Abstract
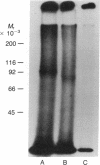
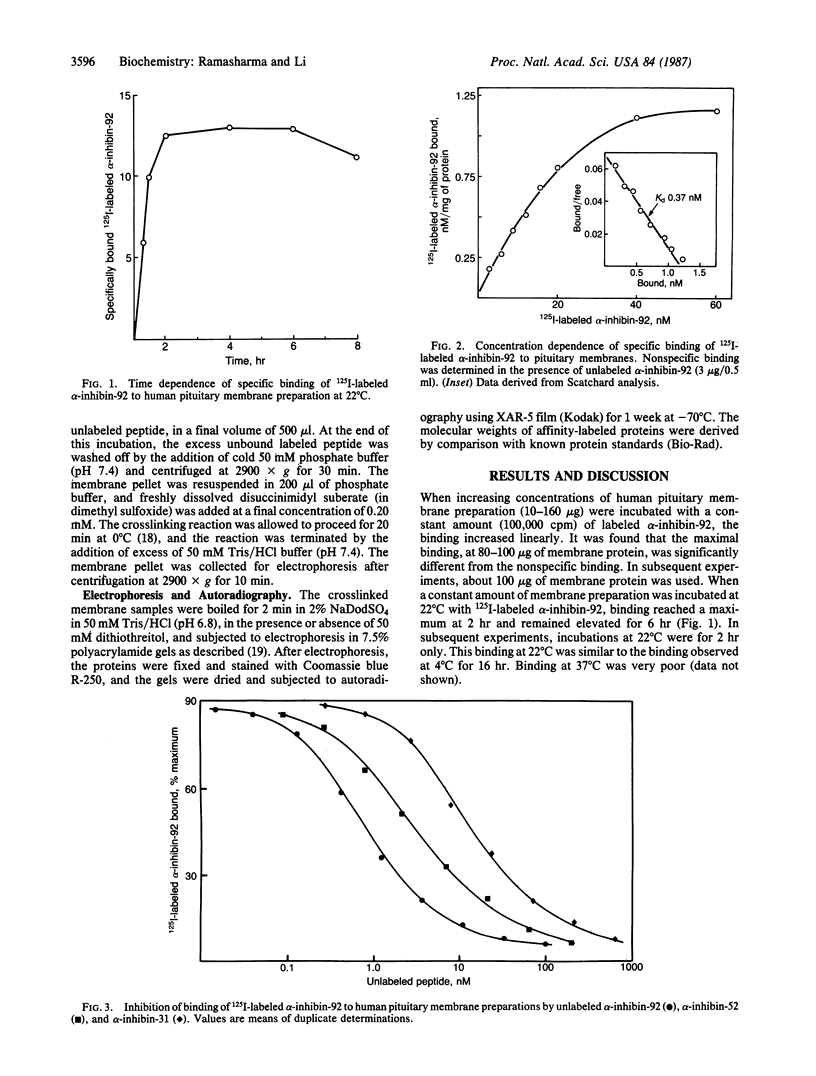
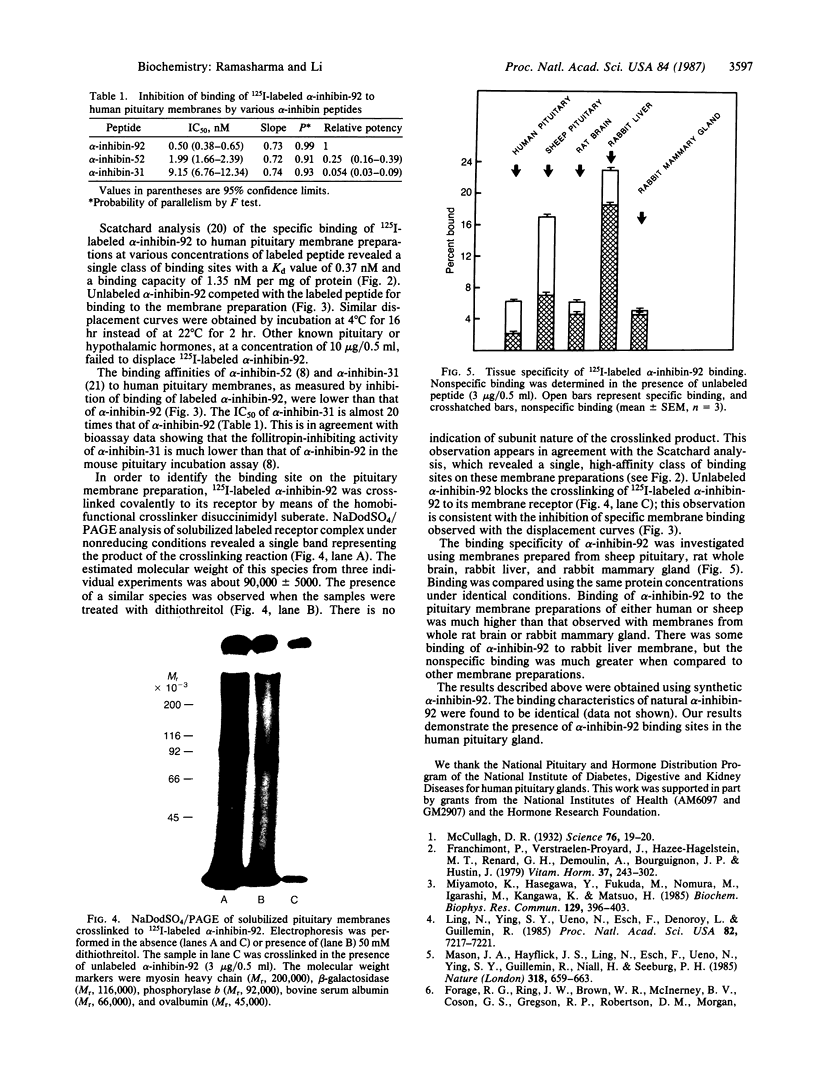
We investigated the binding of 125I-labeled alpha-inhibin-92 (a 92-residue peptide) to human pituitary membrane preparations. Unlabeled alpha-inhibin-92 competed effectively with the labeled peptide for binding to the membranes. Binding was also inhibited by both alpha-inhibin-52 and alpha-inhibin-31, but less effectively. Scatchard analysis of the alpha-inhibin-92 binding data indicated the presence of high-affinity binding sites (1.35 nM/mg of membrane protein) with an apparent Kd of 0.37 nM. When 125I-labeled alpha-inhibin-92 was covalently crosslinked to the pituitary membrane preparation with disuccinimidyl suberate and the solubilized labeled receptor complex was analyzed by NaDodSO4/PAGE under either reducing or nonreducing conditions, a single radioactive band at an apparent molecular weight of 90,000 +/- 5000 was observed. These data suggest that human pituitary has specific binding sites for alpha-inhibins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forage R. G., Ring J. M., Brown R. W., McInerney B. V., Cobon G. S., Gregson R. P., Robertson D. M., Morgan F. J., Hearn M. T., Findlay J. K. Cloning and sequence analysis of cDNA species coding for the two subunits of inhibin from bovine follicular fluid. Proc Natl Acad Sci U S A. 1986 May;83(10):3091–3095. doi: 10.1073/pnas.83.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont P., Verstraelen-Proyard J., Hazee-Hagelstein M. T., Renard C., Demoulin A., Bourguignon J. P., Hustin J. Inhibin: from concept to reality. Vitam Horm. 1979;37:243–302. doi: 10.1016/s0083-6729(08)61071-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li C. H., Hammonds R. G., Jr, Ramasharma K., Chung D. Human seminal alpha inhibins: isolation, characterization, and structure. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4041–4044. doi: 10.1073/pnas.82.12.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Esch F., Denoroy L., Guillemin R. Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7217–7221. doi: 10.1073/pnas.82.21.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Ling N., Esch F., Ueno N., Ying S. Y., Guillemin R., Niall H., Seeburg P. H. Complementary DNA sequences of ovarian follicular fluid inhibin show precursor structure and homology with transforming growth factor-beta. Nature. 