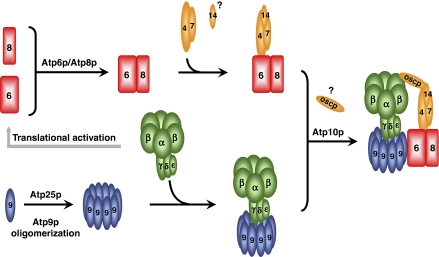
Modular assembly of yeast mitochondrial ATP synthase
The assembly of the mitochondrial F1–FO ATP synthase complex involves two separate assembly intermediates, an Atp6p/Atp8p complex and the Atp9p ring in complex with the F1 sector.
Keywords: ATPase, ATP synthase, biogenesis, mitochondria, Saccharomyces cerevisiae
Abstract
The mitochondrial ATP synthase (F1–F0 complex) of Saccharomces cerevisiae is a composite of different structural and functional units that jointly couple ATP synthesis and hydrolysis to proton transfer across the inner membrane. In organello, pulse labelling and pulse-chase experiments have enabled us to track the mitochondrially encoded Atp6p, Atp8p and Atp9p subunits of F0 and to identify different assembly intermediates into which they are assimilated. Surprisingly, these core subunits of F0 segregated into two different assembly intermediates one of which is composed of Atp6p, Atp8p, at least two stator subunits, and the Atp10p chaperone while the second consists of the F1 ATPase and Atp9p ring. These studies show that assembly of the ATP synthase is not a single linear process, as previously thought, but rather involves two separate but coordinately regulated pathways that converge at the end stage.
Introduction
The mitochondrial ATP synthase (F1–F0 complex) is composed of three distinct structural units, each with its own unique function. F1 is a globular protein with an α3β3γδɛ stoichiometry of its five subunits. It houses the adenine nucleotide binding sites responsible for catalysing ATP synthesis and hydrolysis (Boyer, 1997; Stock et al, 2000). F1 is attached to a set of membrane-embedded proteins comprising the F0 unit of the ATP synthase. In Saccharomyces cerevisiae, the three core subunits of F0: Atp6p, Atp8p and Atp9p are encoded by mitochondrial DNA (mtDNA). Atp9p is a low-molecular weight proteolipid that oligomerizes into a ring structure consisting of 10 copies of the monomer. The other two core subunits, Atp6p and Atp8p, are present in a single copy. The interface between Atp6p and Atp9p has been shown to form the channel through which protons are transferred across the inner membrane (Stock et al, 2000). The physical connection between F1 and F0 is enabled by two separate stalks. The central stalk consists of the γδɛ subunits of F1. The peripheral stalk or stator is composed of four subunits (Atp4p, Atp7p, Atp14p and OSCP) and is the third functional unit of the ATP synthase.
The F1 and F0 sectors of the ATP synthase have been proposed to have evolved from functionally unrelated ancestral proteins (Walker, 1998; Mulkidjanian et al, 2007). This is supported by earlier studies, indicating that F1 assembles independent of F0 (Schatz, 1968; Tzagoloff, 1969). Whether F0 can also assemble as an independent unit has been more difficult to unravel because of the high turnover rate of Atp6p and peripheral stalk subunits in mutants arrested in assembly of the F1–F0 complex (Paul et al, 1989; Helfenbein et al, 2003). To circumvent this problem, we have taken advantage of the relative stability of the core subunits of F0 when they are synthesized in isolated mitochondria and of their ability to assemble into larger complexes (Herrmann et al, 1994b; Tzagoloff et al, 2004; Jia et al, 2007).
In the present study, we have made use of a strain expressing tagged Atp6p to study the pathway by which mitochondria assemble the F0 unit. Unexpectedly, we found that Atp6p and Atp8p form an intermediate inclusive of stator subunits and of the Atp10p chaperone previously shown to promote the interaction of Atp6p with the Atp9p ring (Tzagoloff et al, 2004). This intermediate does not contain the Atp9p ring, which is assembled by another pathway culminating in a complex of the Atp9p ring and F1. Based on these and other data, we propose two separate but coordinately regulated pathways that converge to form the ATP synthase from their respective end products: a complex of Atp6p/Atp8p/stator and of the Atp9p ring with F1. This bifurcated process may have bearing on some of the events that occurred during evolution of this enzyme.
Results
Growth properties of strains expressing tagged Atp6p
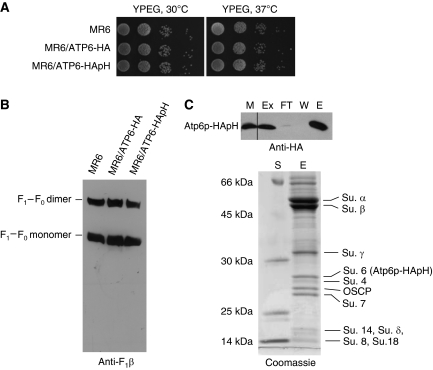
The mitochondrial ATP6 gene was modified so as to express the encoded Atp6p subunit of F0 either with a C-terminal HA tag for detection or with a double HA followed by polyhistidine tag for affinity purification. Transformants with a chromosomally integrated copy of either gene grew as well as wild type on non-fermentable carbon sources at 30 and 37°C (Figure 1A) and their abundance of ATP synthase monomers or dimers was similar to that of the parental strain (Figure 1B).
Figure 1.
Growth properties and characterization of ATP synthase in strains expressing tagged Atp6p. (A) MR6, the parental strain, MR6/ATP6-HA and MR6/ATP6-HApH, expressing Atp6p with a C-terminal HA and double HA and polyhistidine tags, respectively, were grown overnight in liquid YPD media. Serial dilutions were spotted on solid YPEG (rich glycerol/ethanol) and incubated at 30 or 37°C for 2–3 days. (B) Mitochondria from the same strains were grown to exponential phase in rich galactose. Mitochondria (250 μg protein) were extracted with digitonin and separated by BN-PAGE on a 3–13% polyacrylamide gel. Proteins were transferred to a PVDF membrane and probed with polyclonal antibody against the β subunit of F1 (Anti-F1β). The F1–F0 monomer and dimer are identified in the margin (C) Purification of the F1–F0 complex. Upper panel: MR6/ATP6-HApH mitochondria (0.5 mg protein) were extracted with 3% digitonin. The soluble fraction (Ex) obtained after clearing at 90 000 gav for 10 min, was incubated at 4°C for 2 h with 200 μl of Ni-NTA agarose beads. The equivalent of 50 μg of starting mitochondria (M), the fraction that did not adsorbed to Ni-NTA (FT), the 10-mM imidazole wash (W) and proteins eluted with 200 mM imidazole (E) were separated by SDS–PAGE on a 12% polyacrylamide gel, transferred to nitrocellulose and probed with a monoclonal antibody against the HA tag (Anti-HA). All the lanes shown are part of the same gel exposed for the same period of time. Lower panel: The remainder of the 200-mM imidazole eluate was precipitated with 5% trichloroacetic acid, separated on a 12% polyacrylamide gel and stained with Coomassie blue. The subunit polypeptides of the F1–F0-ATP synthase are identified in the right hand margin. Protein standards of known molecular weight were loaded on the lane labelled S.
The polyhistidine tag on Atp6p (Atp6p-HApH) made it possible to affinity purify the F1–F0 complex on Ni-NTA. Mitochondria containing the doubly tagged Atp6p were extracted with digitonin and the extract purified with Ni-NTA beads. Western analysis indicated that Atp6p-HApH in the extract was quantitatively adsorbed on Ni-NTA (Figure 1C, upper panel). The fraction eluted with 200 mM imidazole was highly enriched in subunits of the ATP synthase with only minor contaminating proteins in the high-molecular weight region of the gel (Figure 1C, upper panel).
