Abstract
Here we present the 100% complete assignment chemical shift of non-labile 1H, 15N and 13C nuclei of Calbindin D9k P43G. The assignment includes all non-exchangeable side chain nuclei, including ones that are rarely reported, such as LysNζ as well as the termini. NMR experiments required to achieve truly complete assignments are discussed. To the best of our knowledge our assignments for Calbindin D9k extend beyond previous studies reaching near-completeness (Vis et al. in Biochem 33:14858–14870, 1994; Yamazaki et al. in J Am Chem Soc 116:6464–6465, 1994; Yamazaki et al. in Biochem 32:5656–5669, 1993b).
Keywords: Calbindin D9k, 100% Complete assignment, Assignment strategy, NMR spectroscopy
Biological context
Calbindin D9k is a small monomeric protein (Mr 8.5 kDa, 76 amino acids) which belongs to the EF-hand family, and consists of two helix-loop-helix motifs that bind one calcium ion each (Kretsinger and Nockolds 1973). Calbindin D9k undergoes small structural changes upon calcium-binding, involving the rearrangement of non-polar side chains (Ikura 1996). The protein is predominantly found in the mammalian epithelial cells of the small intestine and placenta, and it has been implicated to facilitate the transport of calcium across the intestinal epithelial cells (Christakos et al. 1989).
High resolution three dimensional structures of Calbindin D9k in various calcium-loaded states have been characterized extensively by X-ray crystallography and solution state NMR spectroscopy (Kordel et al. 1997; Kordel et al. 1993; Szebenyi and Moffat 1986). Although the complete resonance assignment of 13C, 15N and 1H nuclei for this protein was not used for structure determination, it will facilitate a comprehensive study of its dynamics, structure, dihedral angle distributions and electrostatic interactions, as well as supplying data for comparisons with solid-state NMR and chemical shift calculations by quantum chemical methods.
Methods and experiments
MM294 E. coli cells transformed by the PCBWR plasmid containing the calbindin Pro43Gly gene (Bos taurus) were used for protein expression. A single colony was picked from agar plate and grown overnight in 100 mL LB medium with ampicillin at 30°C. 20 mL of the overnight culture was added to 500 mL minimal medium containing U-13C-glucose and U-15N ammonium chloride at 30°C. Protein production was started by ten-fold dilution of the cells into medium containing U-13C glucose, U-15N ammonium chloride and 0.1 mg/mL IPTG at 37°C. Purification of Calbindin D9k P43G was performed as in a previous study (Thulin 2002).
All experiments (see Table 1) were carried out on Varian Unity INOVA 500 and 600 MHz spectrometers equipped with pulsed field gradient probes. The spectra were recorded at 301 K. The NMR sample contained ~2.5 mM [13C,15N]-enriched Calbindin D9k P43G, pH 6.0, 7% D2O. The spectra were processed using NMRPipe (Delaglio et al. 1995) and analyzed using Sparky (Goddard and Kneller 2003).
Table 1.
