Abstract
Maternal smoking during pregnancy increases the risk of respiratory disease in offspring, but surprisingly little is known about the underlying mechanisms. Nicotinic acetylcholine receptors (nAChRs) expressed in bronchial epithelial cells (BECs) mediate the effects of nicotine on lung development and function. Recently, BECs were also shown to express a GABAergic paracrine loop that was implicated in mucus overproduction in asthma. We therefore investigated the interactions between cholinergic and GABAergic signaling in rhesus macaque BECs, and found that nicotine upregulated GABA signaling in BECs through the sequential activation of BEC nAChR and GABA receptors. The incubation of primary cultures of rhesus BECs increased concentrations of GAD, GABAA receptors, and mucin mRNA. The nicotine-induced increase in glutamatic acid decarboxylase (GAD) and GABAA receptor mRNA resulted in increased GABA-induced currents and increased expression of mucin. The ability of nicotine to increase mucin expression was blocked by nicotinic and GABAA antagonists. These results implicate GABA signaling as a middleman in nicotine's effects on mucus overproduction. Similar effects of nicotine on GABA signaling and the expression of mucin were seen in vivo after chronic exposure of rhesus monkeys to nicotine. These data provide a new mechanism linking smoking with the increased mucin seen in asthma and chronic obstructive pulmonary disorder, and suggest a new paradigm of communication between non-neuronal transmitter systems in BECs. The existence of neural-like transmitter interactions in BECs suggests that some drugs active in the central nervous system may possess previously unexpected utility in respiratory diseases.
Keywords: smoking, nicotine, GABA, nicotinic receptor, mucin
CLINICAL RELEVANCE.
Our research suggests a new mechanism by which smoking leads to lung disease, thereby suggesting new therapeutic approaches to smoking-induced lung disease. This research also increases our understanding of the pathophysiologic roles of the classic neurotransmitters expressed in bronchial epithelial cells.
Our laboratory previously showed that fetal and adult lungs express a cholinergic paracrine loop that underlies the ability of prenatal and postnatal nicotine exposure to affect normal lung development and function (1–3). Bronchial epithelial cells express choline acetyltransferase (ChAT), the enzyme needed for acetylcholine (ACh) synthesis, secrete ACh, and express cholinergic receptors and cholinesterase. All these components are necessary for cholinergic signaling (1, 4). In the central and peripheral nervous systems, ACh is a well-characterized excitatory neurotransmitter that acts at nicotinic ACh receptors (nAChR) or muscarinic AChR receptors (mAChR). nAChR are ligand-gated ion channels (ionotropic) and mAChR are G-protein linked receptors (metabotropic). nAChRs are pentameric structures assembled from either a mix of α and β subunits, or a single α subunit. There are 10 different α subunits (α1–α10) and four different β subunits (β1–β4) (5). In airway epithelium, muscarinic M3 receptors play a key role in stimulating the secretion of mucus, and nicotinic receptors modulate normal airway development.
Xiang and colleagues (6) reported that bronchial epithelial cells (BECs) also express a GABAergic paracrine loop. Bronchial epithelial cells express glutamic acid decarboxylase (GAD), the enzyme necessary for GABA synthesis, secrete GABA, and express GABA receptors and GABA transporters. All these components are necessary for GABAergic signaling. In the brain, GABA is a well-characterized inhibitory neurotransmitter that acts at ionotropic (GABAA and GABAC) and metabotropic (GABAB) receptors in the central nervous system. GABAA receptors, like nAChRs, are pentameric structures, assembled by combining homologous subunits from five different classes: α (subunits 1–6), β (subunits 1–4), γ (subunits 1–3), δ, and ρ (subunits 1 and 2). The co-expression of α and β subunits is the minimum requirement for the cell-surface expression of functional GABAA receptors in heterogonous cell lines. The full pharmacologic profile of native GABAA receptors requires the presence of α1, β2, and γ2 subunits (7).
