Abstract
Although individuals exposed to cigarette smoke are more susceptible to respiratory infection, the effects of cigarette smoke on pulmonary defense are incompletely understood. Based on the observation that interactions between bacteria and host cells result in the expression of critical defense genes regulated by NF-κB, we hypothesized that cigarette smoke alters NF-κB function. In this study, primary human tracheobronchial epithelial cells were treated with cigarette smoke extract (CSE) and exposed to Haemophilus influenzae, and the effects of CSE on bacteria-induced signaling and gene expression were assessed. CSE inhibited high concentrations of induced NF-κB activation and the consequent expression of defense genes that occurred in airway epithelial cells in response to H. influenzae. This decreased activation of NF-κB was not attributable to cell loss or cytotoxicity. Glutathione augmentation of epithelial cells decreased the effects of CSE on NF-κB–dependent responses, as well as the effects on the inhibitor of κB and the inhibitor of κB kinase, which are upstream NF-κB regulators, suggesting the involvement of reactive oxygen species. The relevance of these findings for lung infection was confirmed using a mouse model of H. influenzae airway infection, in which decreased NF-κB pathway activation, keratinocyte chemoattractant (KC) chemokine expression, and neutrophil recruitment occurred in animals exposed to cigarette smoke. The results indicate that although cigarette smoke can cause inflammation in the lung, exposure to smoke inhibits the robust pulmonary defense response to H. influenzae, thereby providing one explanation for the increased susceptibility to respiratory bacterial infection in individuals exposed to cigarette smoke.
Keywords: Haemophilus influenzae, airway epithelial cells, glutathione, inflammation
CLINICAL RELEVANCE.
We focus on a mechanism through which cigarette smoke impairs antibacterial defense in the airway. The most novel portions of this work include the identification of direct effects of cigarette smoke on NF-κB signaling, using both cell-based and animal model systems.
A growing body of evidence indicates that cigarette smoke exposure increases susceptibility to bacterial (as well as viral) respiratory infections. Individuals who smoke cigarettes are more likely to experience nasopharyngeal colonization by potential bacterial pathogens, possibly through increased bacterial adherence (1). Cigarette use is an independent risk factor for invasive pneumococcal disease, and the increased risk persists for 5–9 years after the cessation of smoking (2). Infants and children exposed to environmental tobacco smoke are well-known to exhibit an increased incidence and severity of otitis media, bronchitis, and pneumonia (3). Cigarette smoke appears to alter airway antibacterial defenses by affecting multiple components of innate and adaptive immunity (4). For example, cigarette smoke exposure dysregulates lung protease and inflammatory mediator concentrations, and decreases mucociliary clearance, macrophage function, and pulmonary dendritic cell numbers (5–9). Although the available information indicates that cigarette smoke alters respiratory system defenses and increases the incidence, duration, and severity of bacterial respiratory infection, the mechanisms responsible for these effects are incompletely understood.
When constitutive innate defense mechanisms in airway epithelia are overwhelmed by bacteria, an inflammatory response is initiated that recruits leukocytes (particularly neutrophils) to the site of infection (10, 11). The transcription factor NF-κB is critical for the regulation of inflammatory gene expression that controls leukocyte recruitment (e.g., cytokines and cell adhesion molecules), as well as other defense responses that control bacterial infection (11, 12). Under basal conditions, epithelial and other cells sequester NF-κB family members, such as RelA, in the cytoplasm bound to inhibitor of κB (IκB) proteins (13). A variety of stimuli, including bacterial molecules and host mediators, induce the phosphorylation-dependent activation of the inhibitor of κB kinase (IKK), which phosphorylates specific serine residues on IκB proteins, thereby targeting them for ubiquitination. These modifications of IκB lead to degradation by the 26S proteasome, resulting in the release of NF-κB, the unmasking of its nuclear localization signal, and translocation to the cell nucleus, where it mediates defense gene transcription (14). RelA (p65) and NF-κB1 (p50) are NF-κB family members that are activated, and regulate defense gene expression, in airway epithelial cells exposed to Haemophilus influenzae (15). This NF-κB–dependent defense system allows for rapid and efficient airway defense that most often results in the clearance of pulmonary infections.
Based on this information, we questioned whether cigarette smoke has direct effects on NF-κB function and antibacterial defense mechanisms in airway epithelial cells and in the lung. Here, we use both primary human airway epithelial cells with an extract of cigarette smoke and a mouse model of exposure to cigarette smoke with airway H. influenzae infection to demonstrate that cigarette smoke alters pulmonary responses to bacteria. Our results indicate that one key effect of cigarette smoke extract (CSE) on airway epithelial cells and of cigarette smoke exposure in mice is an inhibition of high-level NF-κB activation and function occurring after epithelial cell or lung interactions with bacteria. In addition, the effects of CSE on NF-κB–dependent gene expression in response to bacteria can be decreased by glutathione augmentation of epithelial cells, suggesting that oxidants in cigarette smoke mediate at least a portion of this effect. These findings support the concept that exposure to cigarette smoke in the human airway directly alters antibacterial defense, providing one mechanism for the increased susceptibility to respiratory bacterial infections in individuals exposed to cigarette smoke.
MATERIALS AND METHODS
Airway Epithelial Cell Isolation, Culture, and Treatments
Human trachea and bronchial samples from individuals without lung disease were obtained through the University of Iowa Cell Culture Core Repository under a protocol approved by the University of Iowa Institutional Review Board. Airways were dissected from lung tissue, and primary human tracheobronchial epithelial (hTBE) cells from the surface of airway mucosa were isolated by enzymatic dissociation. Cells were cultured in Laboratory of Human Carcinogenesis–8e medium on plates coated with collagen or albumin, as described previously (15, 16). To ensure reproducible and generalizable results, experiments were repeated at least three times, using hTBE cells from different individuals (n = 12). In some experiments, hTBE cells were treated with the antioxidants N-acetylcysteine (NAC) or glutathione monoethyl ester (GSH-MEE; Sigma-Aldrich, St. Louis, MO).
Cigarette Smoke Extract
Fresh CSE was prepared before each experiment by drawing mainstream smoke from the base of a lighted research reference cigarette (University of Kentucky, Lexington, KY) into a 60-ml syringe containing 10 ml of culture medium. Smoke was drawn into the syringe seven times with syringe capping and 100 shakes after each draw, resulting in combustion of the full length of the cigarette, except for 0.5 cm adjacent to the filter. The consistency of the 100% CSE preparation was monitored by spectrophotometric measurement of absorbance, resulting in an absorbance at 300 nm wavelength of 2.59–2.92 that correlated with an added cigarette smoke nonvolatile mass of 0.48–0.72 mg/ml. CSE was used immediately after generation, and was diluted to 5% or 10% in culture medium (final pH, 7.26–7.31; normal pH of medium, 7.25–7.30) before exposure of hTBE cells.
Bacterial Preparation
Aerated, log-phase cultures of nontypable H. influenzae strain 12 or nontypable strains isolated from the sputum of individuals with COPD were prepared and quantified as described previously (11, 15–17). Epithelial cells were incubated in 108–1010 colony-forming units (CFU)/ml (500–50,000 CFU/epithelial cell) of bacteria that were pretreated with 100 μg/ml gentamicin for 30 minutes, and then incubated with epithelial cells in culture medium containing gentamicin for 1–24 hours.
Enzyme-Linked Immunoassays
Human IL-8 and IL-6 and mouse keratinocyte chemoattractant (KC) protein concentrations were determined using commercial sandwich enzyme-linked immunoassay kits (R&D Systems, Minneapolis, MN), as described previously (11, 16–18). The sensitivity of this assay system for IL-8 is greater than 37.5 pg/ml, its sensitivity for IL-6 is greater than 9.4 pg/ml, and its sensitivity for KC is greater than 2.0 pg/ml. Intercellular adhesion molecule–1 (ICAM-1) concentrations on the surfaces of cell monolayers were determined using an enzyme-linked immunoassay, as described previously (11, 16, 17).
