatial contrast sensitivity in older adults as compared to younger adults from previous work (Owsley et al., 1983). That older adults’ loss in spatial contrast sensitivity under photopic conditions is largely optical in origin was also established through psychophysical studies using laser interferometry to bypass the optics of the eye in generating targets on the retina (see Burton, Owsley & Sloane, 1993 for a critical review of this literature). These studies found that older adults either exhibited no (Dressler & Rassow, 1981; Kayazawa, Yamamoto & Itoi, 1981) or a small yet statistically significant loss in contrast sensitivity (0.1–0.2 log units) (Burton et al., 1993) when the interference fringe targets are utilized. This 0.1–0.2 loss in “neural” contrast sensitivity accounted for less than half of older adults’ photopic contrast sensitivity loss at higher frequencies when sensitivity was measured using conventional viewing techniques where the optics are not bypassed (Derefeldt et al., 1979; Kline et al., 1983; Owsley et al., 1983; Tulunay-Keesey et al., 1988), implying that optical factors make a major contribution to spatial contrast sensitivity deficits in older adults at photopic levels (Figure 1).
Figure 1.
Adapted from Burton et al. (1993). Comparison of older adults’ loss in photopic spatial contrast sensitivity using interference fringes (labeled “Neural” Contrast Sensivity) and older adults’ vision loss in a direct viewing spatial contrast sensitivity task from Owsley et al. (1983) where the optics were not bypassed (“Neural + Optical” Contrast Sensitivity). The contrast sensitivity loss for interference fringes accounts for less than half of the contrast sensitivity loss at higher spatial frequencies in the direct view task. This suggests that older adults’ spatial contrast sensitivity loss under photopic conditions is largely optical in origin.
However, other psychophysical studies, while acknowledging that optical changes in the aged eye contribute to aging-related contrast sensitivity deficits, stress that neural factors play a significant role. One study using laser interferometry (Elliott, 1987) reported a neural contribution to older adults’ spatial contrast sensitivity loss that accounted for about half of older adults’ impairment But this loss occurred only at the highest spatial frequency tested (16.5 cycles/degree). In a recent study (Elliott, Choi, Doble, Hardy, Evans & Werner, 2009), adaptive optics (AO) were used to correct monochromatic higher-order aberrations when measuring older adults’ spatial contrast sensitivity; the basic experimental logic was that if older adults’ contrast sensitivity loss disappeared, this would constitute evidence that optical factors, specifically higher-order aberrations, underlie older adults’ deficit in photopic spatial vision. Results were that on average older adults’ spatial contrast sensitivity with AO improved, but not to the level of younger adults’ sensitivity level when they had no AO compensation. As suggested by the authors, these results might imply neural factors play a role in older adults’ loss in photopic contrast sensitivity, but they could also indicate that optical variables other than monochromatic aberrations (e.g., increased lens density and light scatter) could be playing a larger role once AO corrections are introduced.
Although in our view strong evidence that neural factors have a sizeable influence on older adults’ photopic contrast sensitivity is lacking, it is important to point that Curcio & Drucker (1990) have shown that ganglion cell density was reduced by 25% in the central 11° of retina in donor eyes from older adults, compared to eyes from young adults; Gao and Hollyfield (Gao & Hollyfield, 1992) also report aging-related ganglion cell density declines although less steep than those reported by Drucker & Curcio (1990). However, loss in neural elements does not necessarily correspond to functional deficits as measured psychophysically. Furthermore, although Curcio & Drucker (1990) and colleagues reported decreased aging-related ganglion cell density, Curcio and colleagues found a remarkable stability throughout adulthood in cone photoreceptor density throughout the retina including the fovea and the macula (Curcio, Millican, Allen & Kalina, 1993). With respect to aging-related structural changes later in the visual pathways, research on post-retinal visual pathways has not been extensive, and what does exist has been in animal models. In old monkeys decreased neuron density in both the magnocelllular and parvocellular layers of the lateral geniculate nucleus (LGN) was reported (Ahmad & Spear, 1993), but this was not due to a loss in neurons. Rather, it stemmed from a minor decrease in the number of neurons coupled with an increase in LGN volume. Thus, from at least one study, there is no evidence that LGN deterioration is contributing to spatial vision loss in older adults. Aging effects on visual cortical cells have been more noteworthy. Single-unit recording studies in V1 performed on old monkeys found that cortical neurons exhibited lower optimal spatial and temporal frequencies, less sensitivity to contrast, lower spatial resolution, and lower higher temporal frequency cut-offs as compared to recordings in young monkeys (Yang, Liang, Liang, Glasser, Wang, Zhou & Leventhal, 2008; Zhang, Wang, Wang, Fu, Liang, Ma & Leventhal, 2008). In addition, these cells in older monkeys were accompanied by increased spontaneous, visually-driven activity and decreased signal-to-noise ratio. Many of these effects were more pronounced in area V2 as compared to V1 (Wang, Zhou, Ma & Leventhal, 2005). Results from monkey models suggest that it is conceivable that changes in cortical cells in V1 and V2 could be producing spatial contrast sensitivity deficits observed in older adult humans, however, these studies do not confirm that this is the case.
To summarize, 25 years of research on spatial contrast sensitivity under photopic conditions in older adults converges on the following points. For older adults, spatial contrast sensitivity loss under photopic conditions increases with increasing spatial frequency. Low spatial frequency sensitivity at photopic levels is not impacted by aging, or only minimally, when targets are presented at low temporal frequencies. However, sensitivity for low spatial frequencies is impaired under rapid temporal modulation, which probably stems from the reduced temporal sensitivity of the aging visual system. Optical characteristics of the aged eye reduce spatial contrast sensitivity. These factors include reduced retinal illuminance (either from pupillary miosis, increased lens density, or both), increased intraocular light scatter, and increased aberrations. (Not discussed above, it must be acknowledged that pupillary miosis can also have a positive impact on older adults’ spatial vision by reducing optical aberration.) Although some psychophysical studies provide data implying there is a neural contribution, these studies do not provide definitive evidence that neural deterioration in the visual pathways are practically significant contributions to older adults’ loss in spatial contrast sensitivity. Reduced ganglion cell density in older donor retina from human studies and deleterious changes in the spatial and temporal response properties of visual cortical neurons in old monkey models suggest that neural deterioration in the primary visual pathway could contribute to older adults’ spatial contrast sensitivity loss. However, it remains to be determined whether these structural changes actually do impact photopic spatial vision in older humans.
At this juncture it is constructive to take a look at the magnitude of the threshold elevation effect in older adults for which the field has persistently been searching for a cause for 25 years. Is the young-old difference in contrast sensitivity of such a magnitude that it is practically or theoretically significant? The difference between contrast sensitivity for 20-year-olds and 70-year-olds at approximately 8 cycles/degree ranges from 0.2 to 0.57 log units, depending on which study is used as a source (Elliott, 1987; Elliott et al., 1990; Elliott et al., 2009; Kline et al., 1983; Owsley et al., 1983), with the average young vs. old sensitivity difference across these studies being approximately 0.3 log units. Is the size of this aging effect sufficient to merit persistent investigation? Granted, it appears to be a repeatable effect as conveyed by the number of studies that show statistically significant young-old differences. But does research on the causes of the effect, i.e. mechanisms, have pressing significance? Maybe not, for the following reasons. First, as just reviewed, optical mechanisms largely account for the loss. Furthermore, the size of the young versus old difference itself is small, and in some studies, the methods used for estimating threshold may have had a test-retest reliability similar in size to, or exceeding, the effect size. Although an effect of this size can elevate contrast thresholds for real world targets (Akutsu, Legge, Ross & Schuebel, 1991; Owsley, Sekuler & Boldt, 1981), there are a variety of compensatory strategies that older adults can use to increase the visibility of targets, such as moving closer to the target, scanning the target, increasing viewing time, and if illumination is low, by increasing illumination. Thus, a 0.3 log unit loss in contrast sensitivity is not apt to be visually disabling although it may be modestly visually impairing. The primary cause of the optical degradation in the aged eye is likely to be cataractous lens changes. When visibility problems impinge on quality of life and visual task performance, older adults elect cataract surgery, which is covered by health insurance or government health programs in many countries. Therefore, until the time an older adult undergoes cataract surgery, these aging-related contrast reductions may be operative, but in the longer term, they are largely reversible.
None of this is meant to be an argument that we should not seek to improve our understanding of how aging impacts the visual pathways in the brain. The point is that research over the past 25 years taken as a whole makes a reasonable case that that aging-related spatial contrast sensitivity deficits under photopic conditions are largely optical in origin. Ultimately for many older adults this optical deficit is in large part correctable through cataract surgery and intraocular lens insertion. Therefore, it is reasonable to suggest that going forward the field move beyond studies searching for a neural basis of a phenomenon that is largely optical, is small compared to other types of aging-related visual deficits, and lacks serious detrimental significance to older adults in the longer term.
3. Scotopic Contrast Sensitivity
A common visual complaint described by older adults including those in good eye health is difficulty seeing under low illumination or at night. Older adults cite these problems in both surveys and focus groups. For example they report difficulty reading under dim light (Kosnik, Winslow, Kline, Rasinski & Sekuler, 1988; Mangione, Berry, Spritzer, Janz, Klein, Owsley & Lee, 1998; Owsley, McGwin Jr, Scilley & Kallies, 2006b) and modify their behavior by avoiding night driving (Ball, Owsley, Stalvey, Roenker, Sloane & Graves, 1998; Brabyn, Schneck, Lott & Haegerström-Portnoy, 2005). Poor vision under reduced light levels and at night in the elderly has been linked to their involvement in motor vehicle collisions and falls (Källstrand-Ericson & Hidingh, 2009; Massie, Campbell & Williams, 1990; McMurdo, 1991; Mortimer & Fell, 1989). Older adults who report visual difficulty under poor lighting conditions are also those who are more likely to have scotopic sensitivity impairment as determined psychophysically (Owsley et al., 2006b; Scilley, Jackson, Cideciyan, Maguire, Jacobson & Owsley, 2002). Over the past 25 years two lines of research have addressed low luminance vision problems in older adults – one focus is on diminished spatial contrast sensitivity under low luminance, which will be discussed here, and the other is slowing in the rate of dark adaptation, which will be discussed in the next section.
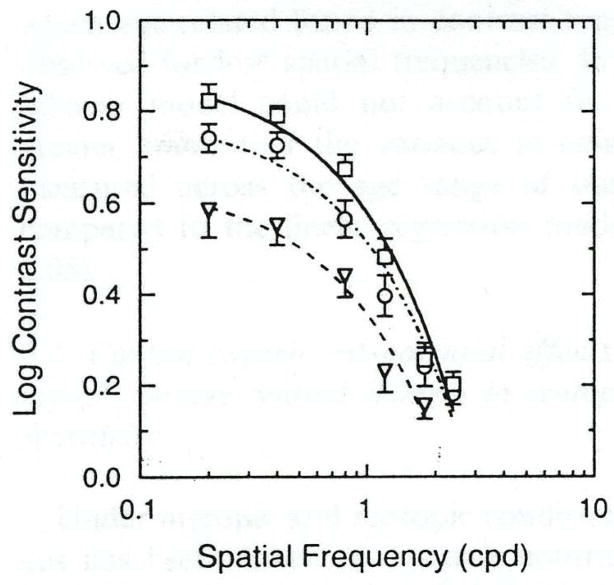
Older adults’ loss of spatial contrast sensitivity at low luminance levels (scotopic and mesopic) is accentuated compared to their loss under photopic conditions including a decline in older adults’ high spatial frequency cut-off as compared to that for young adults (Schefrin, Tregear, Harvey & Werner, 1999; Sloane, Owsley & Alvarez, 1988a; Sloane et al., 1988b). To gain an appreciation for the disadvantage older adults face under scotopic conditions, consider that young and older adults have the same sensitivity for a 0.5 cycles/degree grating in daylight, whereas under scotopic conditions older adults require on average three times the contrast of younger adults in order to discern the target (Schefrin et al., 1999) (Figure 2). There is also evidence that this deficit increases as background adaptation level decreases in the mesopic and scotopic ranges, and the deficit may be the most severe at lower spatial frequencies (Schefrin et al., 1999; Clark, Hardy, Volbrecht & Werner, 2010). Like photopic spatial contrast sensitivity deficits, mesopic and scotopic deficits have an optical contribution, but unlike photopic sensitivity, neural factors appear to play a major role in spatial vision loss under mesopic and scotopic conditions. On first analysis, it would seem that decreased rod photoreceptor density and ganglion cell density during the aging process (Curcio et al., 1993; Drucker & Curcio, 1990; Gao & Hollyfield, 1992) are logical candidate mechanisms underlying decreased spatial vision under low luminance in older adults. It could be the case that the loss in density of these neural elements and/or the enlargement of rod inner segments whereby they occupy the space left by necrotic rods (Curcio et al., 1993) increase receptive field center size for ganglion cells. However psychophysical data have not supported this purported mechanism. Schefrin, Hauser, & Werner (2004) reasoned that if the retinal ganglion cell receptive field centers that receive rod input are increased in size in the older adult, then Weber-like behavior (e.g., contrast sensitivity increases monotonically with mean luminance level for a spatial target) should theoretically occur at a lower luminance level for older adults as compared to young adults. Yet younger and older adults displayed similar mean luminance levels corresponding to Weber-like behavior onset (Schefrin, Hauser & Werner, 2004), implying that ganglion cell receptive field centers do not get larger during aging.