GABA signaling in bronchial epithelium was shown to play a role in allergen-induced asthma (6). The sensitization of mice with ovalbumin markedly increased the expression of GAD and GABAA receptors in airway epithelial cells, leading in turn to the overproduction of mucin observed in asthma, especially in the most severe cases. Similar increases in GAD and GABAA receptors were also observed in bronchoscopic biopsies of humans with asthma after challenge with allergen inhalation. In mice, the activation of mucin production by allergens can be blocked by GABAA receptor antagonists, showing that bronchial epithelial GABA signaling plays a key role in mucin overproduction in asthma (6). Consistent with this stimulatory role, GABA signaling is excitatory, rather than inhibitory, in airway epithelium, causing membrane depolarization. This is in contrast to the effects of GABA in the central nervous system (CNS), and reflects the chloride gradient in which intracellular chloride in BECs is greater than in extracellular chloride. Thus, with the opening of the GABA channel, negatively charged chloride ions flow out of the cell, thereby reducing membrane potential and partly depolarizing the cell. In this way, GABA signaling by bronchial epithelium resembles GABA signaling in the fetal hypothalamus, where GABA is also stimulatory (8).
Mucus hypersecretion is a major cause of pathology in asthma, chronic bronchitis, chronic obstructive pulmonary disease (COPD), and cystic fibrosis. Mucus hypersecretion leads to plugging of the small airways and dramatic reductions of airflow, and is associated with the majority of asthma deaths (9, 10). The airways of healthy individuals contain relatively few mucous cells, but the airways of humans with asthma and animal models of asthma display dramatically increased numbers of mucin-producing cells (9, 10). Thus the production of mucin is a critical component in essentially all chronic pulmonary diseases.
Smoking is intimately related to asthma, chronic bronchitis, and COPD. More than 40% of smokers develop chronic bronchitis (11), which is defined as chronic mucus production from the airways for at least 3 months in each of 2 successive years, provided no other causes of chronic cough are evident (12). Twenty-five percent of smokers will develop COPD, which combines chronic mucus production with decreases in lung function. In adults, smoking is associated with the worsening of asthma and increased wheezing. Mechanisms underlying the increased production of mucin in smoking are still poorly understood. Similarly, in utero exposure to tobacco smoke increases the risk of asthma, and recent studies show a twofold increase in the incidence of asthma in offspring of women who smoked during pregnancy (13). Despite this clear link between smoking and lung disease, much remains unknown about the mechanisms underlying that link.
Our studies of functional cholinergic signaling in bronchial epithelium (2, 3, 14), combined with observations by Xiang and colleagues (6) of functional GABA signaling in lung epithelium, suggest that cholinergic signaling may interact with GABAergic signaling within BECs, reflecting communication between transmitter systems that existed in epithelium before the evolution of synaptic communication (15, 16). Certainly in the CNS, cholinergic signaling commonly modifies GABAergic signaling (17–19), and a similar interaction may occur in bronchial epithelial cells. Therefore, the present study sought to determine if the nicotinic cholinergic activation of GABAergic signaling occurs in BECs, and thus may underlie some of the connections between smoking and lung disease.
MATERIALS AND METHODS
Epithelial Cell Culture
All animal procedures were approved by the Institutional Animal Care and Utilization Committee at the Oregon National Primate Research Center. Bronchial epithelial cell cultures were established as described by Wu and colleagues (20) and Robinson and Wu (21). In brief, lungs were obtained from rhesus macaques (newborn to 1 year old) undergoing necropsy for protocols unrelated to lung function or development. Lungs were immersed in ice-cold Eagle's minimum essential medium (MEM; i.e., MEM + 15 mM Hepes + 50 μg/ml gentamicin + penicillin–streptomycin). The major bronchi were crudely dissected and washed with medium, or opened longitudinally and incubated overnight at 4°C in 0.1% Protease Type 14 (Sigma, St. Louis, MO) in MEM. Cells were then washed from the epithelial cell–side surface of the bronchi and collected by centrifugation at 1,000 × g for 10 minutes at 4°C. Cells were resuspended in MEM with 10% FCS, incubated for 5 minutes, washed, and incubated with bronchial epithelium culture medium (50% Ham's nutrient mixture F12 + 50% DMEM + 1.8 mM calcium chloride, 5.0 μg/ml insulin, 5.0 μg/ml transferrin, 20 ng/ml epidermal growth factor, 0.1 μM dexamethasone, 20 ng/ml cholera toxin, 30 μg/ml bovine hypothalamic extract, and 1.0 μM retinol) containing 2% FCS. The next day, cells were changed to serum-free bronchial epithelium culture medium and incubated in 5% CO2 at 37°C for 7–10 days. For patch-clamp analysis, cells were plated on Thermanox 13-mm plastic coverslips (NUNC, Rochester, NY), coated with 1.5 mg/ml collagen Type I (Sigma).
