Abstract
Originally called retinoid X receptor interacting protein 14 (RIP14), the farnesoid X receptor (FXR) was renamed after the ability of its rat form to bind supra-physiological concentrations of farnesol. In 1999 FXR was de-orphanized since primary bile acids were identified as natural ligands. Strongly expressed in the liver and intestine, FXR has been shown to be the master transcriptional regulator of several entero-hepatic metabolic pathways with relevance to the pathophysiology of conditions such as cholestasis, fatty liver disease, cholesterol gallstone disease, intestinal inflammation and tumors. Furthermore, given the importance of FXR in the gut-liver axis feedbacks regulating lipid and glucose homeostasis, FXR modulation appears to have great input in diseases such as metabolic syndrome and diabetes. Exciting results from several cellular and animal models have provided the impetus to develop synthetic FXR ligands as novel pharmacological agents. Fourteen years from its discovery, FXR has gone from bench to bedside; a novel nuclear receptor ligand is going into clinical use.
Introduction
Nuclear receptors (NRs) are ligand-activated transcription factors that, in response to lipophilic hormones, vitamins, and dietary lipids, regulate many aspects of mammalian physiology, including development, reproduction and metabolism [Chawla et al., 2001; Mangelsdorf et al., 1995].
In 1995, using a two-hybrid yeast system, Seol et al. identified several mouse liver cDNA sequences encoding for proteins able to interact with the ligand binding domain (LBD) of the human nuclear receptor RXRα [Seol et al., 1995]. Among these proteins, a novel protein termed RIP14 (RXR-interacting protein 14) turned out to be unique in its interaction with RXRα. By northern blot analysis, RIP14 expression was detected in kidney and liver, and two isoforms of RIP14 proteins were discriminated. RIP14-1 had a 4-amino acid (MYTG) central insertion, while RIP14-2 presented a 38-amino acid NH2-terminal extension. RIP14 was able to bind as a heterodimer with RXRα to previously identified hormone responsive elements (HREs), such as the retinoic acid response element (RARE) from the promoter of the RARβ2 isoform and the ecdysome response element (EcRE) from the Drosophila hsp27 promoter. The RIP14-RXRα heterodimer was shown to bind to direct repeats with a 5-, 4- and 2-bp spacer (DR5, DR4 and DR2, respectively) and inverted repeats IR0 and IR1, but not to DR0, DR1, DR3, IR2 and IR5. Importantly, the fact that, in reporter assays, RIP14 was unable to transactivate a reporter containing multiple copies of the βRARE, suggested that RIP14 activity was ligand-dependent. However, the natural ligand for RIP14 was unknown at that time. Later, in 1995, Forman et al. were able to clone from a rat liver cDNA library the rat homolog of the mouse RIP14 and they showed that farnesol, an intermediate of the mevalonate pathway, was able to activate rat RIP14 at the concentration of 50 µM [Forman et al., 1995]. For this reason, rat RIP14 was renamed farnesoid X receptor (FXR) and its expression was detected in the liver, kidney, intestine and adrenal cortex. However, supra-physiological concentrations of farnesol were required to induce FXR activity.
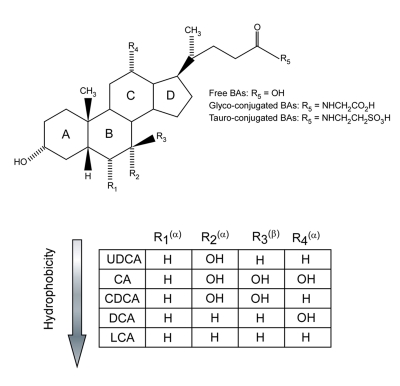
In 1999, FXR was “de-orphanized” when it was demonstrated that primary bile acids (BAs) were the endogenous ligands for FXR [Makishima et al., 1999; Parks et al., 1999; Wang et al., 1999]. Indeed, FXR was also named BAR (bile acid receptor). BAs were shown to activate FXR at physiological concentrations using an in vitro coactivator recruitment assay. BAs are steroid-end products of cholesterol catabolism and are classified as either primary BAs (chenodeoxycholic acid (CDCA) and cholic acid (CA)), synthesized from cholesterol in the liver, or secondary BAs (deoxycholic acid (DCA) and lithocholic acid (LCA)), produced by intestinal bacteria from primary BAs. The majority of circulating BAs are conjugated with glycine or taurine, by a process occurring in the liver (for details about BA homeostasis, see below). The chemical structure of BAs is shown in Figure 1.
Figure 1. Structure and hydrophobic/hydrophilic profile of the most common bile acids.
Cholic acid (CA) and chenodeoxycholic acid (CDCA) are primary BAs. Deoxycholic acid (DCA) and lithocholic acid (LCA) are secondary BAs. Ursodeoxycholic acid (UDCA) is a primary BA predominantly produced in bears. Hydroxyl groups that are in α-orientation are located below the steroid nucleus and are axial to the plane of the steroid nucleus. Hydroxyl groups that are in β-orientation are located above the steroid nucleus and are equatorial to the plane of the steroid nucleus. The hydrophobicity increases as follows: UDCA, CA, CDCA, DCA, LCA.
The generation of FXR knock-out mice revealed a clear role for FXR in vivo as the master regulator of BA homeostasis [Kok et al., 2003; Sinal et al., 2000]. The generation of FXR knock-out mice was a breakthrough for understanding the role of FXR in many BA-related physiological and pathophysiological conditions. Loss of FXR resulted in an enlarged BA pool size as a consequence of increased expression of CYP7A1, the rate-limiting enzyme for the conversion of cholesterol to BAs. Interestingly, FXR knock-out mice also exhibited elevated levels of cholesterol and triglycerides in both plasma and liver. These observations suggested a key role for FXR also in lipid metabolism. Indeed, it was shown in 2004 that FXR activation is required to reduce sterol-regulatory-element-binding-protein-1c (SREBP1-c) expression, a key molecule involved in lipogenesis [Watanabe et al., 2004]. In the same year, it was shown that the loss of FXR in mice predisposes to gallstone disease, while its activation may ameliorate this condition by restoring the right biliary BA:cholesterol ratio [Moschetta et al., 2004]. Before these findings, the use of FXR as a target for the management of cholestasis was also proposed [Liu et al., 2003].
In addition to BA and lipid metabolism, it has been shown that FXR also plays an important role in glucose homeostasis by improving insulin sensitivity and glucose tolerance in diabetic mice [Ma et al., 2006; Zhang et al., 2006a]. Furthermore, in the intestine, FXR is required to protect the epithelium by preventing bacterial overgrowth and consequent mucosa deterioration and bacterial translocation [Inagaki et al., 2006]. Finally, FXR also plays a role in liver regeneration [Huang et al., 2006], and both hepatic [Kim et al., 2007b; Yang et al., 2007] and intestinal tumorigenesis [Maran et al., 2009; Modica et al., 2008].
By regulating BAs, lipid and glucose homeostasis, FXR affects many aspects of our metabolism. This makes FXR an attractive pharmacological target for the management of diseases ranging from hyperlipidemia to diabetes, from cholestasis to enterohepatic tumors.
FXR gene and protein structure
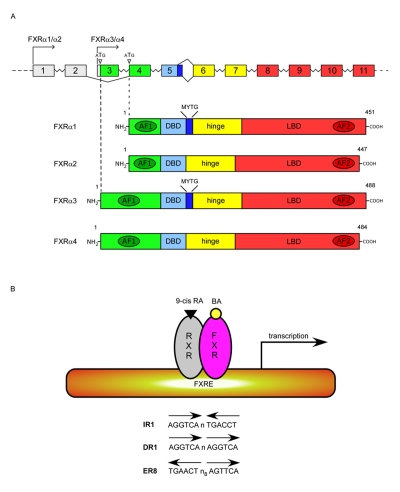
Two FXR genes are known and are referred to as FXRα (NR1H4) and FXRβ (NR1H5). FXRβ is a pseudogene in humans, while it is expressed in rodents, rabbits and dogs. It has been proposed to be a lanosterol sensor, although its physiological function remains to be established [Otte et al., 2003]. The FXRα gene is conserved from fish (teleost fish, Fugu rubriens) to humans, which suggests a crucial role for this gene in many species [Maglich et al., 2003]. In humans and mice, FXRα is highly expressed in the liver, intestine, kidney and adrenal gland, while low expression has been detected in the heart and adipose tissue [Zhang et al., 2003]. The FXRα gene is mapped to human chromosome 12q23.1 and mouse chromosome 10c.2, and it is composed of 11 exons and 10 introns (Figure 2). As a single gene, FXRα encodes four transcript isoforms in both humans and mice, FXRα1 (RIP14-2), FXRα2, FXRα3, and FXRα4 (RIP14-1), as a result of alternative splicing of exon 5 and the use of two distinct promoters that initiate transcription from either exon 1 (5' promoter) or exon 3 (3' promoter) [Huber et al., 2002; Zhang et al., 2003]. The 5' or 3' promoters of the FXRα gene regulate the expression of FXRα1 and FXRα2 or FXRα3 and FXRα4 transcripts, respectively. Unlike FXRα2 and FXRα4, FXRα1 and FXRα3 transcripts contain four amino acids (MYTG) immediately adjacent to the DNA-binding domain (DBD) in a region referred to as the hinge domain. Notably, the four FXRα isoforms are expressed in a tissue-specific manner and few FXRα target genes are regulated in an isoform-dependent manner [Lee et al., 2005; Lee et al., 2006; Zhang et al., 2003]. FXRα1 and FXRα2 are moderately expressed in the adrenal gland and ileum, while FXRα3 and FXRα4 are highly expressed in the ileum, moderately in the kidney and at low levels in the duodenum and jejunum. Some genes such as the ileal bile acid binding protein (IBABP) and fibroblast growth factor 19 (FGF19) are more responsive to the FXRα2 and FXRα4 isoforms (lacking the MYTG motif) than FXRα1 and FXRα3 isoforms [Zhang et al., 2003].
Figure 2. Structure and transcripts of the mouse FXRα gene.
A) Similar to the human FXRα gene (mapped to chromosome 12 (q23.1)), the mouse FXRα gene (mapped to chromosome 10 (c2)) is characterized by 11 exons and two distinct promoters that initiate transcription from either exon 1 or exon 3. Four distinct transcripts, FXRα1, FXRα2, FXRα3, FXRα4 are generated from the same gene, as a consequence of the alternative splicing of exon 5 and the use of two distinct promoters. ATG indicate the translational starting sites. In blue is indicated the alternative splicing of the 12 bp of exon 5 that encode the MYTG motif in the hinge region. The four FXRα transcripts are shown with the different classical NR receptor domains color coded. B) Upon ligand binding, FXRα binds to FXR response elements (FXRE) of its target genes as a heterodimer with RXR. Examples of consensus sequences are shown.
As members of the NR superfamily, the four FXRα proteins (grouped hereafter with the term of FXR) encoded by the single FXRα gene (referred to hereafter as FXR) present a typical NR structure organized in a highly conserved DBD, a poorly conserved N-terminal domain and a moderately conserved C-terminal LBD (Figure 2). In the absence of BAs, FXR sits on the HRE of its target genes as a heterodimer with the obligate partner RXR, in association with corepressor proteins. Located in the DBD, two cysteine-coordinated Zn2+ finger motifs are responsible for DNA binding and dimerization. The FXR/RXR heterodimer mainly binds IR1 (two canonical AGGTCA inverted repeats with one space nucleotide) and can be activated by either FXR- or RXR-specific ligands. Besides IR-1, other HREs for FXR are direct repeated such as DR-1 and everted repeated such as ER-8. Ligand binding induces conformational changes of FXR that determine the release of corepressor proteins such as NCor (nuclear corepressor), and the recruitment of coactivator proteins, such as SRC-1 (steroid receptor coactivator 1), PGC1α (peroxisome-proliferator-receptor (PPAR)-γ coactivator-1α) [Savkur et al., 2005b; Zhang et al., 2004], CARM-1 (coactivator associated arginine (R) methyl transferase-1) [Ananthanarayanan et al., 2004; Bauer et al., 2002], PMRT-1 (protein arginine (R) methyl transferase-1) [Rizzo et al., 2005], and DRIP-205 (vitamin-D-receptor-interacting protein-205) [Pineda Torra et al., 2004]. In addition to the LBD, the C-terminal region of FXR contains a ligand-dependent transactivation domain, AF2, that along with a ligand-independent transactivation domain, AF1, in the N-terminal region, interacts with coregulatory proteins.
The LBD of FXR contains a hydrophobic pocket that accommodates lipophilic molecules such as BAs. Crystal structures of the human and rat FXR LBD in complex with two CDCA derivatives ((3-deoxy-CDCA and 6α-ethyl-chenodeoxycholic acid (6α-ECDCA)), the synthetic ligand fexaramine and a coactivator peptide have been solved [Downes et al., 2003; Mi et al., 2003], showing that the amino acids involved in the ligand binding are conserved between human and rat. Moreover, these studies revealed that, beside the classical features of the LBD of NRs, such as its general organization in a 12-α helix bundle, the LBD of FXR presents peculiar characteristics. Similar to other NRs, the LBD of FXR acts as a molecular switch after ligand binding, undergoing conformational changes that result in the recruitment of coactivator proteins by forming a “charge clamp” and a hydrophobic groove that interacts with the LXXLL motifs of the coactivators [Downes et al., 2003; Mi et al., 2003]. Unlike steroid NRs, the LBD of FXR accommodates the steroid nucleus of its ligands in a reverse orientation. Moreover, a second docking site for the LXXLL motifs has been found in the LBD of FXR [Mi et al., 2003]. Consequently, a coactivator with a single LXXLL motif binds to the FXR LBD with low affinity, while coactivators with two or more LXXLL motifs exhibit higher affinity. The FXR LBD binds the two LXXLL motifs in an anti-parallel manner with one motif located in the classical groove and blocked by a “charge clamp”, and the other motif located in the helix 3, without being secured by a “charge clamp”. It has been suggested that the FXR LBD uses both LXXLL surfaces to interact with a single coactivator protein, with one of them increasing its affinity for the coactivator. However, this is a hypothesis that needs to be confirmed.
Genetic variability of FXR
The paucity of nonsynonymous single nucleotide polymorphisms (SNPs) in FXR indicates that this is an important and evolutionarily conserved gene. Two nonsynonymous SNPs have been detected in exon 6 at the level of the hinge region (C643T and G646T). In Chinese- and Hispanic-Americans, two synonymous polymorphisms have been also detected in exon 7 (C783T) and 11 (C1341T), although with very low genotypic frequency. Conversely, a G-1T polymorphism located in the Kozak consensus motif of the exon 3 sequence flanking the start codon is relatively common among European-, Chinese-, African- and Hispanic-Americans [Marzolini et al., 2007].
Although this SNP results in reduced transactivation activity, it is not clear whether this effect is due to decreased transcription/translation or the formation of a low-activity, translational variant. Consistent with the in vitro studies, reduced hepatic expression of the FXR target gene small heterodimer partner (SHP) was observed [Marzolini et al., 2007]. While it remains unclear how the decreased expression of SHP in subjects with G-1T polymorphism would manifest, studies in SHP knock-out mice showed that the lack of SHP leads to increased hepatic insulin sensitivity [Ballatori et al., 2005]. Moreover, Japanese patients with mutations in the SHP gene present hypertriglyceridemia and mild obesity [Nishigori et al., 2001].
It is worth noting that the expression of another FXR target gene, the ATP-binding cassette (ABC) transporter bile salt export pump (BSEP/ABCB11) was not changed. Thus, if there is an effect of FXR polymorphisms, this seems to be target gene-dependent.
It has been suggested that mutations in the FXR gene may also predispose subjects to intrahepatic cholestasis of pregnancy (ICP). Four heterozygous variants have been identified in patients with ICP [Van Mil et al., 2007], with three of them showing a functional defect in translational efficiency or activity. Moreover, an FXR haplotype (NR1H4_1) in the Mexican population has been associated with gallstone disease [Kovacs et al., 2008]. Taken together, the few data available on FXR polymorphism indicate that this is an important and evolutionarily-conserved gene with rare relevant mutations.
Natural and synthetic FXR ligands
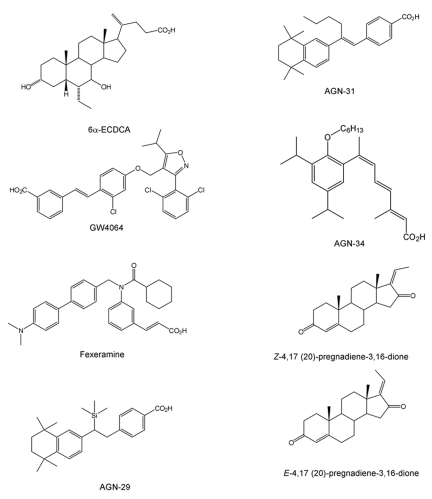
Naturally-occurring BAs differ in minor structural modifications. Nevertheless, these differences have impact on the affinity for FXR and ability to activate it. Taking advantage of the knowledge of the structure-activity relationship of BAs for the FXR LBD, semisynthetic and synthetic molecules have been made to obtain more selective and potent FXR activators than BAs. Examples of semisynthetic, synthetic and natural ligands are reported in Figure 3.
Figure 3. FXR ligands.
Examples of FXR semisynthetic (6α-ECDCA), synthetic (GW4064, Fexeramine, AGN-29, AGN-31, AGN-34) and natural (Z-4,17(20)-pregnadiene-3,16-dione and E-4,17(20)-pregnadiene-3,16-dione) ligands.
Bile acids and semisynthetic bile acid derivatives
The chemical structure of BAs consists of a steroid nucleus with an acidic side chain (Figure 1). They are amphipatic molecules with a unique shape characterized by a concave hydrophilic face (α face) and a convex hydrophobic face (β face). The hydrophobic pocket of the FXR LBD interacts with BAs mainly through the β face. The α face contains several hydroxyl groups in the 3, 7 and/or 12α positions that impacts on the ability to activate FXR [Fujino et al., 2004]. Optimal FXR activation through ligand binding requires the proper positioning of helix 3 versus helix 12 in order to generate the LXXLL docking groove. The 7α-hydroxy group confers high capacity to CDCA and synthetic ligands, such as fexaramine and 6α-ECDCA (see below), to activate FXR by inducing a highly stable interaction between helix 3 and helix 12, resulting in a strong agonist activity. Conversely, the absence of a 7α-hydroxy group, in the case of DCA and LCA, compromises stable coactivator recruitment and results in partial agonistic properties. Finally, the FXR LBD cannot accommodate a 12α-hydroxy group and consequently, CA and DCA have low affinity for FXR. The hydrophobic pocket of the LBD contains a series of hydrophobic cavities that can be exploited to first generate further contacts, and then to increase the affinity of the ligand for the FXR LBD. Thus, 6α-ECDCA has a higher affinity than CDCA for FXR. Notably, the carboxyl extremity of BAs is oriented towards the entry of the hydrophobic pocket of the LBD, indicating why conjugated BAs conserve a high affinity for FXR and the ability to activate it [Pellicciari et al., 2006]. Structural modifications of the carboxyl terminus of BAs may result in altered FXR activity.