Figure 2.
From Schefrin et al. (1999). Average scotopic spatial contrast sensitivity for three age groups. Squares signify 20 to 40-year-olds, circles 41 to 60 –year-olds, and triangles 61 to 88-year-olds. Note that these losses are sizeable at low spatial frequencies and cannot be attributed to optical factors, suggesting a neural origin.
Other research has demonstrated an enlargement in Ricco’s area under scotopic conditions, the largest area over which complete spatial summation holds (Schefrin, Bieber, McLean & Werner, 1998; Schefrin et al., 1999), which cannot be attributed to optical factors. Schefrin et al. (1998) suggest that this change in spatial summation could be attributable to a change in the relative sensitivities of spatially selective channels under scotopic conditions or possibly synaptic re-wiring at either the retinal or cortical levels. It remains to be determined what retinal and cortical mechanisms underlie older adults’ impairment in scotopic contrast sensitivity, and to determine whether this scotopic dysfunction is modifiable through intervention.
4. Dark Adaptation
As far back as the 1940s it has been known that older adults have decreased light sensitivity in the dark and this deficit is larger than for photopic thresholds (Birren & Shock, 1950; Gunkel & Gouras, 1963; Jackson & Owsley, 2000; Jackson, Owsley, Cordle & Finley, 1998; McFarland, Domey, Warren & Ward, 1960; Robertson & Yudkin, 1944; Steven, 1946; Sturr, Zhang, Taub, Hannon, Jackowski, 1997; Weale, 1982b). Although increased optical density of the aged crystalline lens and pupillary miosis contribute to their scotopic threshold elevation (increasing threshold by about a 0.10–0.15 log units), more recently it has been established that these factors are not primarily responsible for this sensitivity loss, with about a half log unit elevation in threshold or more remaining after these factors are taken into account (Jackson et al., 1998; Sturr et al., 1997). Only in the past decade or so has research addressed potential neural mechanisms that underlie the loss. By age 60 to 70 years old, the density of rod photoreceptors decreases dramatically in the peri-macula as indicated by studies on donor retinas (Curcio et al., 1993; Gao, Rayborn, Myers & Hollyfield, 1990). However, scotopic sensitivity loss in older adults occurs in retinal areas where there is negligible rod loss and is not accentuated in the areas of heightened rod loss (Jackson et al., 1998), suggesting that rod loss by itself cannot account for older adults’ sensitivity impairment in the dark. Furthermore, there is little change in the amount of rod photopigment, rhodopsin, throughout adulthood (Liem, Keunen, van Norren & van de Kraats, 1991; Plantner, Barbour & Kean, 1988; van Kuijk, Lewis, Buck, Parker & Kliger, 1991).
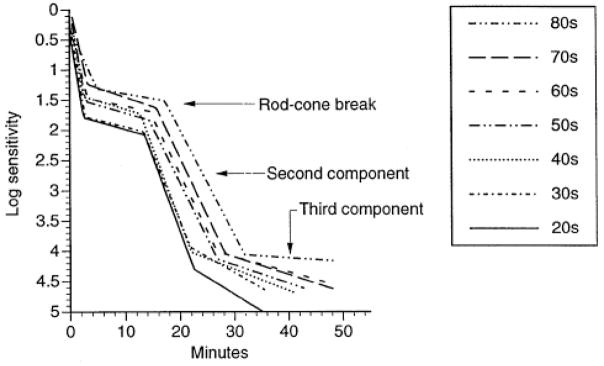
An alternative explanation for older adults’ scotopic sensitivity loss is that the visual cycle, the biochemical pathway responsible for rhodopsin regeneration, is perturbed with age. The visual cycle includes the production of 11-cis-retinal from retinoid and the subsequent regeneration of rhodopsin. Slowing of the visual cycle results in a prolongation of dark adaptation kinetics. Psychophysical dark adaptometry techniques can estimate the time constants associated with the visual cycle by measuring the recovery of light sensitivity after exposing the photopigment to an intense light that bleaches the photopigment (Alpern, 1971; Barlow, 1972; Hecht, Haig & Chase, 1937; Lamb & Pugh, 2004; Rushton & Powell, 1972). Jackson and colleagues (Jackson, Owsley & McGwin, 1999) studied rod-mediated dark adaptation in older adults overcoming the methodological shortcomings of the earlier work. They found that older adults experience substantial delays in adapting to darkness (Figure 3). Specifically, they found that older adults exhibited an increase in the time constant for the second and third components of rod-mediated dark adaptation (Lamb & Pugh, 2004; Leibrock, Reuter & Lamb, 1998), indicating slowing in rhodopsin degeneration. These psychophysical results, later replicated (Owsley, McGwin, Jackson, Kallies & Clark, 2007), are consistent with results obtained by rod densitometry (Liem et al., 1991). From a practical everyday standpoint, the problems faced by older adults in adjusting to darkness are nontrivial (e.g., adjusting to dark indoor environment after being outside on sunny day; searching for object in dark closet or drawer). The time taken for 70-year-olds to reach pre-bleach light sensitivity is over 10 minutes longer than for those in their 20s.
Figure 3.
From Jackson et al. (1999). Dark adaptation as a function of decade. Arrows label the portion of the function representing the rod-cone break and the second and third components of rod-mediated dark adaptation. Note that the funcs shift to the right with increasing decade, indicating a slowing in the rate of dark adaptation during aging.
What might be contributing to slowed rhodopsin regeneration in older adults? Photoreceptor function and survival are critically dependent on the retinal pigment epithelium (RPE) and Bruch’s membrane to regulate the transport of nutrients, fluid, ions, and metabolites to and from the subretinal space (Bok, 1985). During aging there are changes in the RPE-Bruch’s membrane complex such as progressive thickening of Bruch’s membrane (Bird, 1992; Feeney-Burns & Ellersieck, 1985; Newsome, Huh & Green, 1987), accumulation of extracellular material between the RPE and Bruch’s membrane (Curcio, Millican, Bailey & Kruth, 2001; Pauleikhoff, Harper, Marshall & Bird, 1990), reduced hydraulic conductivity of Bruch’s membrane (Starita, Hussain, Pagliarini & Marshall, 1996), and changes in the structure of RPE cells (Kornzweig, 1979). These changes could compromise metabolic exchange by causing a diffusion barrier between the choroid and photoreceptors, causing a localized scarcity of vitamin A, critical for rod photoreceptor function (Dowling & Wald, 1958; Kemp, Jacobson & Faulkner, 1988). Psychophysical data are consistent with this explanation. In a recent study (Owsley et al., 2006a) older adults’ dark adaptation was measured before and after they received a 30-day, high-dose course of retinol (pre-formed vitamin A). Older adults receiving the retinol course had rod-mediated sensitivity recovery that was faster as compared to a placebo-control group. The responsiveness of rod-mediated dark adaptation to a short course of high-dose retinol is consistent with the hypothesis that depositions and other structural changes in the RPE/Bruch’s membrane complex in aging caused a diffusion barrier that disrupts normal metabolic exchange, leading to a local shortage of vitamin A. Although this study does not provide direct evidence of an in vivo localized nutritional deficiency, it does highlight a possible pathway by which rod dysfunction and degeneration could occur during aging, and why dark adaptation is delayed in the elderly. These data cannot inform us of the possible site of the dysfunction that limits the availability of retinoids necessary for visual sensitivity recovery in older adults. As mentioned above, increased systemic vitamin A concentrations may force additional vitamin A across Bruch’s membrane into the RPE cells, via mass action, or, alternatively, increased levels of vitamin A may overcome possible impaired transport between the RPE cells and rod outer segments.
Slowing in cone-mediated dark adaptation in the fovea has also been reported for persons in their 60s and 70s (Coile & Baker, 1992). The slowing of sensitivity recovery was closely related to pigment regeneration rate implying that the latter contributes to older adults’ cone-mediated delay in sensitivity recovery (see also (Keunen, Norren & Meel, 1987; Kilbride, Hutman, Fishman & Read, 1986; van Norren & van Meel, 1985)). However, another study (Eisner, Fleming, Klein & Mauldin, 1987) found that the cone-mediated time constant for sensitivity recovery was unchanged between the 60s to the 80s, suggesting potential individual differences among older adults, possibly a leveling off of aging-related changes after the 70s, or/and methodological differences among studies (e.g., bleach magnitude).
Rod-mediated dark adaptation delays are also a hallmark of early AMD (Haimovici, Owens, Fitzke & Bird, 2002; Owsley, Jackson, White, Feist & Edwards, 2001a; Owsley et al., 2007; Steinmetz, Haimovici, Jubb, Fitzke & Bird, 1993). Histopathological studies on human donor retinas with AMD indicate a predilection for parafoveal loss of rod photoreceptors over cones in early nonexudative disease, with foveal cone loss not being observed until later in AMD progression (Curcio, Medeiros & Millican, 1996; Medeiros & Curcio, 2001). Although both rods and cones in the parafovea degenerate in AMD, rod loss precedes and is more severe than cone loss in most donor retinas evaluated, and even in the exudative form, there is greater retention of cones versus rods. These findings have lead to the question as to whether during the course of aging, accentuated slowing in the recovery during rod-mediated dark adaptation is a marker for those older adults at high risk for the eventual development of AMD (Jackson, Curcio, Sloan & Owsley, 2004; Jackson, Owsley & Curcio, 2002). Prospective data are needed to establish whether rod- delays pre-date the emergence of the disease. If so, this line of research not only sheds light on mechanisms of early AMD pathogenesis, but is also relevant for clinical research. For example, a psychophysical biomarker could be used to identify older adults at high-risk for early AMD and thus most appropriate for enrollment in clinical trials evaluating treatments for early AMD (Jackson & Edwards, 2008). In addition, a functional test could serve as an outcome measure for evaluating the impact of treatment interventions, particularly in early AMD where visual acuity remains good and thus is insensitive indicator of efficacy or proof of concept for early disease or preventative interventions (Csaky, Richman & Ferris III, 2008). Cone-mediated dark adaptation delays have also been reported for older adults with early AMD or for those at high-risk for early AMD because of fundus appearance or exudative disease in the fellow eye (Binns & Margrain, 2007; Dimitrov et al., 2008; Eisner, Klein, Zilis & Watkins, 1992; Sunness, Rubin, Applegate, Bressler, Marsh, Hawkins & Haselwood, 1997; Sunness, Rubin, Broman, Applegate, Bressler & Hawkins, 2008). Thus, prospective work to identify potential aging-related biomarkers for AMD should also address whether cone dysfunction in older adults pre-dates the emergence of AMD.
