Abstract
A cDNA encoding an endogenous inhibitor, termed calpastatin, for calcium-dependent cysteine protease (calpain, EC 3.4.22.17) was cloned by screening rabbit cDNA libraries with a synthetic oligodeoxynucleotide probe based on the partial amino acid sequence of the purified protein. The deduced amino acid sequence contains 718 amino acid residues (Mr, 76,964), and the mature protein corresponds to the deduced sequence from the 80th residue of the primary translation product (resultant Mr, 68,113). This deduced molecular weight is significantly lower than that determined by NaDodSO4/polyacrylamide gel electrophoresis, suggesting the possibility that the inhibitor is post-translationally modified. The sequence of the mature inhibitor contains four consecutive internal repeats approximately 140 amino acid residues long, each of which might be responsible for the inhibitory activity. Calpastatin is apparently different from a typical cysteine protease inhibitor (cystatin), suggesting that the mechanism of inhibition of calcium-dependent cysteine protease by the inhibitor might be different from that of other cysteine proteases by cystatin.
Full text
PDF


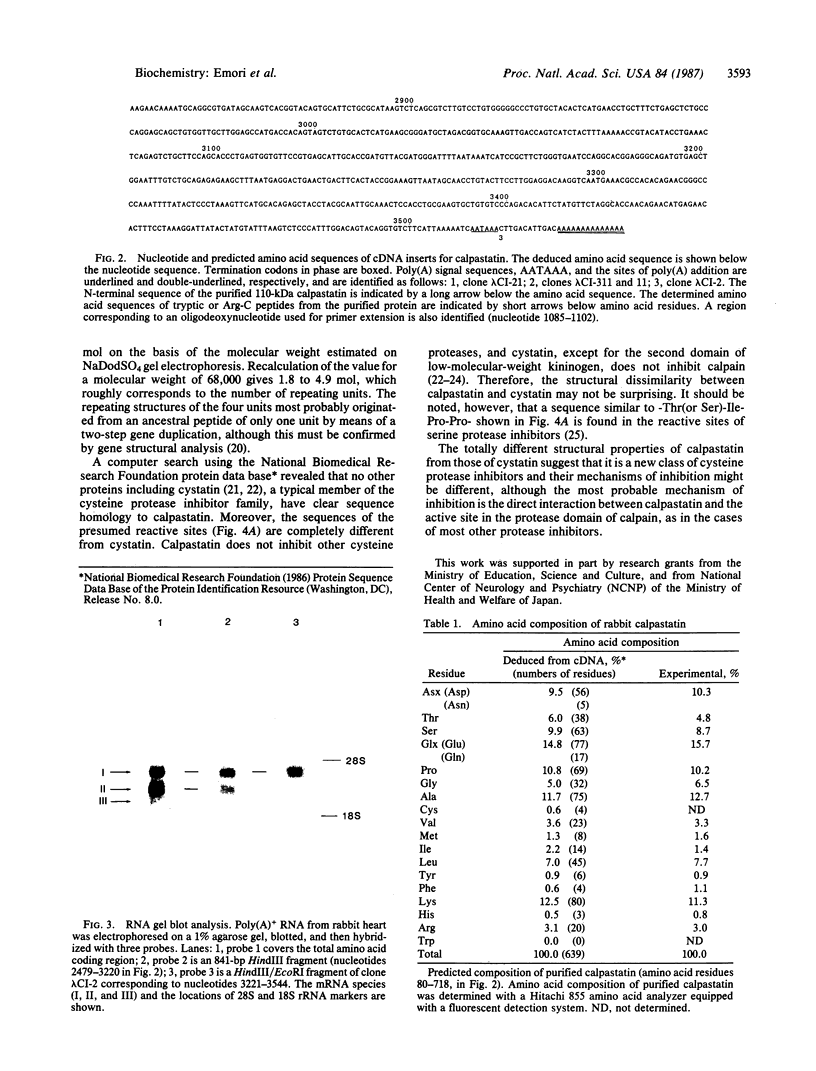
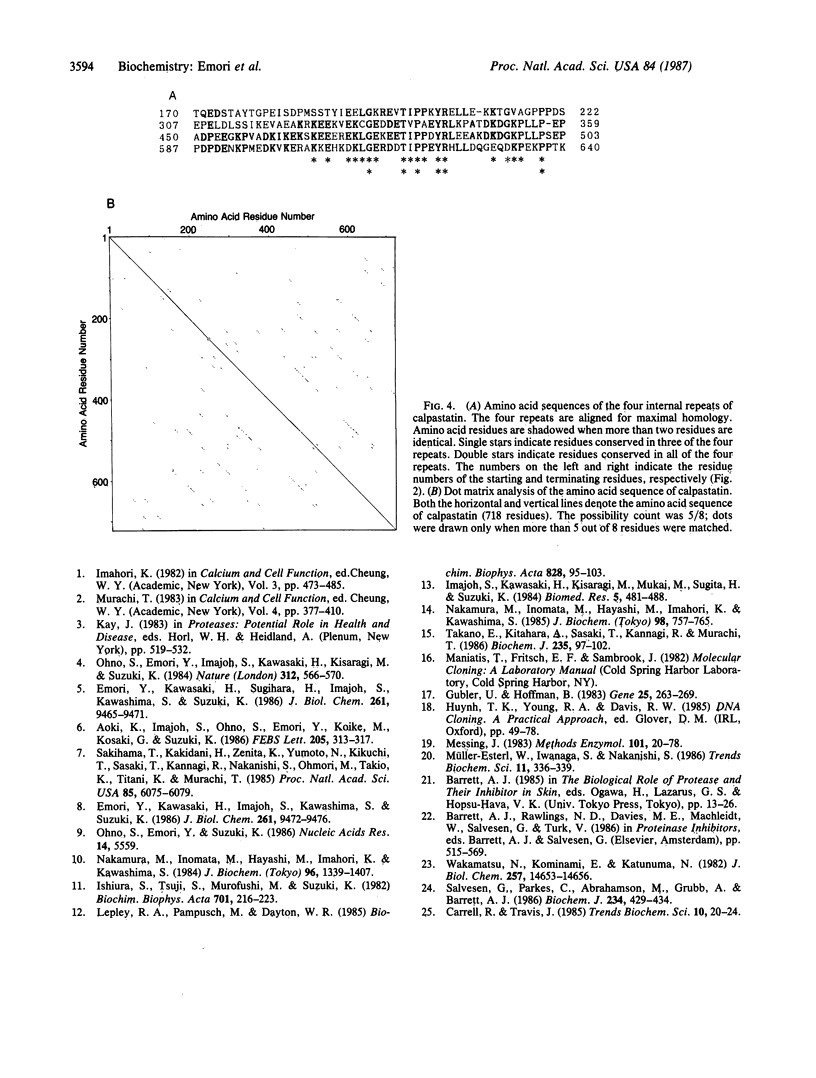
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki K., Imajoh S., Ohno S., Emori Y., Koike M., Kosaki G., Suzuki K. Complete amino acid sequence of the large subunit of the low-Ca2+-requiring form of human Ca2+-activated neutral protease (muCANP) deduced from its cDNA sequence. FEBS Lett. 1986 Sep 15;205(2):313–317. doi: 10.1016/0014-5793(86)80919-x. [DOI] [PubMed] [Google Scholar]
- Emori Y., Kawasaki H., Imajoh S., Kawashima S., Suzuki K. Isolation and sequence analysis of cDNA clones for the small subunit of rabbit calcium-dependent protease. J Biol Chem. 1986 Jul 15;261(20):9472–9476. [PubMed] [Google Scholar]
- Emori Y., Kawasaki H., Sugihara H., Imajoh S., Kawashima S., Suzuki K. Isolation and sequence analyses of cDNA clones for the large subunits of two isozymes of rabbit calcium-dependent protease. J Biol Chem. 1986 Jul 15;261(20):9465–9471. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
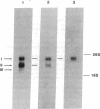