The agonist or antagonist properties of a NR ligand are generally evaluated by the recruitment to the LBD of an SRC-1 derivate peptide containing the typical LXXLL motif in a cell-free fluorescence energy transfer (FRET) assay. In this way, it was shown that CDCA and its glycol- and tauro-conjugates were able to recruit a peptide from the SRC-1 coactivator (EC50=3.5 µM) to the FXR LBD [Makishima et al., 1999; Parks et al., 1999; Pellicciari et al., 2002; Yu et al., 2002]. On the contrary, DCA, LCA, CA and their conjugates were not able to induce significant coactivator recruitment up to the concentration of 1000 µM (100 µM for DCA), but importantly, they interfered in a dose-dependent fashion with the ability of CDCA to induce FXR-SRC-1 interaction [Lew et al., 2004; Makishima et al., 1999; Parks et al., 1999; Yu et al., 2002]. These data indicate that DCA, LCA, CA and their conjugates act as partial antagonists in this assay. Next, LCA was proposed as an FXR antagonist with partial agonist activity because of its ability to antagonize the CDCA promoting association between FXR and SRC-1 in the cell-free assay, which correlated with antagonist activity on FXR transactivation and the partial induction of BSEP [Yu et al., 2002]. Finally, ursodeoxycholic acid (UDCA), a hydrophilic BA found in large quantities in bear, was shown to act as a very weak FXR agonist for the induction of BSEP expression and reduction of CYP7A1 expression [Lew et al., 2004].
In addition to cell-free assays, cell-based assays were carried out to evaluate the ability of different BAs to activate FXR. Initial transfection studies were consistent in indicating CDCA as the most potent FXR activator (EC50=50 µM), while DCA and LCA activated FXR with a lower efficacy than CDCA [Makishima et al., 1999]. Hydrophilic unconjugated BAs, such as CA and UDCA, could not activate FXR, and moreover, UDCA turned out to be a partial antagonist, able to inhibit activation by CDCA [Campana et al., 2005]. Interestingly, in the same transfection assays, the glycol- and tauro-CDCA, -DCA and -LCA were likewise unable to activate FXR. After cotransfection of the apical sodium-dependent BA transporter (ASBT, (SLC10A2)), an apical protein required for the active uptake of conjugated BAs, the ability of glycol- and tauro-CDCA, -DCA and -LCA were likewise unable to activate FXR. After ASBT cotransfection, CA and its conjugates were also able to cross the cell membrane and activate FXR [Wang et al., 1999].
It is worth noting that apart from the cotransfection assays where ASBT was present and a rat form of FXR was used [Wang et al., 1999], there is no convincing evidence showing that CA is a direct ligand of FXR. However, the FXR LBD is not perfectly conserved between species. This makes, for instance, the mouse FXR LBD much more responsive to CA than its human counterpart [Cui et al., 2002]. In line with CA being of more physiological importance in mice, they do not have CDCA, the most potent natural activator of human FXR, which is converted to β-MCA.
Taken together, the cell-free and cell-based assays indicate weak correlation between the affinity of BAs for FXR, their ability to induce FXR LBD coactivator interaction and their biological activity. Thus, cell-free assays for coactivator recruitment analysis are not predictive of the transcriptional outcome or the transcriptional outcome depends on many parameters, such as DNA binding sequences and dimerization partners, as suggested for FXR [Lew et al., 2004] and other NRs [Molavi et al., 2006]. It is also possible that the SRC-1 coactivator assay is not the best investigative model.
Chemical manipulations of the side chain and the steroid nucleus of BAs have been exploited to generate semisynthetic BA derivatives that could be more potent FXR activators. The use of these semisynthetic molecules in transcriptional and ligand-binding assays has generated important information regarding the structure-activity relationship, confirming to some extent the crystallographic studies on the structure of the FXR LBD. The conversion of the 24-carboxyl group of CDCA and CA to the corresponding alcohol, generated compounds with FXR ligand-like activities, similar to CDCA [Fujino et al., 2004]. It is worth noting that while CA can activate FXR only if the ASBT protein is expressed, the corresponding alcohol derivative can activate FXR in the absence of ASBT, indicating that the conversion of the 24-carboxyl group into the corresponding alcohol increases its transport across the cell membrane by passive diffusion.
With respect to the steroid nucleus of BAs, the importance of the C3 and C12 hydroxyl groups was also addressed [Fujino et al., 2004]. The corresponding 3β and 12β epimers of DCA and LCA were shown to be completely inactive in transactivation assays. Similarly, the introduction of a hydrophobic alkyl group at the 3β and 12β position made the BA derivatives inactive. It is noteworthy that 3β,7α-dihydroxy-3α-methyl-5β-cholan-24-oic acid was able to activate FXR as well as CDCA, indicating that the 2α hydroxyl group of CDCA is not involved in FXR activation.
The ability to activate FXR by early intermediates produced in the BA biosynthetic pathway was also evaluated [Nishimaki-Mogami et al., 2004]. The molecules maintaining an intact cholesterol side chain were inactive, while cholestanoic acids, as well as 25- and 26-hydroxylated bile alcohols, were able to activate FXR to the same extent as CDCA. Since these intermediates in the BA biosynthetic pathway are evolutionary precursors of BAs in mammals, it may be that the affinity for them to FXR has been conserved during evolution.
In a small library of BA derivatives, obtained by modification of the ring B to improve the chemical properties/activity relationship, the 6α-methyl derivate of CDCA was identified [Pellicciari et al., 2002]. With respect to CDCA, this compound exhibited a tenfold higher activity as an FXR agonist. This finding indicated the presence of a hydrophobic pocket in the LBD of FXR corresponding to the 6α position of BAs. In line with this hypothesis, 6α-alkyl derivates such as 6α-ethyl, n-propyl and n-butyl derivatives were generated. Among these compounds, 6α-ECDCA (INT-747) was the most potent FXR activator in a FRET assay. Its activity in this transcriptional assay was three times higher than CDCA with EC50=99 nM [Pellicciari et al., 2002]. Other modifications in the ring B or in the acidic side chain of BAs generated an array of interesting derivatives for the modulation of FXR activity, but none was superior to 6α-ECDCA [Pellicciari et al., 2004].
Natural extracts
Guggulipid, the extract from the gum resin of Commiphora mukul, is known from traditional Indian medicine for its hypolipidemic properties consisting of reduced serum levels of cholesterol, low-density lipoprotein (LDL)-cholesterol and triglycerides (TGs). The active principle of guggulipid responsible for these metabolic effects is the guggulsterone, a mixture of Z- and E-4, 17(20)-pregnadiene-3, 16-dione. From the studies on guggulsterone, it is now clear that its metabolic effects are mediated by an array of NRs [Urizar and Moore, 2003]. Thus, both Z- and E-guggulsterone interact with members of the endocrine NR subfamily, including glucocorticoid receptor (GR), mineralocorticoid receptor (MR), androgen receptor (AR), progesterone receptor (PR) and estrogen receptor α (ERα) [Burris et al., 2005], as well as members of the adopted orphan NR subfamily, such as pregnane X receptor (PXR) [Brobst et al., 2004] and FXR. Regarding FXR [Cui et al., 2003; Urizar et al., 2002; Wu et al., 2002], it has been shown by transfection experiments that both Z- and E-guggulsterone are unable to activate this NR, while they can interfere with CDCA activity. These antagonistic properties were confirmed in a cell-free coactivator binding assay, where guggulsterone fails to recruit SRC-1 peptide [Urizar et al., 2002; Wu et al., 2002]. Nevertheless, guggulsterone was shown to enhance CDCA and GW4064 (a selective and potent synthetic FXR agonist) induced BSEP expression [Cui et al., 2003]. Thus, despite its lack of selectivity, guggulsterone might represent an example of a gene-selective modulator for FXR.
Other natural extracts contain FXR modulators. Cafestol, a diterpene isolated from unfiltered coffee brew, has been shown to have agonistic effects on FXR and PXR in HepG2 cells, similar to those of CDCA and pregnenolone 16-α carbonitrile (PCN), but it was unable to induce hepatic FXR target genes in vivo. [Ricketts et al., 2007]. Indeed, the hypercholesterolemic properties of cafestol are due to its ability to induce the fibroblast growth factor 15 (FGF15) in the small intestine, which by repressing CYP7A1, would induce accumulation of cholesterol in the body [Nilsson et al., 2007]. Furthermore, stigmasterol, a soy lipid-derived phytosterol, was able to interfere with the induction of FXR target genes in HepG2 cells [Carter et al., 2007]. Finally, the prenylated chalcone xanthohumol, derived from beer hop, was shown to reduce serum and hepatic TGs levels in animal models of diabetes and to induce BSEP expression in hepatoma cells, suggesting that this molecule may be an FXR agonist [Nozawa, 2005].
Synthetic ligands
Since BAs do not display strong selectivity for FXR, scientists were prompted to develop synthetic molecules with higher affinity and selectivity for this NR. A lead compound in this field is represented by the TTNPB compound, a synthetic retinoid with low affinity for FXR identified in coactivator recruitment assays [Parks et al., 1999; Zavacki et al., 1997]. Starting from the structure of the TTNPB, different stilbene derivatives were synthesized [Love et al., 2002; Maloney et al., 2000]. Among these, GW9047 exhibits good selectivity, but low affinity for FXR. Modulations of the structure of GW9047 led to GW4064, a selective and potent activator of FXR (EC50=90 nM) that is still seen as the reference compound. This synthetic molecule is an FXR agonist active both in vitro and in vivo, but unfortunately it displays a limited bioavailability that precludes the possibility of using it in clinical phases. Starting again from TTNBP, new modifications on its backbone, to avoid retinoic acid receptor (RAR) binding, yielded other FXR ligands, such as AGN29, AGN31 and AGN34 [Dussault et al., 2003]. While AGN29 and AGN31 exhibit agonistic properties for both FXR and RXR with an EC50=1 µM, AGN34 turned out to be an FXR and RXR antagonist. The benzopyran structure was also used as a starting point to generate FXR agonists and the biaryl cinnamate derivates such as fexaramine and fexarine resulted as selective and potent FXR agonists with an EC50=38 nM and EC50=36 nM, respectively [Downes et al., 2003]. Interestingly, gene expression analysis on primary human hepatocytes revealed that CDCA, GW4064 and fexaramine activated FXR target genes differently, suggesting that these FXR ligands may also act as gene–selective FXR modulators. This means that they can modulate the expression of various FXR target genes in different manners, either as agonists or antagonists. This phenomenon could be explored to develop FXR modulators in which therapeutic effects may be separated by unwanted side effects. Finally, 1,1-biphosphonate esters were shown to activate FXR with anti-proliferative and hypocholesterolemic effects [Niesor et al., 2001].
FXR as more than a bile acid sensor
For a long time the physiological effects of BAs were attributed exclusively to their physico-chemical properties, which allow them to form fat-solubilizing micelles for the absorption of lipophilic nutrients. Besides this well-established role in dietary lipid absorption, in the last few years it has been revealed that BAs also behave as signaling molecules (hormones) with systemic endocrine functions. BAs are in fact ligands for both the G-protein-coupled receptor (GPCR) TGR5 [Maruyama et al., 2002; Watanabe et al., 2006] and the nuclear receptor FXR [Makishima et al., 1999; Parks et al., 1999; Wang et al., 1999]. Activation of the BA-TGR5 pathway increases energy expenditure in brown adipose tissue, which would be beneficial against obesity and insulin resistance. Through activation of their sensor FXR, BAs can regulate not only their own homeostasis, but also cholesterol, TG, and glucose metabolism. Thus, the BA-regulated transcriptional pathways via TGR5 and FXR are promising, novel drug targets for the management of metabolic diseases such as diabetes, hyperlipidemia, gallstone disease, cholestasis and enterohepatic tumors.
FXR and bile acid metabolism
BAs are amphipathic molecules synthesized in the liver from cholesterol by multiple enzymatic steps occurring in different hepatic cellular compartments, such as the cytosol, mitochondria, endoplasmic reticulum and peroxisomes [Russell, 2003]. This is the so called “classical” pathway. How the nascent BAs can shuttle from one compartment to another is still unknown. Newly-synthesized BAs are termed primary BAs and consist of CDCA and CA. The “classical” pathway in humans leads to an equal amount of CDCA and CA. An “alternative” pathway is responsible for the production of oxidized cholesterol molecules that are afterwards converted predominantly to CDCA in the liver [Chiang, 2002]. Before active secretion of BAs into the canalicular lumen, primary BAs are conjugated at the C24 carboxyl group with the amino acids taurine or glycine [Falany et al., 1994] (Figure 1). This amidation process is very efficient and it is important to make BAs less hydrophobic, less cytotoxic, and more readily secretable into bile [Vessey et al., 1977]. Thus, the majority of BAs present in bile are conjugated.
After a postprandial stimulus, BAs are released from the gall bladder into the duodenum to promote the absorption of dietary lipids and lipid soluble vitamins. Then, BAs travel along the intestine and at the distal ileum the majority of the BAs (95%) are actively absorbed and returned to the liver via the portal vein, to be re-secreted into the bile [Love and Dawson, 1998]. This process of BA recycling is called enterohepatic circulation and is of extreme importance in terms of energy saving, since BA biosynthesis is an energetically expensive process involving more than 15 enzymes [Love and Dawson, 1998]. Only 0.5 g of BAs per day is lost through feces. This loss is compensated by de novo hepatic synthesis.
During the enterohepatic circulation, conjugated BAs undergo a de-conjugation process in the distal intestine by bacterial enzymes. The de-conjugated BAs are then passively absorbed by enterocytes and returned to the liver where they will be re-conjugated. Primary BAs (CDCA and CA) are transformed from anaerobic bacteria present in the colon to secondary BAs (LCA and DCA, respectively). Also secondary BAs are passively re-absorbed from enterocytes to travel back to the liver where they will be conjugated. Thus, the total BA pool is represented by secondary and, for the majority, primary BAs. LCA, after uptake from the liver, undergoes a double conjugation, with glycine or taurine first, and then with a sulfonyl group. In this way, LCA is not efficiently absorbed in the distal ileum and thereby eliminated with the feces. This is beneficial since LCA is highly cytotoxic.
BAs are able to repress their own synthesis in a feed-back way through a synergistic mechanism that involves FXR in the liver and in the intestine. Under fasting conditions, BAs are synthesized from cholesterol in the liver and stored in the gall bladder, ready to be delivered in the small intestine after a postprandial stimulus. The rate-limiting step enzyme in the biosynthetic pathway for the conversion of cholesterol to BAs is the CYP7A1 enzyme, a member of the cytochrome P450 (CYP) superfamily [Russell, 2003]. The orphan nuclear receptor liver receptor homolog-1 (LRH-1) and liver X receptor (LXR), an oxysterol sensor, are two important inducers of CYP7A1 mRNA levels in rodents [Goodwin et al., 2000; Lee and Moore, 2008; Lehmann et al., 1997; Peet et al., 1998]. In human, on the other hand, LXR represses CYP7A1 [Goodwin et al., 2003]. The inhibition of CYP7A1 by LXR in human may represent a physiological strategy to suppress the absorption of dietary cholesterol in the intestine. BAs are in fact essential for the intestinal absorption of cholesterol. In line with this hypothesis, a high-cholesterol diet reduced the expression of CYP7A1 in the liver of African Green monkeys, a species that is similar to humans in cholesterol and lipoprotein metabolism [Rudel et al., 1994]. Thus, while rodents would deal with excessive cholesterol by stimulating CYP7A1 expression in order to increase the catabolism of cholesterol into BAs, humans may have evolved an alternative strategy, whereby the absorption of cholesterol in the intestine is blunted through a decrease in the BA pool as a result of CYP7A1 repression by LXR.
After feeding, BAs are delivered from the gall bladder in the duodenum, in order to travel through the enterohepatic circulation. Activation of FXR in the liver induces the expression of SHP, which interacts with LRH-1 and represses its activity [Goodwin et al., 2000; Lee and Moore, 2008; Lu et al., 2000]. As a consequence, SHP-LRH-1 interaction results in reduced expression of CYP7A1 and ultimately BAs synthesis. However, the fact that BAs are still able to repress CYP7A1 in SHP knock-out mice indicates that other mechanisms for the repression of this enzyme are present. Indeed, FXR, in the murine distal ileum, induces FGF15 (FGF19 is the human ortholog), a hormone secreted in the portal circulation that reaches the liver to bind to the fibroblast growth factor receptor 4 (FGFR4) and repress CYP7A1 via a c-jun N-terminal kinase-dependent pathway [Inagaki et al., 2005]. This mechanism constitutes a crosstalk between the intestine and liver for the regulation of BA synthesis. The use of tissue-specific FXR knock-out mice for the liver and intestine assessed the relative contribution of hepatic and intestinal FXR in the repression of CYP7A1 and revealed a more determinant role for the intestinal FXR [Kim et al., 2007a].
In addition to CYP7A1, another critical cytochrome, CYP8B1, is involved in the classical pathway of BA synthesis. This enzyme controls the CA:CDCA ratio by regulating the synthesis of CA. Just like CYP7A1, CYP8B1 is repressed by FXR via SHP [Zhang and Chiang, 2001], but in this case SHP interacts and represses the ability of the hepatic nuclear factor 4α (HNF-4α) to induce CYP8B1 expression. Moreover, as shown in hepatic specific FXR knock-out mice, CYP8B1 expression was repressed, probably via FGF15 [Kim et al., 2007a]. Notably, hepatic specific knock-out mice for LRH-1 exhibit reduced expression of CYP8B1, but not of CYP7A1. These findings suggest that hepatic LRH-1 is involved in the control of the composition of the BA pool, but not of its size, which is instead mainly regulated by the FXR-FGF15 pathway [Lee et al., 2008; Mataki et al., 2007].
Newly-synthesized BAs are conjugated with taurine or glycine and then actively secreted in the gall bladder. FXR regulates both of these critical processes. The amidation reaction requires an initial activation of BAs to CoA-thioesters by the BA CoA synthase (BACS). These activated BAs become the substrate for the BA-CoA:amino acid N-acetyltransferase (BAAT) enzyme, which is responsible for the conjugation with glycine or taurine [Solaas et al., 2000]. Conjugated BAs are potent natural activators of FXR [Parks et al., 1999], which positively regulates the expression of BACS and BAAT [Pircher et al., 2003]. Monoanionic- and dianionic-conjugated BAs are then actively secreted in the gall bladder by BSEP and the multidrug related protein 2 (MRP2/ABCC2), respectively. These transporters belong to the ABC transporter family and are both induced by FXR at the transcriptional level. Moreover, FXR activation also induces the expression of the multidrug protein 3/2 (MDR3/Mdr2, ABCB4/Abcb4), another ABC transporter involved in the biliary secretion of phosphatidylcholine [Modica et al., 2009] (Figure 4). The regulation of these ABC transporters is of critical importance in order to avoid BA accumulation in the liver and consequent hepatic injury. Indeed, mutations in the BSEP and MDR3 proteins are responsible for progressive familial intrahepatic cholestasis (PFIC) type 2 [Strautnieks et al., 1998] and 3 [de Vree et al., 1998], respectively (see below).