List of experiments
| No | Experiments | Connectivities | Experimental time (h) |
|---|---|---|---|
| 1 | 1H–15N HSQC-SE-wfba,b,c | NH–HN | 0.2 |
| 2 | Sensivity-enhanced HA(CA)COd,e | Hα–C′ | 0.6 |
| 3 | 3D–HN(C′)Nf | NH(i)–HN(i)–NH(i+1) | 20.5 |
| NH(i)–HN(i)–NH(i) | |||
| 4 | 3D–HNNf | NH(i)–HN(i)–NH(i) | 55.8 |
| NH(i)–HN(i)–NH(i−1) | |||
| NH(i)–HN(i)–NH(i+1) | |||
| 5 | H(N)COg | HN(i)–C′(i−1) | 0.3 |
| 6 | HACA(N)h | Hα(i)–Cα(i) | 1.75 |
| 7 | H2(C)Ni | NH–Hα | 0.3 |
| Nζ–Hε(Lysine) | |||
| N–Hα (Proline) | |||
| 8 | H2(CA)Ni | Nterminus–Hα | 0.6 |
| 9 | 3D 1H–15N–TOCSY−HSQC,j,k,l,m | NH–(i)HN(i)–all aliphatic side chain protons (i) | 8.25 |
| 10 | 3D HCCH–COSYn,o | C(i)–H(i)–H(i) (through one bond coupling of aliphatic resonances) | 17 |
| 11 | 3D C–TOCSY–N(C)H2i | Hε–Nε and all side chain carbons of lysine | 20 |
| 12 | 3D H(CCO)NH–TOCSY | NH(i)–HN(i)–all aliphatic side chain protons (i−1) | 21.25 |
| 13 | 3D (H)C(CO)NH–TOCSYq | NH(i)–HN(i)–all aliphatic side chain carbons (i–1) | 14 |
| 14 | 1H–13C constant time HSQCr | C(i)–H(i) of aliphatic resonances | 0.2 |
| 15 | (HBGCBG)CO(CBGCABCON)Ht | Cγ(i)–HN(i+1)for asparagine and aspartic acid | 4 |
| Cδ(i)–HN(i+1)for glutamine and glutamatic acid | |||
| 16 | H2(C)COu | C′–Hα | 2 |
| Cγ–Hβ (for asparagine and aspartate) | |||
| Cδ–Hγ (for glutamate and glutamine) | |||
| 17 | 3JsNCγ | Cγ(i)–HN(i) | 8.6 |
| Cβ(i)–HN(i) | |||
| Cα(i)–HN(i) | |||
| Cα(i−1)–HN(i)(if i−1 is glycine) | |||
| Cγ(i)–Hε2(i) for glutamine | |||
| Cβ(i)–Hδ2(i) for asparagine | |||
| 18 | 3JsC′Cγ | C′(i−1)–HN(i) | 2.16 |
| Cγ(i−1)–HN(i) | |||
| Cβ(i)–HN(i) | |||
| Cβ(i−1)–HN(i) | |||
| Cα(i−1)–HN(i) (for proline) | |||
| 19 | 3D1H–13C HSQC NOESYv | C(i)–H(i)-all protons within 5Å | 38 |
| 20 | 3D HCCH-COSY aromatico,p | C–H–H | 16.8 |
| 21 | CG(CB)HBw | Cγ–Hβ for aromatic side chain | 9.3 |
| 22 | CB(CGCD)HDx | Cβ–Hδ for aromatic side chain | 10.8 |
| 23 | CB(CGCDCE)HEx | Cβ–Hε for aromatic side chain | 10.8 |
| 24 | 1H–13C HSQC aromaticr | Cδ–Hδ | 0.8 |
| Cε–Hε | |||
| Cζ–Hζ | |||
| for aromatic side chain | |||
| 25 | 1H–13C HSQC CP aroy | Cδ–Hδ | 7 |
| Cδ–Hε | |||
| Cε–Hε | |||
| Cε–Hζ | |||
| Cζ–Hζ | |||
| for aromatic side chain | |||
| 26 | 1H–13C HMQC aromaticz | Cδ–Hδ | 0.3 |
| Cε–Hε | |||
| Cζ–Hζ | |||
| for aromatic side chain |
aCavanagh et al. 1991; b Palmer et al. 1991; c Palmer et al. 1992; d Kay et al. 1990b; e Powers et al. 1991; f Panchal et al. 2001; g Muhandiram and Kay 1994; h Ottiger and Bax 1997; i Andre et al. 2007; j Fesik and Zuiderweg 1990; k Marion et al. 1989a; l Marion et al. 1989b; m Zhang et al. 1994; n Ikura et al. 1991; o Kay et al. 1990a; p Ikura et al. 1991; q Logan et al. 1993; r Vuister and Bax 1992; s Konrat et al. 1997; t Tollinger et al. 2002; u Oda et al. 1994; v Majumdar and Zuiderweg 1993; w Prompers et al. 1998; x Yamazaki et al. 1993a; Y Zuiderweg et al. 1996; z Bax et al. 1990
Assignments and data deposition
Relation to previous assignment (BMRB entry 327): Only 1H signals of Calbindin D9k P43G had been assigned. Here the 100% complete assignment of non-labile 1H, 13C and 15N signals for calcium-loaded D9k P43G was achieved using an extensive suit of standard and non-standard 2D and 3D NMR experiments.