5. Processing of Time-Varying Targets
Many older adults have deficits in visually processing temporal information, which can hamper the visual performance of everyday tasks. For example, slowed visual processing speed in older adults increases their risk for motor vehicle collision involvement even in the absence of impaired visual acuity, contrast sensitivity, and visual field sensitivity (Cross, McGwin, Rubin, Ball, West, Roenker & Owsley, 2009; Owsley, Ball, McGwin, Sloane, Roenker, White & Overly, 1998; Rubin, Ng, Bandeen-Roche, Keyl, Freeman & West, 2007), increases the time it takes them to complete visual tasks of everyday living such as finding an item on a shelf or reading a prescription bottle (Ball, Berch, Helmers, Jobe, Leveck, Marsiske, Morris, Rebok, Smith, Tennstedt, Unverzagt, Willis & Group, 2002; Edwards, Wadley, Myers, Roenker, Cissell & Ball, 2002; Owsley, McGwin, Sloane, Stalvey & Wells, 2001b), and is associated with problems with ambulatory mobility (Owsley & McGwin, 2004). Older drivers with elevated thresholds in a coherent motion task had difficulties with detecting signs and hazards on the road, took longer to drive a route, and had worse performance evaluations as assessed by raters specialized in on-road assessment (Wood, 2002; Wood, Anstey, Kerr, Lacherez & Lord, 2008). Those older adults who reported more difficulty with certain driving maneuvers were also more likely to have impaired performance on speed discrimination, and estimates of the direction of heading and time to collision (Raghuram & Lakshminarayanan, 2006). In this section, we review what we have learned in the past 25 years about how aging impacts temporal sensitivity and motion perception, and in the next section, we address aging and slowed visual processing speed.
It has been known for over 50 years that critical flicker fusion (CFF) is decreased in older adults (Coppinger, 1955; McFarland, Warren & Karis, 1958; Misiak, 1947). But only in the past 25 years has research comprehensively explored older adults’ problems in processing time-varying targets. Temporal contrast sensitivity is impaired in older adults, more so at higher temporal frequencies than at lower. The deficit cannot be wholly accounted for by optical characteristics of the aged eye (Kim & Mayer, 1994; Mayer, Kim, Svingos & Glucs, 1988; Tyler, 1989; Wright & Drasdo, 1985). Measurements of the impulse response function (related to the temporal contrast sensitivity function) in older adults using a double-pulse method showed that the amplitude of both the excitatory and inhibitory responses is reduced in later adulthood (Shinomori & Werner, 2003). There were also individual differences; some older adults age ≥ 70 years old showed an inhibitory phase in the response amplitude while others did not. Although a retinal locus for these changes is likely to be contributory, post-retinal sites(s) may also play a role (Gerth, Sutter & Werner, 2003).
Ball and Sekuler (1986) were the first to demonstrate impaired motion perception in older adults that cannot be accounted for by spatial vision or cognitive differences between young and older observers. Specifically, they found that older adults were less able to discriminate between two directions of motion as depicted by random dot displays as compared to young adults. Since this report 25 years ago, many studies have probed motion-processing deficits in the elderly. For example, elevations in both detection and discrimination thresholds in a variety of motion tasks have been documented for older adults suggesting a reduced sensitivity to motion and impaired abilities to identify the direction of movement and differentiate differences in speed (Anderson & Atchley, 1995; Atchley & Anderson, 1998; Norman, Ross, Hawkes & Long, 2003; Raghuram, Lakshminarayanan & Khanna, 2005; Snowden & Kavanagh, 2006; Tran, Silverman, Zimmerman & Feldon, 1998; Trick & Silverman, 1991). There is preliminary evidence that these deficits do not appear gradually during the course of adulthood, but rather, may emerge in late adulthood around the 70s (Bennett, Sekuler & Sekuler, 2007). Furthermore, increasing the duration of the stimulus allows some older adults to improve their performance to the level of young adults (Bennett et al., 2007; Raghuram et al., 2005), which suggests that extending viewing time for older adults in a real-world performance task dependent on motion processing may at least partly compensate for deficits.
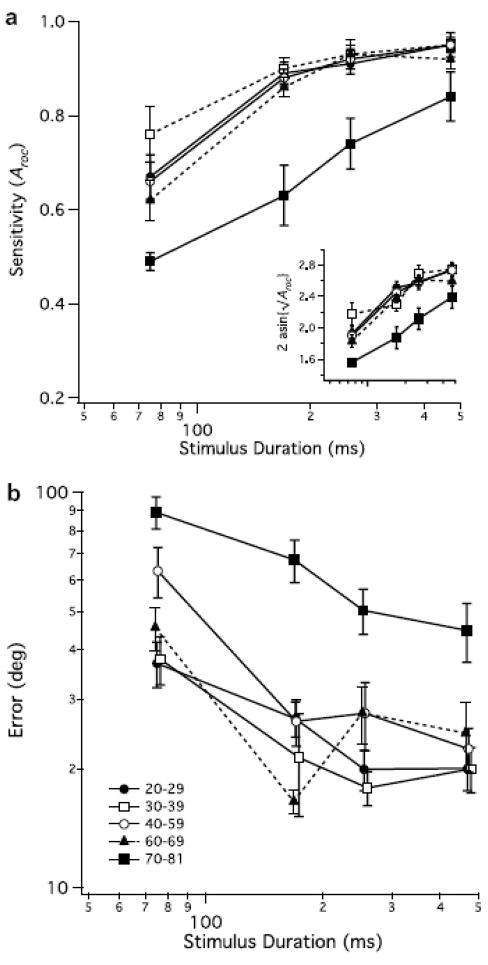
Bennett and colleagues (Bennett et al., 2007) found deficits in both detection sensitivity and direction discrimination for coherent global flow when measured in the same older adults (Figure 4). They found that direction judgments were more difficult for older adults than mere detection, persisted even after adjustment for aging-related motion sensitivity differences, and that there was larger divergence between a percept of global flow and perceiving the actual direction of flow among older adults than among young adults. A higher level of additive intrinsic noise and a wider bandwidth for directionally tuned cortical mechanisms was needed to model the older adult data as compared to modeling the young data. These findings in older adults are consistent with recent neurophysiological studies in old monkeys finding that, compared to young monkeys, neurons in the primary visual cortex of old monkeys had increased internal noise and reduced orientation and directional selectivity (Schmolesky, Wang, Pu & Leventhal, 2000; Wang et al., 2005). The cells’ direction and orientation selectivity could be improved by local administration of GABA and GABA agonists, with some cells improving to the level of cells from young monkeys (Leventhal, Wang, Pu, Zhou & Ma, 2003). These intriguing results not only provide evidence of a decreased inhibition in older visual cortex but that it is also reversible, at least in an animal model.
Figure 4.
From Bennett et al. (2007). Panel A shows sensitivity to motion for various age groups as a function of stimulus duration. The inset shows the data after an arcsine transformation. Note that adults ≥ 70 years old how impaired motion sensitivity. Panel B shows error magnitude in judging direction of motion (on trials for which motion was detected) for various age groups as a function of stimulus duration. Note that adults ≥ 70 years old exhibited larger direction judgment errors.
Psychophysical measurements of motion perception imply that there is indeed decreased visual cortical inhibition in older adults. Betts and colleagues (Betts, Taylor, Sekuler & Bennett, 2005) used a task previously developed (Tadin, Lappin, Gilroy & Blake, 2003) to study surround inhibition when processing a high-contrast moving pattern. Tadin et al. (2003) found that young adults needed longer stimulus durations to discriminate the direction of a moving high-contrast grating, as compared to the shorter durations required for the task when a low contrast grating was used. This effect was interpreted as evidence of center-surround spatial suppression whereby a neuron’s response to a high contrast stimulus is inhibited when the stimulus boundaries extends beyond its receptive field (Angelucci & Bullier, 2003; Sceniak, Ringach, Hawken & Shapley, 1999). When Betts et al. (2005) measured the performance of older adults in this task, they found that older adults needed briefer, not longer, durations to discrimination motion direction for large, high contrast gratings than did young adults. This pattern of findings suggests the existence of decreased visual cortical inhibition in the older adult cortex for motion-sensitive neurons. Following from Leventhal’s work in the senescent monkey model (Leventhal et al., 2003), the candidate mechanism for this decreased suppression could be reduced GABAergic functioning in the older brain. Although the Betts et al. study illustrates conditions where older adults could be characterized as having a superior motion perception ability, there also could be some adverse perceptual consequences to this phenomenon (Tadin & Blake, 2005). Older adults displaying this motion perception advantage may have difficulty in ignoring background motion since it is a large (often high-contrast) stimulus field, while at the same time having a weaker sensitivity to smaller, moving figures. This is a common stimulus condition while driving where the “figure” could be an obstacle (e.g., another car or pedestrian), and the “ground” is the rest of the roadway environment. This perceptual characteristic in older adults might contribute to driving problems faced by some older adults (e.g., turning left across oncoming traffic), an issue worthy of further investigation.
Several studies have found an aging-related deficit in the ability to use visual motion to perceive aspects of a 3-d world and spatial layout. For example, older adults have exhibited deficits in using motion to extract self-motion information (Warren, Blackwell & Morris, 1989), to perceive shape (Blake, Rizzo & McEvoy, 2008; Norman, Bartholomew & Burton, 2008; Norman, Clayton, Shular & Thompson, 2004; Norman, Dawson & Butler, 2000; Wist, Schrauf & Ehrenstein, 2000), and to identify collision paths (Andersen, Cisneros, Saidpour & Atchley, 2000; Anderson & Enriquez, 2006). Under certain stimulus conditions older adults also show deficits in the perception of biological motion (Billino, Bremmer & Gegenfurtner, 2008; Norman, Payton, Long & Hawkes, 2004), which may stem from difficulties in efficiently integrating local motion cues with global motion information (Pilz, Bennett & Sekuler, 2010). All or some of these deficits could be contributing to older adults’ difficulties in the performance of everyday tasks such as mobility, driving, visual search, and objective recognition (Ball et al., 2002; Edwards et al., 2002; Owsley & McGwin, 2004; Owsley et al., 2001b; Wood et al., 2008). However, research to date has largely ignored an examination of links between aging-related deficits in motion processing and perceiving and acting in a 3-d world, with a few noteworthy exceptions on driving and the visibility of pedestrians in the roadway environment (Raghuram & Lakshminarayanan, 2006; Tyrrell, Wood, Chaparro, Carberry, Chu & Marszalek, 2009; Wood, 2002; Wood et al., 2008; Wood, Tyrrell & Carberry, 2005).
6. Visual Processing Speed
Slowing in visual processing speed is a common characteristic of aging, and has been well established as a phenomenon since the 1970s (Kline & Birren, 1975; Walsh, Williams & Hertzog, 1979; Walsh, 1976). Many older adults require more time than younger adults to detect, discriminate, recognize, or identify visual targets, and this slowing contributes to higher-order processing problems characteristic of cognitive aging (e.g., associative learning, working memory, inhibition) (Salthouse, 1991; Salthouse, 1993; Salthouse, 1994; Salthouse & Meinz, 1995). These deficits occur even in older adults who do not have conditions that cause dementia (e.g., Alzheimer’s disease, cerebrovascular accident). Ball’s work has demonstrated that aging-related slowing in visual processing speed is exacerbated by increasing attentional task demands (e.g., divided attention tasks) and by increasing visual clutter (e.g., distracting stimuli) (Ball, Edwards & Ross, 2007; Ball, Roenker & Bruni, 1990). That is, in performing laboratory tasks, the display duration needed by many older adults to complete a task under dual task conditions with distracting stimuli is proportionately greater than what is needed by young adults. Much of the work in this field has made use of a task originally described by Ball, Sekuler, and others called the useful field of view task (Ball, Beard, Roenker, Miller & Griggs, 1988; Ball et al., 2007; Ball, Roenker, Wadley, Edwards, Roth, McGwin, Raleigh, Joyce, Cissell & Dube, 2006; Ball et al., 1990; Edwards, Ross, Wadley, Clay, Crowe, Roenker & Ball, 2006; Sekuler & Ball, 1986; Sekuler, Bennett, & Mamelak, 2000). The task has been refined over the years. The essential features of the task are that the observer is asked to identify a central target while simultaneously determining the location of a peripheral target, which on some trials is embedded in a field of distractors. Performance is measured by the minimum stimulus duration needed to perform the task (there is no motor response component).