Immunoblot Analysis
Whole-cell and nuclear protein extract preparation and immunoblot analysis were performed as described previously (15, 18). Primary antibodies used to detect specific cellular and nuclear proteins included rabbit polyclonal IgG 4915 against human ICAM-1, mouse IgG1 monoclonal antibody (mAb) clone 3G2 against human caspase-3, rabbit IgG mAb clone 5A1E against human cleaved caspase-3, rabbit IgG mAb clone C22B4 against human RelA/p65, rabbit IgG mAb clone C84E11 against human IKKα phosphorylated on Ser-176 and IKKβ phosphorylated on Ser-177, rabbit IgG mAb clone 14D4 against human IκBα phosphorylated on Ser-32 (which also binds mouse phosphorylated IκBα), and mouse IgG1 mAb clone L35A5 against human and mouse IκBα from Cell Signaling Technology (Beverly, MA); mouse IgG2a mAb clone AC-74 against human β-actin (which also binds mouse β-actin) from Sigma-Aldrich (St. Louis, MO); and rabbit polyclonal antiserum against human heat shock protein (HSP)–90 from Assay Designs (Ann Arbor, MI). Primary antibody binding was detected using goat anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA, or Cell Signaling Technology) and an enhanced chemiluminescence detection system (Amersham Biosciences, Uppsala, Sweden). Reprobing of membranes was performed after washing twice in Restore buffer (Pierce, Rockford, IL) for 15 minutes at 37°C. In some experiments, radiographic film images were analyzed using ImageJ software (19). To generate an integrated density level, band area was multiplied by the band mean gray value, and the integrated density for phosphorylated or total IκBα was divided by the corresponding β-actin concentration, creating a ratio for each sample.
mRNA Analysis
Total cellular RNA was isolated and mRNA concentrations determined using real-time RT-PCR analysis with an iCycler iQ Fluorescence Thermocycler (Bio-Rad Laboratories, Hercules, CA), as described previously (15). The primers designed with software by S. Rozen and H. Skaletsky (http://www.genome.wi.mit.edu/genome_software/other/primer3.html) included: (1) human IL-8 sense 5′-TCTGCAGCTCTGTGTGAAGG-3′ and antisense 5′-AATTTCTGTGTTGGCGCAGT-3′; (2) human ICAM-1 sense 5′-CATAGAGACCCCGTTGCCTA-3′ and antisense 5′-GAAATTGGCTCCATGGTGAT-3′; and (3) human hypoxanthine phosphoribosyltransferase (HPRT) sense 5′-GCAGACTTTGCTTTCCTTGG-3′ and antisense 5′-AAGCAGATGGCCACAGAACT-3′. Data were collected and recorded by iCycler iQ software (Bio-Rad Laboratories) and initially quantified as a function of threshold cycle (Ct). The Ct was defined as the cycle at which the fluorescence intensity in a given reaction tube rose above background, which was calculated as 10 times the mean standard deviation of fluorescence in all wells over the baseline cycles. Concentrations of mRNA are expressed relative to control HPRT concentrations, and are calculated as 2ΔCt.
Epithelial Cytotoxicity Assays
Mitochondrial activity was assessed by quantification of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reduction to a colored formazan product in the presence of phenazine ethosulfate, using a commercial kit (Promega, Madison, WI). The determination of plasma membrane permeability to ethidium homodimers in dead cells, and of intracellular esterase activity in live cells, was performed using a commercial fluorescence-based viability and cytotoxicity kit (Molecular Probes, Eugene, OR). Cell numbers in samples were quantified by blinded manual counts of cell numbers per low-power field in four random fields per condition from duplicate samples. Images from this assay were acquired using a fluorescence microscope (Leica Microsystems, Cambridge, UK) linked to a digital camera system interfaced with QCapture Suite software (QImaging, Surrey, BC, Canada). A positive control for 100% cell death was generated by adding the permeabilizing agent 1% saponin to cells. Equal cell numbers in each condition were also estimated at the end of some experiments by measurement of total cell protein in lysate samples, using a Coomassie brilliant blue G-250 binding assay (Bio-Rad Laboratories).
NF-κB Activation Assays
NF-κB–dependent gene activation was determined using a recombinant adenoviral vector that expresses a luciferase gene driven by four tandem NF-κB enhancer sequences, as described previously (11, 18). Photinus pyralis luciferase activity was determined using a commercial luciferase reporter assay kit (Promega) and a Lumat LB 9501 luminometer (Berthold, Bad Wildbad, Germany). Nuclear protein binding to a double-stranded, synthetic oligonucleotide corresponding to the NF-κB consensus sequence (Santa Cruz Biotechnology) was determined using an electrophoretic mobility-shift assay (EMSA), as described previously (15). Proteins in DNA-binding complexes were identified by supershift analysis, using 5 μg of control antibody or rabbit polyclonal IgG sc-109 against human RelA/p65 (Santa Cruz Biotechnology).
Mouse Cigarette Smoke Exposure
Experiments involving mice were performed under a protocol approved by the University of Iowa Institutional Animal Care and Use Committee. C57BL/6J mice, 4–6 weeks old (National Cancer Institute, Frederick, MD) and housed under pathogen-free conditions, underwent nose-only exposure to combined mainstream and sidestream cigarette smoke at a rate of four cigarettes/hour. Chamber carbon monoxide and total particulate matter concentrations, and blood carboxyhemoglobin concentrations, were monitored in selected experiments to provide information on exposure conditions. Details of the mouse cigarette smoke exposure system are provided in the online supplement.
Mouse Airway Bacterial Infection
Mice were anesthetized using ketamine and xylazine, and underwent orotracheal intubation, followed by airway injection of a bacterial inoculum containing 107–108 CFU H. influenzae strain 12 incorporated into and mixed with amorphous agar particles (ranging in size from 30–200 μm) in a 50-μl volume, as described previously (10, 11, 17). After a specified duration of infection, mice were anesthetized and then killed by cervical dislocation, and their lungs were exposed, resected, and placed in a Petri dish at 4°C. To evaluate whole-lung KC and phosphorylated and total IκB concentrations, extracts were generated from the left lung in immunoblot analysis lysis buffer by dissociation using a tissue tearer, by cell disruption using sonication, and by supernatant isolation after centrifugation at 4°C. To assess lung neutrophil recruitment, bronchoalveolar lavage (BAL) was performed in the left lung with 0.5-ml aliquots of PBS, followed by quantitation of total leukocyte counts using a hemocytometer, and differential counts using a modified Wright's staining of cytospin preparations. To verify pulmonary bacterial infection, homogenates from the right lung underwent serial dilution, followed by aseptic inoculation on solid agar medium for the culturing and quantitation of colonies.
Statistical Analysis
Enzyme-linked immunoassays, real-time RT-PCR mRNA analyses, cytotoxicity and luciferase assays, and densitometry analyses were repeated multiple times to assure reproducible results, and were analyzed for statistical significance using ANOVA for a factorial experimental design. The multicomparison significance level for the ANOVA was 0.05. If significance was achieved by one-way analysis, a post-ANOVA comparison of means was performed using the Bonferroni or Tukey tests (20). BAL neutrophil number/lung bacterial load values from animals with and without exposure to cigarette smoke were analyzed for statistical significance using a one-sample t test, and the significance level was 0.05.