Detection of newly translated Atp6p and Atp8p in partially and completely assembled ATP synthase
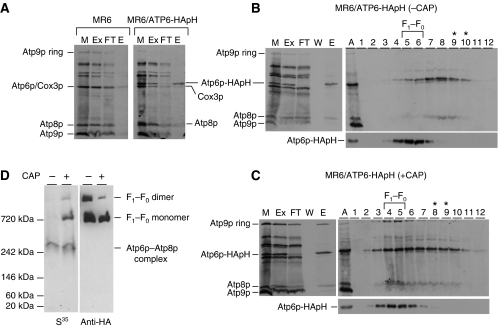
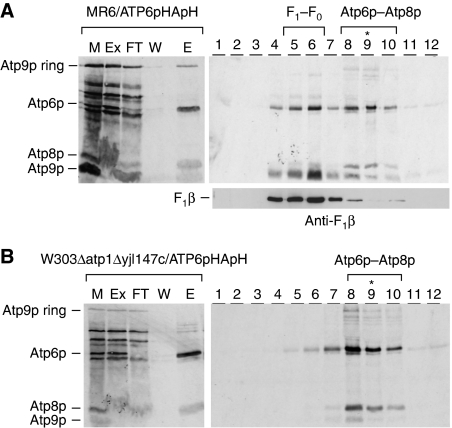
The respiratory competent yeast strain, MR6/ATP6-HApH, with the doubly tagged Atp6p was used to study assembly of the F1–F0 complex in isolated mitochondria. Mitochondria of MR6/ATP6-HApH were labelled with a [35S]methionine, extracted with digitonin and the extract purified with Ni-NTA beads. As a control, mitochondrial translation products were also labelled and fractionated from the parental strain expressing non-tagged Atp6p. A comparison of the starting digitonin extract (Ex), the fraction not adsorbed on the Ni-NTA beads (FT) and the 200-mM eluate (E), indicated that essentially all the Atp6p-HApH in the digitonin extract was recovered in the high imidazole eluate. This fraction was also enriched in Atp8p, although a significant fraction of this subunit was not adsorbed on Ni-NTA (Figure 2A). In contrast to Atp6p-HApH and Atp8p, Atp9p was almost completely absent in the imidazole eluate. The presence of only background levels of the three proteins in the imidazole eluate of mitochondria from control cells expressing untagged Atp6p (Figure 2A), indicated that Atp6p-HApH and Atp8p recovered from Ni-NTA are part of a common complex.
Figure 2.
Newly synthesized Atp6p and Atp8p form a complex. (A) Mitochondria were prepared from galactose grown culture of the wild-type strain MR6 and from MR6/ATP6-HApH expressing Atp6p tagged with HA and polyhistidine. The mitochondria (0.5 mg of protein) labelled for 20 min with a mixture of [35S]methionine and [35S]cysteine were extracted with 3% digitonin. The extract was purified on Ni-NTA agarose as described in Materials and methods section and the different fractions were separated by SDS–PAGE on 17.5% polyacrylamide gel, transferred to nitrocellulose and exposed to X-ray film. (B, C) MR6/ATP6-HApH was grown to exponential phase in rich galactose (− CAP). One half of the culture was incubated for an additional 2 h in fresh galactose medium containing 2 mg/ml chloramphenicol (+ CAP). Mitochondria from both the samples were labelled for 20 min, extracted with digitonin and purified on the Ni-NTA agarose. The equivalent of 50 μg of starting mitochondrial proteins (M) and of the digitonin extract (Ex), the non-adsorbed fraction (FT) and the high imidazole eluate (E), each adjusted to the starting volume of mitochondria, were separated on 17.5% polyacrylamide gel, transferred to nitrocellulose and exposed to X-ray film (left panel in B, C). Imidazole eluates equivalent to 0.4 mg of starting mitochondrial proteins were mixed with lactate dehydrogenase (132 kDa), layered on 4 ml of 10–25% linear sucrose gradients containing 10 mM Tris–Cl, pH 7 and 0.05% digitonin and centrifuged for 3 h at 60 000 r.p.m. in a Beckman SW60Ti rotor. Aliquots of the 12 gradient fractions were separated by SDS–PAGE on 17.5% polyacrylamide gel, transferred to nitrocellulose and exposed to the X-ray film (right panels in B, C). Lane A denotes F1–F0 complex, which was labelled in organello and co-immunoprecipitated with anti-F1 antibody in a separate experiment, was used as a migration standard for Atp6p, Atp8p and the Atp9p monomer and oligomer. The lactate dehydrogenase peaked in fractions 8 and 9 (asterisks) and the F1–F0 complex in fractions 5 and 6. (D) Imidazole eluates equivalent to 50 μg of starting mitochondrial proteins were separated by CN-PAGE on 3–10% polyacrylamide gel. Proteins were transferred to a PVDF membrane and exposed to X-ray film (35S). The F1–F0 monomer, dimer, and the Atp6p/Atp8p complex are identified in the right hand margin.
The physical association of newly translated Atp6p and Atp8p was borne out by sedimentation of the Ni-NTA eluate on a sucrose gradient and by electrophoresis in clear native polyacrylamide gels (CN-PAGE). MR6/ATP6-HApH expressing the double-tagged Atp6p was grown with or without a 2-h preincubation in medium containing chloramphenicol. The chloramphenicol treatment allows an accumulation of nuclear gene products, which enhances expression of the mitochondrially encoded genes (Tzagoloff, 1971). Analysis of the sucrose gradient fractions by SDS–PAGE revealed the presence of radiolabelled F0 subunits in two different-size complexes. The predominant complex, present in cells that had been grown without chloramphenicol, consisted of Atp6p and Atp8p that peaked in fraction 8, one fraction displaced from lactate dehydrogenase (asterisks in Figure 2B). A smaller fraction of the radiolabelled Atp6p and Atp8p was also detected in fractions 5 and 6, corresponding to the peak of unlabelled ATP synthase localized with an antibody against Atp6p (Figure 2B). An inverse distribution of the radiolabelled subunits between the two complexes was observed in the gradient of the Ni-NTA eluate of cells that had been incubated in chloramphenicol. In this instance, a larger fraction of radiolabelled Atp6p-HApH and Atp8p together with Atp9p ring sedimented in fractions 4–6 that also contained most of the unlabelled Atp6p, a marker for the ATP synthase (Figure 2C). The difference in the relative abundance of the Atp6p/Atp8p complex and of the F1–F0 complex in cells grown under the two conditions was confirmed by CN-PAGE. Most of the radioactivity in the Ni-NTA eluate from cells pretreated with chloramphenicol was associated with the monomeric ATP synthase. Some label was detected in a complex that migrated with an apparent mass of 250 kDa and a smaller fraction still migrated as the dimeric ATP synthase (Figure 2D). The 250-kDa complex was also detected in the Ni-NTA eluate of cells that had not been grown in chloramphenicol. However, these cells lacked radiolabelled ATP synthase monomer and dimer (Figure 2D). The identity of the radiolabelled monomer and dimer forms of the ATP synthase was confirmed by western analysis of the complexes separated by CN-PAGE with a monoclonal HA antibody, which detected the steady-state levels of the two forms of the synthase in mitochondria (Figure 2D).