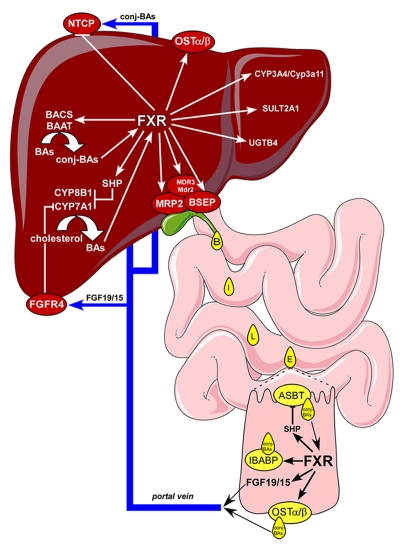
Figure 4. FXR regulates BA metabolism.
BAs that recirculate in the enterohepatic system activate hepatic and intestinal FXR to regulate genes important for BA metabolism. Hepatic FXR 1) represses BA synthesis by reducing CYP7A1 and CYP8B1 expression via SHP and 2) efficiently promotes BA conjugation (conj-BAs) with taurine or glycine via BACS and BAAT. FXR controls BA secretion in the small intestine by upregulating the expression of ABC transporters such as MRP2, BSEP and MDR3/Mdr2. When reaching the distal ileum, unconjugated BAs are passively reabsorbed, while conj-BAs are taken up by ASBT at the luminal membrane, shuttled to the basolateral membrane of the ileal enterocyte by the cytosolic transporter IBABP, then secreted in the portal blood via the OSTα/β transporter to travel back to the liver and be taken up by the NTCP transporter (repressed by FXR) and complete the enterohepatic circulation. In addition to regulating genes involved in active BA reabsorption in the distal ileum, activation of FXR by BAs also induces FGF19/15, a hormone that is secreted in the portal circulation and that signals to the liver through FGFR4 to repress CYP7A1. During cholestasis, when BAs reach high levels in the liver, FXR also induces OSTα/β to allow BAs to spill over from the liver in the systemic circulation for their final elimination with urine. Under these pathological conditions FXR also induces phase I (CYP3A4/Cyp3a11) and phase II (SULT2A1 and UGTB4) reactions to turn BAs into more hydrophilic and less toxic molecules that are efficiently excreted.
After postprandial stimulus, BAs are secreted from the gall bladder into the small intestine to allow the absorption of lipophilic nutrients. In the distal ileum, conjugated BAs are actively reabsorbed in the portal blood, while unconjugated BAs are only partially absorbed by passive diffusion. At the enterocyte brush border membrane, ASBT determines the uptake of BAs [Dawson et al., 2003; Oelkers et al., 1997]. Once BAs are taken up by ASBT, they are shuttled from the apical to the basolateral membrane by IBABP [Gong et al., 1994; Tochtrop et al., 2004; Toke et al., 2006]. Finally, BAs are secreted in the portal blood by the heterodimeric organic solute transporter α/β (OSTα/β [Dawson et al., 2005] (Figure 4). This complex process of active BA absorption is entirely orchestrated by FXR. Activation of FXR in the distal ileum downregulates the expression of ASBT, while expression of IBABP [Grober et al., 1999; Kok et al., 2003] and OSTα/β is induced[Landrier et al., 2006; Rao et al., 2008]. Notably, FXR directly induces IBABP and OSTα/β expression on the level of their promoters, while the expression of human ASBT is negatively regulated via SHP [Neimark et al., 2004].
Similar to the intestine, the active transport system for BAs absorption regulated by FXR also exists in the bile duct and kidney. In cholangiocytes, conjugated BAs are absorbed by ASBT at the canalicular membrane and secreted by MRP3 and OSTα/β at the basolateral membrane [Ballatori et al., 2005]. Also in the kidney, conjugated BAs are absorbed by ASBT, localized at the apical membrane of proximal renal tubular cells, and then secreted by OSTα/β from the basolateral membrane in the systemic circulation [Ballatori et al., 2005; Christie et al., 1996; Craddock et al., 1998]. Indeed, under normal conditions, BAs are not excreted into urine.
Although the adrenal gland cortex is not known to be involved in any aspect of BA metabolism, the expression of FXR has been detected at high levels also in this tissue [Forman et al., 1995]. Herein, OSTα and OSTβ were identified as FXR target genes by in vitro and in vivo approaches [Lee et al., 2006]. It has been proposed that when the serum levels of BAs increase, such as in cholestasis, OSTα and OSTβ would facilitate the uptake of conjugated BAs into the adrenal glands, resulting in a feed-forward activation of OSTα and OSTβ by FXR. Since OSTα and OSTβ form a multifunctional facilitative transporter, this heterodimer may also export specific substrates from the adrenal glands. In frog oocytes, OSTα and OSTβ have been shown to increase the efflux of dehydroepiandrosterone-3-sulfate [Ballatori et al., 2005]. The human adrenal cortex synthesizes and secretes dehydroepiandrosterone-3-sulfate, suggesting that also in humans the OSTα/β heterodimer export conjugated steroid intermediates from the adrenals glands into the blood while exchanging conjugated BAs. It is unknown whether apart from BAs, other endogenous molecules in the adrenal glands could activate FXR. For example, androsterone, a metabolite derived from adrenal steroids, has been reported to activate FXR [Howard et al., 2000]. However, since this activation is relatively weak, it remains unclear whether androsterone or other steroid intermediates are of physiological importance for FXR activation.
BAs are detergent-like molecules. Thus, despite their beneficial role in solubilizing lipophilic nutrients facilitating their intestinal absorption, accumulation of high levels of BAs, especially secondary BAs, can be detrimental. Notably, BA cytotoxicity increases linearly with the hydrophobic index [Moschetta et al., 2001]. Indeed, the order of increasing cytotoxicity is UDCA, CA, CDCA, DCA and LCA (Figure 1). The cytotoxic effects of BAs have been demonstrated in FXR knock-out mice which spontaneously develop hepatic tumors [Kim et al., 2007b; Yang et al., 2007]. Moreover, accumulation of BAs in the liver is believed to play a pivotal role in cholestasis-associated liver damage (see below). Thus, the concentration of BAs needs to be tightly regulated. As a BA sensor, FXR controls not only BAs synthesis, but also their metabolism and clearance. While the first process is evident under physiological conditions during the fast-fed state switch, the ability of FXR to induce BAs biotransformation and elimination is more evident under pathological conditions (e.g., cholestasis).
The biotransformation process of BAs consists of two phases occurring mainly in the liver. Phase I involves oxidation reactions, which generate more polar products. Then, phase II enzymes are responsible for the conjugation of phase I products with endogenous molecules to further increase their water solubility. At this point, conjugated BAs are polar enough to be easily eliminated from the body. However, they cannot exit the liver by passive diffusion. Thus, a phase III of active secretion is required. In this respect, BAs can be secreted into the bile by MDR3/Mdr2 and BSEP or into the systemic circulation by OSTα/β and MRP members for elimination through urine. This latter process occurs when secretion of BAs into bile is somehow impaired, as during cholestasis.
In the liver, CYP3A4/Cyp3a11 is the most important enzyme involved in phase I reactions. It catalyzes hydroxylation of BAs at different positions, leading to 3-oxo-, 1-β-, 6-α-, and 22-hydroxy Bas production [Araya and Wikvall, 1999; Bodin et al., 2005]. Activation of FXR by GW4064 results in the induction of CYP3A4 in HepG2 cells and Cyp3a11 in wild type mice, but not in FXR knock-out mice [Gnerre et al., 2004]. Furthermore, FXR can regulate phase II enzymes involved in glucuronidation and sulfonation reactions. FXR response elements have been found in the promoter of the SULT2A1 gene [Song et al., 2001], whose encoded protein generates 3-α-sulfated BAs. These BAs are low in healthy subjects [Podesta et al., 1980], while high levels are detected in cholestatic patients [Takikawa et al., 1983], indicating the protective role of SULT2A1. However, while FXR activation fails to induce SULT2A1 expression in HepG2 cells [Fang et al., 2005], the expression of UGT2B4 for the glucuronidation of 6α-hydroxilated BAs is upregulated [Barbier et al., 2003]. Interestingly, in colon carcinoma CaCO2 cells, FXR acts as negative regulator of the UGT2B7 gene, whose encoded enzyme is involved in the formation of 3-hydroxy-glucuronidated BAs [Lu et al., 2005]. Thus, FXR seems to control BA glucuronidation in a tissue- and isoform-specific manner.
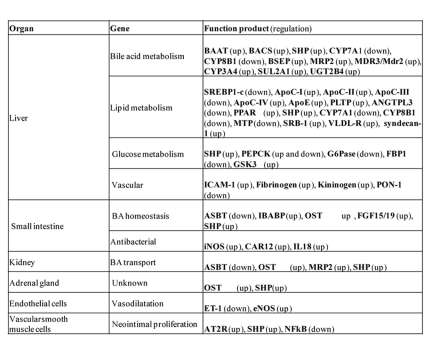
Under physiological conditions, bile is the preferential route used by the liver to secrete BAs. However, under cholestatic conditions, BAs are also secreted into the systemic circulation by an alternative basolateral export system, including OSTα/β, MRP3 and MRP4 [Dawson et al., 2005]. Under normal conditions, FXR induces the expression of canalicular BSEP and MRP2, but when BAs accumulate in the liver at toxic levels, FXR also induces the expression of basolateral OSTα/β for the secretion of BAs into the systemic circulation and finally urinary elimination. In the kidney, BAs are normally reabsorbed by the ASBT transporter localized at the apical membrane of proximal renal tubular cells and then secreted by the basolateral membrane OSTα/β into the systemic circulation [Ballatori et al., 2005; Christie et al., 1996; Craddock et al., 1998]. Thus, under healthy conditions, BAs are not excreted into urine. Conversely, during cholestasis, passive glomerular filtration of serum BAs and repression of ASBT expression [Lee et al., 2001] account for the loss of BAs through urine. Since FXR induces the expression of OSTα/β in the kidney [Lee et al., 2006], the use of a selective antagonist for FXR in the kidney could have a beneficial effect for patients with cholestasis. A list of FXR target genes is reported in Table 1.
Table 1. FXR target genes.
List of genes that are transcriptionally-regulated by FXR, with their tissue distribution and function.
FXR and cholesterol-triglyceride homeostasis
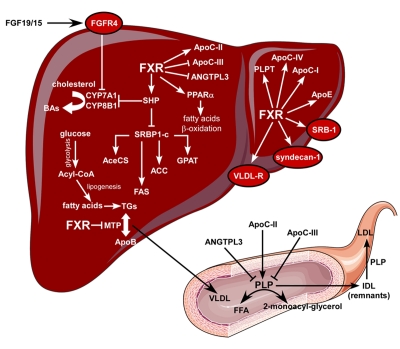
The downregulation of CYP7A1 via FXR signaling leads to decreased cholesterol catabolism with consequent accumulation of hepatic and serum cholesterol. Thus, the use of an FXR antagonist could represent a promising approach to decrease cholesterol levels by enhancing the conversion of cholesterol into BAs. In line with this idea, as mentioned previously, guggulsterone, a plant sterol used in Indian herbal medicine, has been shown to block FXR activity and reduce serum LDL levels, while increasing high-density lipoprotein (HDL) levels [Urizar et al., 2002]. Moreover, adenoviral CYP7A1 over-expression in LDL-receptor knock-out mice, which exhibit high levels of LDL-cholesterol, resulted in reduced LDL-cholesterol levels [Ratliff et al., 2006]. Taken together, these data suggest that FXR antagonists may be used for the management of atherosclerosis. However, the other side of the coin is the role that FXR plays in intestinal cholesterol absorption. It has been shown that FXR knock-out mice exhibit increased intestinal absorption of cholesterol, which indicates a negative regulatory role for FXR in intestinal cholesterol absorption [Lambert et al., 2003]. The increase in intestinal cholesterol absorption is probably due to increased solubilization of cholesterol in the gut via the increased amount of circulating BAs in these mice. Moreover, FXR activation seems to contribute to reverse cholesterol transport, a process that results in the delivery of cholesterol from peripheral tissues to the liver for biliary disposal and consequent fecal elimination [Lambert et al., 2003]. In this metabolic scenario, FXR regulates the expression of phospholipids transfer protein (PLPT), responsible for the transfer of phospholipids and cholesterol from LDL to HDL, hepatic lipoproteins, such as ApoE, ApoC-I, ApoC-IV, and scavenger receptor B1 (SRB1), which is involved in the hepatic uptake of HDL and is one of the major actors in the clearance of serum HDL cholesterol esters (Figure 5).
Figure 5. FXR regulates lipid metabolism.
By repressing CYP7A1 and CYP8B1 via SHP and FGF19, FXR activation may result in hepatic cholesterol accumulation. In the liver, FXR activation results in increased expression of PLPT for the transfer of phospholipids and cholesterol from LDL to HDL, SRB1 for the hepatic uptake of HDL and lipoproteins ApoE, ApoC-I and ApoC-IV. Activation of FXR also leads to the repression of hepatic lipogenesis by reducing the expression of SREBP1-c in a SHP-dependent manner. SREBP1-c induces the expression of key genes for fatty acids synthesis and lipogenesis such as AceCS, FAS, ACC, and GPAT. By increasing the expression of PPARα, FXR also promotes FFA catabolism via β-oxidation. By repressing the expression of MTP, an enzyme that controls VLDL assembly, FXR reduces VLDL production. Activation of FXR increases TG clearance by promoting PLP activity, via induction of ApoC-II, and by upregulating the expression of VLDL-R and syndecan-1 for the hepatic uptake of LDL and IDL (remnants). Activation of FXR also reduces TG clearance by decreasing the expression of ApoC-III and ANGPTL3, two PLP inhibitors.
The BA pool size can also affect TG metabolism. Early clinical studies suggested a direct relationship between BA and TG levels, showing that patients with decreased BA synthesis due to CYP7A1 deficiency presented high plasmatic levels of TGs [Pullinger et al., 2002]. Moreover, subjects with monogenic familial hypertriglyceridemia displayed defects in ileal BA reabsorption [Angelin et al., 1987]. In line with these evidences, dyslipidemic patients treated with BA-sequestering resins (e.g., cholestyramine or cholestipol) presented increased levels of plasma TG and very-low-density lipoprotein (VLDL) [Angelin et al., 1987; Beil et al., 1982; Crouse, 1987]. Conversely, administration of a BA such as CDCA for the management of hypertriglyceridemia and gallstone disease resulted in decreased TG levels [Angelin et al., 1987; Bateson et al., 1978; Bell et al., 1973]. The ability of BAs to act as hypotriglyceridemic agents has also been confirmed in animal models. In this respect, KK-A(y) mice, an animal model of hypertrigliceridemia, exhibit reduced TG serum levels, hepatic TG accumulation and VLDL secretion after treatment with CA [Watanabe et al., 2004].
Following the discovery that FXR is a transcriptional mediator of the physiological effects of BA, it was speculated that the activation of FXR by synthetic ligands could be exploited to modulate TG metabolism. Thus, mice and rats treated with GW4064 showed reduced plasma TG levels [Claudel et al., 2003; Ma et al., 2006; Maloney et al., 2000; Zhang et al., 2006a]. Interestingly, administration of GW4064 was also able to improve hypertriglyceridemia in ob/ob and db/db mice, two animal models of insulin resistance [Cariou et al., 2005; Zhang et al., 2006a]. Moreover, FXR agonists reduced free fatty acids (FFA) levels in insulin-resistant rodents [Bilz et al., 2006; Zhang et al., 2006a]. The important role of FXR in regulating TG homeostasis has been highlighted in FXR knock-out mice which exhibit a lipid profile characterized by high levels of serum TGs and increased hepatic synthesis of VLDL [Sinal et al., 2000].
FXR controls TG metabolism by regulating hepatic de novo lipogenesis and triglyceride clearance. Upon activation by BA or synthetic ligands, FXR downregulates the expression of SREBP-1c [Watanabe et al., 2004], a transcription factor that plays a critical role in stimulating fatty acid synthesis and lipogenesis by inducing the expression of key enzymes such as the fatty acid synthase (FAS), acetyl-CoA synthase (AceCS), acetyl-CoA (ACC) and glycerol-3-phosphate (GPAT) [Horton et al., 2002] (Figure 5). This metabolic scenario has been elucidated in a mouse model of obesity and type 2 diabetes, the KK-A(y) mouse [Watanabe et al., 2004]. When fed with a natural (CA) or synthetic (GW4064) ligand for FXR, these mice exhibit a significant reduction in hepatic and serum TG levels, and VLDL secretion as a consequence of the induced expression of SHP via FXR. By interfering with LRH1, SHP is able to blunt the ability of LXR to induce SREBP-1c expression. Notably, insulin induces SREBP-1c expression and maturation [Foretz et al., 1999; Shimomura et al., 1999], but also LXR [Steffensen and Gustafsson, 2004; Tobin et al., 2002]. Thus, while the LXR-LRH1- SREBP-1c cascade plays a key role in inducing lipogenesis, on the other hand the FXR-SHP-SREBP-1c cascade plays a crucial role in repressing lipogenesis. The central role of SHP in inhibiting SREBP-1c was confirmed by the combined use of natural (CA) or synthetic (GW4064) FXR agonists and SHP knock-out mice [Watanabe et al., 2004]. In these mice, activation of FXR did not result in lower TG levels. Interestingly, the observation that FXR knock-out mice have normal or even reduced SREBP-1c mRNA levels [Duran-Sandoval et al., 2004; Zhang et al., 2004] suggests that FXR-independent mechanisms also must play an important role. However, since FXR knock-out mice exhibit increased serum and hepatic TG levels along with increased production of VLDL, FXR activation may control de novo lipogenesis via SREBP-1c-dependent and -independent mechanisms.
In addition to the reduction of de novo lipogenesis, FXR activation may also modulate TG clearance. This additional TG-lowering effect of FXR is explained at the molecular level by the induction of key genes, such as Apo-C-II [Kast et al., 2001], an activator of lipoprotein lipase (LPL) activity responsible for VLDL-TG hydrolysis, and the VLDL receptor, in both human and mouse livers, which enhances the clearance of TG-rich lipoproteins (Figure 5). Activation of FXR has also been shown to induce the in vitro expression of syndecan-1 [Anisfeld et al., 2003], a transmembrane herpain sulfate proteoglycan responsible for the clearance of remnant particles. Also, activation of FXR represses the expression of Apo-C-III [Claudel et al., 2003] and ANGPTL3 [Watanabe et al., 2004], two PLP inhibitors. Finally, an FXR agonist may induce the expression of peroxisome proliferator activated receptor α (PPARα) and in turn its target gene pyruvate dehydrogenase kinase-4 (PDK-4), to promote fatty acid oxidation [Pineda Torra et al., 2003; Savkur et al., 2005a] (Figure 5). Taken together, these studies propose FXR as a pharmacological target for the management of hypertriglyceridemia and fatty liver disease, two common features of the metabolic syndrome in humans.