1985 Dec 19;318(6047):659–663. doi: 10.1038/318659a0. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Niall H. D., Seeburg P. H. Structure of two human ovarian inhibins. Biochem Biophys Res Commun. 1986 Mar 28;135(3):957–964. doi: 10.1016/0006-291x(86)91021-1. [DOI] [PubMed] [Google Scholar]
- McCullagh D. R. DUAL ENDOCRINE ACTIVITY OF THE TESTES. Science. 1932 Jul 1;76(1957):19–20. doi: 10.1126/science.76.1957.19. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Fukuda M., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of porcine follicular fluid inhibin of 32K daltons. Biochem Biophys Res Commun. 1985 Jun 14;129(2):396–403. doi: 10.1016/0006-291x(85)90164-0. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Ramasharma K., Li C. H. Human seminal alpha-inhibins: detection in human pituitary, hypothalamus, and serum by immunoreactivity. Proc Natl Acad Sci U S A. 1986 May;83(10):3484–3486. doi: 10.1073/pnas.83.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasharma K., Sairam M. R., Seidah N. G., Chrétien M., Manjunath P., Schiller P. W., Yamashiro D., Li C. H. Isolation, structure, and synthesis of a human seminal plasma peptide with inhibin-like activity. Science. 1984 Mar 16;223(4641):1199–1202. doi: 10.1126/science.6422553. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Frazier G. R. Statistical analysis of radioligand assay data. Methods Enzymol. 1975;37:3–22. doi: 10.1016/s0076-6879(75)37003-1. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Ranganathan M. R., Ramasharma K. Binding of an inhibin-like protein from bull seminal plasma to ovine pituitary membranes. Mol Cell Endocrinol. 1981 May;22(2):251–264. doi: 10.1016/0303-7207(81)90095-2. [DOI] [PubMed] [Google Scholar]
- Seethalakshmi L., Steinberger A., Steinberger E. Pituitary binding of 3H-labeled Sertoli cell factor in vitro: a potential radioreceptor assay for inhibin. Endocrinology. 1984 Oct;115(4):1289–1294. doi: 10.1210/endo-115-4-1289. [DOI] [PubMed] [Google Scholar]
- Steinberger A., Seethalakshmi L., Kessler M., Steinberger E. Binding of 3H-Sertoli cell factor to rat anterior pituitary in vitro. Endocrinology. 1982 Aug;111(2):696–698. doi: 10.1210/endo-111-2-696. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]