Backbone and aliphatic side chains
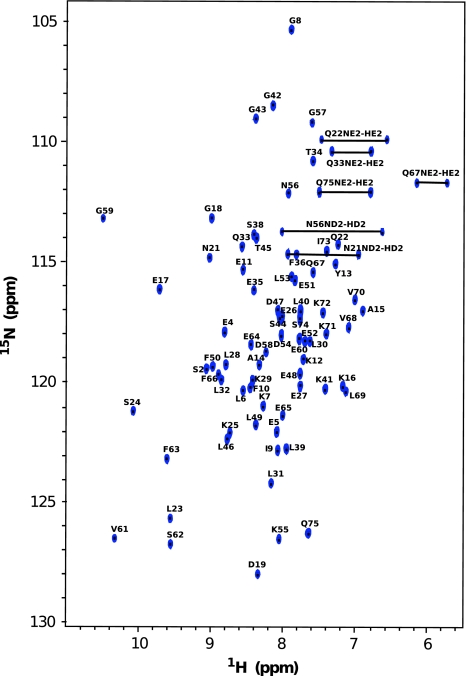
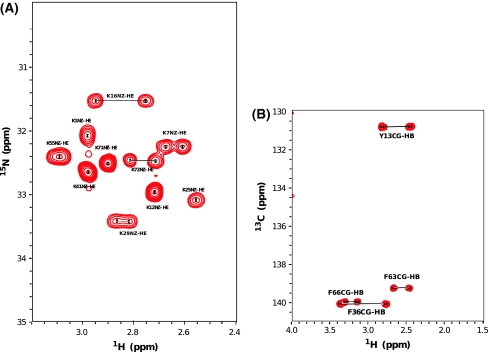
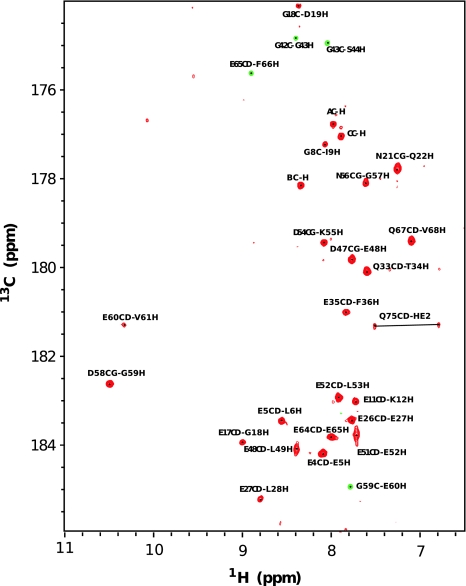
Backbone assignments were obtained using 3D experiments such as HN(C′)N and HNN to obtain the amide proton and nitrogen chemical shifts (Fig. 1 displays the dispersion of NH–HN chemical shift of Calbindin D9k from 1H–15N HSQC). Several 2D projections of triple resonance experiments; H(N)CO, HA(CA)CO, and HACA(N) were recorded to assign the carbonyl, alpha proton and alpha carbon resonances. Most aliphatic side chain signals were assigned using 3D experiments; (H)C(CO)NH-TOCSY and H(CCO)NH-TOCSY, which correlate the backbone nitrogen and amide proton shifts to aliphatic carbon and proton frequencies of residue i − 1. For residues which are preceded by proline, the side chain nuclei were assigned using a 3D 15N–1H TOCSY-HSQC experiment which correlates NH, HN, and proton aliphatic side chain in the same residue. The HCCH-COSY experiment, which gives information about chemical shifts of protons bound to carbon, enabled us to verify the assignment for long side chains like lysine, leucine, glutamate, isoleucine, valine and proline. The specific assignment of lysine Nζ were obtained using the 2D H2(C)N experiment. The signals in this spectrum are well dispersed between 31 and 34 ppm (see Fig. 2a). The 15N shift of the N terminal methionine residue was obtained using 2D H2(C)N pulse sequence where the final shaped carbon inversion pulse was replaced by a full power rectangular 180º pulse (Andre et al. 2007). The carbonyl side chains of glutamate, glutamine, aspragine and aspartate were detected using (HBGCBG)CO(CBGCABCACON)H experiments which correlates the side chain carbonyl of residue i to the amide proton of residue i+1 (see Fig. 3). This experiment is very powerful to get the unambiguous chemical shift assignment of carbonyl/carboxyl side chains due to the excellent dispersion of amide proton chemical shifts in folded proteins. However, the sensitivity of the experiments was not sufficient to detect all signals (Q22 was absent) and a H2(C)CO experiment was used to detect the Q22 Cδ–Hγ correlation.