In summary, the use of FXR agonists would be beneficial for the reduction of TG levels, but detrimental in regard to the induction of LDL-cholesterol and reduction of HDL-cholesterol levels. Instead, the use of FXR antagonists would be beneficial to reduce LDL-cholesterol and increase HDL-cholesterol levels, but disadvantageous because of the increased TG levels. Indeed, the benefit of FXR antagonists or agonists in diseases such as atherosclerosis, hypercholesterolemia and hypertriglyceridemia, remains to be established. For the future, the identification of specific FXR isoforms linked to a specific lipid-metabolic pathway would be extremely helpful for the design of specific FXR (ant-)agonists targeting the desired pathway without undesirable side effects.
FXR and glucose metabolism
A physiological role for FXR in glucose metabolism has emerged in the last few years by the discoveries of the effects of FXR on gluconeogenesis, glycogen synthesis, and insulin sensitivity. The link between FXR and glucose homeostasis has been highlighted by several studies showing that BAs profile is altered by diabetes [Staels and Kuipers, 2007]. The BA pool size is markedly increased in rat models of diabetes, suggesting that insulin may affect BA synthesis and secretion [Hassan et al., 1980; Nervi et al., 1974]. Indeed, insulin administration was able to restore a normal BA pool size in experimental models of diabetes [Nervi et al., 1978; van Waarde et al., 2002]. Moreover, liver-specific insulin receptor knock-out mice showed increased bile volume, suggesting a larger BA pool size [Michael et al., 2000]. Taken together, these observations indicate that the negative feedback mechanism repressing BA synthesis is impaired in diabetes. Concurrently, in rat models of diabetes, hepatic FXR expression is decreased and CYP7A1 consequently induced. Thereby, BA pool size is increased, but notably after insulin administration FXR and CYP7A1 gene expression is restored [Duran-Sandoval et al., 2004]. Moreover, BA synthesis is reduced in vitro in rat hepatocytes treated with a physiological concentration of insulin, due to the reduced expression of CYP7A1 [Twisk et al., 1995].
While insulin regulates FXR expression, FXR has an impact on insulin sensitivity. Compared to wild type mice, FXR knock-out mice exhibit impaired glucose tolerance and insulin sensitivity [Cariou et al., 2006; Ma et al., 2006; Zhang et al., 2006a]. Conversely, mice treated with natural (CA) and synthetic (GW4064) FXR ligands, or infected with an adenovirus expressing a constitutively-active form of FXR (VP16-FXR), showed a marked reduction in plasma glucose levels and improved insulin sensitivity [Cariou et al., 2006; Ma et al., 2006; Zhang et al., 2006a]. These effects were seen in mouse models of diabetes such as db/db, ob/ob and KK-A(y) mice. Since FXR is not expressed in skeletal muscle and very low levels are detected in white adipose tissue, it is conceivable that the molecular mechanisms behind the peripheral insulin sensitizing effect of FXR could be indirect. FXR knock-out mice exhibit elevated circulating FFA, as well as increased content of TGs and FFA in the skeletal muscle [Cariou et al., 2006; Ma et al., 2006]. It is believed that this phenomenon known as “lipotoxicity” could impair insulin signaling at the peripheral level [Savage et al., 2007] (Figure 6). Moreover, a similar mechanism could operate in the liver and pancreas, in the latter case reducing the secretion of insulin. Activation of FXR would reduce the “lipotoxicity” phenomenon by decreasing the levels of TGs and FFA (see FXR and TG metabolism) and restoring insulin secretion and sensitivity. Indeed, in vivo studies on FXR knock-out mice demonstrate a lipoatrophic phenotype that would be responsible for interfering with insulin sensitivity [Cariou et al., 2006; Rizzo et al., 2006]. Also, it has recently been shown that treatment of adipocyte-like 3T3-L1 cells with FGF19 results in increased glucose uptake [Kurosu et al., 2007]. Moreover, resistance to insulin desensitization was observed in FGF19 transgenic mice. However, the FGF19 effects are less important in white adipose tissue than in the liver, suggesting that the extra-hepatic activity of FGF19 was due to supra-physiological concentrations [Tomlinson et al., 2002]. It is conceivable that an increase in fatty acid oxidation, as a consequence of the inhibition of hepatic acetyl CoA carboxylase 2 (ACC2) and steaoryl- CoA desaturase-1 (SCD-1), would account for the reduced hepatic TG levels and the following improved insulin sensitivity. Overall, these studies suggest that the FXR-dependent insulin-sensitizing effects may, to some extent, be mediated by FGF19. In order to better dissect this scenario, studies of tissue-specific FXR knock-out mice are required to demonstrate the relative contribution of the liver, intestine and white adipose tissue in insulin sensitivity. On the other hand, one cannot exclude that FXR activation also might lead to increased synthesis or secretion of insulin by the pancreatic cells, and the higher circulating insulin levels could somewhat counteract the insulin-resistant phenotype of the diabetic animal models.
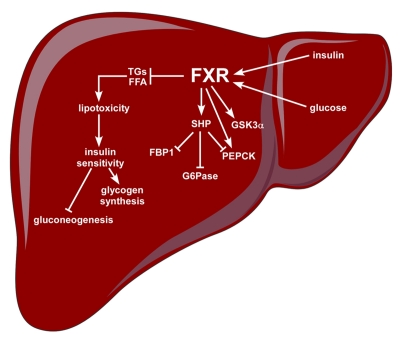
Figure 6. FXR regulates glucose metabolism.
Activation of hepatic FXR can increase insulin sensitivity not only in the liver, but also in peripheral tissues (skeletal muscle and adipose tissue) by reducing the levels of TGs and FFA. High levels of circulating TGs and FFA can impair insulin signaling and reduce pancreatic insulin secretion, a phenomenon known as “lipotoxicity”. The increased insulin sensitivity due to the attenuation of the “lipotoxicity” phenomenon following FXR activation, results in increased glycogen synthesis and reduced gluconeogenesis, two desirable events for the management of diabetes. Activation of FXR induces GSKα expression to promote glycogen synthesis and repress in a SHP-dependent way three key enzymes involved in gluconeogenesis such as PEPCK, FBP1 and G6Pase, but an induction of PEPCK after FXR agonist treatment has been also reported. In this scenario of glucose metabolism regulated by FXR, glucose and insulin increase the expression of FXR.
Another goal in diabetes management, to improve insulin sensitivity, is to limit hepatic output of glucose by reducing gluconeogenesis and glycogenolysis [Moller, 2001]. In this respect, FXR would be a suitable target since it has been demonstrated that the activation of FXR may reduce hepatic gluconeogenesis and induce glycogen synthesis [Cariou et al., 2006; Ma et al., 2006; Zhang et al., 2006a]. Since VP16-FXR over-expression in the liver confirmed the results obtained by pharmacological treatment known to activate FXR in the liver, intestine and kidney, a key role for hepatic FXR in glucose homeostasis, rather than extra-hepatic FXR, is inferred. In the liver of mouse models of diabetes, activation of FXR represses the expression of phosphoenoyl-pyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase) and fructose 1,6-bis phosphatase (FBP1), three rate-limiting enzymes in the glucogenetic process [Ma et al., 2006; Zhang et al., 2006a] (Figure 6). At the molecular level, these findings are explained by the repressive effects of the FXR-SHP pathway on transcription. The expression of PEPCK, G6Pase and FBP1 is positively-regulated by different transcription factors and coactivators. Specifically, HNF4α, GR, forkhead transcription factor Foxo1 and PGC1α cooperate to induce the expression of these three enzymes in the fasting state. Activation of FXR by CA [Ma et al., 2006] or GW4064 [Zhang et al., 2006a] induces the expression of SHP, which in turn disrupts the interaction of PGC1α with GR, HNF4α and Foxo1 with a final decrease of gluconeogenesis in db/db and KK-A(y) diabetic mouse models, but not in FXR knock-out mice [Borgius et al., 2002; Yamagata et al., 2004]. In support of the concept that BAs repress gluconeogenesis via the FXR-SHP pathway, over-expression of SHP reduces gluconeogenic gene expression. Besides the direct role of SHP in reducing the mRNA levels of these three enzymes, it is also possible that FXR may act indirectly by increasing hepatic insulin sensitivity and reducing serum FFA levels. Notably, in contrast with the diabetic mice, studies on wild type mice with FXR agonists showed stimulation [Stayrook et al., 2005; Zhang et al., 2006a] or repression [De Fabiani et al., 2003; Ma et al., 2006; Yamagata et al., 2004] of PEPCK, paralleled by decreased or unchanged glucose levels [Ma et al., 2006; Stayrook et al., 2005; Zhang et al., 2006a]. The explanation for these discrepancies remains to be determined. Moreover, the in vitro use of GW4064 resulted in induction rather than suppression of PEPCK [Stayrook et al., 2005]. In line with this evidence, administration of GW4064 to fed mice also resulted in the upregulation of PEPCK. These differences in the literature may be due to the fast-feeding regulation of this important FXR-driven scenario. Parallel to G6Pase reduced expression, activation of FXR induces glycogen synthesis in the liver of diabetic db/db mice as a consequence of increased phosphorylation of glycogen synthase kinase 3α (GSK3α), a critical regulator of glycogen synthase [Zhang et al., 2006a] (Figure 6). In accordance with this finding, FXR knock-out mice exhibit reduced hepatic glycogen content [Cariou et al., 2005].
Despite the discrepancies in the literature regarding the role of FXR in glucose metabolism, FXR agonists may be useful for the management of type 2 diabetes. Moreover, the observation that activation of FXR results in insulin substrate receptor 1 (IRS-1) phosphorylation in the liver and adipose tissue suggests a potential application of FXR ligands for the management of patients with insulin resistance [Ma et al., 2006]. Studies on human patients are ongoing and the results will shed light on FXR as an anti-diabetic target.
A list of FXR target genes involved in glucose metabolism is reported in Table 1.
FXR and disease
Cholestasis
Cholestasis is a pathological condition characterized by impairment or reduction of bile flow. It results from defects in the process of bile formation in hepatocytes or cholangiocytes (intrahepatic cholestasis), or alternatively from physical obstructions in bile ducts due to tumors or stones (extrahepatic cholestasis). As a consequence, accumulation of potentially toxic biliary constituents leads to initial hepatic fibrosis and inflammation that may result in cirrhosis, cancer and finally liver failure. Alterations in the bile formation process can derive from inflammation, drug administration, pregnancy or hereditary defects in the transport systems needed for bile formation/secretion. In the latter case, PFIC1, also termed Byler disease, affects patients with mutations in FIC1, a putative aminophospholipid transferase on the hepatocyte canalicular membrane, while mutations in BSEP and MDR3 are responsible for PFIC2 and PFIC3 diseases [Bull et al., 1998; de Vree et al., 1998; Strautnieks et al., 1998]. Other forms of cholestasis include primary biliary cirrhosis (PBC) and primary sclerosis cholangitis (PSC), two progressive liver disorders characterized by bile duct proliferation associated with bile flow impairment and liver damage [Bergasa et al., 2004; Levy and Lindor, 2006].
Irrespective of the etiology of cholestasis, accumulation of toxic hydrophobic BAs in the liver is thought to play a key role in cholestasis-associated liver damage. Thus far, the use of UDCA, a polar BA that reduces the hydrophobicity and toxicity of the BA pool, is the only pharmacological intervention recognized for the management of cholestasis [Paumgartner and Beuers, 2002]. However, while endoscopy and surgery are effective therapies for most of the patients with obstructive cholestasis, therapeutic approaches based on UDCA for non-obstructive cholestasis, especially those with a genetic background, remain mostly ineffective [Goulis et al., 1999; Paumgartner and Beuers, 2002]. Therapies aiming at reducing the size and hydrophobic index of the BA pool during cholestasis are still missing.
The discovery that FXR is the master regulator of BA homeostasis makes this NR a suitable pharmacological target for the management of cholestasis. Activation of FXR could reduce BA synthesis by repressing CYP7A1 and CYP8B1 expression, decrease hepatic BA uptake by downregulating the Na+/taurocholate cotransporting polypeptide (NTCP) and increase the expression of MDR3 and BSEP for the secretion of BAs from the liver into bile (Figure 4). The final result would be a reduced hepatic BA overload. It is noteworthy that in those forms of cholestasis that are characterized by compromised activity of BSEP and MDR3 (as in PFIC2 and PFIC3, respectively), BAs can spill over from the liver at the basolateral membrane through transporters such as OSTα/β, which also are FXR targets (Figure 4). Moreover, FXR can induce phase I and phase II reactions to make BAs more hydrophilic and ready for excretion through urine. In line with the important role that FXR plays to keep BA levels under control, FXR knock-out mice present a phenotype similar to Byler disease, a hereditary form of cholestasis with reduced FXR expression. Furthermore, four different heterozygous functional variants of FXR (-1>g, M1V, W80R, M173T) were found in patients affected by intrahepatic cholestasis of pregnancy (ICP), a pathological condition occurring in 1/200 pregnancies in Caucasian women, that can lead to intrauterine fetal death [Heinonen and Kirkinen, 1999; Rioseco et al., 1994]. Characterization of these variants displayed functional defects in either translational efficiency or activity in three of the four variants (-1>g, M1V, M173T) [Van Mil et al., 2007].
The protective role of FXR activation in cholestasis has been investigated in mouse and rat models for this disease. Treatment with GW4064 reduced bile duct proliferation, inflammation and liver damage induced by bile duct ligation (BDL) and α-naphtylisothiocyanate (ANIT), the two experimental setups generally used to induce cholestasis. Moreover, both GW4064 and 6α-ECDCA were able to protect rats from ethinyl estradiol-induced cholestasis. The mechanism proposed for the protective effect of FXR observed in these studies focused only on the liver and was ascribed to the reduced expression of CYP7A1 and NTCP, and the induced expression of MRP2 and BSEP. However, since the reduced BA pool size, as a consequence of the decreased expression of CYP7A1, could play the main role in the protection against cholestasis after FXR activation, and since it was recently shown that the intestinal FXR-FGF15 pathway plays a crucial role in repressing CYP7A1 expression, the relative contribution of hepatic and intestinal FXR activation against cholestasis should be investigated by the use of tissue-specific FXR knock-out mice.
In contrast to the studies reported above, suggesting a protective role of FXR activation against cholestasis, other evidences suggest that FXR inhibition also may have a protective role in cholestasis [Marschall et al., 2006; Stedman et al., 2006]. FXR knock-out mice were shown to be protected from liver injury caused by BDL. These mice adapt to BDL by a significant increased expression of MRP4, suggesting that FXR may negatively regulate this hepatic basolateral transporter. The induction of MRP4 expression represents an important adaptive mechanism against cholestasis to protect the liver from accumulation of BAs during cholestasis, by stimulating their efflux into the systemic circulation for final renal excretion. Thus, MRP4 knock-out mice are susceptible to cholestatic-induced liver damage [Mennone et al., 2006]. Moreover, subjects with PFIC [Jansen et al., 2001] and mice, as well as rats, present a marked upregulation of MRP4 expression after BDL [Denk et al., 2004; Mennone et al., 2006]. Overall, these findings imply that inhibition of hepatic FXR may represent an important adaptive mechanism observed in cholestasis.
In summary, FXR activation reduces the BA pool size, the most important determinant in cholestasis, by downregulating CYP7A1 expression via a synergistic mechanism that involves hepatic and intestinal FXR. However, hepatic FXR activation may also reduce the expression of basolateral transporters, such as MRP4, which are required for BA secretion into systemic circulation and consequent renal elimination. For these reasons, a selective activation of intestinal FXR would be more beneficial than a systemic activation of FXR. Through this selective activation, the total BA pool would be reduced by the FXR-FGF15/19 pathway, while the adaptive hepatic response would normally function. Another option would be the generation of drugs that would act as gene selective modulators for FXR by reducing the expression of CYP7A1, inducing the expression of BSEP, MDR3, OSTα/β and MRP4, and repressing the activity of FXR in the kidney to reduce BA uptake at this level. Finally, it is worth mentioning that the mouse models of cholestasis used so far are not completely representative of the human situation. It would be more relevant to investigate the role of FXR activation in genetic mouse models of cholestasis with the same mutations as those observed in humans (e.g., PFICs).
Cholesterol gallstone disease
The correct ratio of biliary BAs, phospholipids and cholesterol is of crucial importance for maintaining cholesterol in solution in the bile and preventing the precipitation of cholesterol crystals, the first step in cholesterol gallstone formation [Lo Sasso et al., 2008; Portincasa et al., 2006]. Bile with a high BA and phospholipid versus cholesterol ratio protects from gallstone formation, while high cholesterol versus BA and phospholipid ratio predisposes to cholesterol gallstone disease (CGD). Thus, the protective role of FXR against CGD was suggested. Indeed, FXR knock-out mice are more susceptible to cholesterol gallstone formation than wild type mice, after feeding them with a lithogenic diet. This higher susceptibility is a consequence of lower BA and phospholipid versus cholesterol ratio in bile [Moschetta et al., 2004]. At the molecular level, decreased expression of BSEP and Mdr2 were observed in FXR knock-out mice, while the expression of biliary cholesterol transporters, such as ABCG5 and ABCG8, was unchanged. Moreover, treatment with GW4064 of C57L mice, which are susceptible to CGD when fed with a lithogenic diet, prevents gallstone formation [Moschetta et al., 2004]. Compared to vehicle-treated mice, GW4064-treated mice showed an increased expression of BSEP and Mdr2 that was coupled with a higher biliary content of BAs and phospholipids and a consequent reduced cholesterol saturation index. Thus, activation of FXR is crucial for maintaining proper solubilization of cholesterol into bile, by inducing the expression of BSEP and MDR3/Mdr2, and FXR agonists may be useful for the prevention or management of CGD. However, activation of FXR inhibits BA synthesis by reducing CYP7A1 expression, resulting in a decreased BA pool size, the most important determinant of the secretory rate of BAs. This would result in less BA content in bile, increased cholesterol levels, and susceptibility to gallstone formation. Notably, in the mouse models of CGD, the use of the lithogenic diet, containing 0.5% of CA, is a must to induce gallstone formation [Tepperman et al., 1964]. The CA in the lithogenic diet increases the BA pool size and compensates for the reduced expression of CYP7A1 after GW4064 treatment. This would account for the protective effects obtained with GW4064 in this animal model of CGD despite the downregulation of CYP7A1. The situation is quite different in patients with CGD. These subjects may be treated with FXR agonists in association with hydrophilic BAs (e.g., UDCA, a prescription drug in CGD), with the synergistic effect of inducing BA and phospholipid secretion into bile while maintaining BA pool size and decreasing total BA hydrophobicity index.