RESULTS
CSE Decreases H. influenzae–Induced Defense Gene Expression
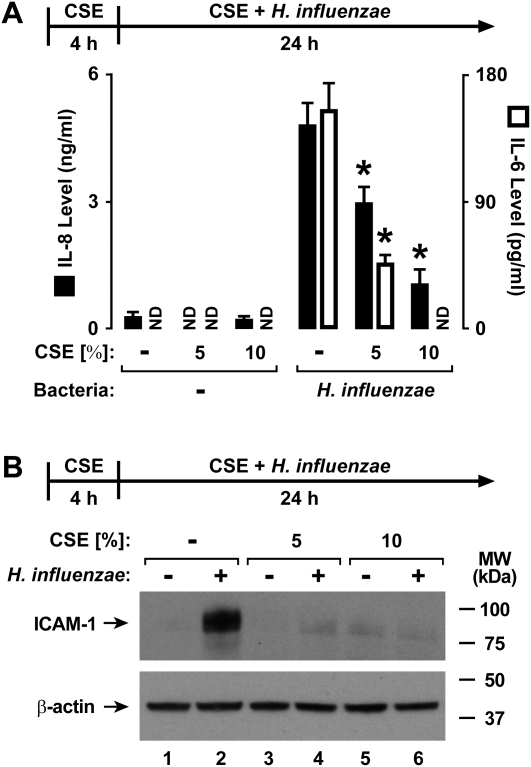
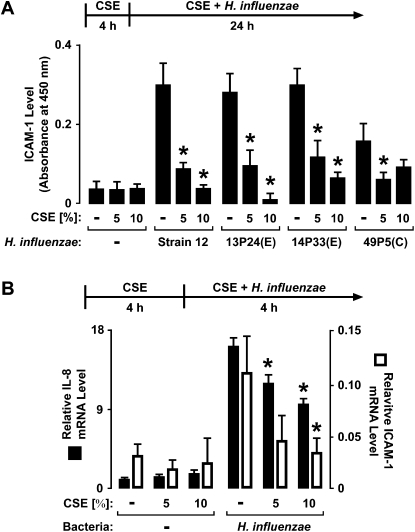
Human airway epithelial cells respond to direct interaction with nontypable H. influenzae by increasing their expression of several defense genes, and many of these genes regulate inflammation, including cytokines and cell adhesion molecules (11, 16). To assess the effects of cigarette smoke on airway responses to this bacterium, a fresh extract of cigarette smoke was generated in culture medium and immediately applied to hTBE cell monolayers. The treatment of hTBE cells with CSE for 4 hours before and during interaction with bacteria decreased the epithelial-cell release of IL-8 and IL-6 in response to H. influenzae (Figure 1A). The inhibitory effects of CSE were also evident in the induction of epithelial-cell ICAM-1 protein expression by H. influenzae (Figure 1B). Different strains of H. influenzae exert variable effects on epithelial-cell defense gene expression, with one correlation of increased inflammatory mediator induction with bacterial isolates from patients with COPD undergoing exacerbation, compared with strains obtained at symptom baseline (11). However, the inhibitory effects of CSE on ICAM-1 concentrations were evident with several nontypable strains of H. influenzae isolated from individuals with COPD, with or without exacerbation (Figure 2A). Furthermore, CSE also inhibited H. influenzae–induced IL-8 and ICAM mRNA expression, suggesting that CSE affects common gene regulation mechanisms that control defense gene transcription or mRNA stability (Figure 2B).
Figure 1.
Cigarette smoke extract (CSE) decreases Haemophilus influenzae–induced defense protein expression. (A) IL-8 and IL-6 secretion levels in culture medium were determined using enzyme-linked immunoassays with human tracheobronchial epithelial (hTBE) cells that were first treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 24 hours in medium containing the same CSE concentration with or without H. influenzae strain 12. Values are expressed as mean ± SD (n = 3). *Significant difference in concentrations from cells treated with and without CSE. ND, none detected. (B) Intercellular adhesion molecule–1 (ICAM-1) and β-actin protein concentrations were assessed using immunoblot analyses of extracts from hTBE cells that were first treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 24 hours in medium containing the same CSE concentrations with or without H. influenzae strain 12. MW, molecular weight.
Figure 2.
CSE decreases H. influenzae–induced defense protein and mRNA expression. (A) ICAM-1 cell surface protein concentrations were determined using an enzyme-linked immunoassay with hTBE cell monolayers that were first treated with medium containing CSE at the indicated concentrations for 4 hours. Cells were then incubated for 24 hours in medium containing the same CSE concentration with or without H. influenzae strain 12 or strains from patients with chronic obstructive pulmonary disease (COPD) isolated from sputum during an exacerbation (E) or that colonized during symptom baseline (C). (B) IL-8 and ICAM-1 mRNA concentrations were determined using real-time RT-PCR analyses of total RNA from hTBE cells that were first treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 4 hours in medium containing the same CSE concentrations with or without H. influenzae strain 12. Values are expressed as mean relative mRNA concentrations compared with control human hypoxanthine phosphoribosyltransferase (HPRT) mRNA. Values are expressed as mean ± SD (n = 3).*Significant difference in concentrations from cells treated with or without CSE.
Cigarette Smoke Extract Causes Minimal Airway Epithelial-Cell Cytotoxicity
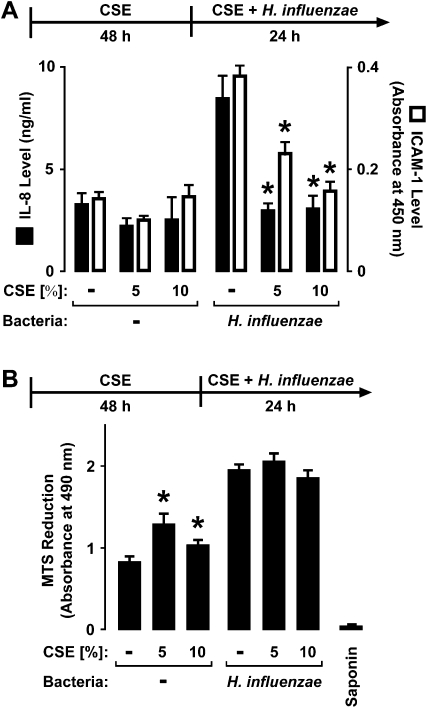
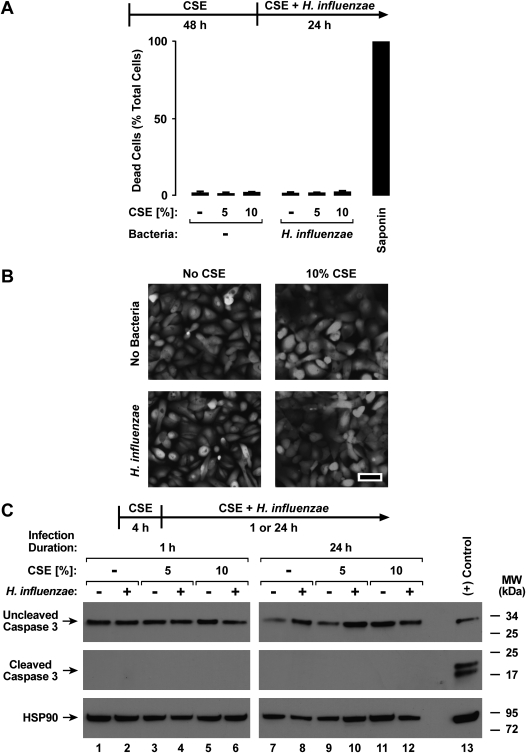
Epithelial-cell exposure to CSE for 48 hours before and during hTBE cell interactions with bacteria still inhibited H. influenzae–induced IL-8 and ICAM protein expression, indicating that the effects of CSE are not transient (Figure 3A). A slight increase in the release of IL-8 from hTBE cells was intermittently observed with CSE treatment alone, but this effect was much less pronounced than that seen after epithelial-cell exposure to bacteria. The modulation by CSE of defense gene expression did not appear to be secondary to epithelial-cell loss or cytotoxicity. For example, no decrease in epithelial-cell numbers was detected in samples treated with CSE for 4 or 48 hours, followed by incubation with CSE without or with H. influenzae for an additional 24 hours (results not shown). Although no significant change occurred in total cellular protein in samples treated with CSE for up to 72 hours (results not shown), an increase in mitochondrial metabolic activity was observed in hTBE cells after interaction with CSE or H. influenzae (Figure 3B). This effect was likely attributable to increased mitochondrial reductase activity rather than increased cell number. The effects of H. influenzae on mitochondrial metabolic activity were not decreased by pretreatment of epithelial cells with CSE for 4 hours (results not shown) or 48 hours (Figure 3B). CSE for 4 hours (results not shown) or 48 hours (Figure 4A), followed by CSE with or without bacteria, did not significantly increase necrotic cell death, as detected by plasma membrane permeability to ethidium homodimers in dead cells and intracellular esterase activity in live cells. We detected little change in epithelial-cell confluence or morphology in hTBE cells exposed to CSE and/or H. influenzae (Figure 4B). Lastly, we found no evidence of caspase-3 cleavage in hTBE cells treated with CSE or bacteria for the short or longer durations used in our experiments (Figure 4C). This result, combined with those of experiments indicating no decrease in MTS reduction under conditions of similar or longer duration (Figure 3B), support our conclusion that altered responses were not attributable to epithelial-cell apoptosis. Thus, based on multiple assays of cell cytotoxicity, minimal, if any, cytotoxic effects of CSE occurred.