The Atp6p/Atp8p complex can be chased into the ATP synthase
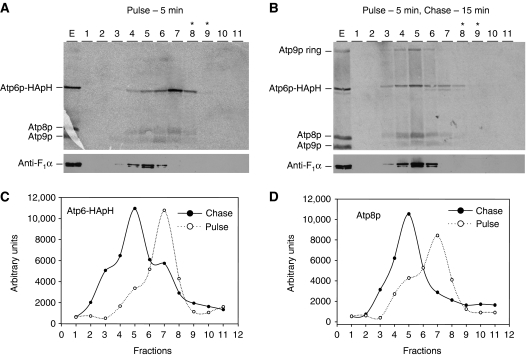
In the experiments described in the previous section, mitochondria of chloramphenicol-treated cells were labelled for 20 min, a period of time sufficient for the newly translated mitochondrial gene products to assemble into the ATP synthase (Figure 2C). To ascertain if the Atp6p/Atp8p complex detected under these conditions is a genuine precursor of the ATP synthase, mitochondria of cells pregrown for 2 h in chloramphenicol, were labelled for 5 min with [35S]methionine and chased for 15 min after addition of excess cold methionine. Almost all the radiolabelled F0 subunits translated during the pulse, sedimented at a position of the sucrose gradient corresponding to the Atp6p/Atp8p intermediate, approximately 2 fractions distal to the peak of the α subunit of F1, a marker of the F1–F0 complex (Figure 3A). This indicates that the 5-min pulse was insufficient to permit appreciable assembly of the native complex. Following 15 min of chase, the peak of the radiolabelled subunits shifted to a position of the gradient corresponding to the ATP synthase (Figure 3B). The visual impression was confirmed by quantification of radiolabelled Atp6p-HApH and Atp8p in the different fractions of the sucrose gradients (Figure 3C and D). The sucrose gradient of the material labelled during the 5-min pulse revealed in addition to the Atp6p-HApH/Atp8p complex, background levels of radiolabelled monomeric Atp9p and Cox3p. We observed that the levels of these subunits in the fraction eluted from Ni-NTA beads are variable and in some experiments they are undetectable (e.g. experiments in Figures 2, 4 and 6). To exclude the presence of Atp9p in the Atp6p/Atp8p intermediate, a strain expressing Atp9p with a haemagglutinin tag (MR6/ATP9-HA) was pregrown for 2 h in medium containing chloramphenicol and mitochondria were pulse labelled for 3 min with [35S]methionine and chased for 30 min after addition of puromycin and excess cold methionine. Mitochondrial proteins were extracted with digitonin, incubated with anti-HA antibody and absorbed to Protein G sepharose (Supplementary Figure S1). During the 3-min pulse, nearly all newly synthesized Atp9p-HA was in the monomeric form and only a small fraction had oligomerized into the ring. Following co-immunoprecipitation, both monomer and ring forms of Atp9p-HA were quantitatively recovered in the eluate from Protein G sepharose. In contrast, only a small fraction of newly synthesized Atp6p and Atp8p co-precipitated with Atp9p-HA. The 30-min chase allowed substantial oligomerization of Atp9p-HA to the ring with a concomitant large increase in co-immunoprecipitation of radiolabelled Atp6p and Atp8p. This is consistent with an exclusive interaction of Atp6p and Atp8p, with Atp9p only after the latter forms a ring and argues against monomeric Atp9p being part of the Atp6p/Atp8p intermediate.
Figure 3.
The Atp6p/Atp8p complex can be chased into the F1–F0 complex. Mitochondria were prepared from MR6/ATP6-HApH and the equivalent of 1 mg of protein was labelled for 5 min with [35S]methionine. Puromycin and excess unlabelled methionine was added to one half of the sample and incubated for an additional 15 min. The radiolabelled proteins were extracted with digitonin, purified on the Ni-NTA agarose and the 200-mM imidazole eluates was mixed with lactate dehydrogenase and centrifuged on linear sucrose gradient as described in Figure 2. The gradients were collected in 11 equal fractions and samples separated by SDS–PAGE on 17.5% polyacrylamide gel. Proteins were transferred to nitrocellulose and exposed to the X-ray films (A, B; upper panels). The blots were then probed with a polyclonal antibody against the α subunit of F1 (A, B; lower panels). The radioactive signals of Atp6p-HApH and Atp8p were quantified with a phosphorimager (C, D).
Figure 4.
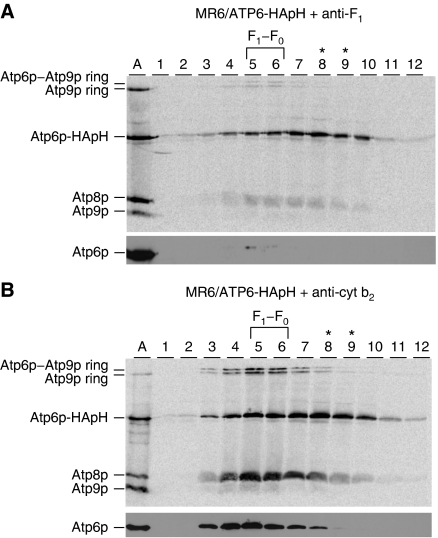
F1 and Atp9p rings are not associated with Atp6p/Atp8p subcomplex. (A) Mitochondria (1 mg) from MR6/ATP6-HApH, expressing double-tagged Atp6p were labelled with [35S]methionine/cysteine for 10 min as in Figure 2. The radiolabelled proteins were extracted with 3% digitonin one half of which was treated with antibody against yeast F1 (A) and the other half with antibody against cytochrome b2 (B) for 2 h at room temperature. The antibody–antigen complexes were removed by centrifugation at 14 000 r.p.m. for 15 min and the supernatants were purified on the Ni-NTA agarose as described in the Materials and methods section. The Ni-NTA eluates equivalent to 0.4 mg of starting mitochondrial protein were centrifuged in 10–25% linear sucrose gradients containing 0.05% digitonin and were analysed by SDS–PAGE on a 17.5% polyacrylamide gel as in Figure 2. The lane labelled A was loaded with a sample of the F1–F0 complex containing radiolabelled Atp6p, Atp8p and Atp9p as in Figure 2.
F1 is not associated with the Atp6p/Atp8p intermediate
The size of the Atp6p/Atp8p intermediate was too small to accommodate F1 with a mass of 360 kDa. This was confirmed by an antibody depletion experiment. Mitochondria isolated from MR6/ATP6-HApH pregrown for 2 h in chloramphenicol were labelled with [35S]methionine/cysteine and extracted with digitonin. The extract was divided into two equal parts, one of which was treated with a polyclonal antibody against yeast F1 under conditions that precipitate F1 and the F1–F0 complex. As a control, the other half was treated with a polyclonal antibody against cytochrome b2. Following fractionation on Ni-NTA, the high imidazole eluates were analysed by sucrose gradient centrifugation (Figure 4A and B). The gradient of the Ni-NTA eluate obtained from the sample treated with cytochrome b2 antibody contained both the F1–F0 complex peaking in fractions 5 and 6 and the Atp6p/Atp8p intermediate in fractions 7–10. The anti-F1 antibody treatment resulted in substantial loss of radiolabel Atp6p, Atp8p and Atp9p ring associated with the F1–F0 complex but did not significantly reduce the radiolabel in fractions 7–10 containing the Atp6p/Atp8p intermediate. The effectiveness of the depletion with the F1 antibody was confirmed by western analysis with an Atp6p antibody, which indicated an almost complete absence of this ATP synthase marker (Figure 4A, lower panel).