Prenatal Exposure to Nicotine
For studies of the in vivo effects of nicotine, timed-pregnant rhesus monkeys received nicotine (0.7 mg/kg/day, subcutaneously), using osmotic minipumps from Days 26–160 of gestation (term, 165 days), as described previously (22).
RT-PCR and Quantitative Real-Time PCR
Total RNA was extracted from monkey lungs, brains, and cultured BECs (5 × 106 cells), using TriReagent (Invitrogen, Carlsbad, CA). Reverse transcription and PCR were performed as previously described (2), using 5′ and 3′ specific primers for monkey GAD1, GAT1–3, GABAA receptors α1, α5, β1–3, ρ, and γ2, GABAB receptor R2, and GABAA-associated protein. All amplified bands were the correct size, consistent with the chosen primers. Taqman real-time PCR was used to quantify GAD1, GAT1, GAT3, GABAA receptors α5, β1, β2, and ρ, and GABAA-associated protein, as described previously (14). Primers and probes (14) were designed using Primer Express software (Applied Biosystems; for further details, see Table E1 in the online supplement). For the quantification of mucin-5AC mRNA, the 5′, 3′ primers and probe comprised GACCCCGGGAACCACTGT, CCACGAGCCCGTCCAA, and CCT ATG AGT GTG AGA AGC. All probes were purchased from Applied Biosystems (Foster City, CA). A standard curve was drawn on the basis of the log of the input RNA versus the critical threshold cycle, and samples were normalized to levels of 18S RNA in each sample for relative quantification. All real-time PCR reactions were run in triplicate.
Immunofluorescence for GABAA Receptor and nACh Receptor Subunits
Immunohistochemistry was performed on lung tissue, as previously described (2). Several primary antibodies were used, including (1) anti-glutamate decarboxylase65/67 (GAD65/67) (rabbit, polyclonal, 1:200 dilution; Chemicon, Billerica, MA); (2) anti-GABAA receptor β3 subunit (rabbit, polyclonal, 1:200 dilution; Chemicon) and anti-GABAA receptor α5 subunit (rabbit, polyclonal, 1:200 dilution; Abcam, Cambridge, MA); (3) anti-nAChR α7 (mouse, mAb 306, 1:200 dilution; Sigma); and (4) anti-muc5ac mucin (mouse, monoclonal, 1:300; Chemicon). The secondary antibodies consisted of Texas red goat anti-mouse IgG (1:400 dilution) and FITC-conjugated goat anti-rabbit IgG (1:400 dilution; Jackson Immunoresearch Laboratories, West Grove, PA). All immunofluorescence experiments were repeated at least three times. Samples were viewed under an Axioskop 2 fluorescence microscope (Zeiss, Oberkochen, Germany), and images were acquired with an AxioCam digital camera and analyzed with Axiovision 4.2 software. Mucus and mucus-containing goblet cells in the bronchial epithelium were stained with periodic acid–Schiff (PAS) reagents (Sigma).
Electrophysiology
For electrophysiologic recordings, cells were grown on coverslips and transferred to a recording chamber mounted on the stage of an Axioskop2 microscope (Zeiss). Currents were recorded from confluent cultured monkey BECs (7–10 days). The majority of electrophysiologic experiments involved the gramicidin perforated patch and whole-cell patch-clamp technique (6, 23). The external Krebs solution bathing the BECs contained 130 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM NaHCO3, 5 mM Hepes, and 10 mM glucose, pH 7.35–7.4. To isolate GABA inward currents, an internal pipette solution was used, containing 155 mM KCl, 15 mM KOH, 10 mM Hepes, 2 mM MgCl2, 1 mM CaCl2, and 2 mM tetraethylammonium and gramicidin (15–20 μg). The pH was adjusted to 7.3 (6). The pipette resistance was 3–5 MΩ, and access resistance was less than or equal to 15 MΩ. The chamber, which had a volume of 1 ml, was perfused continuously with Krebs solution at a rate of 6–7 ml/minute. All recordings were performed using cells at room temperature.
Drugs were added to the perfusate, and their delivery to cells was controlled by separate valves. The several drugs in this study obtained from Sigma included GABA, picrotoxin, bicuculline, mecamylamine, and methyllycaconitine (MLA). A stock solution (1–10 mM) of all drugs was prepared on the day of the experiment in distilled water and diluted with Krebs solution to their final concentration before use.