Figure 3.
CSE selectively alters the effects of H. influenzae on airway epithelial cells. (A) IL-8 secretion into culture medium and ICAM-1 cell-surface protein concentrations were determined using enzyme-linked immunoassays with hTBE cells that were first treated with medium containing CSE at the indicated concentrations for 48 hours, followed by incubation for 24 hours in medium containing the same CSE concentrations with or without H. influenzae strain 12. (B) Mitochondrial activity was determined using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)–based assay with hTBE cells that were first treated with medium containing CSE at the indicated concentrations for 48 hours, followed by incubation for 24 hours in medium containing the same CSE concentrations, with or without H. influenzae strain 12. Control samples were treated with 1% saponin to induce 100% cell death. Values are expressed as mean ± SD (n = 3). *Significant difference in concentrations from cells treated without compared with CSE.
Figure 4.
CSE causes minimal airway epithelial cell cytotoxicity. (A and B) Dead and live cell numbers were quantified using hTBE cells that were first treated with medium containing CSE at the indicated concentrations for 48 hours, followed by incubation for 24 hours in medium containing the same CSE concentrations, with or without H. influenzae strain 12. Plasma membrane permeability to ethidium homodimers was detected in dead cells, and intracellular esterase activity was identified in live cells. (A) Control samples were treated with 1% saponin to induce 100% cell death. Values were calculated as mean percent dead cells/total cells ± SD for four random low-power fields (∼ 500–750 cells/field) from duplicate samples. No significant difference between conditions was evident. (B) Photomicrographs show cell morphology after cell treatments and esterase activity staining in this assay. Bar, 50 μm. (C) Uncleaved and cleaved caspase-3 and heat shock protein (HSP)–90 protein concentrations were assessed using immunoblot analyses of extracts from hTBE cells that were first treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 1 hour or 24 hours in medium containing the same CSE concentrations, with or without H. influenzae strain 12. As a positive control for caspase cleavage, Jurkat cell extract was treated with cytochrome c (Cell Signaling Technology).
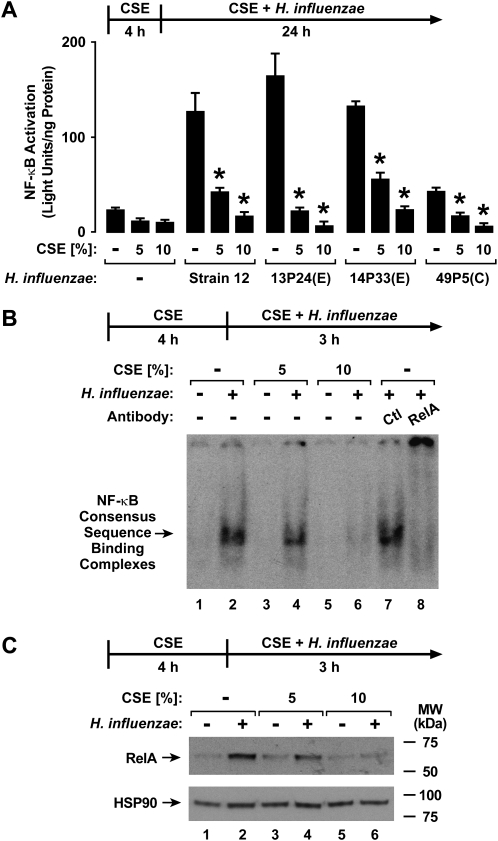
CSE Inhibits H. influenzae–Induced NF-κB Activation
The NF-κB transcription factor binds characteristic enhancer sequences that regulate the expression of IL-8, IL-6, ICAM-1, and other defense genes in response to bacteria (11, 15, 21, 22). In our hTBE cell system, the induction of IL-8 and ICAM-1 in response to H. influenzae is mediated through an increase in the rate of gene transcription, requires NF-κB binding enhancer sequences in the gene 5′-flanking regions, and is inhibited by blocking the activation of NF-κB (11, 15) (results not shown). Therefore, we questioned whether CSE affected H. influenzae–induced NF-κB activation or function in airway epithelial cells. The treatment of hTBE cells with CSE for 4 hours (Figure 5A) or 48 hours (results not shown) before and during interaction with bacteria inhibited NF-κB–dependent gene activation in response to H. influenzae, and this effect was seen with multiple bacterial isolates from patients with COPD. Altered NF-κB–dependent gene expression appeared attributable to decreased transcription factor activation, because EMSA analysis showed decreased RelA binding to an NF-κB consensus sequence, using nuclear extracts from hTBE cells pretreated with CSE before the interaction with H. influenzae (Figure 5B). Similarly, immunoblot analyses indicated a decreased concentration of RelA in the same nuclear extract from cells treated with CSE and H. influenzae, whereas nuclear concentrations of control HSP-90 changed minimally (Figure 5C). Based on these results, it appears that CSE exerts direct effects on NF-κB function in airway epithelial cells.
Figure 5.
CSE inhibits H. influenzae–induced NF-κB activation. (A) NF-κB–dependent reporter gene activation was assessed using luciferase activity assays of extracts from hTBE cells that were initially infected with an adenoviral vector expressing a luciferase gene driven by four tandem NF-κB sites. Cells were then treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 24 hours in medium containing the same CSE concentrations, with or without H. influenzae strain 12 or strains from patients with COPD isolated from sputum during an exacerbation (E) or that colonized during symptom baseline (C). Values are expressed as mean ± SD (n = 3–6). *Significant difference in concentrations from cells treated with or without CSE. (B) Transcription factor binding to an NF-κB consensus sequence was assessed using electrophoretic mobility-shift assay (EMSA) with nuclear extracts from hTBE cells that were first treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 3 hours in medium containing the same CSE concentrations, with or without H. influenzae strain 12. Supershift analysis was performed by the addition of control antibodies or antibodies against NF-κB RelA. (C) NF-κB RelA and HSP-90 nuclear protein concentrations in hTBE cells were assessed using immunoblot analyses of nuclear extracts from B.