Intestinal diseases
The levels of BAs in the intestine have to be tightly regulated. Reduced intestinal BA concentration, due to bile flow obstruction, results in bacterial overgrowth and translocation across the mucosal barrier with possible systemic infection. On the other hand, an excessive level of BAs in the intestine, resulting from defects in BA reabsorption, results in chronic diarrhea, inflammatory bowel disease and promotion of intestinal tumorigenesis.
BAs have long been known to have surfactant properties responsible for keeping intestinal bacterial proliferation under control. Moreover, it has been recently shown that part of the antibacterial activity of BAs is mediated by FXR [Inagaki et al., 2006]. FXR knock-out mice exhibit hyper-proliferation of intestinal bacteria and evidence of a compromised epithelial barrier with consequent inflammation due to bacteria infiltration and recruitment of activated macrophages/neutrophils [Inagaki et al., 2006; Modica et al., 2008]. Notably, treatment of wild type mice with GW4064 was able to reverse both intestinal bacterial overgrowth and the integrity of the epithelial barrier caused by BDL [Inagaki et al., 2006]. These protective effects of FXR activation were seen in neither sham operated nor BDL FXR knock-out mice. Under physiological conditions, activation of intestinal FXR by BAs is important for controlling bacterial proliferation and maintaining the integrity of the epithelial barrier. At the transcriptional level, FXR induces the expression of genes involved in intestinal mucosa defense such as interleukin 18 (IL18), inducible nitric oxide synthase (iNOS) and carbonic anhydrase 12 (CAR12) (Table 1). Moreover, induction of FGF15 by FXR could also protect the intestine. FGF15 knock-out mice present an altered intestinal morphology, suggesting that some of the functions of FXR in the intestine are mediated by FGF15 [Inagaki et al., 2005]. The relevance of this scenario for the regulation of intestinal inflammatory disorders is an active research field for several laboratories.
In humans, reduced intestinal BA reabsorption and increased fecal BA excretion contributes to chronic diarrhea occurring in different clinical contexts, including Crohn’s disease, post-gastrectomy syndrome, and short bowel syndrome. In most cases, the molecular mechanisms underlying the disruption of the enterohepatic circulation are unknown. However, mutations in the ASBT gene is one cause for intestinal BA malabsorption in humans [Oelkers et al., 1997]. Indeed, ASBT knock-out mice recapitulate key aspects of BA malabsorption in humans [Dawson et al., 2003]. This mouse model exhibits impaired FXR activity in both intestine and liver. Thus, FGF15 and SHP expression is reduced with consequent upregulation of CYP7A1 expression that results in a vicious cycle of increased BA production. Currently, a major drug against BA malabsorption is cholestyramine, a resin that, sequestering BAs in the intestine, protects the bowel from exposure to the increased levels of colonic BAs. However, cholestyramine is unpalatable and exhibits adverse side effects including constipation, vitamin deficiency [Hathcock, 1985], and hypertrigliceridemia [Crouse, 1987]. In addition, by sequestering BAs in the intestine, treatment with cholestyramine could result in increased BA production by interrupting the normal feedback repression of hepatic BA synthesis. Using ASBT knock-out mice, it has been shown that GW4064-dependent FXR reactivation results in reduced BA pool size and fecal excretion as a consequence of the decreased CYP7A1 expression via hepatic SHP and intestinal FGF15. This finding may be of important therapeutic potential in diseases associated with BA malabsorption. Moreover, FXR agonists decrease circulating TG levels, protecting against one of the major side effects of cholestyramine and other resins. Indeed, by breaking the cycle of increased BA synthesis in BA malabsorption-related diseases, the use of synthetic FXR ligands or a hormonal therapy based on FGF19 would be beneficial for patients with either temporary or long-term interruption of enterohepatic circulation due to BA malabsorption.
Liver regeneration and tumorigenesis
It has been known for a long time that BAs are able to induce hepatocyte proliferation and liver regeneration [Barone et al., 1996]. Conversely, blocking the enterohepatic circulation of BAs results in the inhibition of liver regeneration [Ueda et al., 2002]. Recently, a key role for FXR in mediating BA-induced liver regeneration has been suggested. Administration of a diet containing 0.2% CA stimulated liver regeneration in partial hepatectomized mice, while a diet containing the BA sequestrant resin cholestyramine delayed liver regeneration [Huang et al., 2006]. In line with these observations, FXR knock-out mice presented impaired liver regeneration ability [Huang et al., 2006]. This data suggests a direct involvement of FXR in BA-induced liver regeneration. However, unlike BAs that have FXR-dependent and -independent activities, the use of a selective synthetic ligand for FXR such as GW4064 was unable to induce the liver regeneration process [Huang et al., 2006]. It is possible that a normal BA pool size can blunt the hepatic proliferative effect of FXR activation by a synthetic ligand. To investigate the hepatic proliferative effects of synthetic FXR agonists, a mouse model of partial hepatectomy with a decreased BA pool size (such as during cholestyramine treatment) should be used. The importance of BAs and FXR in hepatic proliferation is of great interest for the pathogenesis of hepatocellular carcinoma (HCC).
HCC is the primary liver tumor and is one of the most common forms of cancer in the world. The etiology of this disease mainly includes hepatitis B and C infections, alcoholic and non-alcoholic fatty liver diseases, chemicals and, to a lesser extent, genetic defects such as hematochromatosis and α-1-antitrypsin deficiency [Deugnier and Turlin, 2001; Propst et al., 1994]. Regardless of the liver cancer etiology, irregular liver regeneration represents a general mechanism of hepatocarcinogenesis [Shibata et al., 1998; Ueno et al., 2001]. Common pathological changes in the development of liver tumors consist of inflammation and chronic liver injury, which can lead to a continuous proliferation of hepatocytes at a higher rate than normal liver cells. In contrast to the normal liver regeneration, a process where the liver resumes its normal size after an injury and stops proliferating within a short period of time, the hepatocarcinoma process consists of an uncontrolled proliferation that does not stop because of chronic injury.
Recently it has been shown that BAs can also promote liver tumorigenesis. A BA-supplied diet in a Hepatitis B Virus (HBV) transgenic mouse model was able to promote hepatic tumors, an effect also observed in Mdr2 knock-out mice [Barone et al., 2003; Katzenellenbogen et al., 2006; Mauad et al., 1994]. Moreover, a CA diet has been shown to promote chemical-induced hepatocarcinogenesis [Yang et al., 2007]. Since FXR tightly regulates BA concentration, thereby protecting from detrimental effects of BA toxicity, a role for FXR in HCC can be supposed. In accordance with this, FXR knock-out mice, which have chronic high levels of circulating BAs, spontaneously develop HCC between 12-15 months of age [Kim et al., 2007b; Yang et al., 2007]. These findings suggest a protective role of FXR during the life span against hepatocarcinoma development. However, whether and how FXR directly suppresses liver tumorigenesis remains to be established. It is likely that FXR acts as a metabolic oncosuppressor by keeping BA concentration under physiological levels or by reducing the toxic properties of BAs. These levels of BAs would otherwise increase to toxic levels in the absence of FXR and induce chronic hepatic injury, ultimately resulting in liver tumorigenesis. However, it is interesting to note that FXR is required for both liver regeneration and protection against hepatic tumorigenesis, suggesting an intrinsic link between these two cellular processes. This link should be exploited to find new therapies for the management of HCC.
Intestinal tumorigenesis
The role of FXR in carcinogenesis is not restricted only to the liver. Recent studies have highlighted an oncosuppressive role for FXR in intestinal tumorigenesis [Maran et al., 2009; Modica et al., 2008; Yang et al., 2007]. Colorectal cancer (CRC) is a primary intestinal tumor, and is nowadays a leading cause of cancer-related death in the Western world [Arnold et al., 2005; Potter, 1999]. CRC is a long multistep process that arises from a combination of genetic and environmental causes. Mutations in the tumor suppressor adenomatous polyposis coli (APC) gene are detectable in dysplastic aberrant crypt foci (ACFs), the smallest neoplastic lesion in the CRC development process, and are believed to be the primum movens for the initiation of the majority of sporadic and hereditary forms of CRC. These observations make APC the gatekeeper in CRC development. However, patients with APC mutations do not necessarily develop CRC; additional mutations are required in order to develop tumors and in this respect environmental factors are likely to have an important contribution [Kinzler and Vogelstein, 1996]. Epidemiological studies indicate that a high fat/cholesterol diet may increase the susceptibility to CRC, while a low fat/high fiber diet can protect against CRC [Willett, 2001]. One possible mechanism by which a long term “Western-style” diet may contribute to CRC is through a chronic exposure to toxic high levels of BAs derived from excessive cholesterol catabolism. Consistent with this hypothesis, animal studies link BAs, especially secondary BAs, to CRC, and human epidemiological studies indicate that the tumor promoting activity of a “Western-style” diet is accompanied by an increase in colonic BA concentrations with higher levels in the feces [Bianchini et al., 1989; Reddy et al., 1978]. Furthermore, cholecystectomy, which leads to an increased intestinal exposure to BAs, has been suggested as a predisposing factor for the development of CRC [Giovannucci et al., 1993; Lagergren et al., 2001].
Although BAs are tumor-promoting agents, FXR is not involved in mediating their tumorigenic effects. On the contrary, FXR expression is reduced in CRC after an APC mutation, as in APC min/+ mice [Maran et al., 2009; Modica et al., 2008], a mutant murine lineage carrying a nonsense mutation of the APC gene [Moser et al., 1990], and patients with familial adenomatous polyposis (FAP) [Modica et al., 2008], a hereditary form of colon cancer which just like APC min/+ mice possess inactivating nonsense mutations in the APC gene [Kinzler et al., 1991; Powell et al., 1993]. Moreover, crossing FXR knock-out mice with APC min/+ resulted in increased number and size of intestinal tumors and earlier mortality than APC min/+ mice [Maran et al., 2009; Modica et al., 2008]. Finally, as in the APC min/+ model, the lack of FXR was able to promote tumorigenesis in a chemical model of colon carcinogenesis associated with chronic colitis [Maran et al., 2009; Modica et al., 2008; Popivanova et al., 2008]. It is worth noting that, unlike liver tumorigenesis, the susceptibility of FXR knock-out mice to intestinal tumorigenesis does not relate to the elevated levels of BAs, as shown by treatment with cholestyramine, indicating that the loss of FXR, and not merely elevated BA concentrations, promotes intestinal tumors. It has been demonstrated that activated macrophages further promote WNT/β-CATENIN signaling and intestinal tumor development in APC min/+ mice through the tumor necrosis factor α (TNFα) [Oguma et al., 2008]. Moreover, it has been shown that FXR knock-out mice have a compromised intestinal architecture, with consequent bacterial translocation across the epithelial barrier and recruitment of macrophages/neutrophils [Inagaki et al., 2006]. Thus, the loss of FXR may result in increased susceptibility to CRC by further promoting WNT signaling via TNFα released by macrophages infiltrating into the intestinal mucosa because of altered gap-junctions [Modica et al., 2008].
Besides acting as an indirect oncosuppressor gene and maintaining the integrity of the intestinal mucosa barrier, activation of FXR also leads to a more direct oncosuppressing activity by blocking tumor growth in a xenograph mouse model [Modica et al., 2008]. Activated FXR reduces proliferation and induces apoptosis in colorectal cancer cells by increasing the mRNA levels of pro-apoptotic genes, such as P21, BCL-2 antagonist killer 1 (BAK1), FAS, Fas-associated death domain (FADD), and by decreasing the expression of anti-apoptotic genes, such as B cell lymphoma gene-2 (BCL2) and TNFα. Likewise, it has been shown that FXR also induces apoptosis in breast cancer [Swales et al., 2006] and in the vasculature [Bishop-Bailey et al., 2004].
In summary, it is unlikely that the intestinal tumor promoting activity of BAs is mediated by FXR, whose expression is reduced in ACFs. For this reason, FXR may be considered a new early diagnostic marker for CRC development. Moreover, the absence of FXR contributes to the increase of CRC susceptibility both directly, by inducing apoptotic events, and indirectly, by maintaining the integrity of the intestinal mucosa barrier. Thus, FXR may also be considered an intestinal oncosuppressor. Although the loss of FXR, and not merely elevated BA concentrations, increase susceptibility to intestinal tumorigenesis under a normal diet, FXR-independent tumor promoting activity of toxic high levels of BAs because of a long-term “Western-style” diet should also be considered for the pathogenesis of CRC. After mutation of the APC gene, FXR expression would be decreased with further promotion of intestinal tumorigenesis. In this scenario, a long-term “Western-style” diet would produce high levels of toxic BAs that will not be buffered by the detoxification activity of FXR and will be free to promote CRC via FXR-independent mechanisms. As a result, a high-fat diet will reduce the time window for the manifestation of CRC.
Atherosclerosis
The initial finding that FXR knock-out mice exhibit a pro-atherogenic lipid profile, having increased plasma TGs, FFA and LDL levels [Sinal et al., 2000], suggests a protective role for FXR in atherosclerosis. However, these mice did not show features of atherosclerosis even when fed high-fat/cholesterol diets [Hanniman et al., 2005; Zhang et al., 2006b]. To investigate the role of FXR in atherosclerosis, FXR knock-out mice were crossed with two genetic mouse models of accelerated atherosclerosis, the ApoE- [Guo et al., 2006; Hanniman et al., 2005] and the LDLR-deficient mice [Zhang et al., 2006b] and fed with a Western diet. Female FXR-/-/ApoE-/- mice presented less severe atherosclerosis compared to ApoE-/- control mice [Guo et al., 2006]. In contrast, male FXR-/-/ApoE-/- mice presented more severe atherosclerosis than ApoE-/- control mice [Hanniman et al., 2005]. Indeed, compared to LDLR-/- mice, male FXR-/-/LDLR-/- exhibit decreased aortic atherosclerotic lesions [Zhang et al., 2006b]. The atheroprotective effects of FXR deficiency in female FXR-/-/ApoE-/- mice and male FXR-/-/LDLR-/- mice may be due to decreased LDL-cholesterol, although the mechanism resulting in this reduction remains unclear. In line with this hypothesis, female FXR-/-/LDLR-/- mice did not display reduced atherosclerotic lesions and did not have reduced LDL-cholesterol levels. Taken together, these data suggest that the effect of the loss of FXR on atherosclerosis is dependent on the genetic background and on the sex of the animal.
In addition to the impact of FXR on cardiovascular risk factors such as dyslipidemia and hyperglycemia, FXR may also have an effect on the arterial wall. It has been demonstrated by western blot and immunohistochemical analysis that FXR is expressed in vascular smooth muscle cells (VSMCs) in human vessels and in atherosclerotic lesions [Bishop-Bailey et al., 2004]. Activation of FXR by GW4064 and 6α-ECDCA resulted in the induction of SHP and PLTP in rat smooth muscle cells (RASMCs) [Li et al., 2007; Zhang et al., 2008]. However, since these effects were not always confirmed with CDCA [Bishop-Bailey et al., 2004; Zhang et al., 2008], it cannot be excluded that FXR ligands, other than BAs, may exist outside the enterohepatic circulation. Interestingly, 6α-ECDCA and GW4064 blunt the VSMCs inflammatory response and migration by inhibiting IL1β-induced expression of cyclooxygenase-2 (COX2) and iNOS in RASMCs, an effect achieved by decreasing NF-κB in a SHP-dependent way [Li et al., 2007] (Table 1). Furthermore, activation of FXR promotes apoptosis in RASMC cells and reduces their migration induced by platelet-derived growth factor (PDGF)-β [Li et al., 2007]. Finally, FXR activation induces the expression of angiotensin type 2 receptor (AT2R) (Table 1) in RASMCs, which results in inhibition of angiotensin II-mediated ERK signaling [Zhang et al., 2008]. Notably, overexpression of AT2R and inhibition of the ERK signaling has been proposed to partially mediate the hypotensive effects of AT1R blockers [Savoia et al., 2007] and prevent neointimal proliferation [Nakajima et al., 1995]. Although FXR activity does not impact on AT1R expression in RASMCs, CDCA represses angiotensinogen in a SHP-dependent fashion in hepatocytes [Shimamoto et al., 2004]. Overall, FXR activation in VSMCs may have some benefit on blood pressure, vascular inflammation and neointimal proliferation.
Low expression levels of FXR were also detected in several endothelial cells such as human pulmonary artery endothelial cells (HPAEC) [Qin et al., 2006], rat (RPAEC) [He et al., 2006], bovine aortic endothelial cells (BAEC) [Li et al., 2008] and primary human umbilical vein endothelial cells (HUVEC) [Qin et al., 2006]. Despite the low expression levels, activation of endothelial FXR by CDCA seems to be functional. FXR activation reduced the expression of endothelin-1 (ET-1) (Table 1), a vasoconstrictive peptide, with possible beneficial effects against atherosclerosis [He et al., 2006]. Further beneficial effects on the vascular tone may derive from the fact that FXR directly induced the endothelial nitric oxide synthase (eNOS) (Table 1) gene promoter to increase the NO production in vascular endothelial cells [Li et al., 2008]. In line with these observations, reduced activity of vasoconstrictors and arterial hypotension has been reported in cirrhotic patients with high levels of circulating BAs [MacGilchrist et al., 1991]. On the other hand, CDCA treatment increases the expression of the adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which promote macrophage recruitment to the endothelium, a crucial process for atherosclerosis development [Qin et al., 2006]. However, GW4064 treatment only increases the expression of adhesion molecules at high concentrations and still to a lesser extent than CDCA. This suggests an FXR-independent activity of CDCA on ICAM-1 and VCAM-1 expression.
Taken together, the data concerning the role of FXR in the vasculature tissue should be carefully judged. The levels of FXR expression are in fact very low in this tissue making it unlikely that BAs would activate FXR in vivo under physiological conditions. Indeed, FXR activation in this tissue requires high doses of its specific synthetic ligand GW4064.
FXR can also affect vascular function through its hepatic activity. In vitro experiments on human hepatocytes displayed that FXR activation is able to induce the expression of the human ICAM-1 promoter [Qin et al., 2006] (Table 1). Moreover, mice treated with CA exhibit increased levels of ICAM-1 and VCAM-1. These findings suggest that hepatic FXR activation may be associated with promotion of the atherogenic process. On the other hand, recent data indicate that FXR activation in human hepatocytes induces the expression of kininogen [Zhao et al., 2003] and bradykinin [Margolius, 1996] (Table 1). Kininogen is a precursor of the two-chain kinin free kininogen, which has anti-inflammation, anti-adhesion and anti-platelet aggregation activities, and bradykinin is a potent vasodilator. Therefore, induction of these two molecules may contribute to the antithrombotic activity of FXR.