It is important to emphasize that slowed visual processing speed during later adulthood is not inevitable in that there are wide individual differences, with some older adults exhibiting processing speeds like those of young adults, and others having serious slowing (Ball, Owsley, Sloane, Roenker & Bruni, 1993; Rubin et al., 2007). Unlike other types of visual psychophysical deficits discussed above, a great deal of research has already demonstrated that slowed processing speed in older adults has negative implications for their everyday life. Slowed visual processing in the elderly is associated with increased crash risk (Ball et al., 2006; Owsley et al., 1998; Rubin et al., 2007; Cross et al., 2009), increased fall risk (Sims, Owsley, Allman, Ball & Smoot, 1998; Staplin, Gish & Wagner, 2003), mobility problems such as transitioning from sitting to standing (Owsley & McGwin, 2004; Riolo, 2003), and increased time needed to complete visual tasks typical of everyday life (Owsley et al., 2001b; Owsley, Sloane, McGwin & Ball, 2002). What is particularly promising is that for some older adults, processing speed can be improved, i.e. “speeded up”, through practice (Ball et al., 1988; Ball et al., 2002; Edwards et al., 2002; Roenker, Cissell, Ball, Wadley & Edwards, 2003; Sekuler & Ball, 1986). This training intervention, described in detail elsewhere (Ball et al., 2007), basically consists of computer-based nonverbal exercises that are visually presented very briefly and involve practice in the detection, identification, discrimination, and localization of visual targets. Speed of processing training focuses on improving the speed of visual search and the ability to perform one or more attentional tasks quickly. During training, the stimulus duration of the visual display in each visual task (e.g., discrimination) is systematically reduced and the tasks are made increasingly more difficult (discrimination task in central vision alone versus discrimination and localization of a peripheral target).
The speed of processing gains by older adults have been shown in one study to be enduring up to five years (Willis, Tennstedt, Marsiske, Ball, Elias, Koepke, Morris, Rebok, Unverzagt, Stoddard, Wright & the ACTIVE Study Group, 2006). Most importantly, intervention evaluations including multi-site randomized trials have demonstrated that faster processing speed in older adults, or prevention of further slowing in processing speed as one ages, enhances several aspects of everyday functioning and health in older adults. Specifically, visual processing speed training led to more efficient completion of everyday visual tasks (less time needed) (Edwards et al., 2002; Edwards, Wadley, Vance, Roenker & Ball, 2005), reduced motor vehicle collision risk (Ball, Edwards, Ross & McGwin, in press), improved health-related quality of life (Wolinsky, Unverzagt, Smith, Jones, Wright & Tennstedt, 2006), reduced risk of clinical depression or depressive symptoms (Wolinsky, Mahncke, Weg, Martin, Unverzagt, Ball, Jones & Tennstedt, 2009; Wolinsky, Vander Weg, Martin, Unverzagt, Ball, Jones & Tennstedt, 2009), and improvements in self-rated health (Wolinsky, Mahncke, Vander Weg, Martin, Unverzagt, Ball, Jones & Tennstedt, 2010).
The modifiability of visually processing speed in the context of reading speed in older adults is relevant for understanding whether it is reasonable to expect that older adults with a central scotoma due to AMD can be trained to increase their reading speed in the retinal periphery. The term “visual span” with respect to reading refers to a spatial property of the visual field defined as the number of characters that can be recognized with no eye movement (Legge, Ahn, Klitz & Luebker, 1997; O”Regan, 1990). The visual span is smaller in the retinal periphery and has been shown to limit reading speed (Legge, Mansfield & Chung, 2001). Perceptual training can enlarge the visual span in young adults with normal peripheral vision which is accompanied by an increase in reading speed (Chung, Legge & Cheung, 2004). More recently, this research group (Yu, Cheung, Legge & Chung, 2010) has extended these findings to older adults with normal vision (Figure 5). The training benefits were smaller than those observed for young adults, which may be related to older adults’ reduced retention of training gains from session to session (Yu et al., 2010). In light of robust training gains seen in the visual processing speed training studies described above, future work should address whether larger reading speed benefits may be possible if a speed-of-processing training component were incorporated into a perceptual training program to improve reading speed in the periphery.
Figure 5.
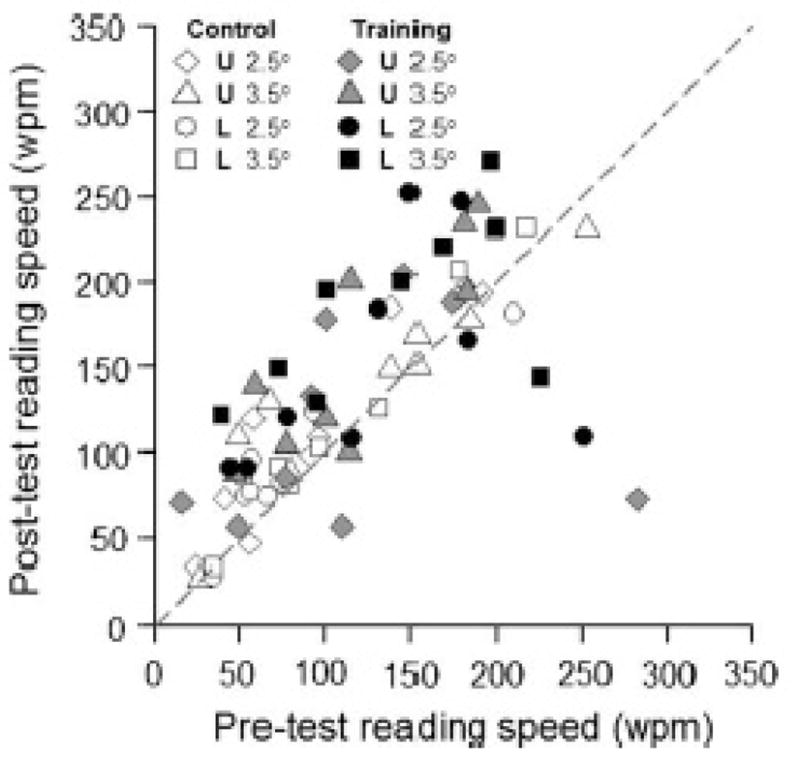
From Yu et al. (2010). Scatterplot showing relationship between pre-training reading speed and post-training reading speed for older adults with normal vision. The reading task was performed at 10° in the periphery. Those undergoing training are solid symbols, controls are open symbols. U and L stand for upper and lower visual field, respectively, for location of the test. 2.5° and 3.5° refer to print size. Note that most filled symbols from the training group are above the diagonal indicating improved reading speeds in older adults following training.
7. Other Intriguing Issues in Vision and Aging Research
Older adults’ threshold elevations for visually complex stimuli, i.e., second-order stimuli, are more accentuated than for simpler stimuli, i.e. first order stimuli (Faubert & Bellefeuille, 2002; Habak & Faubert, 2000; Habak, Wilkinson & Wilson, 2009; Tang & Zhou, 2009). In vision research, particularly in the study of motion perception, first- and second-order stimuli have been exploited to improve our understanding of early visual processing, with first-order stimuli defined by luminance modulation, and second-order by features such as contrast, texture, or depth. At least partly different mechanisms underlie the processing of first versus second-order visual stimuli (e.g., (Baker, 1999; Lu & Sperling, 2001)), and the computational models developed to account for their visual processing are also different (e.g., Chubb & Sperling, 1989; Wilson, Ferrar & Yo, 1992). Although there are varying reports in the literature, both types of stimuli appear to be processed within the same retinotopically-organized areas of cortex (e.g., Nishida, Sadaki, Murakami, Watanabe & Tootell, 2003), yet some areas may have cells that prefer second-order stimuli (e.g., Dumoulin, Baker, Hess & Evans, 2003). Findings of aging-related visual deficits that are more severe for second-order stimuli as compared to first-order stimuli have prompted the proposal (Faubert, 2002; Habak & Faubert, 2000) that older adults’ visual abilities using second-order stimuli are more vulnerable because larger, more complex, and simultaneously engaged neural networks are required; in essence, second-order stimulus processing is more vulnerable than first-order processing because the computational load is increased. Further research is needed to evaluate the testable implications of this hypothesis for visual tasks and behaviors in everyday life. More recently, Tang and Zhou (2009) have pointed out, based on cross-sectional data, that aging-related deficits for second-order stimuli emerge earlier during the course of adulthood than do deficits to first-order stimuli, although the time course of decline is slower for first-order stimuli. A question that arises from this body of work is what sorts of visual tasks or behaviors (e.g., object recognition, face recognition, reading, route finding, driving) would be hampered at what point during adulthood given these reported selective vulnerabilities in visual processing, and what tasks are immune to aging effects. Furthermore, it is possible that the identification of such tasks, their characteristics, and their neural loci could help uncover the mechanistic underpinnings of visual processing deficits in the older brain, or at least set the field on a course to uncover these mechanisms. For example, Habak, Wilkinson & Wilson (2008) showed that older adults have a processing speed similar to that of young adults when making same-view face discriminations, yet their processing speed is slowed when the task involves different views of the face. Their performance does not reach young adult levels even by increasing stimulus duration. The authors (Habak et al., 2008) point out that cortical activation during same-view face discrimination involves more extensive regions in older adults than young adults (Cabeza, Anderson, Locantore & McIntosh, 2002; Grady, 2002; Grady, McIntosh, Horwitz & Rapoport, 2000). They (Habak et al., 2008) argue, however, that the increase in cortical activation areas may not be sufficient for visually complex face tasks such as integrating face information across multiple facial views, or, alternatively, older adults’ broader cortical activation may commandeer networks that would normally have been used for more complex face processing.
Research has suggested that there is considerable neural adaptation in the aging visual system that supports the preservation, or maintenance, of certain aspects of visual perception during the adult life-course. As the discussion above implies, the field has largely been focused on how visual function is impaired as we grow older. But equally interesting is what aspects of visual processing and visual behavior are preserved and how does the brain “accomplish” this. Studying the instances of stability of visual function during aging could provide clues as to mechanisms of neural adaptation and plasticity in the central nervous system that mitigate the negative ramifications of aging-related structural and physiological changes in the brain. Examples of the preservation of function are thus quite interesting, and suggest that if the mechanisms supporting maintenance of function are identified, this potentially could inform the development of interventions to preserve visual functions that normally succumb to aging-associated degeneration. McIntosh and colleagues (McIntosh, Sekuler, Penpeci, Rahah, Grady, Sekuler & Bennett, 1999) showed that both young and older adults were equally adept at a visual short-term memory task requiring the discrimination of two sine wave gratings. Yet positron emission tomography (PET) suggested that the neural systems used by young versus old adults were different in some respects. Occipital, temporal and inferior cortices and caudate were involved regardless of age. But for older adults, the functional interconnections between these regions were much weaker and the older adults recruited areas not used by younger adults to perform the task, namely medial temporal and dorsolateral prefrontal cortices (see also Bennett, Sekuler, McIntosh & Della-Maggiore, 2001; Della-Maggiore, Sekuler, Grady & Bennett, 2000).
Along similar lines, there are a number of examples in the vision and aging literature where a key aspect of visual perception remains constant throughout adulthood even though related aspects of the visual function are impaired -- for example orientation tuning as estimated psychophysically, even though older adults require more contrast to discriminate the orientation of gratings (Delahunt, Hardy & Werner, 2008). Other examples include the renormalization of chromatic mechanisms after cataract surgery (Hardy, Frederick, Kay & Werner, 2005; Delahunt, Webster, & Ma, & Werner, 2004), perceived contrast at suprathreshold levels (Beard, Yager & Neufeld, 1994), and blur perception (Elliott, Hardy, Webster & Werner, 2007; Jung & Kline, 2010) (see also Enoch, Werner, Haegerstrom-Portnoy, Lakshminarayanan & Rynders, 1999; Werner, 1996 for a discussion of these issues). These phenomena highlight a potentially remarkable tendency of the aging nervous system, at least under some circumstances, to reorganize or adapt itself to preserve visual perception and the visual behaviors it supports; these examples also highlight the possibility that deteriorating functions not spontaneously re-calibrated by the aging brain could be modified by perceptual learning through training or practice regimens. As discussed earlier, visual processing speed has already been shown to be amenable to improvement in older adults through practice (Ball et al., 2007; Yu et al., 2010), and thus, there may be reason for optimism that other aspects of visual processing can also be enhanced in older adults.