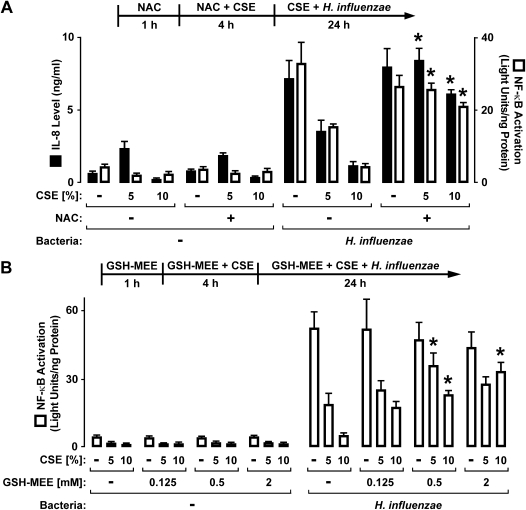
Antioxidants Inhibit Effects of CSE on Epithelial-Cell Antibacterial Defense
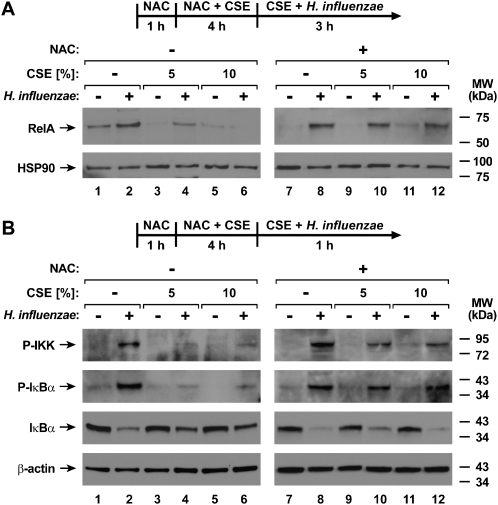
Cigarette smoke contains a variety of free radicals and other highly reactive species that can affect cell function (23). Therefore, we tested selected compounds that augment cell glutathione concentrations, for their capacity to inhibit the effects of CSE. The antioxidant NAC significantly decreased the inhibitory effects of CSE on both the H. influenzae induction of IL-8 release and NF-κB–dependent gene activation (Figure 6A). In these experiments, epithelial cells were exposed to NAC before and during pretreatment with CSE, but NAC was removed during bacterial interaction with epithelial cells due to the observation that this compound modestly inhibited H. influenzae–induced effects (results not shown). The effects of CSE on NF-κB–dependent gene activation were also decreased by the antioxidant GSH-MEE, with a clear dose-dependent response (Figure 6B). In contrast to NAC, GSH-MEE was left in the medium with H. influenzae because it had no clear effect on bacteria-induced NF-κB–dependent gene activation in the absence of CSE. The effects of NAC on NF-κB function correlated with results indicating no CSE-induced decrease in the H. influenzae–dependent nuclear localization of RelA in cells treated with NAC, as assessed by immunoblot analyses of nuclear extracts (Figure 7A). Upstream regulators of NF-κB activation were also examined in hTBE cells exposed to CSE with and without NAC treatment. Treatment with CSE inhibited the phosphorylation of IKK and IκBα in response to H. influenzae interaction with epithelial cells (Figure 7B). This result correlated with th loss of H. influenzae–induced IκBα degradation in CSE-treated hTBE cells. However, the treatment of epithelial cells with NAC before and during CSE pretreatment decreased the effects of CSE on IKK and IκBα phosphorylation, and allowed H. influenzae–induced IκBα degradation in CSE-treated cells. These results indicate that antioxidants may offer an effective strategy for inhibiting the effects of cigarette smoke on airway defense.
Figure 6.
Glutathione augmentation inhibits effects of CSE on airway epithelial cells. (A) IL-8 secretion concentration in hTBE cell culture medium was determined using an enzyme-linked immunoassay. NF-κB–dependent reporter gene activation was assessed using the reporter gene adenoviral vector and luciferase activity assays of hTBE cell extracts. Cells were treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 24 hours in medium containing the same CSE concentrations, with or without H. influenzae strain 12. Some samples were also incubated with 20 mM N-acetylcysteine (NAC) from 1 hour before the addition of CSE until incubation with bacteria. (B) NF-κB–dependent reporter gene activation was assessed using the reporter gene adenoviral vector and luciferase activity assays of extracts from hTBE cells that were treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 24 hours in medium containing the same CSE concentrations, with or without H. influenzae strain 12. Some samples were also incubated with the indicated concentrations of glutathione monoethyl ester (GSH-MEE) from 1 hour before the addition of CSE until the end of the experiment. Values are expressed as mean ± SD (n = 3). *Significant difference in comparable samples treated with or without NAC or GSH-MEE.
Figure 7.
Glutathione augmentation inhibits effects of CSE on H. influenzae–induced cell signaling. (A) NF-κB RelA and HSP-90 nuclear protein concentrations were assessed using immunoblot analyses of nuclear extracts from hTBE cells that were treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 3 hours in medium containing the same concentrations of CSE, with or without H. influenzae strain 12. (B) Phosphorylated (P) inhibitor of κB kinase (IKK) and of κB-inhibitor (IκB)–α, total IκBα, and β-actin protein concentrations were assessed using immunoblot analyses of extracts from hTBE cells that were treated with medium containing CSE at the indicated concentrations for 4 hours, followed by incubation for 1 hour in medium containing the same concentrations of CSE, with or without H. influenzae strain 12. Some samples were also incubated with 20 mM NAC from 1 hour before the addition of CSE until incubation with bacteria.
Cigarette Smoke Inhibits Airway Antibacterial Defense
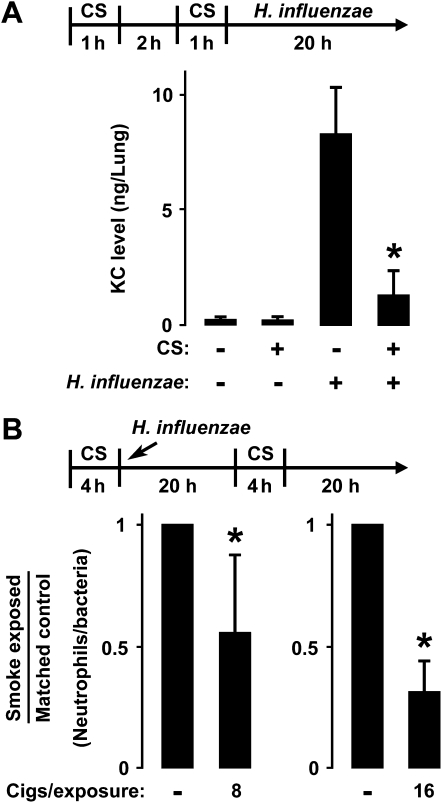
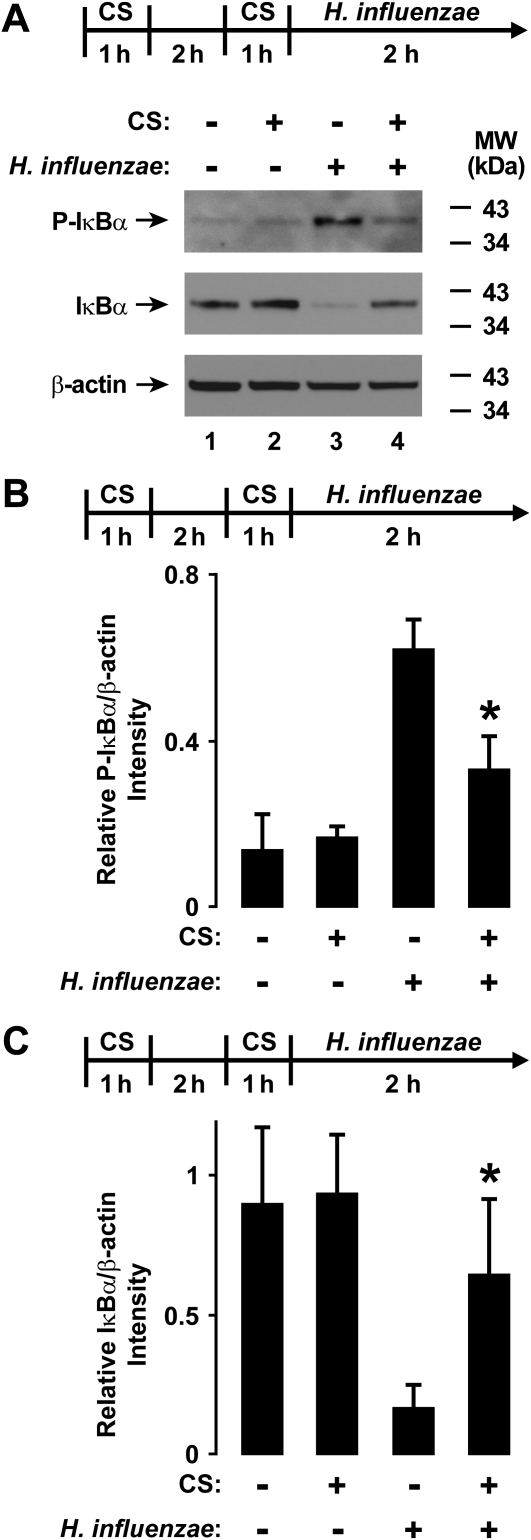
To assess the relevance of cell-based studies of cigarette smoke's effects on lung infection, we established a mouse model of nose-only exposure to cigarette smoke. This exposure system produced transient peak ambient mean CO concentrations of 417 ± 63 ppm, mean total particulate matter concentrations of 187.5 ± 12.6 mg/m3, and mean mouse carboxyhemoglobin concentrations of 7.6% ± 2.2%. These values are similar to those in studies of humans and animals (24, 25). We also found that the relatively short exposures used in our experiments resulted in minimal effects on whole-lung KC or BAL leukocyte concentrations (results not shown). This cigarette smoke exposure system was combined with a mouse model of airway bacterial infection in which agar particles were mixed with bacteria and injected into the airway. This model resulted in persistent high pulmonary bacterial loads for at least 48 hours, with peak CXC chemokine KC expression at 24 hours, and peak BAL neutrophil concentrations at 48 hours (10, 11, 17). Mice exposed to cigarette smoke exhibited significantly lower lung KC concentrations after airway infection with H. influenzae for 24 hours (Figure 8A). No significant difference in lung bacterial load was evident at this earlier time point before bacterial clearance is typically observed, thereby excluding this cause for the altered response in smoke-exposed animals (results not shown). Although this model of respiratory bacterial infection is characterized by variability in bacterial load among infected animals, a clear correlation exists between BAL neutrophil numbers and bacterial load in individual animals. Therefore, to control for this variability, the number of BAL neutrophils per lung bacteria was calculated for each animal, and mice were paired according to approximately equivalent bacterial loads. Mice exposed to eight cigarettes per day before and 24 hours after bacterial inoculation demonstrated a significant decrease in neutrophils per bacteria after 48 hours of infection, compared with animals infected with H. influenzae without exposure to cigarette smoke (Figure 8B). When the exposure to cigarettes was increased to 16 per day, mice exposed to cigarette smoke exhibited a large decrease in the concentration of BAL neutrophils per lung bacteria compared with mice not exposed to smoke. To correlate these findings concerning the effects of cigarette smoke on bacteria-induced lung inflammation with effects on NF-κB signaling, upstream regulators of NF-κB activation were examined in whole-lung extracts. The phosphorylation of IκBα was increased in lung extracts obtained 2 hours after airway inoculation with H. influenzae, but animals exposed to cigarette smoke before inoculation demonstrated significantly less IκBα phosphorylation (Figures 9A and 9B). Furthermore, total IκBα concentrations decreased after airway infection with H. influenzae, but cigarette smoke exposure before infection inhibited this effect (Figures 9A and 9C). The parallel findings in both the in vitro and in vivo experimental models support our conclusion that cigarette smoke inhibits NF-κB–dependent signaling in response to H. influenzae.