Assembly of the Atp6p/Atp8p intermediate does not depend on F1
The almost complete absence of Atp6p and Atp8p translation in F1 mutants (Rak and Tzagoloff, 2009) precluded such strains from being used to assess a possible requirement of F1 for assembly of the Atp6p/Atp8p intermediate. This problem was solved by constructing the double-mutant W303/ATP6-HApH/ΔATP1,ΔYJL147c with null alleles in atp1 (α subunit of F1) and in reading frame YJL147c. The latter mutation suppresses the Atp6p and Atp8p translation defect of F1 mutants (unpublished data). The Δyjl147c null mutation by itself does not affect biogenesis of the ATP synthase and growth on non-fermentable carbon sources (Supplementary Figure S2). The wild type and the Δatp1 Δyjl147c double mutant, each expressing Atp6p with the double HA and polyhistidine tag, were grown in rich galactose medium and incubated for 2 h in fresh medium containing chloramphenicol. Following in organello labelling, newly translated mitochondrial gene products were extracted with digitonin, purified on Ni-NTA and further fractionated on sucrose gradients. The sucrose gradient of the Ni-NTA-purified fraction from respiratory competent mitochondria of MR6/ATP6-HApH displayed the presence Atp6p in fractions that included both the fully assembled F1–F0 complex and the Atp6p/Atp8p intermediate (Figure 5A). Western analysis of the gradient fraction confirmed that the faster sedimenting radiolabelled Atp6p-HApH in fractions 4–6 of the respiratory competent control strain, co-sedimented with Atp2p, a marker for the mitochondrial F1–F0 complex while the Atp6p-HApH and Atp8p peaking in fractions 7–9 corresponded to the intermediate complex. Sucrose gradient analysis of the Ni-NTA-purified fraction from the Δatp1 Δyjl147c double mutant revealed the presence of the slower sedimenting Atp6p/Atp8p intermediate but not of the ATP synthase, which is not assembled in this strain because of the absence of F1 (Figure 5B).
Figure 5.
F1 is not required for the interaction of Atp6p and Atp8p. Mitochondria were prepared from MR6/ATP6-HApH and W303ΔATP1ΔYJL147/ATP6-HApH, both expressing the double-tagged Atp6p. The latter strain is respiratory deficient because of the null mutation in ATP1 coding for the α subunit of F1. The absence of F1 imposes a block in translation of Atp6p and Atp8p (Rak and Tzagoloff, 2009), which is relieved by the null mutation in reading frame YJL147C (unpublished data). (Left panels) Mitochondria were labelled for 20 min, extracted with digitonin and the extract purified on Ni-NTA as described in the legend to Figure 2. Mitochondria (M), the digitonin extract (Ex), the non-adsorbed fraction (FT), the 10-mM imidazole wash (W) and 200 mM imidazole eluate (E) were analysed by SDS–PAGE on 17.5% polyacrylamide gels. The mitochondrial gene products of the ATP synthase are identified in the left hand margin. (Right panels) The 200-mM imidazole eluates were centrifuged on 10–25% linear sucrose gradient containing 0.05% digitonin and 12 equal fractions were collected. Proteins were precipitated with 5% TCA and analysed by SDS–PAGE. In this experiment, all the Atp9p ring is converted to the monomer by TCA. The F1–F0 complex in the sucrose gradient of MR6/ATP6-HApH mitochondria was localized by western analysis with an antibody against Atp2p (Anti-F1β). Because of the absence of the α subunit of F1, the F1–F0 complex is not detected in the atp1 mutant.
In this experiment, the sucrose gradient fractions were precipitated with trichloracetic acid, which quantitatively converts the Atp9p ring to the monomer. This accounts for the large amount Atp9p monomer that co-sediments with Atp6p-HApH and Atp8p in the gradient of the respiratory competent strain MR6/ATP6-HApH (Figure 5A). Because of the absence of the F1–F0 complex in the Δatp1 Δyjl147c mutant, only background levels of radiolabelled Atp9p is detected in the sucrose gradient. The disparity in the signal strength of Atp9p ring in the elution fraction (E) and the gradient fractions after TCA precipitation is explained by the much more efficient transfer of the Atp9p monomer compared with the high-molecular weight and hydrophobic Atp9p ring (see Figure 8A).
Are stator subunits associated with the Atp8p/Atp6p intermediate?
Based on its sedimentation in sucrose gradients and migration on CN-PAGE, the apparent mass of the Atp6p/Atp8p complex was estimated to be approximately five times larger than the composite mass of the two subunits (36 kDa). To account for this difference, we probed for the presence of stator subunits in the Atp6p/Atp8p complex.
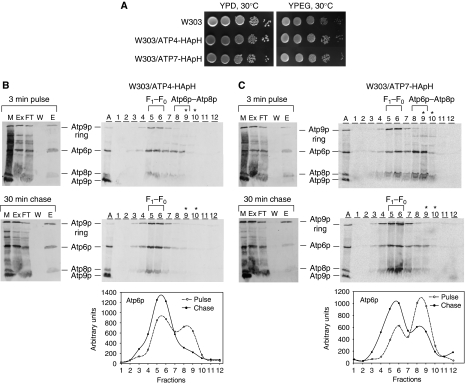
Stator subunits were expressed with an HA or myc plus polyhistidine double tag from genes that had been integrated in single copy into chromosomal DNA. The presence of the tags did not affect growth on non-fermentable carbon sources (shown for W303/ATP4-HApH and W303/ATP7-HApH in Figure 6A). Isolated mitochondria were labelled for 3 min and one half of the translation mixture was chased in the presence of puromycin and excess unlabelled methionine and cysteine for 30 min. The mitochondria were extracted with digitonin, purified on Ni-NTA and analysed on sucrose gradients. When this protocol was used in conjunction with strains expressing double-tagged Atp4p-HApH and Atp7p-HApH, both radiolabelled Atp6p and Atp8p in the digitonin extracts of the pulsed labelled and pulse-chased mitochondria, were eluted from Ni-NTA with 200 mM imidazole. A weak signal of the Atp9p ring was also present in Ni-NTA eluate of mitochondria that had been labelled for 3 min, indicating that some ATP synthase had been assembled during the pulse (Figure 6B). The radiolabelled Atp9p ring signal was increased after the chase, consistent with its incorporation into the fully assembled ATP synthase. This was confirmed by the distribution of the three radiolabelled F0 subunits in the sucrose gradients (Figure 6C). Gradients of the Ni-NTA eluate of mitochondria that had been pulse labelled but not chased, indicated the presence of Atp9p ring predominantly in fractions 5 and 6, corresponding to the fully assembled F1–F0 complex. In contrast, Atp6p and Atp8p were more broadly distributed with a significant proportion in fractions 8 and 9 corresponding to the partially assembled intermediate. Following the 30-min chase, most of the radiolabelled Atp6p and Atp8p in the Ni-NTA eluate sedimented as the F1–F0 complex (Figure 6C). The incorporation of the Atp6p/Atp8p precursor into the ATP synthase was confirmed by quantification of the radiolabelled Atp6p in the sucrose gradient fractions (Figure 6B and C, lower panels).
Figure 6.