8. Conclusion
The motivation for research on vision and aging should spring from significance. Research on vision and aging over the next 25 years would best serve science and society if motivated by a theoretical framework or a practical need. It is not enough to describe and catalog all the various ways that older adults compares or fails to compare to younger adult’s vision, and unfortunately there have been some instances of this approach to research in the past 25 years. Of course there is always the possibility of a serendipitous discovery when engaged in description, but it can be argued that the most exciting and efficient scientific efforts will be those that programmatically build a basic evidence-base that also has the potential to address public health priorities. Significance in aging and vision research can assume a few different forms, and when we plan future scientific endeavors on this topic, it is useful to ask ourselves the following questions. First, how does the research contribute to our understanding of the fundamental processes involved in visual perception? Not just about aging per se, but theories and models about how we see. Just as human development is part of a comprehensive understanding of a visual phenomenon, so is aging. Second, is the research directed at a fundamental visual process that is critical in performing a task or behavior important in everyday life? Here it is important to keep in mind that just because one can demonstrate an aging-related deficit or difference in some task in the psychophysics laboratory, this does not necessarily mean that this deficit is relevant to understanding older adults’ everyday visual performance or behavior difficulties. Whether a deficit negatively impacts everyday function and well-being is actually an empirical question in that everyday ramifications of the deficit must be established. Third, does an aging-associated deficit, particularly if it is accentuated in some individuals, serve as an advance “warning sign” of a degenerative condition of aging (e.g., AMD, glaucomatous optic neuropathy)? How could knowledge of this putative signal and its underlying causes be exploited to help understand the pathogenesis of the condition and ultimately to prevent or arrest the development of the disease? By this third point, we are not arguing that all aging and vision research has to have clinical significance. Yet researchers in aging and vision should always be mindful that degenerative conditions of the eye and brain are prevalent in later adulthood, and discoveries in aging and vision process can be relevant to these pathogenetic processes.
In general, there is an under-appreciation of individual differences in the aging of visual function. The most popular study design in the field to date involves the comparison of young versus old group differences. While there are undoubtedly “general principles” in the aging of visual function (some of which are discussed in this article), older adults are individuals with varying lifestyle, genetic, and environmental exposures during the life-course that can theoretically impact ocular and brain structure and function in later life in different ways. For this reason, there is little theoretical basis for assuming that a visual processing deficit demonstrated in a handful of older adults is in fact universal. Differences in genetic, environmental and lifestyle factors in older adults could increase or reduce the risk of certain types of aging-associated visual deficits, an issue that has received scant attention in previous research. By methodological necessity such studies typically require large sample sizes, yet some of the most exciting discoveries in vision and aging research from an etiologic perspective may be those that identify the characteristics of those who visually age well versus those who exhibit more serious visual losses that interfere with visual performance and quality of life. Once those markers, causes, and/or risks are identified, research can focus on using this information to develop interventions to prevent, reduce, or side-step their impact, the ultimate goal being to preserve a high-level of visual function into the advanced years of adulthood.
Acknowledgments
Preparation of this article was made possible through support from the National Institutes of Health (R01AG04212, P30AG22838, R01EY18966, R21EY16801), the EyeSight Foundation of Alabama, Research to Prevent Blindness, the Alberta J. Schueler Trust, and the Able Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration. AREDS Report No. 17. Archives of Ophthalmology. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Spear PD. Effects of aging on the size, density, and number of Rhesus monkey lateral geniculate neurons. Journal of Comparative Neurology. 1993;334:631–643. doi: 10.1002/cne.903340410. [DOI] [PubMed] [Google Scholar]
- Akutsu H, Legge GE, Ross JA, Schuebel KJ. Psychophysics of reading -X. Effects of aged related changes in vision. Journal of Gerontology: Psychological Sciences. 1991;46:325–331. doi: 10.1093/geronj/46.6.p325. [DOI] [PubMed] [Google Scholar]
- Alpern M. Rhodopsin kinetics in the human eye. Journal of Physiology. 1971;217:447–471. doi: 10.1113/jphysiol.1971.sp009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen GJ, Cisneros J, Saidpour A, Atchley P. Age-related differences in collision detection during deceleration. Psychology and Aging. 2000;15(2):241–252. doi: 10.1037//0882-7974.15.2.241. [DOI] [PubMed] [Google Scholar]
- Anderson GJ, Atchley P. Age-related differences in the detection of thre-dimensional surfaces from optic flow. Psychology and Aging. 1995;10:650–658. doi: 10.1037//0882-7974.10.4.650. [DOI] [PubMed] [Google Scholar]
- Anderson GJ, Enriquez A. Aging and the detection of observer and moving object collisions. Psychology and Aging. 2006;21:74–85. doi: 10.1037/0882-7974.21.1.74. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Bullier J. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons. Journal of Physiology (Paris) 2003;97:141–154. doi: 10.1016/j.jphysparis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Artal P, Ferro M, Miranda I, Navarro R. Effects of aging in retinal image quality. Journal of the Optical Society of America A. 1993;10(7):1656–1662. doi: 10.1364/josaa.10.001656. [DOI] [PubMed] [Google Scholar]
- Artal P, Guirao A, Berrio E, Piers P, Norrby S. Optical aberrations and the aging eye. International Ophthalmology Clinics. 2003;43:63–77. doi: 10.1097/00004397-200343020-00008. [DOI] [PubMed] [Google Scholar]
- Atchley P, Anderson GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychology and Aging. 1998;13(2):297–308. doi: 10.1037//0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Baker CL., Jr Central neural mechanisms for detecting second-order motion. Current Opinion in Neurobiology. 1999;9:461–466. doi: 10.1016/S0959-4388(99)80069-5. [DOI] [PubMed] [Google Scholar]
- Ball K, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: expanding the useful field of view. Journal of the Optical Society of America A. 1988;5:2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL for the ACTIVE Study Group. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Edwards J, Ross L. The impact of speed of processing training on cognitive and everyday functions. Journals of Gerontology: Psychological Sciences. 2007;62B:19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Investigative Ophthalmology & Visual Science. 1993;34(11):3110–3123. [PubMed] [Google Scholar]
- Ball K, Owsley C, Stalvey B, Roenker DL, Sloane M, Graves M. Driving avoidance and functional impairment in older drivers. Accident Analysis & Prevention. 1998;30:313–322. doi: 10.1016/s0001-4575(97)00102-4. [DOI] [PubMed] [Google Scholar]
- Ball K, Roenker D, Wadley V, Edwards J, Roth D, McGwin G, Raleigh R, Joyce J, Cissell G, Dube T. Can high-risk older drivers be identified through performance-based measures in a department of motor vehicles setting? Journal of the American Geriatric Society. 2006;54:77–84. doi: 10.1111/j.1532-5415.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Improving visual perception in older observers. Journal of Gerontology. 1986;41:176–182. doi: 10.1093/geronj/41.2.176. [DOI] [PubMed] [Google Scholar]
- Ball KK, Edwards JD, Ross LA, McGwin G. Cognitive training decreases risk for motor vehicle crash involvement among older drivers. Journal of the American Geriatrics Society. doi: 10.1111/j.1532-5415.2010.03138.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Roenker DL, Bruni JR. Developmental changes in attention and visual search throughout adulthood. In: Enns JT, editor. The Development of Attention: Research and Theory. North-Holland: Elsevier Science Publishers B. V.; 1990. pp. 489–507. [Google Scholar]
- Barlow HB. Dark and light adaptation: Psychophysics. In: Jameson D, Hurvich LM, editors. Handbook of Sensory Physiology: Visual Psychophysics. Vol. 7. New York: Springer-Verlag; 1972. pp. 1–55. [Google Scholar]
- Beard BL, Yager D, Neufeld S. Contrast detection and discrimination in young and older adults. Optometry and Vision Science. 1994;71(12):783–791. doi: 10.1097/00006324-199412000-00009. [DOI] [PubMed] [Google Scholar]
- Bennet PJ, Sekuler AB, McIntosh AR, Della-Maggiore V. The effects of aging on visual memory: evidence for functional reorganisatoin of cortical networks. Acta Psychologica. 2001;107:249–273. doi: 10.1016/s0001-6918(01)00037-3. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler R, Sekuler AB. The effects of aging on motion detection and direction identification. Vision Research. 2007;47:799–809. doi: 10.1016/j.visres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005;45:361–366. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Billino J, Bremmer F, Gegenfurtner K. Differential aging of motion processing mechanisms: Evidene against general perceptual decline. Vision Research. 2008;48:1254–1261. doi: 10.1016/j.visres.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Binns A, Margrain T. Evaluating retinal function in age-related maculopathy with the ERG photostress test. Investigative Ophthalmology & Visual Science. 2007;48(6):2806–2813. doi: 10.1167/iovs.06-0392. [DOI] [PubMed] [Google Scholar]
- Bird AC. Bruch’s membrane change with age. British Journal of Ophthalmology. 1992;763:166–168. doi: 10.1136/bjo.76.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren JE, Shock NW. Age changes in rate and level of visual dark adaptation. Journal of Applied Physiology. 1950;2:407–411. [Google Scholar]
- Blake R, Rizzo M, McEvoy S. Aging and perception of visual form from temporal structure. Psychology and Aging. 2008;23:181–189. doi: 10.1037/0882-7974.23.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Investigative Ophthalmology and Visual Science. 1985;26:1659–1694. [PubMed] [Google Scholar]
- Brabyn JA, Schneck ME, Lott LA, Haegerström-Portnoy G. Night driving self-restriction: vision function and gender differences. Optometry & Vision Science. 2005;82:755–764. doi: 10.1097/01.opx.0000174723.64798.2b. [DOI] [PubMed] [Google Scholar]
- Burton KB, Owsley C, Sloane ME. Aging and neural contrast sensitivity: Photopic vision. Vision Research. 1993;33:939–946. doi: 10.1016/0042-6989(93)90077-a. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G. Two motion perception mechanisms revealed through distance-driven reversal of apparent motion. Proceedings of the National Academy of Sciences, USA. 1989;86:2985–2989. doi: 10.1073/pnas.86.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Legge GE, Cheung S. Letter-recognition and reading speed in peripheral vision benefit from perceptual learning. Vision Research. 2004;44:695–709. doi: 10.1016/j.visres.2003.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chylack LT, Jr, Wolfe JK, Singer DM, Leske C, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY the Longitudinal Study of Cataract Group. The lens opacities classification system III. Archives of Ophthalmology. 1993;111:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- Clark CL, Hardy JL, Volbrecht VJ, Werner JS. Scotopic spatiotemporal sensitivity differences between young and old adults. Ophthalmic & Physiological Optics. 2010;30:339–350. doi: 10.1111/j.1475-1313.2010.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coile DC, Baker HD. Foveal dark adaptation, photopigment regeneration, and aging. Visual Neuroscience. 1992;8:27–39. doi: 10.1017/s0952523800006465. [DOI] [PubMed] [Google Scholar]