Figure 8.
Cigarette smoke modulates in vivo airway antibacterial defense. (A) keratinocyte chemoattractant (KC) concentrations were determined using an enzyme-linked immunoassay with lung extracts from C57BL/6J mice that were sham-exposed or exposed to cigarette smoke (CS) for 2 out of 4 hours. Whole-lung samples were isolated 20 hours after endotracheal injection with agar particles combined with H. influenzae strain 12. Values are expressed as mean ± SD (n = 3–5 in each group). *Significant difference between bacterial infection with or without exposure to CS. (B) The ratio of bronchoalveolar lavage neutrophil number to lung bacterial load was determined in C57BL/6J mice that were sham-exposed or exposed to eight or 16 cigarettes over 4 hours, followed by endotracheal injection with agar particles combined with H. influenzae strain 12. After 20 hours, animals underwent a second exposure to CS, and lungs were isolated after 44 hours of infection. Smoke-exposed and control animals were matched by approximately equivalent bacterial loads, and animals were exposed to either eight or 16 cigarettes in independent experiments. Values are expressed as mean smoke-exposed/control animal ratio ± SD (n = 7 in each group). *Significant difference between animals with or without exposure to CS.
Figure 9.
CS inhibits H. influenzae–induced NF-κB pathway signaling in vivo. (A) Phosphorylated and total IκBα and β-actin protein concentrations were assessed using immunoblot analyses of lung extracts from C57BL/6J mice that were sham-exposed or exposed to CS for 2 out of 4 hours. Whole-lung samples were isolated 2 hours after an endotracheal injection with agar particles combined with H. influenzae strain 12. (B) Whole-lung phosphorylated IκBα protein concentrations in A were quantified using results from band densitometry of immunoblot analyses, with inclusion of samples from additional animals. (C) Whole-lung total IκBα protein concentrations in A were quantified using results from band densitometry of immunoblot analyses, with inclusion of samples from additional animals. In B and C, values are expressed as mean ± SD (n = 3–5 in each group). *Significant difference between bacterial infection with or without exposure to CS.
DISCUSSION
Epithelial cells are often the first to encounter bacteria in the airway, and are responsive to intact bacteria and bacterial products as well as soluble mediators generated by other cells (16, 17). A complex system of signaling pathways allows for the modulation of cellular defense gene expression at multiple control levels, permitting a rapid and precise regulation of expression in response to several stimuli. A critical pathway that leads from the detection of bacteria (including H. influenzae) to the increased expression of many inflammatory genes requires the induction of the transcription-activating complex NF-κB (11, 12, 15). The NF-κB/Rel family includes RelA (p65), NF-κB1 (p50), NF-κB2 (p52), RelB, and Rel (c-Rel), but RelA and NF-κB1 appear to be the important transactivating forms of NF-κB in human airway epithelial cells after interaction with H. influenzae, likely acting as RelA homodimers or RelA–NF-κB1 heterodimers (14, 15). Moreover, respiratory epithelia often have first contact with and defend against inhaled chemicals, and cigarette smoke could directly affect epithelial-cell functions. Our results indicate that cigarette smoke inhibits the expression of defense genes induced in airway epithelial cells exposed to H. influenzae. CSE markedly inhibited the high-level NF-κB pathway activation observed after bacterial interaction with epithelial cells, thereby providing a mechanism for the effects of CSE on gene expression. The relevance of these in vitro findings to lung infection was strengthened by using a murine model of airway infection with H. influenzae in which we found evidence of decreased NF-κB pathway activation, KC chemokine expression, and neutrophil recruitment in animals exposed to cigarette smoke. These results suggest that cigarette smoke directly alters the inflammatory response in the airway, and this effect may increase the incidence, duration, and severity of respiratory bacterial infections.
Cigarette smoke is estimated to contain as many as 4,700 chemical compounds, including a variety of free radicals and other highly reactive species (23, 26). The numerous compounds in cigarette smoke combine to exert multiple effects on lung function and defense that alter epithelial barrier function, mucociliary clearance, inflammatory and immune responses, and the killing of bacteria (4, 5, 8, 27). Because cigarette smoke is a complex combination of many compounds that could affect epithelial-cell functions in different ways, we believed it most valid to study complete mixtures initially, for a better understanding of the overall effects of cigarette smoke on airway defense. Some reports demonstrate significant cytotoxicity of cigarette smoke, particularly with subconfluent cells, and thus we chose well-defined conditions and smoke concentrations, and also monitored to avoid cytotoxicity (28–30). In addition, in some experiments, epithelial cells were exposed to CSE for 48 hours before treatment, to verify the effects and assess cytotoxicity, because humans are often passively or actively exposed to cigarette smoke for longer durations. However, it is unclear if our results correlate with the effects induced by exposure to cigarette smoke for months or years. Prolonged exposure to cigarette smoke exerts additional effects on lung epithelia, and more studies will be needed to dissect these effects and their modulation of host responses to infection.
Several models of cigarette smoke generation and isolated cell exposure were used in studies that assessed biological effects. These varied from mixtures of filtered or unfiltered cigarette smoke with medium, the solubilization of smoke material collected on a filter, direct cell exposure to cigarette smoke, and the testing of individual components (28, 31–35). The fresh CSE used in our studies has the advantages of ease of preparation and epithelial-cell exposure, but may vary in concentrations (or even lack) some volatile components of the gaseous phase of cigarette smoke inhaled by humans. Similarly, animal models of smoke-induced lung effects vary in the number of cigarettes used and recovery time, the duration of exposure, whether sidestream or mainstream smoke is used, and whether only the respiratory system or the whole body is in contact with smoke (7, 9, 24, 25, 36, 37). These differences may account for the variability in findings, with each model having advantages and disadvantages that should be taken into consideration when interpreting experimental results (38). Our studies used both in vitro and in vivo exposure to cigarette smoke before the introduction of bacteria, based on the concept that epithelial cells in the airway are most often exposed to smoke before respiratory bacterial infection. We propose that our correlation of findings using both models strengthens the conclusion that cigarette smoke inhibits NF-κB–dependent effects in airway epithelial cells in response to H. influenzae.