In contrast, it has been shown that FXR activation induces the expression of fibrinogen, a key molecule in the coagulation pathway proposed as a cardiovascular risk factor [Scarabin et al., 2003], in an isoform-dependent manner [Anisfeld et al., 2005]. Furthermore, FXR activation decreases hepatic paraoxonase 1 (PON1) expression (Table 1), an enzyme associated with HDL that prevents LDL oxidation, with consequent anti-inflammatory atheroprotective effects [Gutierrez et al., 2006; Shih et al., 2006]. It has been shown that, as for CYP7A1, FXR-mediated repression of PON1 involves the induction of FGF19 for the activation of the FGFR4-JNK pathway [Gutierrez et al., 2006; Shih et al., 2006]. Thus, intestinal FXR activation may promote atherosclerosis by repressing both CYP7A1 and PON1 via FGF19.
Conclusions
Starting from its initial cloning in 1995, the knowledge of the different functions of FXR has expanded rapidly. It is now recognized that activation of FXR not only has an impact on the homeostasis of BAs, which are the endogenous FXR ligands, but also affects lipid and glucose metabolism. Therefore, modulation of FXR activity may be exploited for the pharmacologic management of metabolic disorders related to BA and lipid/glucose metabolism, such as cholestasis, CGD, intestinal bacterial overgrowth, hepatic and intestinal tumorigenesis, dyslipidemia, hypertriglyceridemia, diabetes and atherosclerosis. From a theoretical point of view, targeting FXR may also be beneficial to treat all aspects of the metabolic syndrome, a complex cluster of cardiovascular disease risk factors including dyslipidemia, insulin-resistance, increased blood pressure, visceral obesity and hypercoagubility. However, activation of FXR may have several possible undesirable effects. For instance, activation of FXR in order to reduce TG levels in hypertriglyceridemic patients would also result in decreased HDL levels and accumulation of cholesterol in the body, as a consequence of the inhibition of BA synthesis. Also, treatment of cholestatic subjects with an FXR activator would have some beneficial effects by reducing BA synthesis, and increasing BA secretion into bile, as well as some side effects by reducing hepatic basolateral secretion of BAs. In this complex scenario, where activation of FXR integrates beneficial effects and potentially undesirable side effects, the identification of tissue-specific gene-selective FXR modulators represent an intriguing pharmacologic approach to target a specific cluster of genes avoiding detrimental side effects. Differences in the docking of the FXR ligands in the four FXR subtype receptors account for differences in corepressor dislodgement and/or coactivator recruitment in a tissue-specific manner, which could be responsible for the ability of a certain ligand to act as an agonist or antagonist for a certain gene. In the near future, more studies focusing on this aspect of FXR activation will be required to develop ad hoc FXR modulators. Nevertheless, we now have selective FXR agonists in the pipeline, but data obtained in human subjects in relation to the metabolic diseases are needed to verify the long list of positive or negative results observed in the different animal models. The toxicity issues have now been covered and at 14 years from the cloning of FXR, a novel nuclear receptor ligand is ready to enter the clinic.
Acknowledgments
We thank Drs. C. De Girolamo, C. Gardmo, and M. Petruzzelli for critically reading the manuscript. This work was funded by European Research Council Starting Independent Grant - IDEAS - Italian Ministry of Health and Education (Finanziamenti per la Ricerca di Base RBID08C9N7), Italian Association for Cancer Research (AIRC, Milan, Italy), European Community’s Seventh Framework Programme FP7/2007 – 2013 under Grant Agreement No. 202272 (LipidomicNet), Telethon Foundation (GPP08259), Cariplo Foundation Milan, University of Bari, Italy.
Abbreviations
- ABC
ATP-binding cassette
- ACC
acetyl-CoA
- ACC2
acetyl CoA carboxylase 2
- AceCS
acetyl-CoA synthase
- ACFs
aberrant crypt foci
- ANIT
α-naphtylisothiocyanate
- APC
adenomatous polyposis coli
- AR
androgen receptor
- ASBT
sodium-dependent BA transporter
- BAAT
BA-CoA: amino acid N-acetyltransferase
- BAK1
Bcl-2 antagonist killer 1
- BAR
bile acid receptor
- BACS
CoA-thioesters by the BA CoA synthase
- BAs
bile acids
- BCL2
B cell lymphoma gene-2
- BDL
bile duct ligation
- BSEP
bile salt export pump
- CA
cholic acid
- CAR
constitutive androstane receptor
- CAR12
carbonic anhydrase 12
- CARM-1
coactivator associated arginine (R) methyl transferase-1
- CDCA
chenodeoxycholic acid
- CGD
cholesterol gallstone disease
- COX2
cyclooxygenase-2
- CRC
colorectal cancer
- CYP
cytochrome P450
- DBD
DNA binding domain
- DCA
deoxycholic acid
- DR
direct repeats
- DRIP-205
vitamin-D-receptor-interacting protein-205
- EcRE
ecdysome response element
- eNOS
endothelial nitric oxide synthase
- ER
everted repeats
- ERα
estrogen receptor α
- ET-1
endothelin-1
- FADD
Fas-associated death domain
- FAP
familial adenomatous polyposis
- FAS
fatty acid synthase
- FBP1
fructose 1,6-bis phosphatase
- FFA
free fatty acids
- FGF19/15
fibroblast growth factor 19/15
- FGFR4
fibroblast growth factor receptor 4
- FRET
fluorescence energy transfer
- FXR
farnesoid X receptor
- GPAT
glycerol-3-phosphate
- GR
glucocorticoid receptor
- GSK3α
glycogen synthase kinase 3α
- G6Pase
glucose-6-phosphatase
- HCC
hepatocellular carcinoma
- HDL
high-density lipoprotein
- HNF-4α
hepatic nuclear factor 4α
- HPAEC
human pulmonary artery endothelial cells
- HRE
hormone responsive element
- HUVEC
human umbilical vein endothelial cells
- IBABP
ileal bile acid binding protein
- ICAM-1
intracellular adhesion molecule-1
- ICP
intrahepatic cholestasis of pregnancy
- IL18
interleukin 18
- iNOS
inducible nitric oxide synthase
- IR
inverted repeats
- IRS-1
insulin substrate 1
- LBD
ligand binding domain
- LCA
lithocholic acid
- LDL
low-density lipoprotein
- LPL
lipoprotein lipase
- LRH-1
liver receptor homolog-1
- LXR
liver X receptor
- MDR
multidrug protein
- MR
mineralocorticoid receptor
- MRP
multidrug related protein
- NCor
nuclear corepressor
- NOR1
neuron-derived receptor 1
- NRs
nuclear receptors
- NTCP
Na+/taurocholate cotransporting polypeptide
- NURR1
nuclear receptor related 1
- OSTα/β
organic solute transporter α/β
- PBC
primary biliary cirrhosis
- PCN
pregnenolone 16-α carbonitrile
- PDK-4
pyruvate dehydrogenase kinase-4
- PEPCK
phosphoenoyl-pyruvate carboxykinase
- PFIC
familial intrahepatic cholestasis
- PGC1α
peroxisome-proliferator-receptor (PPAR)-γ coactivator-1α
- PLPT
phospholipids transfer protein
- PMRT-1
protein arginine (R) methyl transferase-1
- PON1
paraoxonase 1
- PR
progesterone receptor
- PSC
primary sclerosis cholangitis
- PXR
pregnane X receptor
- RAR
retinoic acid receptor
- RARE
retinoic acid response element
- RASMCs
rat smooth muscle cells
- RIP14
RXR-interacting protein 14
- RPAEC
rat pulmonary artery endothelial cells
- RXR
retinoid X receptor
- SCD-1
stearoyl-CoA desaturase-1
- SHP
small heterodimer partner
- 6α-ECDCA
6α-ethyl-chenodeoxycholic acid
- SNPs
single-nucleotide polymorphisms
- SRC-1
steroid receptor coactivator 1
- SREBP1-c
sterol-regulatory-element-binding-protein-1c
- TGs
triglycerides
- TNFα
tumor necrosis factor α
- TR
thyroid receptor
- UDCA
ursodeoxycholic acid
- VCAM-1
vascular cell adhesion molecule-1
- VDR
vitamin D receptor
- VLDL
very low-density lipoprotein
- VSMCs
vascular smooth muscle cells
References
- Ananthanarayanan M., Li S., Balasubramaniyan N., Suchy F. J., Walsh M. J. Ligand-dependent activation of the farnesoid X-receptor directs arginine methylation of histone H3 by CARM1. J Biol Chem. 2004;279:54348–57. doi: 10.1074/jbc.M410021200. [DOI] [PubMed] [Google Scholar]
- Angelin B., Hershon K. S., Brunzell J. D. Bile acid metabolism in hereditary forms of hypertriglyceridemia: evidence for an increased synthesis rate in monogenic familial hypertriglyceridemia. Proc Natl Acad Sci U S A. 1987;84:5434–8. doi: 10.1073/pnas.84.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisfeld A. M., Kast-Woelbern H. R., Lee H., Zhang Y., Lee F. Y., Edwards P. A. Activation of the nuclear receptor FXR induces fibrinogen expression: a new role for bile acid signaling. J Lipid Res. 2005;46:458–68. doi: 10.1194/jlr.M400292-JLR200. [DOI] [PubMed] [Google Scholar]
- Anisfeld A. M., Kast-Woelbern H. R., Meyer M. E., Jones S. A., Zhang Y., Williams K. J., Willson T., Edwards P. A. Syndecan-1 expression is regulated in an isoform-specific manner by the farnesoid-X receptor. J Biol Chem. 2003;278:20420–8. doi: 10.1074/jbc.M302505200. [DOI] [PubMed] [Google Scholar]
- Araya Z., Wikvall K. 6alpha-hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochim Biophys Acta. 1999;1438:47–54. doi: 10.1016/s1388-1981(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Arnold C. N., Goel A., Blum H. E., Boland C. R. Molecular pathogenesis of colorectal cancer: implications for molecular diagnosis. Cancer. 2005;104:2035–47. doi: 10.1002/cncr.21462. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Christian W. V., Lee J. Y., Dawson P. A., Soroka C. J., Boyer J. L., Madejczyk M. S., Li N. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–9. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- Barbier O., Torra I. P., Sirvent A., Claudel T., Blanquart C., Duran-Sandoval D., Kuipers F., Kosykh V., Fruchart J. C., Staels B. FXR induces the UGT2B4 enzyme in hepatocytes: a potential mechanism of negative feedback control of FXR activity. Gastroenterology. 2003;124:1926–40. doi: 10.1016/s0016-5085(03)00388-3. [DOI] [PubMed] [Google Scholar]
- Barone M., Maiorano E., Ladisa R., Cuomo R., Pece A., Berloco P., Caruso M. L., Valentini A. M., Iolascon A., Francavilla A., Di Leo A., Ierardi E. Influence of ursodeoxycholate-enriched diet on liver tumor growth in HBV transgenic mice. Hepatology. 2003;37:880–6. doi: 10.1053/jhep.2003.50175. [DOI] [PubMed] [Google Scholar]
- Barone M., Francavilla A., Polimeno L., Ierardi E., Romanelli D., Berloco P., Di Leo A., Panella C. Modulation of rat hepatocyte proliferation by bile salts: in vitro and in vivo studies. Hepatology. 1996;23:1159–66. doi: 10.1053/jhep.1996.v23.pm0008621149. [DOI] [PubMed] [Google Scholar]
- Bateson M. C., Maclean D., Evans J. R., Bouchier I. A. Chenodeoxycholic acid therapy for hypertriglyceridaemia in men. Br J Clin Pharmacol. 1978;5:249–54. doi: 10.1111/j.1365-2125.1978.tb01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U. M., Daujat S., Nielsen S. J., Nightingale K., Kouzarides T. Methylation at arginine 17 of histone H3 is linked to gene activation. EMBO Rep. 2002;3:39–44. doi: 10.1093/embo-reports/kvf013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beil U., Crouse J. R., Einarsson K., Grundy S. M. Effects of interruption of the enterohepatic circulation of bile acids on the transport of very low density-lipoprotein triglycerides. Metabolism. 1982;31:438–44. doi: 10.1016/0026-0495(82)90231-1. [DOI] [PubMed] [Google Scholar]
- Bell G. D., Lewis B., Petrie A., Dowling R. H. Serum lipids in cholelithiasis: effect of chenodeoxycholic acid therapy. Br Med J. 1973;3:520–3. doi: 10.1136/bmj.3.5879.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergasa N. V., Mason A., Floreani A., Heathcote J., Swain M. G., Jones D. E., Lindor K. M., Bassendine M. F., Worman H. J. Primary biliary cirrhosis: report of a focus study group. Hepatology. 2004;40:1013–20. doi: 10.1002/hep.20446. [DOI] [PubMed] [Google Scholar]
- Bianchini F., Caderni G., Dolara P., Fantetti L., Kriebel D. Effect of dietary fat, starch and cellulose on fecal bile acids in mice. J Nutr. 1989;119:1617–24. doi: 10.1093/jn/119.11.1617. [DOI] [PubMed] [Google Scholar]
- Bilz S., Samuel V., Morino K., Savage D., Choi C. S., Shulman G. I. Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab. 2006;290:E716–22. doi: 10.1152/ajpendo.00355.2005. [DOI] [PubMed] [Google Scholar]
- Bishop-Bailey D., Walsh D. T., Warner T. D. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci U S A. 2004;101:3668–73. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin K., Lindbom U., Diczfalusy U. Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta. 2005;1687:84–93. doi: 10.1016/j.bbalip.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Borgius L. J., Steffensen K. R., Gustafsson J. A., Treuter E. Glucocorticoid signaling is perturbed by the atypical orphan receptor and corepressor SHP. J Biol Chem. 2002;277:49761–6. doi: 10.1074/jbc.M205641200. [DOI] [PubMed] [Google Scholar]
- Brobst D. E., Ding X., Creech K. L., Goodwin B., Kelley B., Staudinger J. L. Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J Pharmacol Exp Ther. 2004;310:528–35. doi: 10.1124/jpet.103.064329. [DOI] [PubMed] [Google Scholar]
- Bull L. N., van Eijk M. J., Pawlikowska L., DeYoung J. A., Juijn J. A., Liao M., Klomp L. W., Lomri N., Berger R., Scharschmidt B. F., Knisely A. S., Houwen R. H., Freimer N. B. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219–24. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- Burris T. P., Montrose C., Houck K. A., Osborne H. E., Bocchinfuso W. P., Yaden B. C., Cheng C. C., Zink R. W., Barr R. J., Hepler C. D., Krishnan V., Bullock H. A., Burris L. L., Galvin R. J., Bramlett K., Stayrook K. R. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol Pharmacol. 2005;67:948–54. doi: 10.1124/mol.104.007054. [DOI] [PubMed] [Google Scholar]
- Campana G., Pasini P., Roda A., Spampinato S. Regulation of ileal bile acid-binding protein expression in Caco-2 cells by ursodeoxycholic acid: role of the farnesoid X receptor. Biochem Pharmacol. 2005;69:1755–63. doi: 10.1016/j.bcp.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T. H., Grefhorst A., Abdelkarim M., Caron S., Torpier G., Fruchart J. C., Gonzalez F. J., Kuipers F., Staels B. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–49. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T., Grefhorst A., Bouchaert E., Fruchart J. C., Gonzalez F. J., Kuipers F., Staels B. Transient impairment of the adaptive response to fasting in FXR-deficient mice. FEBS Lett. 2005;579:4076–80. doi: 10.1016/j.febslet.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Carter B. A., Taylor O. A., Prendergast D. R., Zimmerman T. L., Von Furstenberg R., Moore D. D., Karpen S. J. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62:301–6. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- Chawla A., Repa J. J., Evans R. M., Mangelsdorf D. J. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–70. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chiang J. Y. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 2002;23:443–63. doi: 10.1210/er.2000-0035. [DOI] [PubMed] [Google Scholar]
- Christie D. M., Dawson P. A., Thevananther S., Shneider B. L. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271:G377–85. doi: 10.1152/ajpgi.1996.271.2.G377. [DOI] [PubMed] [Google Scholar]
- Claudel T., Inoue Y., Barbier O., Duran-Sandoval D., Kosykh V., Fruchart J., Fruchart J. C., Gonzalez F. J., Staels B. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–55. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- Craddock A. L., Love M. W., Daniel R. W., Kirby L. C., Walters H. C., Wong M. H., Dawson P. A. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157–69. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- Crouse J. R., 3rd. Hypertriglyceridemia: a contraindication to the use of bile acid binding resins. Am J Med. 1987;83:243–8. doi: 10.1016/0002-9343(87)90692-9. [DOI] [PubMed] [Google Scholar]
- Cui J., Huang L., Zhao A., Lew J. L., Yu J., Sahoo S., Meinke P. T., Royo I., Pelaez F., Wright S. D. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem. 2003;278:10214–20. doi: 10.1074/jbc.M209323200. [DOI] [PubMed] [Google Scholar]
- Cui J., Heard T. S., Yu J., Lo J. L., Huang L., Li Y., Schaeffer J. M., Wright S. D. The amino acid residues asparagine 354 and isoleucine 372 of human farnesoid X receptor confer the receptor with high sensitivity to chenodeoxycholate. J Biol Chem. 2002;277:25963–9. doi: 10.1074/jbc.M200824200. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Haywood J., Craddock A. L., Wilson M., Tietjen M., Kluckman K., Maeda N., Parks J. S. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–7. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Hubbert M., Haywood J., Craddock A. L., Zerangue N., Christian W. V., Ballatori N. The heteromeric organic solute transporter α-β, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–8. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fabiani E., Mitro N., Gilardi F., Caruso D., Galli G., Crestani M. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem. 2003;278:39124–32. doi: 10.1074/jbc.M305079200. [DOI] [PubMed] [Google Scholar]
- de Vree J. M., Jacquemin E., Sturm E., Cresteil D., Bosma P. J., Aten J., Deleuze J. F., Desrochers M., Burdelski M., Bernard O., Oude Elferink R. P., Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A. 1998;95:282–7. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk G. U., Soroka C. J., Takeyama Y., Chen W. S., Schuetz J. D., Boyer J. L. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol. 2004;40:585–91. doi: 10.1016/j.jhep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Deugnier Y., Turlin B. Iron and hepatocellular carcinoma. J Gastroenterol Hepatol. 2001;16:491–4. doi: 10.1046/j.1440-1746.2001.02430.x. [DOI] [PubMed] [Google Scholar]
- Downes M., Verdecia M. A., Roecker A. J., Hughes R., Hogenesch J. B., Kast-Woelbern H. R., Bowman M. E., Ferrer J. L., Anisfeld A. M., Edwards P. A., Rosenfeld J. M., Alvarez J. G., Noel J. P., Nicolaou K. C., Evans R. M. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–92. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Sandoval D., Mautino G., Martin G., Percevault F., Barbier O., Fruchart J. C., Kuipers F., Staels B. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53:890–8. doi: 10.2337/diabetes.53.4.890. [DOI] [PubMed] [Google Scholar]
- Dussault I., Beard R., Lin M., Hollister K., Chen J., Xiao J. H., Chandraratna R., Forman B. M. Identification of gene-selective modulators of the bile acid receptor FXR. J Biol Chem. 2003;278:7027–33. doi: 10.1074/jbc.M209863200. [DOI] [PubMed] [Google Scholar]
- Falany C. N., Johnson M. R., Barnes S., Diasio R. B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J Biol Chem. 1994;269:19375–9. [PubMed] [Google Scholar]