Co-purification of Atp4p and Atp7p with the Atp6p/Atp8p complex. (A) The parental strain W303-1A, W303/ATP4-HApH and W303/ATP7-HApH, expressing, respectively, Atp4p and Atp7p, each with a C-terminal double HA and polyhistidine tag were grown overnight in liquid YPD. Serial dilutions were spotted on solid YPD and YPEG and were incubated at 30°C for 2–3 days. (B) Mitochondria of W303/ATP4-HApH were labelled with [35S]methionine/cysteine for 3 min as in Figure 2. One half of the labelled mitochondria were incubated for an additional 30 min after addition of puromycin and excess cold methionine and cysteine. The mitochondria were extracted with digitonin and solubilized proteins were purified on Ni-NTA as described in the Materials and methods section. A sample of mitochondria (M), the digitonin extract (Ex), the fraction that did not adsorb to Ni-NTA (FT), the 10-mM imidazole wash (W) and the 200-mM imidazole eluate (E) were separated by SDS–PAGE on a 17.5% polyacrylamide gel. All the fractions were adjusted to the starting volume of mitochondria (50 μg protein). The radiolabelled subunits of the ATP synthase are identified in the margin. The high imidazole eluates were mixed with lactate dehydrogenase and analysed on sucrose gradients containing 0.05% digitonin as described in Figure 2. The peak fractions with the fully assembled F1–F0 complex and the Atp6p/Atp8p intermediate are indicated in the figure. The lactate dehydrogenase peaked in fractions 9 and 10 (asterisks). The gradient fractions were analysed by SDS–PAGE on a 17.5% polyacrylamide gel. The radiolabelled mitochondrial gene products of the ATP synthase are identified in the margin. Radiolabelled Atp6p in the fractions of each gradient was quantified with a phosphorimager (lower panel). (C) Same as (B) except that the strain used was W303/ATP7-HApH. The aberrant migration of Atp8p in the gradient of the pulse-labelled mitochondria from W303/ATP7-HApH (C, upper panel) is due to the high sucrose in the samples. The F1–F0 complex containing radiolabelled Atp6p, Atp8p and Atp9p was loaded in the lanes labelled A as in Figure 2.
In contrast, we were unable to obtain any evidence for the association of OSCP (Atp5p) with the Atp6p/Atp8p/stator complex. A similar experiment with mitochondria from a strain expressing Atp5p-HApH when pulsed for 3 min revealed only a small amount of fully assembled ATP synthase, which was increased during the chase but was not discernibly altered in its sedimentation properties (Supplementary Figure S3).
In order to verify if Atp14p, the fourth subunit of the peripheral stalk is part of the intermediate, we constructed a strain expressing Atp14p with a double Myc and polyhistidine tag. The presence of the tag on Atp14p, however, resulted in a partial assembly defect as evidenced by a reduction of the F1–F0 monomer and dimer detectable by BN-PAGE and a concomitant large amount of free Atp9p ring (Supplementary Figure S4). This precluded an unambiguous interpretation of the pulse-chase experiments. At present, therefore, the presence the Atp14p stator subunit in the Atp6p/Atp8p/stator complex must remain an open question.
The Atp6p/Atp8p/stator intermediate is associated with Atp10p but not Atp23p
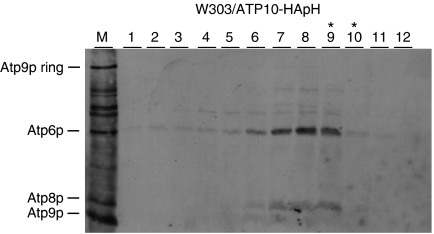
The Atp10p and Atp23p chaperones have previously been reported to interact with Atp6p and to be required for assembly of F0 (Tzagoloff et al, 2004; Osman et al, 2007; Zeng et al, 2007). Both proteins, therefore, were candidates to interact with the Atp6/Atp8p/stator intermediate. The presence of Atp10p in the Atp6p/Atp8p complex was tested by the same approach used to detect stator subunits. Mitochondrial gene products of a strain expressing Atp10p doubly tagged with HA and polyhistidine were radiolabelled in organello and extracted with digitonin. The extract was purified on Ni-NTA and analysed on sucrose gradients. The gradient of the 200-mM Ni-NTA eluate (E) indicated the presence of radiolabelled Atp6p and Atp8p in fractions of the sucrose gradient range inclusive of the Atp6p/Atp8p intermediate (Figure 7). Co-purification of the Atp6p/Atp8p intermediate but not the ATP synthase confirms earlier evidence that Atp10p is not part of the mature enzyme but interacts with newly translated Atp6p.
Figure 7.
Atp10p is present in the Atp6p/Atp8p complex. Mitochondria were prepared from W303/ATP10-HApH, a respiratory competent strain expressing Atp10p with a C-terminal double HA and polyhistidine tag. Following labelling with [35S]methionine/cysteine for 10 min, the mitochondria were extracted with digitonin and Atp10p-HApH was purified on Ni-NTA as described in the legend to Figure 2. The 200-mM imidazole eluate was mixed with lactate dehydrogenase and applied to a 4-ml linear 10–25% sucrose gradient containing 0.05% digitonin. The gradient was centrifuged and analysed as in Figure 2. A sample of the labelled mitochondria was loaded on lane M. The peak lactate dehydrogenase fractions are indicated by the asterisks.
A similar experiment with a strain expressing Atp23p-HApH failed to detect this protein in the Atp6p/Atp8p complex (not shown).
Assembly of the Atp9p ring
Although the Atp9p ring is known to be resistant to denaturation by SDS, the fraction of ring remaining under conditions used to separate proteins by SDS–PAGE has not been examined. This was estimated with a preparation of radiolabelled ATP synthase. The amount of Atp9p recovered in the monomeric and oligomeric form before and after treatment with TCA, which dissociates the ring to the monomer quantitatively (Herrmann et al, 1994b), indicated that <10% of the oligomer is depolymerized by SDS (not shown).
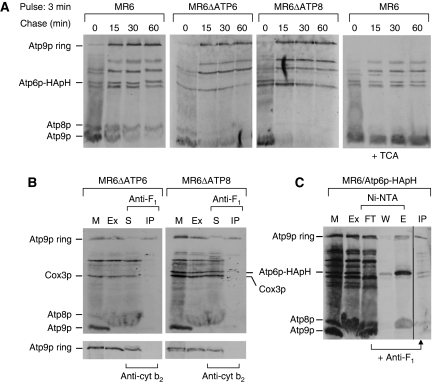
The kinetics of ring formation was studied in mitochondria of the respiratory competent strain MR6. Mitochondria were pulsed for 3 min and chased in the presence of cold methionine for different times. Although a small fraction of the Atp9p translated during the 3-min pulse was converted to the ring, >90% remained as the monomer (Figure 8A; Supplementary Figure S5). Most of the monomer oligomerized to the ring after 15–30 min of chase. Similar kinetics of Atp9p oligomerization were observed in an atp6 and atp8 null mutant, indicating that ring formation is not contingent on subunits encoded by these mitochondrial genes, either individually or as part of the Atp6p/Atp8p/stator intermediate (Figure 8A).
Figure 8.
Kinetics of Atp9p ring oligomerization and detection of an F1/Atp9p ring complex (A) Mitochondria were prepared from the respiratory competent parental strain MR6, from MR6ΔATP6, a mitochondrial mutant with an atp6 null allele and MR6ΔATP8/ATP6-HApH, a mitochondrial mutant with an atp8 null allele expressing Atp6p doubly tagged with HA and polyhistidine. Mitochondria (0.5 mg) were pulse labelled for 3 min (time 0), and were chased for 15, 30 and 60 min after addition of puromycin and excess cold methionine plus cysteine as in Figure 3. Total mitochondrial proteins were separated by SDS–PAGE on 17.5% polyacrylamide gel, transferred to nitrocellulose and exposed to the X-ray film. In the extreme right hand panel, the same samples of MR6 shown in the left panel were treated with 5% TCA before depolymerization in sample buffer. The conversion of the Atp9 ring to the monomer by this treatment increases the transfer efficiency causing a significant enhancement of the signal. (B) Mitochondria (1 mg) of MR6ΔATP6 and MR6ΔATP8/ATP6-HApH were labelled for 20 min, extracted with digitonin and the extracts were incubated with polyclonal antibody against F1 or cytochrome b2 for 1 h at room temperature. The immunoprecipitates were removed by centrifugation at 14 000 r.p.m. for 15 min. Mitochondria (M), the digitonin extract (Ex), the supernatant from the antibody precipitate (S) and the antibody precipitate (IP) were analysed by SDS–PAGE as in (A). (C) Mitochondria of MR6/ATP6-HApH were labelled for 20 min, extracted with 3% digitonin and purified on Ni-NTA. The protein fraction that was not adsorbed to the Ni-NTA beads was incubated with antibody against F1 and the resultant antibody precipitate (IP) was analysed by SDS–PAGE as in (B). All the lanes shown are part of the same gel exposed for the same period of time.