Reports on the effects of cigarette smoke on NF-κB activation and function have not been uniform in their findings. Some reports demonstrated induction, whereas others found inhibition or no effect. Low-level NF-κB induction in isolated cells by cigarette smoke was often demonstrated with subconfluent cell testing, low smoke preparation concentrations, or shorter durations of exposure (28, 33, 39, 40). In contrast, the impairment of NF-κB activation or function was often associated with confluent cells or treatment with higher levels of exposure to cigarette smoke, alone or in combination with other stimuli (28, 41–43). However, other reports showed that cigarette smoke alone exerts little effect on NF-κB activation or function (44, 45). One of those reports demonstrated an inhibition of the LPS induction of IL-8 and granulocyte/macrophage colony–stimulating factor through the suppression of activator protein–1 (AP-1) activation in a bronchial epithelial cell line (44). The investigators reported that their results were not reproduced in primary human bronchial epithelial cells, and our previous work indicates that AP-1 is not required for the H. influenzae induction of IL-8 and ICAM-1 in hTBE cells (15, 44) (results not shown). Animal models of smoke exposure demonstrated similar evidence of the induction or inhibition of NF-κB (9, 25, 46). In one example, alveolar macrophages from smoke-exposed mice demonstrated little change in baseline NF-κB activation, but demonstrated decreased activation after exposure to LPS (36). The lack of uniformity in cigarette smoke's effects on NF-κB is consistent with studies of inflammation using in vivo models, in which cigarette smoke was shown to induce or augment airway or lung inflammation, particularly with exposures of longer duration, whereas others reported minimal or no effects on inflammation (7, 9, 24, 25, 37, 47–49). The variability between studies likely results from differences in the method of generating cigarette smoke, the concentrations of smoke, durations of exposure, and the cell or animal models tested. Based on the available information and our results, we propose that lower levels of cigarette smoke exposure of short duration can cause mild NF-κB induction, whereas higher concentrations of smoke inhibit the robust induction of NF-κB that occurs in response to bacteria in the lung.
The glutathione antioxidant system is pivotal for cellular defense against oxidant stress. In our study, the inhibition by CSE of bacteria-induced NF-κB activation and antibacterial protein expression was decreased by glutathione augmentation, suggesting a role for oxidants and providing one strategy to alter the effects of cigarette smoke. Cigarette smoke can increase intracellular glutathione concentrations by stimulating their production, or decrease glutathione concentrations by decreasing production or overwhelming the capacity of this system to detoxify reactive species (29, 50). We found that concentrations of the reduced form of intracellular glutathione were increased by exposure of hTBE cells to 5% CSE treatment, but were decreased by 10% CSE treatment (results not shown). However, some inhibition of bacterial effects was evident with 5% CSE in our experiments, even with increased cellular glutathione concentrations, suggesting that the effects of cigarette smoke may not be completely attributable to reactive oxygen species. Furthermore, polymorphisms in genes that control the glutathione and other antioxidant systems, as well as environmental factors, influence the capacity to detoxify cigarette smoke (51). Thus, significant individual variability likely occurs in the effects of cigarette smoke.
Multiple factors interact in the human airway to determine the outcome of respiratory infection. Cigarette smoke apparently exerts several effects, some not completely defined, which modify epithelial antibacterial defense in the airway. The modulation by cigarette smoke of specific signal transduction pathways, such as the important NF-κB system, can have profound consequences for pulmonary defense. However, significant variability is evident in the effects of cigarette smoke, based on factors including the number of and manner in which cigarettes are smoked, the presence of underlying lung disease, and differences in antioxidant concentrations and the competence of defense mechanisms among individuals (both humans as well as mouse strains) (25). A better understanding of the mechanisms through which cigarette smoke impairs the host response to infection may allow for the development of therapeutic strategies that restore normal airway defense in individuals exposed to cigarette or other smoke.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the University of Iowa Cell and Tissue Core for providing hTBE cells, T. Murphy and S. Sethi (State University of New York at Buffalo) for providing bacterial isolates from patients with COPD, P. McCray and the Vector Core of the University of Iowa Center for Gene Therapy for providing reagents, A. Adamcakova-Dodd, A. Levitz, T. Nyunoya, and M. Wilson for technical assistance, and G. Buettner, M. McCormick, G. Hunninghake, and D. Spitz for helpful discussions.
This work was supported by grants from the National Institutes of Health (R01-HL082505 and R01-HL075559 to D.C.L., and P30-ES005605 to P.S.T.). The University of Iowa Center for Gene Therapy is supported by grants from the National Institutes of Health and the Cystic Fibrosis Foundation. The contents of this manuscript are solely the responsibility of the authors, and do not necessarily represent the official views of the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0454OC on March 26, 2010
Author Disclosure: D.C.L. received research grants from AstraZeneca, GlaxoSmithKline, and the National Institutes of Health, all for more than $100,001, and a clinical study grant from Altana Pharmaceuticals for $1,001–$5,000. D.C.L. also serves on a grant review study section for the National Institutes of Health, and receives $1,001–$5,000. L.J.M. received royalties from the Coley Pharmaceutical Group for US Patent 6479504 for $1,001–$5,000. P.S.T. received book royalties from McGraw-Hill Publishers for less than $1,000, along with sponsored grants from the National Institutes of Health, the Centers for Disease Control, and the Environmental Protection Agency, all for more than $100,001. None of the other authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Brook I, Gober AE. Recovery of potential pathogens and interfering bacteria in the nasopharynx of smokers and nonsmokers. Chest 2005;127:2072–2075. [DOI] [PubMed] [Google Scholar]
- 2.Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, Breiman RF. Cigarette smoking and invasive pneumococcal disease: Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–689. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the surgeon general. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006.
- 4.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002;2:372–377. [DOI] [PubMed] [Google Scholar]
- 5.Stanley PJ, Wilson R, Greenstone MA, MacWilliam L, Cole PJ. Effect of cigarette smoking on nasal mucociliary clearance and ciliary beat frequency. Thorax 1986;41:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King TE Jr, Savici D, Campbell PA. Phagocytosis and killing of Listeria monocytogenes by alveolar macrophages: smokers versus nonsmokers. J Infect Dis 1988;158:1309–1316. [DOI] [PubMed] [Google Scholar]
- 7.Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, Cox G, Stämpfli MR. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol 2004;30:202–211. [DOI] [PubMed] [Google Scholar]
- 8.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stämpfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2004;170:1164–1171. [DOI] [PubMed] [Google Scholar]
- 9.Vlahos R, Bozinovski S, Jones JE, Powell J, Gras J, Lilja A, Hansen MJ, Gualano RC, Irving L, Anderson GP. Differential protease, innate immunity, and NF-κB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L931–L945. [DOI] [PubMed] [Google Scholar]
- 10.Humlicek AL, Pang L, Look DC. Modulation of airway inflammation and bacterial clearance by epithelial cell ICAM-1. Am J Physiol Lung Cell Mol Physiol 2004;287:L598–L607. [DOI] [PubMed] [Google Scholar]
- 11.Chin CL, Manzel LJ, Lehman EE, Humlicek AL, Shi L, Starner TD, Denning GM, Murphy TF, Sethi S, Look DC. Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers. Am J Respir Crit Care Med 2005;172:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuto T, Xu H, Wang B, Han J, Kai H, Gu X, Murphy TF, Lim DJ, Li J. Activation of NF-κB by nontypeable Hemophilus influenzae is mediated by Toll-like receptor 2-TAK1–dependent NIK-IKKα/β-IκBα and MKK3/6-p38 MAP kinase signaling pathway in epithelial cells. Proc Natl Acad Sci USA 2001;98:8774–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen-Heininger YM, Poynter ME, Aesif SW, Pantano C, Ather JL, Reynaert NL, Ckless K, Anathy V, van der Velden J, Irvin CG, et al. Nuclear factor kappaB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc 2009;6:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tak PP, Firestein GSNF. -κB: a key role in inflammatory diseases. J Clin Invest 2001;107:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manzel LJ, Chin CL, Behlke MA, Look DC. Regulation of bacteria-induced intercellular adhesion molecule–1 by CCAAT/enhancer binding proteins. Am J Respir Cell Mol Biol 2009;40:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldallal N, McNaughton EE, Manzel LJ, Richards AM, Zabner J, Ferkol TW, Look DC. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am J Respir Crit Care Med 2002;166:1248–1256. [DOI] [PubMed] [Google Scholar]
- 17.Frick AG, Joseph TD, Pang L, Rabe AM, St. Geme JW III, Look DC. Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J Immunol 2000;164:4185–4196. [DOI] [PubMed] [Google Scholar]
- 18.Humlicek AL, Manzel LJ, Chin CL, Shi L, Excoffon KJ, Winter MC, Shasby DM, Look DC. Paracellular permeability restricts airway epithelial responses to selectively allow activation by mediators at the basolateral surface. J Immunol 2007;178:6395–6403. [DOI] [PubMed] [Google Scholar]
- 19.Rasband WS. ImageJ. Bethesda, MD: National Institutes of Health; 2009, http://rsbinfonihgov/ij/.