- Fang H. L., Strom S. C., Cai H., Falany C. N., Kocarek T. A., Runge-Morris M. Regulation of human hepatic hydroxysteroid sulfotransferase gene expression by the peroxisome proliferator-activated receptor α transcription factor. Mol Pharmacol. 2005;67:1257–67. doi: 10.1124/mol.104.005389. [DOI] [PubMed] [Google Scholar]
- Foretz M., Pacot C., Dugail I., Lemarchand P., Guichard C., Le Liepvre X., Berthelier-Lubrano C., Spiegelman B., Kim J. B., Ferre P., Foufelle F. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol Cell Biol. 1999;19:3760–8. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Goode E., Chen J., Oro A. E., Bradley D. J., Perlmann T., Noonan D. J., Burka L. T., McMorris T., Lamph W. W., Evans R. M., Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–93. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- Fujino T., Une M., Imanaka T., Inoue K., Nishimaki-Mogami T. Structure-activity relationship of bile acids and bile acid analogs in regard to FXR activation. J Lipid Res. 2004;45:132–8. doi: 10.1194/jlr.M300215-JLR200. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Colditz G. A., Stampfer M. J. A meta-analysis of cholecystectomy and risk of colorectal cancer. Gastroenterology. 1993;105:130–41. doi: 10.1016/0016-5085(93)90018-8. [DOI] [PubMed] [Google Scholar]
- Gnerre C., Blattler S., Kaufmann M. R., Looser R., Meyer U. A. Regulation of CYP3A4 by the bile acid receptor FXR: evidence for functional binding sites in the CYP3A4 gene. Pharmacogenetics. 2004;14:635–45. doi: 10.1097/00008571-200410000-00001. [DOI] [PubMed] [Google Scholar]
- Gong Y. Z., Everett E. T., Schwartz D. A., Norris J. S., Wilson F. A. Molecular cloning, tissue distribution, and expression of a 14-kDa bile acid-binding protein from rat ileal cytosol. Proc Natl Acad Sci U S A. 1994;91:4741–5. doi: 10.1073/pnas.91.11.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B., Jones S. A., Price R. R., Watson M. A., McKee D. D., Moore L. B., Galardi C., Wilson J. G., Lewis M. C., Roth M. E., Maloney P. R., Willson T. M., Kliewer S. A. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Goodwin B., Watson M. A., Kim H., Miao J., Kemper J. K., Kliewer S. A. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-α. Mol Endocrinol. 2003;17:386–94. doi: 10.1210/me.2002-0246. [DOI] [PubMed] [Google Scholar]
- Goulis J., Leandro G., Burroughs A. K. Randomised controlled trials of ursodeoxycholic-acid therapy for primary biliary cirrhosis: a meta-analysis. Lancet. 1999;354:1053–60. doi: 10.1016/S0140-6736(98)11293-X. [DOI] [PubMed] [Google Scholar]
- Grober J., Zaghini I., Fujii H., Jones S. A., Kliewer S. A., Willson T. M., Ono T., Besnard P. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem. 1999;274:29749–54. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- Guo G. L., Santamarina-Fojo S., Akiyama T. E., Amar M. J., Paigen B. J., Brewer B., Jr., Gonzalez F. J. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim Biophys Acta. 2006;1761:1401–9. doi: 10.1016/j.bbalip.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A., Ratliff E. P., Andres A. M., Huang X., McKeehan W. L., Davis R. A. Bile acids decrease hepatic paraoxonase 1 expression and plasma high-density lipoprotein levels via FXR-mediated signaling of FGFR4. Arterioscler Thromb Vasc Biol. 2006;26:301–6. doi: 10.1161/01.ATV.0000195793.73118.b4. [DOI] [PubMed] [Google Scholar]
- Hanniman E. A., Lambert G., McCarthy T. C., Sinal C. J. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J Lipid Res. 2005;46:2595–604. doi: 10.1194/jlr.M500390-JLR200. [DOI] [PubMed] [Google Scholar]
- Hassan A. S., Ravi Subbiah M. T., Thiebert P. Specific changes of bile acid metabolism in spontaneously diabetic Wistar rats. Proc Soc Exp Biol Med. 1980;164:449–52. doi: 10.3181/00379727-164-40894. [DOI] [PubMed] [Google Scholar]
- Hathcock J. N. Metabolic mechanisms of drug-nutrient interactions. Fed Proc. 1985;44:124–9. [PubMed] [Google Scholar]
- He F., Li J., μ Y., Kuruba R., Ma Z., Wilson A., Alber S., Jiang Y., Stevens T., Watkins S., Pitt B., Xie W., Li S. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res. 2006;98:192–9. doi: 10.1161/01.RES.0000200400.55539.85. [DOI] [PubMed] [Google Scholar]
- Heinonen S., Kirkinen P. Pregnancy outcome with intrahepatic cholestasis. Obstet Gynecol. 1999;94:189–93. doi: 10.1016/s0029-7844(99)00254-9. [DOI] [PubMed] [Google Scholar]
- Horton J. D., Goldstein J. L., Brown M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard W. R., Pospisil J. A., Njolito E., Noonan D. J. Catabolites of cholesterol synthesis pathways and forskolin as activators of the farnesoid X-activated nuclear receptor. Toxicol Appl Pharmacol. 2000;163:195–202. doi: 10.1006/taap.1999.8869. [DOI] [PubMed] [Google Scholar]
- Huang W., Ma K., Zhang J., Qatanani M., Cuvillier J., Liu J., Dong B., Huang X., Moore D. D. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–6. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- Huber R. M., Murphy K., Miao B., Link J. R., Cunningham M. R., Rupar M. J., Gunyuzlu P. L., Haws T. F., Kassam A., Powell F., Hollis G. F., Young P. R., Mukherjee R., Burn T. C. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene. 2002;290:35–43. doi: 10.1016/s0378-1119(02)00557-7. [DOI] [PubMed] [Google Scholar]
- Inagaki T., Choi M., Moschetta A., Peng L., Cummins C. L., McDonald J. G., Luo G., Jones S. A., Goodwin B., Richardson J. A., Gerard R. D., Repa J. J., Mangelsdorf D. J., Kliewer S. A. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Inagaki T., Moschetta A., Lee Y. K., Peng L., Zhao G., Downes M., Yu R. T., Shelton J. M., Richardson J. A., Repa J. J., Mangelsdorf D. J., Kliewer S. A. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–5. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen P. L., Muller M., Sturm E. Genes and cholestasis. Hepatology. 2001;34:1067–74. doi: 10.1053/jhep.2001.29625. [DOI] [PubMed] [Google Scholar]
- Kast H. R., Nguyen C. M., Sinal C. J., Jones S. A., Laffitte B. A., Reue K., Gonzalez F. J., Willson T. M., Edwards P. A. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol. 2001;15:1720–8. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen M., Pappo O., Barash H., Klopstock N., Mizrahi L., Olam D., Jacob-Hirsch J., Amariglio N., Rechavi G., Mitchell L. A., Kohen R., Domany E., Galun E., Goldenberg D. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006;66:4001–10. doi: 10.1158/0008-5472.CAN-05-2937. [DOI] [PubMed] [Google Scholar]
- Kim I., Ahn S. H., Inagaki T., Choi M., Ito S., Guo G. L., Kliewer S. A., Gonzalez F. J. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007b;48:2664–72. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- Kim I., Morimura K., Shah Y., Yang Q., Ward J. M., Gonzalez F. J. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007a;28:940–6. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kok T., Hulzebos C. V., Wolters H., Havinga R., Agellon L. B., Stellaard F., Shan B., Schwarz M., Kuipers F. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem. 2003;278:41930–7. doi: 10.1074/jbc.M306309200. [DOI] [PubMed] [Google Scholar]
- Kovacs P., Kress R., Rocha J., Kurtz U., Miquel J. F., Nervi F., Mendez-Sanchez N., Uribe M., Bock H. H., Schirin-Sokhan R., Stumvoll M., Mossner J., Lammert F., Wittenburg H. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol. 2008;48:116–24. doi: 10.1016/j.jhep.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Kurosu H., Choi M., Ogawa Y., Dickson A. S., Goetz R., Eliseenkova A. V., Mohammadi M., Rosenblatt K. P., Kliewer S. A., Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–95. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagergren J., Ye W., Ekbom A. Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis? Gastroenterology. 2001;121:542–7. doi: 10.1053/gast.2001.27083. [DOI] [PubMed] [Google Scholar]
- Lambert G., Amar M. J., Guo G., Brewer H. B., Jr., Gonzalez F. J., Sinal C. J. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–70. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- Landrier J. F., Eloranta J. J., Vavricka S. R., Kullak-Ublick G. A. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-α and -β genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–85. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- Lee J., Azzaroli F., Wang L., Soroka C. J., Gigliozzi A., Setchell K. D., Kramer W., Boyer J. L. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121:1473–84. doi: 10.1053/gast.2001.29608. [DOI] [PubMed] [Google Scholar]
- Lee F. Y., Kast-Woelbern H. R., Chang J., Luo G., Jones S. A., Fishbein M. C., Edwards P. A. α-crystallin is a target gene of the farnesoid X-activated receptor in human livers. J Biol Chem. 2005;280:31792–800. doi: 10.1074/jbc.M503182200. [DOI] [PubMed] [Google Scholar]
- Lee H., Zhang Y., Lee F. Y., Nelson S. F., Gonzalez F. J., Edwards P. A. FXR regulates organic solute transporters α and β in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47:201–14. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- Lee Y. K., Schmidt D. R., Cummins C. L., Choi M., Peng L., Zhang Y., Goodwin B., Hammer R. E., Mangelsdorf D. J., Kliewer S. A. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol. 2008b;22:1345–56. doi: 10.1210/me.2007-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. K., Moore D. D. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci. 2008a;13:5950–8. doi: 10.2741/3128. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Kliewer S. A., Moore L. B., Smith-Oliver T. A., Oliver B. B., Su J. L., Sundseth S. S., Winegar D. A., Blanchard D. E., Spencer T. A., Willson T. M. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–40. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- Levy C., Lindor K. D. Primary sclerosing cholangitis: epidemiology, natural history, and prognosis. Semin Liver Dis. 2006;26:22–30. doi: 10.1055/s-2006-933560. [DOI] [PubMed] [Google Scholar]
- Lew J. L., Zhao A., Yu J., Huang L., De Pedro N., Pelaez F., Wright S. D., Cui J. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem. 2004;279:8856–61. doi: 10.1074/jbc.M306422200. [DOI] [PubMed] [Google Scholar]
- Li Y. T., Swales K. E., Thomas G. J., Warner T. D., Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606–11. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- Li J., Wilson A., Kuruba R., Zhang Q., Gao X., He F., Zhang L. M., Pitt B. R., Xie W., Li S. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc Res. 2008;77:169–77. doi: 10.1093/cvr/cvm016. [DOI] [PubMed] [Google Scholar]
- Liu Y., Binz J., Numerick M. J., Dennis S., Luo G., Desai B., MacKenzie K. I., Mansfield T. A., Kliewer S. A., Goodwin B., Jones S. A. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–87. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Sasso G., Petruzzelli M., Moschetta A. A translational view on the biliary lipid secretory network. Biochim Biophys Acta. 2008;1781:79–96. doi: 10.1016/j.bbalip.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Love M. W., Dawson P. A. New insights into bile acid transport. Curr Opin Lipidol. 1998;9:225–9. doi: 10.1097/00041433-199806000-00007. [DOI] [PubMed] [Google Scholar]
- Love J. D., Gooch J. T., Benko S., Li C., Nagy L., Chatterjee V. K., Evans R. M., Schwabe J. W. The structural basis for the specificity of retinoid-X receptor-selective agonists: new insights into the role of helix H12. J Biol Chem. 2002;277:11385–91. doi: 10.1074/jbc.M110869200. [DOI] [PubMed] [Google Scholar]
- Lu Y., Heydel J. M., Li X., Bratton S., Lindblom T., Radominska-Pandya A. Lithocholic acid decreases expression of UGT2B7 in Caco-2 cells: a potential role for a negative farnesoid X receptor response element. Drug Metab Dispos. 2005;33:937–46. doi: 10.1124/dmd.104.003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T. T., Makishima M., Repa J. J., Schoonjans K., Kerr T. A., Auwerx J., Mangelsdorf D. J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–15. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- MacGilchrist A. J., Sumner D., Reid J. L. Impaired pressor reactivity in cirrhosis: evidence for a peripheral vascular defect. Hepatology. 1991;13:689–94. [PubMed] [Google Scholar]
- Ma K., Saha P. K., Chan L., Moore D. D. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–9. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich J. M., Caravella J. A., Lambert M. H., Willson T. M., Moore J. T., Ramamurthy L. The first completed genome sequence from a teleost fish (Fugu rubripes) adds significant diversity to the nuclear receptor superfamily. Nucleic Acids Res. 2003;31:4051–8. doi: 10.1093/nar/gkg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Maloney P. R., Parks D. J., Haffner C. D., Fivush A. M., Chandra G., Plunket K. D., Creech K. L., Moore L. B., Wilson J. G., Lewis M. C., Jones S. A., Willson T. M. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–4. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R. M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran R. R., Thomas A., Roth M., Sheng Z., Esterly N., Pinson D., Gao X., Zhang Y., Ganapathy V., Gonzalez F. J., Guo G. L. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328:469–77. doi: 10.1124/jpet.108.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolius H. S. Kallikreins and kinins. Molecular characteristics and cellular and tissue responses. Diabetes. 1996;45 Suppl 1:S14–9. doi: 10.2337/diab.45.1.s14. [DOI] [PubMed] [Google Scholar]
- Marschall H. U., Wagner M., Bodin K., Zollner G., Fickert P., Gumhold J., Silbert D., Fuchsbichler A., Sjovall J., Trauner M. Fxr(-/-) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–92. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Nakamura T., Itadani H., Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–9. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- Marzolini C., Tirona R. G., Gervasini G., Poonkuzhali B., Assem M., Lee W., Leake B. F., Schuetz J. D., Schuetz E. G., Kim R. B. A common polymorphism in the bile acid receptor farnesoid X receptor is associated with decreased hepatic target gene expression. Mol Endocrinol. 2007;21:1769–80. doi: 10.1210/me.2007-0025. [DOI] [PubMed] [Google Scholar]
- Mataki C., Magnier B. C., Houten S. M., Annicotte J. S., Argmann C., Thomas C., Overmars H., Kulik W., Metzger D., Auwerx J., Schoonjans K. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27:8330–9. doi: 10.1128/MCB.00852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauad T. H., van Nieuwkerk C. M., Dingemans K. P., Smit J. J., Schinkel A. H., Notenboom R. G., van den Bergh Weerman M. A., Verkruisen R. P., Groen A. K., Oude Elferink R. P. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–45. [PMC free article] [PubMed] [Google Scholar]
- Mennone A., Soroka C. J., Cai S. Y., Harry K., Adachi M., Hagey L., Schuetz J. D., Boyer J. L. Mrp4-/- mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43:1013–21. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- Michael M. D., Kulkarni R. N., Postic C., Previs S. F., Shulman G. I., Magnuson M. A., Kahn C. R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Mi L. Z., Devarakonda S., Harp J. M., Han Q., Pellicciari R., Willson T. M., Khorasanizadeh S., Rastinejad F. Structural basis for bile acid binding and activation of the nuclear receptor FXR. Mol Cell. 2003;11:1093–100. doi: 10.1016/s1097-2765(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Modica S., Bellafante E., Moschetta A. Master regulation of bile acid and xenobiotic metabolism via the FXR, PXR and CAR trio. Front Biosci. 2009;14:4719–45. doi: 10.2741/3563. [DOI] [PubMed] [Google Scholar]
- Modica S., Murzilli S., Salvatore L., Schmidt D. R., Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–94. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- Molavi B., Chen J., Mehta J. L. Cardioprotective effects of rosiglitazone are associated with selective overexpression of type 2 angiotensin receptors and inhibition of p42/44 MAPK. Am J Physiol Heart Circ Physiol. 2006;291:H687–93. doi: 10.1152/ajpheart.00926.2005. [DOI] [PubMed] [Google Scholar]
- Moller D. E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–7. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- Moschetta A., vanBerge-Henegouwen G. P., Portincasa P., Renooij W. L., Groen A. K., van Erpecum K. J. Hydrophilic bile salts enhance differential distribution of sphingomyelin and phosphatidylcholine between micellar and vesicular phases: potential implications for their effects in vivo. J Hepatol. 2001;34:492–9. doi: 10.1016/s0168-8278(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Moschetta A., Bookout A. L., Mangelsdorf D. J. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10:1352–8. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- Moser A. R., Pitot H. C., Dove W. F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Hutchinson H. G., Fujinaga M., Hayashida W., Morishita R., Zhang L., Horiuchi M., Pratt R. E., Dzau V. J. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci U S A. 1995;92:10663–7. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimark E., Chen F., Li X., Shneider B. L. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 2004;40:149–56. doi: 10.1002/hep.20295. [DOI] [PubMed] [Google Scholar]
- Nervi F. O., Severin C. H., Valdivieso V. D. Bile acid pool changes and regulation of cholate synthesis in experimental diabetes. Biochim Biophys Acta. 1978;529:212–23. doi: 10.1016/0005-2760(78)90064-4. [DOI] [PubMed] [Google Scholar]
- Nervi F. O., Gonzalez A., Valdivieso V. D. Studies on cholesterol metabolism in the diabetic rat. Metabolism. 1974;23:495–503. doi: 10.1016/0026-0495(74)90077-8. [DOI] [PubMed] [Google Scholar]
- Niesor E. J., Flach J., Lopes-Antoni I., Perez A., Bentzen C. L. The nuclear receptors FXR and LXRalpha: potential targets for the development of drugs affecting lipid metabolism and neoplastic diseases. Curr Pharm Des. 2001;7:231–59. doi: 10.2174/1381612013398185. [DOI] [PubMed] [Google Scholar]
- Nilsson L. M., Abrahamsson A., Sahlin S., Gustafsson U., Angelin B., Parini P., Einarsson C. Bile acids and lipoprotein metabolism: effects of cholestyramine and chenodeoxycholic acid on human hepatic mRNA expression. Biochem Biophys Res Commun. 2007;357:707–11. doi: 10.1016/j.bbrc.2007.03.196. [DOI] [PubMed] [Google Scholar]