Fig. 1.
2D 1H–15N HSQC spectrum of uniformly 15N/13C-labelled Calbindin D9K. All peaks are annotated with the one letter amino acid symbol and their position in the sequence. All amide proton and nitrogen nuclei in the backbone of Calbindin D9k were observed. The 15Nε and 1Hε chemical shits of glutamine and the chemical shifts of 15Nδ and 1Hδ of asparagines are also indicated
Fig. 2.
Some specific assignments of Calbindin D9k side chains. a 2D H2(C)N spectrum showing Lys Nζ–Hε correlations. b 2D CG(CB)HB spectrum to assign aromatic side chain resonances
Fig. 3.
2D 1H–13C (HBGCBG)CO(CBGCABCACON)H spectrum of uniformly labelled 15N/13C Calbindin D9k. Correlation can be observed for carboxyl/carbonyl side chain 13C′ of glutamate, glutamine, asparagine and aspartate of residue i with the amide proton of residue i+1. Peaks labeled AC–H, BC–H and CC–H refer to carbonyl and amide proton peaks from the soluble cyclic enterobacterial common antigen, ECACYC (Erbel et al. 2003)
Aromatic side chains
Calbindin D9k P43G contains 5 phenylalanines and 1 tyrosine residue. Some specific strategies were required to assign the aromatic ring 1H and 13C resonances. Aromatic Cγ resonances were assigned using a combination of CG(CB)HB, 3JNCγ and 3JC′Cγ experiments. The sequence-specific side chain 1H assignment of the aromatic side chains was obtained via CB(CGCD)HD and CB(CGCDCE)HE experiments. The information of Hδ and Hε in the aromatic rings from these experiments were used to assign 1H–13C HSQC CP aro, aromatic 1H–13C CT HSQC and 1H–13C HMQC aromatic experiment. F10 Cδ–Hδ was only observed in the non constant time 1H–13C HSQC or 1H–13C HMQC experiment due to the strong coupling within the aromatic ring. A 3D 1H–13C HSQC-NOESY experiment was used to verify the assignment of the aromatic side chains.
To summarize, the 1H, 13C and 15N resonance assignments of Calbindin D9k P43G have been deposited in the BioMagResBank (accession number 16340). Although complete side chain resonance assignments were obtained for Calbindin D9k P43G, it should be mentioned that it does not contain any cysteine, arginine, histidine and tryptophan residues. Those residues, in particular, require specific strategies for their side chain assignment. For arginine guanidine groups, sequence specific assignment of 15N and 1H chemical shifts have been presented by Yamazaki et al. 1995, and for histidine and tryptophan ring, sequence specific assignment of 1H, 13C, 15N have been established by Löhr et al. (2005).