- 20.Hassard TH. Understanding biostatistics. St. Louis, MO: Mosby-Year Book, Inc.; 1991.
- 21.Zhang Y, Broser M, Rom WN. Activation of the interleukin 6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by nuclear factors NF-IL6 and NF-kappa B. Proc Natl Acad Sci USA 1994;91:2225–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe T, Jono H, Han J, Lim DJ, Li J. Synergistic activation of NF-κB by nontypeable Haemophilus influenzae and tumor necrosis factor α. Proc Natl Acad Sci USA 2004;101:3563–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pryor WA, Stone K. Oxidants in cigarette smoke: radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci 1993;686:12–28. [DOI] [PubMed] [Google Scholar]
- 24.Hodge-Bell KC, Lee KM, Renne RA, Gideon KM, Harbo SJ, McKinney WJ. Pulmonary inflammation in mice exposed to mainstream cigarette smoke. Inhal Toxicol 2007;19:361–376. [DOI] [PubMed] [Google Scholar]
- 25.Yao H, Edirisinghe I, Rajendrasozhan S, Yang S, Caito S, Adenuga D, Rahman I. Cigarette smoke–mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 2008;294:L1174–L1186. [DOI] [PubMed] [Google Scholar]
- 26.Eiserich JP, van der Vliet A, Handelman GJ, Halliwell B, Cross CE. Dietary antioxidants and cigarette smoke–induced biomolecular damage: a complex interaction. Am J Clin Nutr 1995;62:1490S–1500S. [DOI] [PubMed] [Google Scholar]
- 27.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med 1999;159:473–479. [DOI] [PubMed] [Google Scholar]
- 28.Hellermann GR, Nagy SB, Kong X, Lockey RF, Mohapatra SS. Mechanism of cigarette smoke condensate-induced acute inflammatory response in human bronchial epithelial cells. Respir Res 2002;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kode A, Yang S, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res 2006;7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YC, Chuang CY, Lee PK, Lee JS, Harper RW, Buckpitt AB, Wu R, Oslund K. TRX–ASK1–JNK signaling regulation of cell density–dependent cytotoxicity in cigarette smoke–exposed human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2008;294:L921–L931. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar P, Hayes BE. Induction of COX-2 by acrolein in rat lung epithelial cells. Mol Cell Biochem 2007;301:191–199. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Forman HJ. Acrolein induces heme oxygenase–1 through PKC-delta and PI3K in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2008;38:483–490. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Togo S, Al-Mugotir M, Kim H, Fang Q, Kobayashi T, Wang X, Mao L, Bitterman P, Rennard S. NF-kappaB mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respir Res 2008;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groskreutz DJ, Monick MM, Babor EC, Nyunoya T, Varga SM, Look DC, Hunninghake GW. Cigarette smoke alters respiratory syncytial virus-induced apoptosis and replication. Am J Respir Cell Mol Biol 2009;41:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petecchia L, Sabatini F, Varesio L, Camoirano A, Usai C, Pezzolo A, Rossi GA. Bronchial airway epithelial cell damage following exposure to cigarette smoke includes disassembly of tight junction components mediated by the extracellular signal-regulated kinase 1/2 pathway. Chest 2009;135:1502–1512. [DOI] [PubMed] [Google Scholar]
- 36.Gaschler GJ, Zavitz CCJ, Bauer CMT, Skrtic M, Lindahl M, Robbins CS, Chen B, Stämpfli MR. Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages. Am J Respir Cell Mol Biol 2008;38:218–226. [DOI] [PubMed] [Google Scholar]
- 37.Gaschler GJ, Skrtic M, Zavitz CCJ, Lindahl M, Onnervik PO, Murphy TF, Sethi S, Stämpfli MR. Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am J Respir Crit Care Med 2009;179:666–675. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro SD. Smoke gets in your cells. Am J Respir Cell Mol Biol 2004;31:481–482. [DOI] [PubMed] [Google Scholar]
- 39.Zhao J, Harper R, Barchowsky A, Di YP. Identification of multiple MAPK-mediated transcription factors regulated by tobacco smoke in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L480–L490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Syed DN, Afaq F, Kweon MH, Hadi N, Bhatia N, Spiegelman VS, Mukhtar H. Green tea polyphenol EGCG suppresses cigarette smoke condensate–induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene 2007;26:673–682. [DOI] [PubMed] [Google Scholar]
- 41.Birrell MA, Wong S, Catley MC, Belvisi MG. Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. J Cell Physiol 2007;214:27–37. [DOI] [PubMed] [Google Scholar]
- 42.Pace E, Ferraro M, Siena L, Melis M, Montalbano AM, Johnson M, Bonsignore MR, Bonsignore G, Gjomarkaj M. Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 2008;124:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer CM, Dewitte-Orr SJ, Hornby KR, Zavitz CC, Lichty BD, Stämpfli MR, Mossman KL. Cigarette smoke suppresses type I interferon–mediated antiviral immunity in lung fibroblast and epithelial cells. J Interferon Cytokine Res 2008;28:167–179. [DOI] [PubMed] [Google Scholar]
- 44.Laan M, Bozinovski S, Anderson GP. Cigarette smoke inhibits lipopolysaccharide-induced production of inflammatory cytokines by suppressing the activation of activator protein–1 in bronchial epithelial cells. J Immunol 2004;173:4164–4170. [DOI] [PubMed] [Google Scholar]
- 45.Castro SM, Kolli D, Guerrero-Plata A, Garofalo RP, Casola A. Cigarette smoke condensate enhances respiratory syncytial virus–induced chemokine release by modulating NF-kappa B and interferon regulatory factor activation. Toxicol Sci 2008;106:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong CY, Zhou YM, Pinkerton KE. NF-kappaB inhibition is involved in tobacco smoke–induced apoptosis in the lungs of rats. Toxicol Appl Pharmacol 2008;230:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robbins CS, Bauer CM, Vujicic N, Gaschler GJ, Lichty BD, Brown EG, Stämpfli MR. Cigarette smoke impacts immune inflammatory responses to influenza in mice. Am J Respir Crit Care Med 2006;174:1342–1351. [DOI] [PubMed] [Google Scholar]
- 48.Playbouth V, Wang S, Hutt JA, McDonald JD, Harrod KS, Barrett EG. Cigarette smoke suppresses Th1 cytokine production and increases RSV expression in a neonatal model. Am J Physiol Lung Cell Mol Physiol 2006;290:L222–L231. [DOI] [PubMed] [Google Scholar]
- 49.Kang M, Lee C, Lee J, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 2008;118:2771–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman I, Smith CAD, Lawson MF, Harrison DJ, MacNee W. Induction of γ-glutamylcysteine synthetase by cigarette smoke is associated with AP-1 in human alveolar epithelial cells. FEBS Lett 1996;396:21–25. [DOI] [PubMed] [Google Scholar]
- 51.Breton CV, Vora H, Salam MT, Islam T, Wenten M, Gauderman WJ, Van Den Berg D, Berhane K, Peters JM, Gilliland FD. Variation in the GST mu locus and tobacco smoke exposure as determinants of childhood lung function. Am J Respir Crit Care Med 2009;179:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.