- Nishigori H., Tomura H., Tonooka N., Kanamori M., Yamada S., Sho K., Inoue I., Kikuchi N., Onigata K., Kojima I., Kohama T., Yamagata K., Yang Q., Matsuzawa Y., Miki T., Seino S., Kim M. Y., Choi H. S., Lee Y. K., Moore D. D., Takeda J. Mutations in the small heterodimer partner gene are associated with mild obesity in Japanese subjects. Proc Natl Acad Sci U S A. 2001;98:575–80. doi: 10.1073/pnas.021544398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaki-Mogami T., Une M., Fujino T., Sato Y., Tamehiro N., Kawahara Y., Shudo K., Inoue K. Identification of intermediates in the bile acid synthetic pathway as ligands for the farnesoid X receptor. J Lipid Res. 2004;45:1538–45. doi: 10.1194/jlr.M400102-JLR200. [DOI] [PubMed] [Google Scholar]
- Nozawa H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-A(y) mice. Biochem Biophys Res Commun. 2005;336:754–61. doi: 10.1016/j.bbrc.2005.08.159. [DOI] [PubMed] [Google Scholar]
- Oelkers P., Kirby L. C., Heubi J. E., Dawson P. A. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99:1880–7. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Oshima H., Aoki M., Uchio R., Naka K., Nakamura S., Hirao A., Saya H., Taketo M. M., Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-α in gastric tumour cells. Embo J. 2008;27:1671–81. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte K., Kranz H., Kober I., Thompson P., Hoefer M., Haubold B., Remmel B., Voss H., Kaiser C., Albers M., Cheruvallath Z., Jackson D., Casari G., Koegl M., Paabo S., Mous J., Kremoser C., Deuschle U. Identification of farnesoid X receptor β as a novel mammalian nuclear receptor sensing lanosterol. Mol Cell Biol. 2003;23:864–72. doi: 10.1128/MCB.23.3.864-872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., Lehmann J. M. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Paumgartner G., Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–31. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- Peet D. J., Turley S. D., Ma W., Janowski B. A., Lobaccaro J. M., Hammer R. E., Mangelsdorf D. J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR α. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- Pellicciari R., Fiorucci S., Camaioni E., Clerici C., Costantino G., Maloney P. R., Morelli A., Parks D. J., Willson T. M. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–72. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- Pellicciari R., Gioiello A., Costantino G., Sadeghpour B. M., Rizzo G., Meyer U., Parks D. J., Entrena-Guadix A., Fiorucci S. Back door modulation of the farnesoid X receptor: design, synthesis, and biological evaluation of a series of side chain modified chenodeoxycholic acid derivatives. J Med Chem. 2006;49:4208–15. doi: 10.1021/jm060294k. [DOI] [PubMed] [Google Scholar]
- Pellicciari R., Costantino G., Camaioni E., Sadeghpour B. M., Entrena A., Willson T. M., Fiorucci S., Clerici C., Gioiello A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559–69. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- Pineda Torra I., Claudel T., Duval C., Kosykh V., Fruchart J. C., Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–72. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- Pineda Torra I., Freedman L. P., Garabedian M. J. Identification of DRIP205 as a coactivator for the Farnesoid X receptor. J Biol Chem. 2004;279:36184–91. doi: 10.1074/jbc.M405126200. [DOI] [PubMed] [Google Scholar]
- Pircher P. C., Kitto J. L., Petrowski M. L., Tangirala R. K., Bischoff E. D., Schulman I. G., Westin S. K. Farnesoid X receptor regulates bile acid-amino acid conjugation. J Biol Chem. 2003;278:27703–11. doi: 10.1074/jbc.M302128200. [DOI] [PubMed] [Google Scholar]
- Podesta M. R., Murphy G. M., Dowling R. H. Measurement of faecal bile acid sulphates. J Chromatogr. 1980;182:293–300. doi: 10.1016/s0378-4347(00)81477-2. [DOI] [PubMed] [Google Scholar]
- Popivanova B. K., Kitamura K., Wu Y., Kondo T., Kagaya T., Kaneko S., Oshima M., Fujii C., Mukaida N. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–70. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portincasa P., Moschetta A., Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–9. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- Potter J. D. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–32. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Petersen G. M., Krush A. J., Booker S., Jen J., Giardiello F. M., Hamilton S. R., Vogelstein B., Kinzler K. W. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993;329:1982–7. doi: 10.1056/NEJM199312303292702. [DOI] [PubMed] [Google Scholar]
- Propst T., Propst A., Dietze O., Judmaier G., Braunsteiner H., Vogel W. α-1-antitrypsin deficiency and liver disease. Dig Dis. 1994;12:139–49. doi: 10.1159/000171447. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., Eng C., Salen G., Shefer S., Batta A. K., Erickson S. K., Verhagen A., Rivera C. R., Mulvihill S. J., Malloy M. J., Kane J. P. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–17. doi: 10.1172/JCI15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Tang X., Elloso M. M., Harnish D. C. Bile acids induce adhesion molecule expression in endothelial cells through activation of reactive oxygen species, NF-kappaB, and p38. Am J Physiol Heart Circ Physiol. 2006;291:H741–7. doi: 10.1152/ajpheart.01182.2005. [DOI] [PubMed] [Google Scholar]
- Rao A., Haywood J., Craddock A. L., Belinsky M. G., Kruh G. D., Dawson P. A. The organic solute transporter α-β, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–6. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff E. P., Gutierrez A., Davis R. A. Transgenic expression of CYP7A1 in LDL receptor-deficient mice blocks diet-induced hypercholesterolemia. J Lipid Res. 2006;47:1513–20. doi: 10.1194/jlr.M600120-JLR200. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Hedges A. R., Laakso K., Wynder E. L. Metabolic epidemiology of large bowel cancer: fecal bulk and constituents of high-risk North American and low-risk Finnish population. Cancer. 1978;42:2832–8. doi: 10.1002/1097-0142(197812)42:6<2832::aid-cncr2820420644>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Ricketts M. L., Boekschoten M. V., Kreeft A. J., Hooiveld G. J., Moen C. J., Muller M., Frants R. R., Kasanmoentalib S., Post S. M., Princen H. M., Porter J. G., Katan M. B., Hofker M. H., Moore D. D. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21:1603–16. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- Rioseco A. J., Ivankovic M. B., Manzur A., Hamed F., Kato S. R., Parer J. T., Germain A. M. Intrahepatic cholestasis of pregnancy: a retrospective case-control study of perinatal outcome. Am J Obstet Gynecol. 1994;170:890–5. doi: 10.1016/s0002-9378(94)70304-3. [DOI] [PubMed] [Google Scholar]
- Rizzo G., Disante M., Mencarelli A., Renga B., Gioiello A., Pellicciari R., Fiorucci S. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Mol Pharmacol. 2006;70:1164–73. doi: 10.1124/mol.106.023820. [DOI] [PubMed] [Google Scholar]
- Rizzo G., Renga B., Antonelli E., Passeri D., Pellicciari R., Fiorucci S. The methyl transferase PRMT1 functions as co-activator of farnesoid X receptor (FXR)/9-cis retinoid X receptor and regulates transcription of FXR responsive genes. Mol Pharmacol. 2005;68:551–8. doi: 10.1124/mol.105.012104. [DOI] [PubMed] [Google Scholar]
- Rudel L., Deckelman C., Wilson M., Scobey M., Anderson R. Dietary cholesterol and downregulation of cholesterol 7 α-hydroxylase and cholesterol absorption in African green monkeys. J Clin Invest. 1994;93:2463–72. doi: 10.1172/JCI117255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- Savage D. B., Petersen K. F., Shulman G. I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–20. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savkur R. S., Thomas J. S., Bramlett K. S., Gao Y., Michael L. F., Burris T. P. Ligand-dependent coactivation of the human bile acid receptor FXR by the peroxisome proliferator-activated receptor γ coactivator-1alpha. J Pharmacol Exp Ther. 2005a;312:170–8. doi: 10.1124/jpet.104.072124. [DOI] [PubMed] [Google Scholar]
- Savkur R. S., Bramlett K. S., Michael L. F., Burris T. P. Regulation of pyruvate dehydrogenase kinase expression by the farnesoid X receptor. Biochem Biophys Res Commun. 2005b;329:391–6. doi: 10.1016/j.bbrc.2005.01.141. [DOI] [PubMed] [Google Scholar]
- Savoia C., Touyz R. M., Volpe M., Schiffrin E. L. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007;49:341–6. doi: 10.1161/01.HYP.0000253968.95136.b8. [DOI] [PubMed] [Google Scholar]
- Scarabin P. Y., Arveiler D., Amouyel P., Dos Santos C., Evans A., Luc G., Ferrieres J., Juhan-Vague I. Plasma fibrinogen explains much of the difference in risk of coronary heart disease between France and Northern Ireland. The PRIME study. Atherosclerosis. 2003;166:103–9. doi: 10.1016/s0021-9150(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Seol W., Choi H. S., Moore D. D. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- Shibata M., Morizane T., Uchida T., Yamagami T., Onozuka Y., Nakano M., Mitamura K., Ueno Y. Irregular regeneration of hepatocytes and risk of hepatocellular carcinoma in chronic hepatitis and cirrhosis with hepatitis-C-virus infection. Lancet. 1998;351:1773–7. doi: 10.1016/S0140-6736(97)08002-1. [DOI] [PubMed] [Google Scholar]
- Shih D. M., Kast-Woelbern H. R., Wong J., Xia Y. R., Edwards P. A., Lusis A. J. A role for FXR and human FGF-19 in the repression of paraoxonase-1 gene expression by bile acids. J Lipid Res. 2006;47:384–92. doi: 10.1194/jlr.M500378-JLR200. [DOI] [PubMed] [Google Scholar]
- Shimamoto Y., Ishida J., Yamagata K., Saito T., Kato H., Matsuoka T., Hirota K., Daitoku H., Nangaku M., Yamagata K., Fujii H., Takeda J., Fukamizu A. Inhibitory effect of the small heterodimer partner on hepatocyte nuclear factor-4 mediates bile acid-induced repression of the human angiotensinogen gene. J Biol Chem. 2004;279:7770–6. doi: 10.1074/jbc.M310577200. [DOI] [PubMed] [Google Scholar]
- Shimomura I., Bashmakov Y., Ikemoto S., Horton J. D., Brown M. S., Goldstein J. L. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1999;96:13656–61. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–44. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Solaas K., Ulvestad A., Soreide O., Kase B. F. Subcellular organization of bile acid amidation in human liver: a key issue in regulating the biosynthesis of bile salts. J Lipid Res. 2000;41:1154–62. [PubMed] [Google Scholar]
- Song C. S., Echchgadda I., Baek B. S., Ahn S. C., Oh T., Roy A. K., Chatterjee B. Dehydroepiandrosterone sulfotransferase gene induction by bile acid activated farnesoid X receptor. J Biol Chem. 2001;276:42549–56. doi: 10.1074/jbc.M107557200. [DOI] [PubMed] [Google Scholar]
- Staels B., Kuipers F. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs. 2007;67:1383–92. doi: 10.2165/00003495-200767100-00001. [DOI] [PubMed] [Google Scholar]
- Stayrook K. R., Bramlett K. S., Savkur R. S., Ficorilli J., Cook T., Christe M. E., Michael L. F., Burris T. P. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005;146:984–91. doi: 10.1210/en.2004-0965. [DOI] [PubMed] [Google Scholar]
- Stedman C., Liddle C., Coulter S., Sonoda J., Alvarez J. G., Evans R. M., Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci U S A. 2006;103:11323–8. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen K. R., Gustafsson J. A. Putative metabolic effects of the liver X receptor (LXR) Diabetes. 2004;53 Suppl 1:S36–42. doi: 10.2337/diabetes.53.2007.s36. [DOI] [PubMed] [Google Scholar]
- Strautnieks S. S., Bull L. N., Knisely A. S., Kocoshis S. A., Dahl N., Arnell H., Sokal E., Dahan K., Childs S., Ling V., Tanner M. S., Kagalwalla A. F., Nemeth A., Pawlowska J., Baker A., Mieli-Vergani G., Freimer N. B., Gardiner R. M., Thompson R. J. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–8. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- Swales K. E., Korbonits M., Carpenter R., Walsh D. T., Warner T. D., Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66:10120–6. doi: 10.1158/0008-5472.CAN-06-2399. [DOI] [PubMed] [Google Scholar]
- Takikawa H., Otsuka H., Beppu T., Seyama Y., Yamakawa T. Serum concentrations of bile acid glucuronides in hepatobiliary diseases. Digestion. 1983;27:189–95. doi: 10.1159/000198952. [DOI] [PubMed] [Google Scholar]
- Tepperman J., Caldwell F. T., Tepperman H. M. Induction of Gallstones in Mice by Feeding a Cholesterol-Cholic Acid Containing Diet. Am J Physiol. 1964;206:628–34. doi: 10.1152/ajplegacy.1964.206.3.628. [DOI] [PubMed] [Google Scholar]
- Tobin K. A., Ulven S. M., Schuster G. U., Steineger H. H., Andresen S. M., Gustafsson J. A., Nebb H. I. Liver X receptors as insulin-mediating factors in fatty acid and cholesterol biosynthesis. J Biol Chem. 2002;277:10691–7. doi: 10.1074/jbc.M109771200. [DOI] [PubMed] [Google Scholar]
- Tochtrop G. P., DeKoster G. T., Covey D. F., Cistola D. P. A single hydroxyl group governs ligand site selectivity in human ileal bile acid binding protein. J Am Chem Soc. 2004;126:11024–9. doi: 10.1021/ja047589c. [DOI] [PubMed] [Google Scholar]
- Toke O., Monsey J. D., DeKoster G. T., Tochtrop G. P., Tang C., Cistola D. P. Determinants of cooperativity and site selectivity in human ileal bile acid binding protein. Biochemistry. 2006;45:727–37. doi: 10.1021/bi051781p. [DOI] [PubMed] [Google Scholar]
- Tomlinson E., Fu L., John L., Hultgren B., Huang X., Renz M., Stephan J. P., Tsai S. P., Powell-Braxton L., French D., Stewart T. A. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–7. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- Twisk J., Hoekman M. F., Lehmann E. M., Meijer P., Mager W. H., Princen H. M. Insulin suppresses bile acid synthesis in cultured rat hepatocytes by down-regulation of cholesterol 7 α-hydroxylase and sterol 27-hydroxylase gene transcription. Hepatology. 1995;21:501–10. [PubMed] [Google Scholar]
- Ueda J., Chijiiwa K., Nakano K., Zhao G., Tanaka M. Lack of intestinal bile results in delayed liver regeneration of normal rat liver after hepatectomy accompanied by impaired cyclin E-associated kinase activity. Surgery. 2002;131:564–73. doi: 10.1067/msy.2002.123008. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Moriyama M., Uchida T., Arakawa Y. Irregular regeneration of hepatocytes is an important factor in the hepatocarcinogenesis of liver disease. Hepatology. 2001;33:357–62. doi: 10.1053/jhep.2001.21902. [DOI] [PubMed] [Google Scholar]
- Urizar N. L., Liverman A. B., Dodds D. T., Silva F. V., Ordentlich P., Yan Y., Gonzalez F. J., Heyman R. A., Mangelsdorf D. J., Moore D. D. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–6. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- Urizar N. L., Moore D. D. GUGULIPID: a natural cholesterol-lowering agent. Annu Rev Nutr. 2003;23:303–13. doi: 10.1146/annurev.nutr.23.011702.073102. [DOI] [PubMed] [Google Scholar]
- Van Mil S. W., Milona A., Dixon P. H., Mullenbach R., Geenes V. L., Chambers J., Shevchuk V., Moore G. E., Lammert F., Glantz A. G., Mattsson L. A., Whittaker J., Parker M. G., White R., Williamson C. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507–16. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- van Waarde W. M., Verkade H. J., Wolters H., Havinga R., Baller J., Bloks V., Muller M., Sauer P. J., Kuipers F. Differential effects of streptozotocin-induced diabetes on expression of hepatic ABC-transporters in rats. Gastroenterology. 2002;122:1842–52. doi: 10.1053/gast.2002.33582. [DOI] [PubMed] [Google Scholar]
- Vessey D. A., Crissey M. H., Zakim D. Kinetic studies on the enzymes conjugating bile acids with taurine and glycine in bovine liver. Biochem J. 1977;163:181–3. doi: 10.1042/bj1630181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–53. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., Schoonjans K., Bianco A. C., Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Houten S. M., Wang L., Moschetta A., Mangelsdorf D. J., Heyman R. A., Moore D. D., Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–18. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W. C. Diet and cancer: one view at the start of the millennium. Cancer Epidemiol Biomarkers Prev. 2001;10:3–8. [PubMed] [Google Scholar]
- Wu J., Xia C., Meier J., Li S., Hu X., Lala D. S. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol. 2002;16:1590–7. doi: 10.1210/mend.16.7.0894. [DOI] [PubMed] [Google Scholar]
- Yamagata K., Daitoku H., Shimamoto Y., Matsuzaki H., Hirota K., Ishida J., Fukamizu A. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279:23158–65. doi: 10.1074/jbc.M314322200. [DOI] [PubMed] [Google Scholar]
- Yang F., Huang X., Yi T., Yen Y., Moore D. D., Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–7. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- Yu J., Lo J. L., Huang L., Zhao A., Metzger E., Adams A., Meinke P. T., Wright S. D., Cui J. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem. 2002;277:31441–7. doi: 10.1074/jbc.M200474200. [DOI] [PubMed] [Google Scholar]
- Zavacki A. M., Lehmann J. M., Seol W., Willson T. M., Kliewer S. A., Moore D. D. Activation of the orphan receptor RIP14 by retinoids. Proc Natl Acad Sci U S A. 1997;94:7909–14. doi: 10.1073/pnas.94.15.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., Willson T. M., Edwards P. A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006a;103:1006–11. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang X., Vales C., Lee F. Y., Lee H., Lusis A. J., Edwards P. A. FXR deficiency causes reduced atherosclerosis in Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2006b;26:2316–21. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- Zhang Q., He F., Kuruba R., Gao X., Wilson A., Li J., Billiar T. R., Pitt B. R., Xie W., Li S. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc Res. 2008;77:560–9. doi: 10.1093/cvr/cvm068. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kast-Woelbern H. R., Edwards P. A. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem. 2003;278:104–10. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Castellani L. W., Sinal C. J., Gonzalez F. J., Edwards P. A. Peroxisome proliferator-activated receptor-γ coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–69. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Chiang J. Y. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem. 2001;276:41690–9. doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- Zhao A., Lew J. L., Huang L., Yu J., Zhang T., Hrywna Y., Thompson J. R., de Pedro N., Blevins R. A., Pelaez F., Wright S. D., Cui J. Human kininogen gene is transactivated by the farnesoid X receptor. J Biol Chem. 2003;278:28765–70. doi: 10.1074/jbc.M304568200. [DOI] [PubMed] [Google Scholar]