Currents were recorded with a multiClamp700B amplifier (Molecular Devices/Axon Instruments, Foster, CA). Data were filtered at 5 kHz. Voltage and current clamp protocols, data acquisition, and analyses were performed using pClamp9 software and DigiData 1322A interface (Molecular Devices/Axon Instruments). All values are given as mean ± SEM, and statistical comparisons were performed using an unpaired, two-tailed Student t test.
RESULTS
GAD and GABAA Receptor Expression in Cultured Monkey BECs
Primary cultures of rhesus BECs were prepared from rhesus monkey lungs obtained from necropsies of animals without lung disease, as described in Materials and Methods. PCR indicated the presence of mRNAs of all needed components for GABAergic paracrine signaling in cultured BECs, including GAD, GABAA receptor subunits, and GABA transporters (Figure 1A). GABAA receptor subunits expressed included the α, β, ρ, and γ subunits. Transporters included GABA transporter isoforms 1 and 3 (GATs 1 and 3). In addition, the GABAA receptor-associated protein (GABARAP) (Figure 1A) was expressed. No GABAA receptor α2 or γ1 or GAT2 transcripts were detected in BECs. As we previously reported, primary cultures of BECs also express all needed components for nicotinic signaling, including ChAT and nAChR subunits α1, α3, α4, α7, α9, α10, β2, and β4 (3).
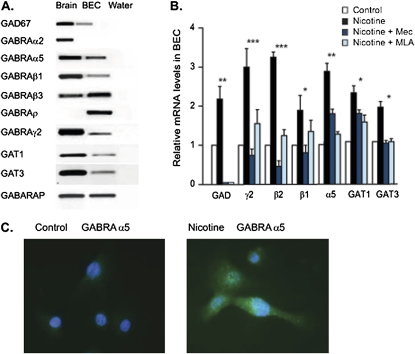
Figure 1.
Detection and expression of GABAA receptors in monkey in cultured bronchial epithelial cells (BECs) in vivo and in vitro. (A) RT-PCR indicated the presence of glutamate decarboxylase (GAD)67, GABAA receptors α2, α5, β1, β3, ρ, and γ2, GAT1, GAT3, and GABAA receptor-associated protein (GABARAP). Monkey cortex was included as a positive control. No bands were evident in negative control (water), which lacked input RNA. (B) Real-time PCR analysis of the effect of nicotine exposure (1 μM for 48 hours) on GAD, GABAA receptors γ2, β2, β1, and α5, GAT1, and GAT3 mRNA levels in BECs. The nicotinic acetylcholine receptor (nAChR) antagonists mecamylamine (Mec; 25 μM) and methyllycaconitine (MLA; 30 nM) inhibited the nicotine-induced increases in GAD, GABAA receptors γ2, β2, β1, and α5, GAT1, and GAT3 mRNA concentrations in BECs. *P < 0.05, **P < 0.01, and ***P < 0.005 for nicotine exposure compared with controls. (C) Immunohistochemical detection of GABAA receptor α5 expression in control cultured BECs and in cultured BECs incubated with 1 μM nicotine for 48 hours. GABRA, GABA receptor A.
Given the co-expression of the nAChR and the GABAA receptor and the ability of nicotine to increase GABAergic signaling in the CNS, we reasoned that nicotine might similarly increase GABAergic signaling in BECs. As shown in Figure 1B, the incubation of cultured BECs with 1 μM nicotine for 48 hours increased the expression of mRNA concentrations of GAD, GABAA receptor α, β, and γ subunits, GAT1, and GAT3. These increases were blocked by the nicotinic receptor antagonists mecamylamine (25 μM) and MLA (30 nM) (Figure 1B). The increased RNA translated into increased protein expression, as shown by immunostaining for the α5 GABAA receptor. Cultured BECs were incubated with or without nicotine (1 μM for 48 hours), stained with GABAA receptor α5 antibody, and counted in four randomly selected fields of view per culture. After this chronic nicotine exposure, the GABAAR α5 subunit had increased its expression from 5% (± 3.0%) of cells to 30% (± 10.4%) of cells (Figure 1 C). As described below, increased receptor expression translated into increased GABA-induced currents.
Nicotine Increases GABAA-Induced Currents in Cultured BECs
The functionality of GABAA receptor in cultured BECs was determined by electrophysiology. During a fast perfusion of GABA, approximately 21% of BECs (33 of 160 BECs) responded with the rapid activation of an inward current, followed by a slower desensitization (Figure 2A, a–c), at a holding potential of 60 mV under a voltage clamp. The amplitude of GABA-induced inward currents was inhibited by the GABAA receptor antagonists bicuculline and picrotoxin. Because GABAA receptors are Cl− selective ion channels, gramicidin perforated patch recording is routinely used to preserve intracellular [Cl−] levels and prevent artifactual changes in the chloride equilibrium potential (24, 25).