- Ishiura S., Tsuji S., Murofushi H., Suzuki K. Purification of an endogenous 68,000-dalton inhibitor of Ca2+-activated neutral protease from chicken skeletal muscle. Biochim Biophys Acta. 1982 Feb 18;701(2):216–223. doi: 10.1016/0167-4838(82)90116-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Inomata M., Hayashi M., Imahori K., Kawashima S. Purification and characterization of 210,000-dalton inhibitor of calcium-activated neutral protease from rabbit skeletal muscle and its relation to 50,000-dalton inhibitor. J Biochem. 1985 Sep;98(3):757–765. doi: 10.1093/oxfordjournals.jbchem.a135333. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Inomata M., Hayashi M., Imahori K., Kawashima S. Purification and characterization of an inhibitor of calcium-activated neutral protease from rabbit skeletal muscle: purification of 50,000-dalton inhibitor. J Biochem. 1984 Nov;96(5):1399–1407. doi: 10.1093/oxfordjournals.jbchem.a134968. [DOI] [PubMed] [Google Scholar]
- Ohno S., Emori Y., Imajoh S., Kawasaki H., Kisaragi M., Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984 Dec 6;312(5994):566–570. doi: 10.1038/312566a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Emori Y., Suzuki K. Nucleotide sequence of a cDNA coding for the small subunit of human calcium-dependent protease. Nucleic Acids Res. 1986 Jul 11;14(13):5559–5559. [PMC free article] [PubMed] [Google Scholar]
- Sakihama T., Kakidani H., Zenita K., Yumoto N., Kikuchi T., Sasaki T., Kannagi R., Nakanishi S., Ohmori M., Takio K. A putative Ca2+-binding protein: structure of the light subunit of porcine calpain elucidated by molecular cloning and protein sequence analysis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6075–6079. doi: 10.1073/pnas.82.18.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G., Parkes C., Abrahamson M., Grubb A., Barrett A. J. Human low-Mr kininogen contains three copies of a cystatin sequence that are divergent in structure and in inhibitory activity for cysteine proteinases. Biochem J. 1986 Mar 1;234(2):429–434. doi: 10.1042/bj2340429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano E., Kitahara A., Sasaki T., Kannagi R., Murachi T. Two different molecular species of pig calpastatin. Structural and functional relationship between 107 kDa and 68 kDa molecules. Biochem J. 1986 Apr 1;235(1):97–102. doi: 10.1042/bj2350097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu N., Kominami E., Katunuma N. Comparison of properties of thiol proteinase inhibitors from rat serum and liver. J Biol Chem. 1982 Dec 25;257(24):14653–14656. [PubMed] [Google Scholar]