The interaction of F1 with the Atp9p ring has been proposed to be an early step in assembly of the ATP synthase (Hadikusumo et al, 1988; Tzagoloff et al, 2004), although this intermediate has not been demonstrated directly. We first tried to detect an F1–Atp9p ring intermediate in atp6 and atp8 null mutants that are blocked in assembly of the ATP synthase (the atp8 mutant contained the mitochondrial ATP6-HApH allele). Digitonin extracts of mitochondria that had been pulsed with [35S]methionine/cysteine were treated with a polyclonal antibody against F1. Different fractions including the antibody precipitate and supernatant were separated by SDS–PAGE and radiolabelled products were visualized by autoradiography. The 20-min pulse used in this experiment allowed a substantial fraction of Atp9p to oligomerize into the ring. Since mitochondrial gene products continued to be translated during the entire pulse period, a large fraction of Atp9p had not yet oligomerized and migrated as the monomer (mitochondria in Figure 8B). More than half of the Atp9p ring, corresponding to an F1/Atp9p ring complex was recovered in the immunoprecipitate with F1 antibody. In view of the almost complete depletion of F1 by the antibody, the residual Atp9p ring remaining in the supernatant after the antibody treatment probably represents a fraction of the Atp9p ring that either had dissociated from the complex or had not yet interacted with F1. The co-precipitation of Atp9p ring with F1 was specific as a similar experiment done with an antibody against cytochrome b2 did not pull down any Atp9p ring (Figure 8B, lower panel).
It was also of interest to see if the F1/Atp9p ring intermediate is detected in the respiratory competent strain capable of assembling the F1–F0 complex normally. A digitonin extract of MR6/ATP6-HApH mitochondria that had been pulsed with [35S]methionine/cysteine for 20 min was extracted with digitonin and purified on Ni-NTA. Virtually all the F1–F0 complex and Atp6p/Atp8p/stator was adsorbed on Ni-NTA. This was not true of the Atp9p ring, which was found either in the fraction adsorbed to the Ni-NTA and corresponding to F1–F0 ATP synthase (E) or the fraction that was not adsorbed to the beads (FT) (Figure 8C). When the non-adsorbed fraction (FT) was treated with the polyclonal antibody against F1 most of the Atp9p ring was recovered in the precipitate confirming that the F1/Atp9p ring is also formed in a strain capable of assembling the ATP synthase.
Discussion
The F0 unit of the ATP synthase is composed of both nuclear and mitochondrial gene products. In S. cerevisiae, two of the three F0 subunits encoded by mtDNA are part of the F0 core that constitutes the channel through which protons transit during the operation of the enzyme (Fillingame et al, 2002). A serious obstacle to the use of mutants to study assembly of F0 under steady-state conditions is the extensive turnover of some subunits. This is especially true of the membrane-embedded Atp6p (Paul et al, 1989; Helfenbein et al, 2003). This problem has been avoided in the present study by labelling and tracking the mitochondrial gene products of F0 in isolated mitochondria. Newly translated proteins labelled in organello in pulse and pulse-chase experiments are sufficiently stable to permit detection and in combination with a tagged version of Atp6p to study their assembly into the F1–F0 complex.
Our results indicate that the ATP synthase is formed from different modules. In addition to F1, which was already known to assemble as an independent unit (Schatz, 1968; Tzagoloff, 1969), these modules include the Atp9p ring and the Atp6p/Atp8p complex. The interaction of At6p with Atp8p is rapid as we were unable to detect any uncomplexed Atp6p even in 3 min pulses. After an additional 15 min of incubation in the presence of cold methionine, the Atp6/Atp8 complex was chased into the full ATP synthase, indicating that it is a bona fide assembly intermediate. It should be pointed out, however, that this was true of strains in which Atp6p has a C-terminal double HA and polyhistidine tag. In strains expressing the wild-type Atp6p subunit, assembly of ATP synthase was somewhat faster as ∼50% of Atp6p was in a complex with Atp8p and the rest had already been incorporated into the full ATP synthase. Our evidence indicates that the Atp6p/Atp8p complex is a true assembly intermediate that can be chased into the fully assembled ATP synthase. The Atp6p/Atp8p intermediate sediments as a 150–200 kDa complex, suggesting the presence of additional proteins. The Atp6p/Atp8p complex of strains expressing polyhistidine tagged Atp4p and Atp7p co-fractionated with each of these stator subunits on Ni-NTA, indicating that they are part of the intermediate. The results of pulse-chase experiments with a strain expressing Atp14p doubly tagged with Myc and polyhistidine were difficult to interpret because of the presence of a substantial amount of free Atp9p ring. On the other hand, we did not detect OSCP, the fourth stator subunit, in the Atp6p/Atp8p complex.
The Atp10p chaperone, previously shown to interact physically with Atp6p and implicated in assembly of Atp6p with the Atp9p ring (Tzagoloff et al, 2004), is also associated with the Atp6p/Atp8p complex. Although sucrose gradient centrifugation achieves a good separation of the intermediate from the native F1–F0 complex, it is not sufficiently resolving to separate proteins of sizes that differ by <50%. We therefore cannot exclude the possibility that the fractions containing the Atp6p/Atp8p intermediate may be heterogeneous with respect to the stator subunits or other proteins. Even though our data points to the Atp6p/Atp8p complex as the attachment site for the peripheral stalk, they do not distinguish between an interaction with the Atp6p/Atp8p complex of the stator components as individual subunits or as a preassembled structure. In view of this, we cannot exclude that part of the peripheral stalk is also a separate module.
The third module is formed by the oligomerization of Atp9p, a relatively slow step compared with the interaction of Atp6p with Atp8p. As already indicated, all of Atp6p translated in a 3-min pulse is detected in the Atp6p/Atp8p and the fully assembled ATP synthase complexes. Under the same conditions of labelling, most of the Atp9p is still in the monomeric form. Complete conversion of the Atp9p monomer to the oligomer requires 15–30 min. In atp6 or atp8 mutants that are arrested in F0 assembly, the Atp9p ring co-precipitates with F1 antibody, indicating that the interaction with F1 to form the F1/Atp9p ring intermediate is rapid and occurs independently of the Atp6p/At8p complex. The F1/Atp9p ring intermediate is also detected in respiratory competent yeast, further substantiating the notion that it is as a bona fide assembly intermediate.
We propose that the two partial intermediates are the immediate precursors of the holoenzyme (Figure 9). The segregation of Atp6p from the Atp9p ring until the very last stage of assembly insures that the proton conductive channel, at the interface of Atp6p and Atp9p ring, is formed concomitant with a coupled ATP synthase thereby preventing proton leakage across the membrane. The Atp6p/Atp8p/stator and F1/Atp9p ring intermediates are products of two different pathways that are subject to coordinate regulation. In a previous study we reported that translation of Atp6p and Atp8p is strictly dependent on F1 (Rak and Tzagoloff, 2009). According to the scheme of Figure 9, activation of Atp6p and Atp8p translational by F1, limits the kinetically more rapid of the two pathways and provides a mechanism for a balanced production of the two intermediates.
Figure 9.