- Coppinger NW. The relationship between critical flicker frequency and chronological age for varying levels of stimulus brightness. Journal of Gerontology. 1955;10:48–52. doi: 10.1093/geronj/10.1.48. [DOI] [PubMed] [Google Scholar]
- Cross JM, McGwin G, Jr, Rubin GS, Ball KK, West SK, Roenker DL, Owsley C. Visual and medical risk factors for motor vehicle collision involvement among older drivers. British Journal of Ophthalmology. 2009;93:400–404. doi: 10.1136/bjo.2008.144584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaky KG, Richman EA, Ferris FL., III Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Investigative Ophthalmology & Visual Science. 2008;49:479–489. doi: 10.1167/iovs.07-1132. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Investigative Ophthalmology & Visual Science. 1996;37(7):1236–1249. [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investigative Ophthalmology & Visual Science. 1993;34(12):3278–3296. [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Bailey T, Kruth H. Accumulation of cholesterol with age in human Bruch’s membrane. Investigative Ophthalmology & Visual Science. 2001;42(1):265–274. [PubMed] [Google Scholar]
- Delahunt PB, Hardy JL, Werner JS. The effect of senescence on orientation discrimination and mechanism tuning. Journal of Vision. 2008;8:1–9. doi: 10.1167/8.3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahunt PB, Webster MA, Ma L, Werner JS. Long-term renormalizatoin of chromatic mechanisms following cataract surgery. Visual Neuroscience. 2004;21:301–307. doi: 10.1017/S0952523804213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Sekuler AB, Grady CL, Bennett PJ. Corticolimbic interactions associated with performance on a short-term memory task are modified by age. Journal of Neuroscience. 2000;20:8410–8416. doi: 10.1523/JNEUROSCI.20-22-08410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derefeldt G, Lennerstrand G, Lundh B. Age variations in normal human contrast sensitivity. Acta Ophthalmologica. 1979;57:679–690. doi: 10.1111/j.1755-3768.1979.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Dimitrov PN, Guymer RH, Zele AJ, Anderson AJ, Vingrys AJ. Measuring rod and cone dynamics in age-related maculopathy. Investigative Ophthalmology & Visual Science. 2008;49(1) doi: 10.1167/iovs.06-1048. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Wald G. Vitamin A deficiency and night blindness. Proceedings of the National Academy of Sciences, USA. 1958;44:648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler M, Rassow B. Neural contrast sensitivity measurements with a laser interference system for clinical and screening application. Investigative Ophthalmology & Visual Science. 1981;21:737–744. [PubMed] [Google Scholar]
- Drucker DN, Curcio CA. Retinal ganglion cells are lost with aging but not in Alzheimer’s disease. Investigative Ophthalmology & Visual Science. 1990;31:356. [Google Scholar]
- Dumoulin SO, Baker CLJ, Hess RF, Evans AC. Cerebral specialization for processing first- and second-order motion. Cerebral Cortex. 2003;13:1375–1385. doi: 10.1093/cercor/bhg085. [DOI] [PubMed] [Google Scholar]
- Edwards J, Ross L, Wadley V, Clay O, Crowe M, Roenker D, Ball K. The useful field of view test: normative data for older adults. Archives of Clinical Neuropsychology. 2006;21:275–286. doi: 10.1016/j.acn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Edwards J, Wadley V, Myers R, Roenker D, Cissell G, Ball K. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. 2002;48:329–340. doi: 10.1159/000065259. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging & Mental Health. 2005;9:262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Eisner A, Fleming SA, Klein ML, Mauldin M. Sensitivities in older eyes with good acuity: cross-sectional norms. Investigative Ophthalmology & Visual Science. 1987;28:1824–1831. [PubMed] [Google Scholar]
- Eisner A, Klein ML, Zilis JD, Watkins MD. Visual function and the subsequent development of exudative age-related macular degeneration. Investigative Ophthalmology and Visual Science. 1992;33(11):3091–3102. [PubMed] [Google Scholar]
- Elliott DB. Contrast sensitivity decline with aging: A neural or optical phenomenon? Ophthalmic & Physiological Optics. 1987;7:415–419. [PubMed] [Google Scholar]
- Elliott DB, Gilchrist J, Whitaker D. Contrast sensitivity and glare sensitivity changes with three types of cataract morphology: Are these techniques necessary in a clinical evaluation of cataract? Ophthalmic & Physiological Optics. 1989;9:25–30. doi: 10.1111/j.1475-1313.1989.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Elliott DB, Whitaker D, MacVeigh D. Neural contribution to spatiotemporal contrast sensitivity decline in healthy eyes. Vision Research. 1990;30:541–547. doi: 10.1016/0042-6989(90)90066-t. [DOI] [PubMed] [Google Scholar]
- Elliott SL, Choi SS, Doble N, Hardy JL, Evans JW, Werner JS. Role of high-order aberrations in senescent changes in spatial vision. Journal of Vision. 2009;9:1–16. doi: 10.1167/9.2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SL, Hardy JL, Webster MA, Werner JS. Aging and blur adaptation. Journal of Vision. 2007;7:1–9. doi: 10.1167/7.6.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SL, Werner JS. Age-related changes in contrast gain related to the M and P pathways. Journal of Vision. 2010;10:1–15. doi: 10.1167/10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch JM, Werner JS, Haegerstrom-Portnoy G, Lakshminarayanan V, Rynders M. Forever young: Visual functions not affected or minimally affected by aging: A review. Journal of Gerontology: Biological Sciences. 1999;54A(8):B336–B351. doi: 10.1093/gerona/54.8.b336. [DOI] [PubMed] [Google Scholar]
- Faubert J. Visual perception and aging. Canadian Journal of Experimental Psychology. 2002;56:164–176. doi: 10.1037/h0087394. [DOI] [PubMed] [Google Scholar]
- Faubert J, Bellefeuille A. Aging effects on intra- and inter-attribute spatial frequency information for luminance, color, and working memory. Vision Research. 2002;42:369–378. doi: 10.1016/s0042-6989(01)00292-9. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L, Ellersieck MR. Age-related changes in the ultrastructure of Bruch’s membrane. American Journal of Ophthalmology. 1985;100:686–697. doi: 10.1016/0002-9394(85)90625-7. [DOI] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Aging of the human retina: Differential loss of neurons and retina epithelial cells. Investigative Ophthalmology & Visual Science. 1992;33:1–17. [PubMed] [Google Scholar]
- Gao H, Rayborn ME, Myers KM, Hollyfield JG. Differential loss of neurons during aging of human retina. Investigative Ophthalmology & Visual Science. 1990;31:357. [PubMed] [Google Scholar]
- Gerth C, Sutter EE, Werner JS. mfERG response dynamics of the aging retina. Investigative Ophthalmology & Visual Science. 2003;44:4443–4450. doi: 10.1167/iovs.02-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser A, Campbell MCW. Presbyopia and the optical changes in the human crystalline lense with age. Vision Research. 1998;38:209–229. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- Grady CL. Age-related differences in face processing: A meta-analysis of three functional neuroimaging experiments. Canadian Journal of Experimental Psychology. 2002;56:208–220. doi: 10.1037/h0087398. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degraded and on-degraded face processing. Cognitive Neuropsychology. 2000;17:165–186. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Guirao A, Gonzalez C, Redondo M, Geraghty E, Norrby S, Artal P. Average optical performance of the human eye as a function of age in a normal population. Investigative Ophthalmology & Visual Science. 1999;40(1):203–213. [PubMed] [Google Scholar]
- Gunkel RD, Gouras P. Changes in scotopic visibility thresholds with age. Archives of Ophthalmology. 1963;69:38–43. doi: 10.1001/archopht.1963.00960040010003. [DOI] [PubMed] [Google Scholar]
- Habak C, Faubert J. Larger effect of aging on the perception of higher-order stimuli. Vision Research. 2000;40:943–950. doi: 10.1016/s0042-6989(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Habak C, Wilkinson F, Wilson HR. Aging disrupts the neural transformatoins that link facial identity across views. Vision Research. 2008;48:9–15. doi: 10.1016/j.visres.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habak C, Wilkinson F, Wilson HR. Preservation of shape discrimination in aging. Journal of Vision. 2009;9:1–8. doi: 10.1167/9.12.18. [DOI] [PubMed] [Google Scholar]
- Haimovici R, Owens SL, Fitzke FW, Bird AC. Dark adaptation in age-related macular degeneration: relationship to the fellow eye. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2002;240:90–95. doi: 10.1007/s00417-001-0417-z. [DOI] [PubMed] [Google Scholar]
- Hardy JL, Frederick CM, Kay P, Werner JS. Color naming, lens aging, and grue. Psychological Science. 2005;16(4):321–327. doi: 10.1111/j.0956-7976.2005.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S, Haig C, Chase AM. The influence of light adaptation on subsequent dark adaptation of the eye. Journal of General Physiology. 1937;20:831. doi: 10.1085/jgp.20.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins KE, Jaffe MJ, Caruso RC, deMonasterio FM. Spatial contrast sensitivity: effects of age, test-retest, and psychophysical method. Journal of the Optical Society of America. 1988;5(12):2173–2180. doi: 10.1364/josaa.5.002173. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Curcio CA, Sloan KR, Owsley C. Photoreceptor degeneration in aging and age-related maculopathy. In: Penfold PL, Provis JM, editors. Macular Degeneration: Science and Medicine in Practice. New York: Springer-Verlag Inc; 2004. pp. 45–62. [Google Scholar]
- Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. Journal of Ocular Biology, Diseases, and Informatics. 2008;1:7–11. doi: 10.1007/s12177-008-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, Owsley C. Scotopic sensitivity during adulthood. Vision Research. 2000;40:2467–2473. doi: 10.1016/s0042-6989(00)00108-5. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Owsley C, Cordle EP, Finley CD. Aging and scotopic sensitivity. Vision Research. 1998;38:3655–3662. doi: 10.1016/s0042-6989(98)00044-3. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Aging Research Reviews. 2002;1:381–386. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Owsley C, McGwin G., Jr Aging and dark adaptation. Vision Research. 1999;39:3975–3982. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- Jung GH, Kline DW. Resolution of blur in the older eye: Neural compensation in addition to optics? Journal of Vision. 2010;10:1–9. doi: 10.1167/10.5.7. [DOI] [PubMed] [Google Scholar]
- Källstrand-Ericson J, Hidingh C. Visual impairment and falls: a register study. Journal of Clinical Nursing. 2009;18:366–372. doi: 10.1111/j.1365-2702.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- Kayazawa F, Yamamoto T, Itoi M. Clinical measurement of contrast sensitivity function using laser generated sinusoidal grating. Japanese Journal of Ophthalmology. 1981;25:229–236. [Google Scholar]
- Kemp CM, Jacobson SG, Faulkner DJ. The effects of vitamin A deficiency on human visual function. Experimental Eye Research. 1988;46:185–197. doi: 10.1016/s0014-4835(88)80076-9. [DOI] [PubMed] [Google Scholar]
- Keunen JEE, Norren DV, Meel GJV. Density of foveal cone pigments at older age. Investigative Ophthalmology & Visual Science. 1987;28:985–991. [PubMed] [Google Scholar]
- Kilbride PE, Hutman LP, Fishman M, Read JS. Foveal cone pigment density differences in the aging human eye. Vision Research. 1986;26:321–325. doi: 10.1016/0042-6989(86)90029-5. [DOI] [PubMed] [Google Scholar]
- Kim CBY, Mayer MJ. Foveal flicker sensitivity in healthy aging eyes. II. Cross-sectional aging trends from 18 through 77 years of age. Journal of the Optical Society of America A. 1994;11(7):1958–1969. doi: 10.1364/josaa.11.001958. [DOI] [PubMed] [Google Scholar]
- Kleiner RC, Enger C, Alexander MF, Fine SL. Contrast sensitivity in age-related macular degeneration. Archives of Ophthalmology. 1988;106:55–57. doi: 10.1001/archopht.1988.01060130061028. [DOI] [PubMed] [Google Scholar]
- Kline DW. Ageing and the spatiotemporal discrimination performance of the visual system. Eye. 1987;1:323–329. doi: 10.1038/eye.1987.52. [DOI] [PubMed] [Google Scholar]