Our study shows that complete assignments of all NMR-active nuclei in small protein can be obtained and describes a suitable strategy for this purpose. In particular, lysine Nζ chemical shifts appear to be difficult to get correct, as witnessed by the BMRB database. Currently (grid update of August 16th, 2010) 110 chemical shift assignments for lysine Nζ are available, but this list contains as many as 25 erroneous assignments. In three cases, a 1H chemical shift was entered for Nz and in 22 instances chemical shifts between 67 and 133 ppm have been listed, either as a result of exchanging the assignment with that of backbone nuclei, or by the incorrect account of spectral aliasing. Even today, the lysine Nζ statistics are heavily polluted and yield 47.8 ± 32.9 ppm for the full set. A restricted set of 14 entries now gives 34.1 ± 3.0 ppm, as opposed to 73.8 ± 50.3 ppm for 7 entries in 2004.
Acknowledgments
We thank Eldon Ulrich at the BioMagRes Bank for helpful discussion.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Andre I, Linse S, Mulder FAA. Residue-specific pKa determination of lysine and arginine side chains by indirect 15N and 13C NMR spectroscopy: application to apo calmodulin. J Am Chem Soc. 2007;129:15805–15813. doi: 10.1021/ja0721824. [DOI] [PubMed] [Google Scholar]
- Bax A, Ikura M, Kay LE, Torchia DA, Tschudin R. Comparison of different modes of two-dimensional reverse-correlation nmr for the study of proteins. J Magn Reson. 1990;86:304–318. [Google Scholar]
- Cavanagh J, Palmer AG, Wright PE, Rance M. Sensitivity improvement in proton-detected two-dimensional heteronuclear relay spectroscopy. J Magn Reson. 1991;91:429–436. [Google Scholar]
- Christakos S, Gabrielides C, Rothen WB. Vitamin D-dependent calcium binding proteins: chemistry, distribution, functional considerations, and molecular biology. Endocr Rev. 1989;10:3–26. doi: 10.1210/edrv-10-1-3. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRpipe—a multidimensional spectral processing system based on unix pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Erbel PJA, Barr K, Gao N, Gerwig GJ, Rick PD, Gardner KH. Identification and biosynthesis of cyclic enterobacterial common antigen in escherichia coli. J Bacteriol. 2003;185:1995–2004. doi: 10.1128/JB.185.6.1995-2004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesik SW, Zuiderweg ERP. Heteronuclear three-dimensional nmr-spectroscopy of isotopically labeled biological macromolecules. Q Rev Biophys. 1990;23:97–131. doi: 10.1017/S0033583500005515. [DOI] [PubMed] [Google Scholar]
- Goddard TD, Kneller DG (2003) SPARKY 3. University of California, San Francisco
- Ikura M. Calcium binding and conformational response in EF-hand proteins. Trends in Biochem Sci. 1996;21:14–17. [PubMed] [Google Scholar]
- Ikura M, Kay LE, Bax A. Improved three-dimensional 1H–13C-1H correlation spectroscopy of a 13C-labeled protein using constant-time evolution. J Biomol NMR. 1991;1:299–304. doi: 10.1007/BF01875522. [DOI] [PubMed] [Google Scholar]
- Kay LE, Ikura M, Bax A. Proton proton correlation via carbon carbon couplings—a three-dimensional NMR approach for the assignment of aliphatic resonances in proteins labeled with carbon-13. J Am Chem Soc. 1990;112:888–889. doi: 10.1021/ja00158a070. [DOI] [Google Scholar]
- Kay LE, Ikura M, Tschudin R, Bax A. Three-dimensional triple-resonance NMR-spectroscopy of isotopically enriched proteins. J Magn Reson. 1990;89:496–514. doi: 10.1016/j.jmr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Konrat R, Muhandiram DR, Farrow NA, Kay LE. Pulse schemes for the measurement of 3JC′Cγ and 3JNCγ in 15N, 13C uniformly labeled proteins. J Biomol NMR. 1997;9:409–422. doi: 10.1023/A:1018354712430. [DOI] [PubMed] [Google Scholar]