Assembly of the ATP synthase. The diagram shows the two separate pathways for assembling the immediate precursors of the ATP synthase. In this scheme, the ATP synthase is composed of at least three different modules, F1, the Atp9p ring and the Atp6p/Atp8p/stator subcomplex. At present we cannot exclude the possibility that the stator is also a separate module that interacts with the Atp6p/Atp8p subcomplex as a preformed unit. Activation of Atp6p and Atp8p translation by F1 is denoted by the grey arrow. Atp25p is a chaperone with two separate functions, one of which is to promote oligomerization of Atp9p (Zeng et al, 2008).
The modules and the proposed pathway for ATP synthase assembly may recapitulate some of the evolutionary events that gave rise to this enzyme. There is compelling evidence that F1 evolved from an ATP-dependent helicase (Gomis-Rüth et al, 2001) while the Atp9p ring has been proposed to have been derived from an ion channel (Walker and Cozens, 1986; Mulkidjanian et al, 2007). The F1/Atp9p ring intermediate could be the product of an evolutionary event, which enabled a passive channel to be converted to an active ion transporter. The function of the ancestral protein from which the Atp6p/Atp8p/stator complex evolved is more difficult to envision. Its function may have been adapted to further modify the ATP-dependent ion pump into the present day energy transforming mechanochemical machine.
Materials and methods
Strains and growth media
The genotypes and sources of the strains of S. cerevisiae used in this study are listed in Table I. The compositions of the solid and liquid media have been described previously (Myers et al, 1985).
Table 1. Genotypes and sources of Saccharomyces cerevisiae strains.
| Strain | Genotype | mtDNA | Source |
|---|---|---|---|
| W303-1A | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 | ρ+ | a |
| MR6 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 arg8∷HIS3 | ρ+ | Rak et al (2007) |
| DFS160 | MATα kar1-1 ade2-10 leu2Δ ura3-52 arg8∷URA3 | ρ0 | Steele et al (1996) |
| MR10 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 arg8∷HIS3 | ρ+ Δatp6∷ARG8m | Rak et al (2007) |
| MR6ΔATP8 | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 arg8∷HIS3 | ρ+ Δatp8∷ARG8m | Rak and Tzagoloff (2009) |
| MR6/ATP6-HA | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 arg8∷HIS3 | ρ+ ATP6-HA | This study |
| MR6/ATP9-HA | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 arg8∷HIS3 | ρ+ ATP9-HA | This study |
| MR6/ATP6-HApH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 arg8∷HIS3 | ρ+ ATP6-HApH | This study |
| W303/ATP4-HApH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 atp4∷URA3 leu2∷ATP4/ST7 | ρ+ | This study |
| W303/ATP7-HApH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 atp7∷URA3 leu2∷ATP7/ST5 | ρ+ | This study |
| W303/ATP14-MycpH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 atp14∷URA3 ATP14/ST22 | ρ+ | This study |
| MLY001/ATP5-HApH | MATα ade5 leu2-3,112 ura3-1 atp5∷LEU2 ura3∷ATP5/ST6 | ρ+ | This study |
| W303/ATP10-HApH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 atp10∷LEU2 ura3∷ATP10/ST22 | ρ+ | This study |
| W303/ATP23-HApH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 atp23∷HIS3 ura3∷ATP23/ST18 | ρ+ | Briere JJ (unpublished data) |
| W303ΔYJL147C | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 yjl147c∷URA3 | ρ+ | This study |
| W303ΔATP1ΔYJL147C/ ATP6-HApH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 atp1∷HIS3 yjl147c∷URA3 | ρ+ ATP6-HApH | This study |
| aRothstein, Department of Genetics and Development, Columbia University. | |||
Construction of mitochondrial genes expressing Atp6p with a single HA and a double HA plus polyhistidine tag
ATP6 with a HA tag was constructed by PCR amplification of 300 bp of the 5′-flanking region plus the coding sequence and separately of the sequence coding for the HA tag plus 300 bp of 3′-flanking region. The two fragments were amplified from mtDNA of the respiratory competent haploid strain MR6 as the template with the primer pairs 5′-ccgagctcgcggaccccaaaggaggag/5′-ccgggtaccatgta-agtatactgcatctt and 5′-ggcggtaccta-cccatacgacgtcccagactacgcttaaatta-taaaataaaattataaaataaaataatttacaatatgg/5′-ggcggatccggc-cgaactccgaaggagtaag. The PCR products were digested with a SacI/KpnI and KpnI/BamHI, respectively, and ligated to the SacI and BamH1 sites of pJM2 (Steele et al, 1996). The resultant plasmid pATP6/ST12 was introduced into the kar1 strain αDFS160ρ0 by biolistic transformation (Bonnefoy and Fox, 2007), with the PDS-1000/He particle delivery system (Bio-Rad, Hercules, CA). Transformants were selected for their ability to rescue the cox2 mutation of M9-96-3A (Tzagoloff et al, 1975). A transformant verified to have acquired the modified ATP6 gene (αDFS160/ATP6/ST12) was crossed to the respiratory-deficient mutants MR10 harbouring the mitochondrial Δatp6∷ARG8m allele (Rak et al, 2007). Recombinants in which atp6∷ARG8m had been replaced by ATP6-HA were identified by their arginine auxotrophy and ability to grow on ethanol/glycerol. The same strategy was used to construct a yeast mutant expressing the double-tagged Atp6p-HApH except that the primer used to amplify the tag was 5′-ggcggtacctacccatacgacgtcccagactacgctcaccatcatcaccatcattaaattataaaataaaattataaaataaaataattac.
Growth of yeast, isolation of mitochondria, and in organello labelling of mitochondrial translation products
Unless otherwise indicated, wild-type and mutant yeast were grown in YPGal (2% galactose, 2% peptone, 1% yeast extract) to mid-log phase. They were harvested, inoculated into fresh YPGal containing 2 mg/ml chloramphenicol and grown for an additional 2 h. Mitochondria were isolated by the method of Herrmann et al (1994a) and their gene products labelled in organello with [35S]methionine/cysteine (1000 Ci/mmol; MP Biochemicals, Solon, OH) for 3–20 min at 30°C as described previously (Hell et al, 2001). The reaction was terminated by addition of puromycin at final concentration of 50 μg/ml and an excess of unlabelled methionine and cysteine.
Purification of polyhistidine tagged proteins on Ni-NTA
Mitochondria that had been radiolabelled were suspended at a protein concentration of 10 mg/ml in a buffer containing 3% digitonin, 150 mM potassium acetate, 2 mM α-aminocaproic acid, 20 mM Hepes pH 7. The supernatant obtained after centrifugation 90 000 gav for 10 min was adjusted to pH 9 with Tris and was incubated for 2 h at 4°C with Ni-NTA agarose beads (Qiagen, Valencia, CA) in binding buffer containing 400 mM sodium chloride, 10 mM imidazole, 10 mM Tris–Cl, pH 9. After centrifugation at 1000 r.p.m. for 30 s, non-adsorbed proteins were removed and the beads were washed three times with 400 mM sodium chloride, 10 mM imidazole, 10 mM Tris–Cl, pH 7.4. Tagged proteins were eluted with high imidazole buffer containing 200 mM imidazole, 50 mM sodium chloride, and 20 mM potassium phosphate, pH 7.4. All steps were carried out in a 1.5-ml Eppendorf tube.
Gel electrophoresis
Two different SDS–PAGE systems were used to separate the radiolabelled mitochondrial gene products. Atp6p was separated from Cox3p on a 12% polyacrylamide gel containing 4 M urea and 25% glycerol. Atp8p was separated from monomeric Atp9p on 17.5% polyacrylamide gel (Laemmli, 1970). Proteins were transferred to a nitrocellulose membrane and exposed to X-ray film. Where indicated, radiolabelled bands were quantified with a phoshorimager.