- Kline DW, Birren JE. Age differences in backwards dichoptic masking. Experimental Aging Research. 1975;1:17–25. doi: 10.1080/03610737508257943. [DOI] [PubMed] [Google Scholar]
- Kline DW, Schieber F. Vision and aging. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. New York: Van Nostrand Reinhold Co; 1985. pp. 296–331. [Google Scholar]
- Kline DW, Schieber F, Abusamra LC, Coyne AC. Age, the eye, and visual channels: Contrast sensitivity and response speed. Journal of Gerontology. 1983;38:211–216. doi: 10.1093/geronj/38.2.211. [DOI] [PubMed] [Google Scholar]
- Kornzweig AL. Aging of the retinal pigment epithelium. In: Marmor MF, Zinn KM, editors. The Retinal Pigment Epithelium. London: Harvard University Press; 1979. pp. 478–495. [Google Scholar]
- Kosnik W, Winslow L, Kline D, Rasinski K, Sekuler R. Visual changes in daily life throughout adulthood. Journal of Gerontology: Psychological Sciences. 1988;43(3):63–70. doi: 10.1093/geronj/43.3.p63. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Progress in Retinal and Eye Research. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Legge GE, Ahn SJ, Klitz TS, Luebker A. Psychophysics of reading-XVI. The visual span in normal and low vision. Vision Research. 1997;37(14):1999–2010. doi: 10.1016/s0042-6989(97)00017-5. [DOI] [PubMed] [Google Scholar]
- Legge GE, Mansfield JS, Chung STL. Psychophysics of reading: XX. Linking letter recognition to reading speed in central and peripheral vision. Vision Research. 2001;41:725–734. doi: 10.1016/s0042-6989(00)00295-9. [DOI] [PubMed] [Google Scholar]
- Leibrock CS, Reuter T, Lamb TD. Molecular basis of dark adaptation in rod photoreceptors. Eye. 1998;12:511–520. doi: 10.1038/eye.1998.139. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang U, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Liem ATA, Keunen JEE, van Norren D, van de Kraats J. Rod densitometry in the aging human eye. Investigative Ophthalmology and Visual Science. 1991;32(10):1676–2682. [PubMed] [Google Scholar]
- Loewenfeld IE. Pupillary changes related to age. In: Thompson HS, editor. Topics in Neuro-Ophthalmolgy. Baltimore: Williams & Wilkins; 1979. pp. 124–150. [Google Scholar]
- Lu ZL, Sperling G. Three-systems theory of human visual motion perception: Review and update. Journal of the Optical Society of America A. 2001;18:2331–2370. doi: 10.1364/josaa.18.002331. [DOI] [PubMed] [Google Scholar]
- Mangione CM, Berry S, Spritzer K, Janz NK, Klein R, Owsley C, Lee PP. Identifying the content area for the 51-item National Eye Institute visual function questionnaire (NEIVFQ-51) Archives of Ophthalmology. 1998;116:227–233. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- Massie DL, Campbell KL, Williams AF. Traffic accident involvement rates by driver age and gender. Accident Analysis and Prevention. 1990;27:73–87. doi: 10.1016/0001-4575(94)00050-v. [DOI] [PubMed] [Google Scholar]
- Mayer MJ, Kim CBY, Svingos A, Glucs A. Foveal flicker sensitivity in healthy aging eyes. I. Compensating for pupil variation. Journal of the Optical Society of America. 1988;5:2201–2209. doi: 10.1364/josaa.5.002201. [DOI] [PubMed] [Google Scholar]
- McFarland RA, Domey RG, Warren AB, Ward DC. Dark adaptation as a function of age: I. A statistical analysis. Journal of Gerontology. 1960;15:149–154. doi: 10.1093/geronj/15.3.267. [DOI] [PubMed] [Google Scholar]
- McFarland RA, Warren B, Karis C. Alterations in flicker frequency as a function of age and light: dark ratio. Journal of Experimental Psychology. 1958;56:529–538. doi: 10.1037/h0049128. [DOI] [PubMed] [Google Scholar]
- McGwin G, Khoury R, Cross JM, Owsley C. Vision impairment and eye care utilization among Americans 50 and older. Current Eye Research. 2010;35:451–458. doi: 10.3109/02713681003664931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Sekuler AB, Penpeci C, Rahah MN, Grady CL, Sekuler R, Bennett PJ. Recruitment of unique neural systems to support visual memory in normal aging. Current Biology. 1999;9:1275–1278. doi: 10.1016/s0960-9822(99)80512-0. [DOI] [PubMed] [Google Scholar]
- McMurdo MET. Dark adaptation and falls in the elderly. Gerontology. 1991;37:221–224. doi: 10.1159/000213264. [DOI] [PubMed] [Google Scholar]
- Medeiros NE, Curcio CA. Preservation of ganglion cell layer neurons in age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2001;42:795–803. [PubMed] [Google Scholar]
- Misiak H. Age and sex differences in critical flicker frequency. Journal of Experimental Psychology. 1947;37:318–332. doi: 10.1037/h0061531. [DOI] [PubMed] [Google Scholar]
- Mortimer RG, Fell JC. Older drivers: their night fatal crash involvement and risk. Accident Analysis and Prevention. 1989;21:273–282. doi: 10.1016/0001-4575(89)90018-3. [DOI] [PubMed] [Google Scholar]
- Newsome DA, Huh W, Green WR. Bruch’s membrane age-related changes vary by region. Current Eye Research. 1987;6(10):1211–1221. doi: 10.3109/02713688709025231. [DOI] [PubMed] [Google Scholar]
- Nishida S, Sadaki Y, Murakami I, Watanabe T, Tootell RB. Neuroimaging of directional-selective mechanisms for second-order motion. Journal of Neurophysiology. 2003;90:3242–3254. doi: 10.1152/jn.00693.2003. [DOI] [PubMed] [Google Scholar]
- Norman JF, Bartholomew AN, Burton CL. Aging preserves the ability to perceive 3d object shape from static but no deforming boundary contours. Acta Psychologica (Amsterdam) 2008;129:198–207. doi: 10.1016/j.actpsy.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Norman JF, Clayton AM, Shular CF, Thompson SR. Aging and the perception of depth and 3-D shape from motion parallax. Psychology and Aging. 2004;19:506–514. doi: 10.1037/0882-7974.19.3.506. [DOI] [PubMed] [Google Scholar]
- Norman JF, Dawson TE, Butler AK. The effects of age upon the perception of depth and 3-d shape from differential motion and binocular disparity. Perception. 2000;29:1335–1359. doi: 10.1068/p3111. [DOI] [PubMed] [Google Scholar]
- Norman JF, Payton SM, Long JR, Hawkes LM. Aging and the perception of biological motion. Psychology and Aging. 2004;19:219–225. doi: 10.1037/0882-7974.19.1.219. [DOI] [PubMed] [Google Scholar]
- Norman JF, Ross HE, Hawkes LM, Long JR. Aging and the perception of speed. Perception. 2003;32:85–96. doi: 10.1068/p3478. [DOI] [PubMed] [Google Scholar]
- O’Regan JK. Eye movements and reading. In: Kowler E, editor. Eye movements and their role in visual and cognitive processes. New York: Elsevier; 1990. pp. 395–454. [Google Scholar]
- Ordy JM, Wengenack TM, Dunlap WP. Visual acuity, aging, and environmental interactions: A neuroscience perspective. In: Armstrong DA, editor. The Effects of Aging and Environment on Vision. New York: Plenum Press; 1991. pp. 1–12. [Google Scholar]
- Owsley C, Ball K, McGwin G, Jr, Sloane ME, Roenker DL, White MF, Overly ET. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Owsley C, Jackson GR, White MF, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001a;108:1196–1202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G, Jackson G, Kallies K, Clark M. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007;114(9):1728–1735. doi: 10.1016/j.ophtha.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G, Jackson GR, Heimburger DC, Piyathilake CJ, Klein R, White MF, Kallies K. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Investigative Ophthalmology & Visual Science. 2006a;47:1310–1318. doi: 10.1167/iovs.05-1292. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G., Jr Association between visual attention and mobility in older adults. Journal of the American Geriatric Society. 2004;52(11):1901–1906. doi: 10.1111/j.1532-5415.2004.52516.x. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G, Jr, Sloane M, Stalvey BT, Wells J. Timed instrumental activities of daily living tasks: Relationship to visual function in older adults. Optometry and Vision Science. 2001b;78(5):350–359. doi: 10.1097/00006324-200105000-00019. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G, Jr, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Investigative Ophthalmology & Visual Science. 2006b;47:528–535. doi: 10.1167/iovs.05-1222. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Boldt C. Aging and low contrast vision: Face perception. Investigative Ophthalmology & Visual Science. 1981;21(2):362–365. [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Research. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane M, McGwin G, Jr, Ball K. Timed instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane ME. Vision and Aging. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 4. Amsterdam: Elsevier; 1990. pp. 229–249. [Google Scholar]
- Pauleikhoff D, Harper C, Marshall J, Bird A. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology. 1990;97:171–178. [PubMed] [Google Scholar]
- Pilz KS, Bennett PJ, Sekuler AB. Effects of aging on biological motion discrimination. Vision Research. 2010;50:211–219. doi: 10.1016/j.visres.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Plantner JJ, Barbour HL, Kean EL. The rhodopsin content of the human eye. Current Eye Research. 1988;7(11):1125–1129. doi: 10.3109/02713688809001883. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Smith VC, Lutze M. Aging of the human lens. Applied Optics. 1987;26:1437–1440. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- Raghuram A, Lakshminarayanan V. Motion perception tasks as potential correlates to driving difficulty in the elderly. Journal of Modern Optics. 2006;53:1343–1362. [Google Scholar]
- Raghuram A, Lakshminarayanan V, Khanna R. Psychophysical estimation of speed discrimination. II. Aging effects. Journal of the Optical Society of America A. 2005;22:2269–2280. doi: 10.1364/josaa.22.002269. [DOI] [PubMed] [Google Scholar]
- Riolo L. Attention contributes to functional reach test scores in older adults with history of falling. Physical and Occupational Therapy in Geriatrics. 2003;22:15–29. [Google Scholar]
- Robertson GW, Yudkin J. Effect of age upon dark adaptation. Journal of Physiology. 1944;103:1–8. doi: 10.1113/jphysiol.1944.sp004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenker DL, Cissell GM, Ball KK, Wadley VG, Edwards JG. Speed-of-processing and driving simulator training result in improved driving performance. Human Factors. 2003;45:218–233. doi: 10.1518/hfes.45.2.218.27241. [DOI] [PubMed] [Google Scholar]
- Rubin GS, Adamsons IA, Stark WJ. Comparison of acuity, contrast sensitivity, and disability glare before and after cataract surgery. Archives of Ophthalmology. 1993;111:56–61. doi: 10.1001/archopht.1993.01090010060027. [DOI] [PubMed] [Google Scholar]
- Rubin GS, Ng ES, Bandeen-Roche K, Keyl PM, Freeman EE, West SK. A prospective, population-based study of the role of visual impairment in motor vehicle crashes among older drivers: the SEE study. Investigative Ophthalmology & Visual Science. 2007;48:1483–1491. doi: 10.1167/iovs.06-0474. [DOI] [PubMed] [Google Scholar]
- Rushton WAH, Powell DS. The rhodopsin content and the visual threshold of human rods. Vision Research. 1972;12:1073–1081. doi: 10.1016/0042-6989(72)90098-3. [DOI] [PubMed] [Google Scholar]
- Said FS, Weale RA. The variation with age of the spectral transmissivity of the living human crystalline lens. Gerontologia. 1959;3:213–231. doi: 10.1159/000210900. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mediation of adult age differences in cognition by reductions in working memory and speed of processing. Psychological Science. 1991;2(3):179–183. [Google Scholar]
- Salthouse TA. Speed mediation of adult age differences in cognition. Developmental Psychology. 1993;29(4):722–738. [Google Scholar]
- Salthouse TA. Aging associations: Influence of speed on adult age differences in associative learning. Journal of Experimental Psychology: Learning Memory, and Cognition. 1994;20(6):1486–1503. doi: 10.1037//0278-7393.20.6.1486. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ. Aging, inhibition, working memory, and speed. Journal of Gerontology: Psychological Sciences. 1995;50B(6):297–306. doi: 10.1093/geronb/50b.6.p297. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast’s effect on spatial summation by macaque V1 neurons. Nature Neuroscience. 1999;2:733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- Schefrin B, Hauser M, Werner J. Evidence against age-related enlargements of ganglion cell receptive field centers under scotopic conditions. Vision Research. 2004;44:423–428. doi: 10.1016/j.visres.2003.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefrin BE, Bieber ML, McLean R, Werner JS. The area of complete scotopic spatial summation enlarges with age. Journal of the Optical Society of America A. 1998;15(2):340–348. doi: 10.1364/josaa.15.000340. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Tregear SJ, Harvey LO, Jr, Werner JS. Senescent changes in scotopic contrast sensitivity. Vision Research. 1999;39:3728–3736. doi: 10.1016/s0042-6989(99)00072-3. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature Neuroscience. 2000;3:384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109(7):1235–1242. doi: 10.1016/s0161-6420(02)01060-6. [DOI] [PubMed] [Google Scholar]