- Kordel J, Skelton NJ, Akke M, Chazin WJ. High-resolution solution structure of calcium-loaded calbindin-D9k. J Mol Biol. 1993;231:711–734. doi: 10.1006/jmbi.1993.1322. [DOI] [PubMed] [Google Scholar]
- Kordel J, Pearlman DA, Chazin WJ. Protein solution structure calculations in solution: solvated molecular dynamics refinement of calbindin D9 k. J Biomol NMR. 1997;10:231–243. doi: 10.1023/A:1018383102870. [DOI] [PubMed] [Google Scholar]
- Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein: II. Structure determination and general description. J Biol Chem. 1973;248:3313–3326. [PubMed] [Google Scholar]
- Logan TM, Olejniczak ET, Xu RX, Fesik SW. A general-method for assigning NMR-spectra of denatured proteins using 3D HC(CO)NH-TOCSY triple resonance experiments. J Biomol NMR. 1993;3:225–231. doi: 10.1007/BF00178264. [DOI] [PubMed] [Google Scholar]
- Löhr F, Rogov VV, Shi M, Bernhard F, Dötsch V. Triple-resonance methods for complete resonance assignment of aromatic protons and directly bound heteronuclei in histidine and tryptophan residues. J Biomol NMR. 2005;32:309–328. doi: 10.1007/s10858-005-1195-4. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Zuiderweg ERP. Improved 13C-Resolved HSQC-NOESY spectra in H2O, using pulsed-field gradients. J Magn Reson Series B. 1993;102:242–244. doi: 10.1006/jmrb.1993.1093. [DOI] [Google Scholar]
- Marion D, Driscoll PC, Kay LE, Wingfield PT, Bax A, Gronenborn AM, Clore GM. Overcoming the overlap problem in the assignment of proton nmr spectra of larger proteins by use of three-dimensional heteronuclear proton and nitrogen hartmann-hahn multiple quantum coherence and nuclear overhauser multiple quantum coherence spectroscopy—application to interleukin 1.beta. Biochemistry. 1989;28:6150–6156. doi: 10.1021/bi00441a004. [DOI] [PubMed] [Google Scholar]
- Marion D, Kay LE, Sparks SW, Torchia DA, Bax A. Three dimensional heteronuclear NMR of nitrogen-15 labeled proteins. J Am Chem Soc. 1989;111:1515–1517. doi: 10.1021/ja00186a066. [DOI] [Google Scholar]
- Muhandiram DR, Kay LE. Gradient-enhanced triple-resonance 3-dimensional NMR experiments with improved sensitivity. J Magn Reson Series B. 1994;103:203–216. doi: 10.1006/jmrb.1994.1032. [DOI] [Google Scholar]
- Oda Y, Yamazaki T, Nagayama K, Kanaya S, Kuroda Y, Nakamura H. Individual ionization-constants of all the carboxyl groups in ribonuclease HI from Escherichia-Coli determined by NMR. Biochemistry. 1994;33:5275–5284. doi: 10.1021/bi00183a034. [DOI] [PubMed] [Google Scholar]
- Ottiger M, Bax A. An empirical correlation between amide deuterium isotope effects on 13Cα chemical shifts and protein backbone conformation. J Am Chem Soc. 1997;119:8070–8075. doi: 10.1021/ja9707466. [DOI] [Google Scholar]
- Palmer AG, Cavanagh J, Wright PE, Rance M. Sensitivity improvement in proton-detected two-dimensional heteronuclear correlation NMR-spectroscopy. J Magn Reson. 1991;93:151–170. [Google Scholar]
- Palmer AG, Fairbrother WJ, Cavanagh J, Wright PE, Rance M. Improved resolution in three-dimensional constant-time triple resonance NMR spectroscopy of proteins. J Biomol NMR. 1992;2:103–108. doi: 10.1007/BF02192804. [DOI] [PubMed] [Google Scholar]