For western analysis, proteins were separated by SDS–PAGE on a 12 or 17.5% polyacrylamide gel (Laemmli, 1970) and by CN-PAGE (Wittig et al, 2007). Proteins were transferred to a nitrocellulose and polyvinylidene difluoride (PVDF) membrane, respectively, and probed with mouse monoclonal antibodies against the HA tag or rabbit polyclonal antibodies against yeast α or β subunit of F1, OSCP or Atp9p. The antibody complexes were visualized with the Super Signal Chemiluminescent substrate kit (Pierce Chemical Co., Rockford, IL).
Miscellaneous procedures
Yeast cells were transformed by the LiAc procedure of Schiestl and Gietz (1989). Standard techniques were used for DNA cloning and for transformation and purification of plasmid DNA from Escherichia coli (Sambrook et al, 1989). Protein concentration was determined by the procedure of Lowry et al (1951). In gel ATPase activity was measured according to Wittig et al (2007).
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health Research grant HL-22174. We thank Dr David Mueller for providing us with the atp5 null mutant MLY001 and anti-OSCP antibody; Dr Jean-Paul di Rago (IBGC, Université Victor Segalen Bordeaux 2, France) for the yeast strains RKY26, MR6, MR10 and DFS160; and Dr Jean Velours for the gift of the Atp6p and Atp9p antibodies.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bonnefoy N, Fox TD (2007) Directed alteration of Saccharomyces cerevisiae mitochondrial DNA by biolistic transformation and homologous recombination. Methods Mol Biol 372: 153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer PD (1997) The ATP synthase—a splendid molecular machine. Annu Rev Biochem 66: 717–749 [DOI] [PubMed] [Google Scholar]
- Fillingame RH, Angevine CM, Dmitriev OY (2002) Coupling proton movements to c-ring rotation in F(1)F(o) ATP synthase: aqueous access channels and helix rotations at the a-c interface. Biochim Biophys Acta 1555: 29–36 [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth FX, Moncalián G, Pérez-Luque R, González A, Cabezón E, de la Cruz F, Coll M (2001) The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature 409: 637–641 [DOI] [PubMed] [Google Scholar]
- Hadikusumo RG, Meltzer S, Choo WM, Jean-Francois MJ, Linnane AW, Marzuki S (1988) The definition of mitochondrial H+ ATPase assembly defects in mit-mutants of Saccharomyces cerevisiae with a monoclonal antibody to the enzyme complex as an assembly probe. Biochim Biophys Acta 933: 212–222 [DOI] [PubMed] [Google Scholar]
- Helfenbein K, Ellis T, Dieckmann C, Tzagoloff A (2003) ATP22, a nuclear gene required for expression of the F0 sector of mitochondrial ATPase in Saccharomyces cerevisiae. J Biol Chem 278: 19751–19756 [DOI] [PubMed] [Google Scholar]
- Hell K, Neupert W, Stuart RA (2001) Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J 15: 1281–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JM, Foelsch H, Neupert W, Stuart RA (1994a) Isolation of yeast mitochondria and study of mitochondrial protein translation. In Cell Biology: A Laboratory Handbook, Celis JE (ed) Vol. I, pp 538–544. San Diego: Academic Press [Google Scholar]
- Herrmann JM, Stuart RA, Craig EA, Neupert W (1994b) Mitochondrial heat shock protein 70, a molecular chaperone for proteins encoded by mitochondrial DNA. J Cell Biol 127: 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Dienhart MK, Stuart RA (2007) Oxa1 directly interacts with Atp9 and mediates its assembly into the mitochondrial F1Fo-ATP synthase complex. Mol Biol Cell 18: 1897–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Mulkidjanian AY, Makarova KS, Galperin MY, Koonin EV (2007) Inventing the dynamo machine: the evolution of the F-type and V-type ATPases. Nat Rev Microbiol 5: 892–899 [DOI] [PubMed] [Google Scholar]
- Myers AM, Pape KL, Tzagoloff A (1985) Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J 4: 2087–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C, Wilmes C, Tatsuta T, Langer T (2007) Prohibitins interact genetically with Atp23, a novel processing peptidase and chaperone for the F1F0-ATP synthase. Mol Biol Cell 18: 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MF, Velours J, Arselin de Chateaubodeau G, Aigle M, Guerin B (1989) The yeast ATP synthase subunit 4, structure and function. Eur J Biochem 185: 163–171 [DOI] [PubMed] [Google Scholar]
- Rak M, Tetaud E, Godard F, Sagot I, Salin B, Duvezin-Caubet S, Slonimski PP, Rytka J, di Rago JP (2007) Yeast cells lacking the mitochondrial gene encoding the ATP synthase subunit 6 exhibit a selective loss of complex IV and unusual mitochondrial morphology. J Biol Chem 282: 10853–10864 [DOI] [PubMed] [Google Scholar]
- Rak M, Tzagoloff A (2009) F1-dependent translation of mitochondrially encoded Atp6p and Atp8p subunits of yeast ATP synthase. Proc Natl Acad Sci USA 106: 18509–18514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Schatz G (1968) Impaired binding of mitochondrial adenosine triphosphatase in the cytoplasmic ‘petite' mutant of Saccharomyces cerevisiae. J Biol Chem 243: 2192–2199 [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16: 339–346 [DOI] [PubMed] [Google Scholar]
- Steele DF, Butler CA, Fox TD (1996) Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc Natl Acad Sci USA 93: 5253–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock D, Gibbons C, Arechaga I, Leslie AG, Walker JE (2000) The rotary mechanism of ATP synthase. Curr Opin Struct Biol 10: 672–679 [DOI] [PubMed] [Google Scholar]
- Tzagoloff A (1969) Assembly of the mitochondrial membrane system. II. Synthesis of the mitochondrial adenosine triphosphatase. F1. J Biol Chem 244: 5027–5033 [PubMed] [Google Scholar]
- Tzagoloff A (1971) Assembly of the mitochondrial membrane system. Role of mitochondrial and cytoplasmic protein synthesis in the biosynthesis of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem 246: 3050–3056 [PubMed] [Google Scholar]
- Tzagoloff A, Akai A, Needleman RB, Zulch G (1975) Assembly of the mitochondrial membrane system. Cytoplasmic mutants of Saccharomyces cerevisiae with lesions in enzymes of the respiratory chain and in the mitochondrial ATPase. J Biol Chem 250: 8236–8242 [PubMed] [Google Scholar]
- Tzagoloff A, Barrientos A, Neupert W, Herrmann JM (2004) Atp10p assists assembly of Atp6p into the F0 unit of the yeast mitochondrial ATPase. J Biol Chem 279: 19775–19780 [DOI] [PubMed] [Google Scholar]
- Walker JE (1998) ATP synthesis by rotary catalysis (Nobel Lecture). Angew Chem Int Ed Engl 37: 2309–2313 [DOI] [PubMed] [Google Scholar]
- Walker JE, Cozens AL (1986) Evolution of ATP synthase. Chem Scr 26B: 263–272 [Google Scholar]
- Wittig I, Karas M, Schägger H (2007) High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol Cell Proteomics 6: 1215–1225 [DOI] [PubMed] [Google Scholar]
- Zeng X, Barros MH, Shulman T, Tzagoloff A (2008) ATP25, a new nuclear gene of Saccharomyces cerevisiae required for expression and assembly of the Atp9p subunit of mitochondrial ATPase. Mol Biol Cell 19: 1366–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Neupert W, Tzagoloff A (2007) The metalloprotease encoded by ATP23 has a dual function in processing and assembly of subunit 6 of mitochondrial ATPase. Mol Biol Cell 18: 617–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.