- Sekuler AB, Bennett PJ, Mamelak M. Effects of aging on the useful field of view. Experimental Aging Research. 2000;26:103–120. doi: 10.1080/036107300243588. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Ball K. Visual localization: Age and practice. Journal of the Optical Society of America A. 1986;3(6):864–867. doi: 10.1364/josaa.3.000864. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Kline D, Dismukes K. Aging and human visual function. New York: Alan R. Liss, Inc; 1982. [Google Scholar]
- Sekuler R, Sekuler AB. Vision and aging. In: Kazdin AE, editor. Encyclopedia of Psychology. Washington DC: American Psychological Association; 2000a. pp. 180–183. [Google Scholar]
- Sekuler R, Sekuler AB. Visual perception and cognition. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, editors. Oxford Textbook of Geriatric Medicine. 2. Oxford: Oxford University Press; 2000b. [Google Scholar]
- Shinomori K, Werner JS. Senescence of the temporal impulse response to a luminous pulse. Vision Research. 2003;43:617–627. doi: 10.1016/S0042-6989(03)00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RV, Owsley C, Allman RM, Ball K, Smoot TM. A preliminary assessment of the medical and functional factors associated with vehicle crashes in older adults. Journal of the American Geriatrics Society. 1998;46:556–561. doi: 10.1111/j.1532-5415.1998.tb01070.x. [DOI] [PubMed] [Google Scholar]
- Sloane ME, Owsley C, Alvarez SL. Aging, senile miosis and spatial contrast sensitivity at low luminance. Vision Research. 1988a;28:1235–1246. doi: 10.1016/0042-6989(88)90039-9. [DOI] [PubMed] [Google Scholar]
- Sloane ME, Owsley C, Jackson CA. Aging and luminance-adaptation effects in spatial contrast sensitivity. Journal of the Optical Society of America A. 1988b;5:2181–2190. doi: 10.1364/josaa.5.002181. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Kavanagh E. Motion perception in the ageing visual system: minimum motion, motion coherence, and speed discrimination thresholds. Perception. 2006;35:9–24. doi: 10.1068/p5399. [DOI] [PubMed] [Google Scholar]
- Spear PD. Minireview: Neural bases of visual deficits during aging. Vision Research. 1993;33(18):2589–2609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- Staplin LK, Gish KW, Wagner EK. MaryPODS revisted: Updated crash analysis and implications for screening program implementation. Journal of Safety Research. 2003;34:389–397. doi: 10.1016/j.jsr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Starita C, Hussain AA, Pagliarini S, Marshall J. Hydrodynamics of aging Burch’s membrane: implications for macular disease. Experimental Eye Research. 1996;62:565–572. doi: 10.1006/exer.1996.0066. [DOI] [PubMed] [Google Scholar]
- Steinmetz RL, Haimovici R, Jubb C, Fitzke FW, Bird AC. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch’s membrane change. British Journal of Ophthalmology. 1993;77:549–554. doi: 10.1136/bjo.77.9.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven DM. Relation between dark adaptation and age. Nature. 1946;157:376–377. doi: 10.1038/157376b0. [DOI] [PubMed] [Google Scholar]
- Sturr JF, Zhang L, Taub HA, Hannon DJ, Jackowski MM. Psychophysical evidence for losses in rod sensitivity in the aging visual system. Vision Research. 1997;37:475–481. doi: 10.1016/s0042-6989(96)00196-4. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Rubin GS, Applegate CA, Bressler NM, Marsh MJ, Hawkins BS, Haselwood D. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–1691. doi: 10.1016/s0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Visual dysfunction as a predictor of subsequent visual acuity loss resulting from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1480–1488. doi: 10.1016/j.ophtha.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadin D, Blake R. Motion perception getting better with age? Neuron. 2005;45:325–332. doi: 10.1016/j.neuron.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature. 2003;424:312–315. doi: 10.1038/nature01800. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhou Y. Age-related decline of contrast sensitivity for second order stimuli: Earlier onset, but slower progression, than for first-order stimuli. Journal of Vision. 2009;9:1–15. doi: 10.1167/9.7.18. [DOI] [PubMed] [Google Scholar]
- Tran DB, Silverman SE, Zimmerman K, Feldon SE. Age-related deterioriation of motion perception and detection. Graefes Archive for Clinical & Experimental Ophthalmology. 1998;236:269–273. doi: 10.1007/s004170050076. [DOI] [PubMed] [Google Scholar]
- Trick GL, Silverman SE. Visual sensitivity ot motion: age-related changes and deficits in senile dementia of the Alzheimer type. Neurology. 1991;41:1437–1440. doi: 10.1212/wnl.41.9.1437. [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U, Ver Hoeve JN, Terkla-McGrane C. Threshold and suprathreshold spatiotemporal response throughout adulthood. Journal of the Optical Society of America. 1988;A5:2191–2200. doi: 10.1364/josaa.5.002191. [DOI] [PubMed] [Google Scholar]
- Tyler CW. Two processes control variations in flicker sensitivity over the lifespan. Journal of the Optical Society of America A. 1989;6:481–490. doi: 10.1364/josaa.6.000481. [DOI] [PubMed] [Google Scholar]
- Tyrrell RA, Wood JM, Chaparro A, Carberry TP, Chu BS, Marszalek RP. Seeing pedestrians at night: Visual clutter does not mask biological motion. Accident Analysis & Prevention. 2009;41:506–512. doi: 10.1016/j.aap.2009.02.001. [DOI] [PubMed] [Google Scholar]
- van Kuijk FJGM, Lewis JW, Buck P, Parker KR, Kliger DS. Spectrophotometric quantitation of Rhodopsin in the human retina. Investigative Ophthalmology and Visual Science. 1991;32:1962–1967. [PubMed] [Google Scholar]
- van Norren D, van Meel GJ. Density of human cone photopigments as a function of age. Investigative Ophthalmology and Visual Science. 1985;26:1014–1016. [PubMed] [Google Scholar]
- Vinding T. Occurrence of drusen, pigmentary changes and exudative changes in the macula with reference to age-related macular degeneration: An epidemiological study of 1000 aged individuals. Acta Ophthalmologica. 1990;68:410–414. doi: 10.1111/j.1755-3768.1990.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Walsh D, Williams MV, Hertzog CK. Age-related differences in two stages of central perceptual processes: the effcts of short duration targets and criterion differences. Journal of Gerontology. 1979;34:234–241. doi: 10.1093/geronj/34.2.234. [DOI] [PubMed] [Google Scholar]
- Walsh DA. Age differences in central perceptual processing: a dichoptic backward masking investigation. Journal of Gerontology. 1976;31:181–188. doi: 10.1093/geronj/31.2.178. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Ma Y, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 in senescent monkeys. Cerebral Cortex. 2005;15:403–408. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Warren WHJ, Blackwell AW, Morris MW. Age differences in perceiving the direction of self-motion from optical flow. Journal of Gerontology: Psychological Sciences. 1989;44:147–153. doi: 10.1093/geronj/44.5.p147. [DOI] [PubMed] [Google Scholar]
- Weale RA. Retinal illumination and age. Transactions Illuminating Engineering Society. 1961;26(2):95–100. [Google Scholar]
- Weale RA. The aging eye. London: H K Lewis; 1963. [Google Scholar]
- Weale RA. A biography of the eye. London: H.K. Lewis; 1982a. [Google Scholar]
- Weale RA. Senile ocular changes, cell death, and vision. In: Sekuler R, Kline D, Dismukes K, editors. Aging and Human Visual Function. New York: Liss; 1982b. pp. 161–171. [Google Scholar]
- Weale RA. Aging and vision. Vision Research. 1986;26(9):1507–1512. doi: 10.1016/0042-6989(86)90170-7. [DOI] [PubMed] [Google Scholar]
- Werner JS. Progress in Retinal and Eye Research. Vol. 15. Great Britain: Elsevier Science Ltd; 1996. Visual problems of the retina during aging: Compensation mechanisms and colour constancy across the life span; pp. 621–645. [Google Scholar]
- Werner JS, Peterzell D, Scheetz AJ. Light, vision, and aging. Optometry and Vision Science. 1990;67:214–229. doi: 10.1097/00006324-199003000-00013. [DOI] [PubMed] [Google Scholar]
- Werner JS, Schefrin BE, Bradley A. Optics and vision of the aging eye. In: Bass M, Enoch JM, Lakshminarayanan V, editors. Handbook of Optics, Third Edition, Volume III, Vision and Vision Optics. New York: McGraw-Hill; 2010. pp. 14.11–14.38. [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E for the ACTIVE Study Group. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Ferrar VP, Yo C. A psychophysically motivated model for two dimensional motion perception. Visual Neuroscience. 1992;9:79–97. doi: 10.1017/s0952523800006386. [DOI] [PubMed] [Google Scholar]
- Wist ER, Schrauf M, Ehrenstein WH. Dynamic vision based on motion-contrast: changes with age in adults. Experimental Brain Research. 2000;134:295–300. doi: 10.1007/s002210000466. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke H, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, Jones RN, Tennstedt SL. Speed of processing training protects self-rated health in older adults: enduring effects observed in the multi-site ACTIVE randomized controlled trial. International Psychogeriatrics. 2010;22:470–478. doi: 10.1017/S1041610209991281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke H, Weg MW, Martin R, Unverzagt FW, Ball KK, Jones RN, Tennstedt SL. The ACTIVE cognitive training interventions and the onset of and recovery from suspected clinical depression. Journal of Gerontology: Psychological Sciences. 2009;64:577–585. doi: 10.1093/geronb/gbp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Wright E, Tennstedt SL. The effects of the ACTIVE cognitive training tiral on clinical relevant declines in health-related quality of life. Journal of Gerontology: Social Sciences. 2006;61:S281–S287. doi: 10.1093/geronb/61.5.s281. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, Jones RN, Tennstedt SL. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64A(4):468–472. doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JM. Age and visual impairment decrease driving performance as measured on a closed-road circuit. Human Factors. 2002;44:482–494. doi: 10.1518/0018720024497664. [DOI] [PubMed] [Google Scholar]
- Wood JM, Anstey KJ, Kerr GK, Lacherez PF, Lord S. A multidomain approach for predicting older driver safety under in-traffic conditions. Journal of the American Geriatrics Society. 2008;56:986–993. doi: 10.1111/j.1532-5415.2008.01709.x. [DOI] [PubMed] [Google Scholar]
- Wood JM, Tyrrell RA, Carberry TP. Limitations in drivers’ ability to recognize pedestrians at night. Human Factors. 2005;47:644–653. doi: 10.1518/001872005774859980. [DOI] [PubMed] [Google Scholar]
- Wright CE, Drasdo N. The influence of age on the spatial and temporal contrast sensitivity function. Documenta Ophthalmologica. 1985;59:385–395. doi: 10.1007/BF00159172. [DOI] [PubMed] [Google Scholar]
- Xu J, Pokorny J, Smith VC. Optical density of the human lens. Journal of the Optical Society of America A. 1997;14:953–960. doi: 10.1364/josaa.14.000953. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liang Z, Liang Z, Glasser A, Wang Y, Zhou Y, Leventhal AG. Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neuroscience. 2008;156:748–757. doi: 10.1016/j.neuroscience.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Yu D, Cheung SH, Legge GE, Chung STL. Reading speed in the peripheral visual field of older adults: Does it benefit from perceptual learning? Vision Research. 2010;50:860–869. doi: 10.1016/j.visres.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang X, Wang Y, Fu Y, Liang Z, Ma Y, Leventhal AG. Spatial and temporal sensitivity degradation of primary visual cortical cells in senescent rhesus monkeys. European Journal of Neuroscience. 2008;28:201–207. doi: 10.1111/j.1460-9568.2008.06300.x. [DOI] [PubMed] [Google Scholar]