- Panchal SC, Bhavesh NS, Hosur RV. Improved 3D triple resonance experiments, HNN and HN(C)N, for HN and 15 N sequential correlations in (13C, 15N) labeled proteins: application to unfolded proteins. J Biomol NMR. 2001;20:135–147. doi: 10.1023/A:1011239023422. [DOI] [PubMed] [Google Scholar]
- Powers R, Gronenborn AM, Clore GM, Bax A. 3-Dimensional triple-resonance NMR of 13C/15N enriched proteins using constant-time evolution. J Magn Reson. 1991;94:209–213. [Google Scholar]
- Prompers JJ, Groenewegen A, Hilbers CW, Pepermans HAM. Two-dimensional NMR experiments for the assignment of aromatic side chains in 13C-labeled proteins. J Magn Reson. 1998;130:68–75. doi: 10.1006/jmre.1997.1277. [DOI] [PubMed] [Google Scholar]
- Szebenyi DM, Moffat K. The refined structure of vitamin D-dependent calcium-binding protein from bovine intestine. Molecular details, ion binding, and implications for the structure of other calcium-binding proteins. J Biol Chem. 1986;261:8761–8777. [PubMed] [Google Scholar]
- Thulin E. Purification of recombinant calbindin D9k. Totowa: Humana Press inc; 2002. [DOI] [PubMed] [Google Scholar]
- Tollinger M, Forman-Kay JD, Kay LE. Measurement of side-chain carboxyl pKa values of glutamate and aspartate residues in an unfolded protein by multinuclear NMR spectroscopy. J Am Chem Soc. 2002;124:5714–5717. doi: 10.1021/ja020066p. [DOI] [PubMed] [Google Scholar]
- Vis H, Boelens R, Mariani M, Stroop R, Vorgias CE, Wilson KS, Kaptein R. 1H, 13C, and 15 N resonance assignments and secondary structure analysis of the HU protein from Bacillus stearothermophilus using two- and three-dimensional double- and triple-resonance heteronuclear magnetic resonance spectroscopy. Biochemistry. 1994;33:14858–14870. doi: 10.1021/bi00253a025. [DOI] [PubMed] [Google Scholar]
- Vuister GW, Bax A. Resolution enhancement and spectral editing of uniformly 13C enriched proteins by homonuclear broad-band 13C decoupling. J Magn Reson. 1992;98:428–435. [Google Scholar]
- Yamazaki T, Forman-Kay JD, Kay LE. 2-Dimensional NMR experiments for correlating carbon-13Beta and proton-delta/epsilon chemical-shifts of aromatic residues in 13C-labeled proteins via scalar couplings. J Am Chem Soc. 1993;115:11054–11055. doi: 10.1021/ja00076a099. [DOI] [Google Scholar]
- Yamazaki T, Yoshida M, Nagayama K. Complete assignments of magnetic resonances of ribonuclease-H from escherichia coli by double-resonance and triple-resonance 2D and 3D NMR spectroscopies. Biochemistry. 1993;32:5656–5669. doi: 10.1021/bi00072a023. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Lee W, Revington M, Mattiello DL, Dahlquist FW, Arrowsmith CH, Kay LE. An HNCA pulse scheme for the backbone assignment of 15N, 13C, 2H-labeled proteins—application to a 37-kDa trp repressor DNA complex. J Am Chem Soc. 1994;116:6464–6465. doi: 10.1021/ja00093a069. [DOI] [Google Scholar]
- Yamazaki T, Pascal SM, Singer AU, Forman-Kay JD, Kay LE. NMR pulse scheme for the sequence-specific assignment of arginine guanidino 15N and 1H chemical shifts in proteins. J Am Chem Soc. 1995;117:3556–3564. doi: 10.1021/ja00117a025. [DOI] [Google Scholar]
- Zhang OW, Kay LE, Olivier JP, Forman-Kay JD. Backbone 1H and 15N resonance assignments of the N-terminal Sh3 Domain of drk in folded and unfolded states using enhanced-sensitivity pulsed-field gradient NMR techniques. J Biomol NMR. 1994;4:845–858. doi: 10.1007/BF00398413. [DOI] [PubMed] [Google Scholar]
- Zuiderweg ERP, Zeng L, Brutscher B, Morshauser RC. Band-selective hetero- and homonuclear cross-polarization using trains of shaped pulses. J Biomol NMR. 1996;8:147–160. doi: 10.1007/BF00211161. [DOI] [PubMed] [Google Scholar]