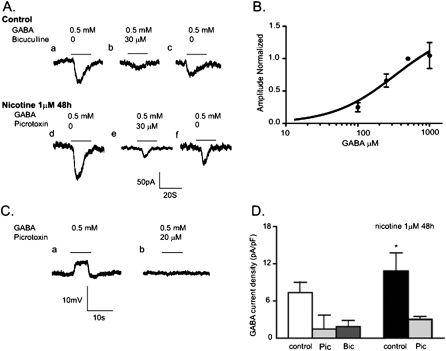
Figure 2.
Effects of nicotine on GABA-induced current in cultured BECs. (A, a–c) The inward current induced by 0.5 mM GABA was partially blocked by the GABAA receptor antagonist bicuculline (20 μM), and GABA induced a current after the bicuculline had been washed out. (A, d–f) Incubation with 1 μM nicotine for 48 hours increased the amplitude of GABA-induced current increased by approximately 33%, compared with no nicotine. (B) Dose–response curve of BEC responses to concentrations of GABA shown. Peak currents evoked at concentrations shown are expressed relative to the peak current evoked by 0.5 mM of GABA and plotted against the log of GABA concentration. Mean response was taken from 6–12 cells. Experimental data were fitted to the Hill equation with an EC50 of 109 μM and a Hill coefficient of 1.1. (C, a and b) Under a current clamp, 0.5 mM GABA depolarized the membrane potential in BECs. Picrotoxin (25 μM) inhibited the GABA-induced depolarized membrane potential. (D) Comparison of control and nicotine-treated groups for GABA current density in BECs, obtained by dividing peak currents by whole-cell capacitance. For GABA-induced currents, the holding potential was −60 mV. *P < 0.05 for nicotine exposure compared with control. Bic, bicuculline; Pic, picrotoxin.
As shown in Figure 2B, the GABA-induced inward currents in BECs were concentration-dependent. To obtain the dose–response relationship, the mean peak current at each GABA concentration was normalized to that elicited by 0.5 mM GABA. The EC50 for activation by GABA was 108 ± 27 μM (n = 4), and the Hill coefficient was 1.1 ± 0.3 (n = 4) (Figure 2B). Under the current clamp, during the application of GABA, BECs responded with a rapid depolarization of approximately 5.1 ± 0.5 mV (n = 6), from a resting potential of −41 ± 3 mV (n = 20) (Figure 2C). The GABA-induced depolarization was blocked by 30 μM of picrotoxin (n = 5) (Figure 2C). The reversal potential of GABA current (IGABA) in cultured BECs was −11 ± 3 mV (n = 3), and the resting potential of these cells was −42.1 ± 4 mV (n = 3). This shows that GABAA receptors modulate anionic efflux in BECs, and that GABA can be considered an excitatory transmitter for bronchial epithelium.
Consistent with the increased expression of the GABAA receptor shown in Figure 1B, 48-hour incubation with 1 μM nicotine significantly increased GABA-induced inward currents compared with controls (Figures 2A and 2D). The mean current induced by 0.5 mM GABA was 109.4 ± 8.7 pA (n = 20) for BECs incubated without nicotine, and 161.3 ± 15.7 pA (n = 7) for BECs incubated with nicotine. The nicotine-increased amplitude of GABA-induced inward currents was significantly inhibited by 30 μM of picrotoxin. Moreover, chronic nicotine exposure increased the number of BECs responding to GABA by 30% (27 of 90 BECs). Chronic exposure to nicotine significantly increased GABA current densities by approximately 31%. The mean densities of GABA currents in BECs increased from 7.3 ± 1.7 pA pF−1 (n = 17) to 10.8 ± 1.7 pA pF−1 (n = 7) after a 48-hour incubation with 1 μM nicotine, at a holding potential of −60 mV (Figure 2D). The inhibitory effects of the GABAA receptor antagonists picrotoxin and bicuculline on GABA current densities are shown in Figure 2D. Thus, chronic exposure to nicotine upregulates GABAA receptor activity in BECs.
Chronic In Vivo Exposure to Nicotine Increases Components of GABA Signaling in Airway Epithelium
To determine if chronic exposure to nicotine increases components of GABA signaling in vivo as well as in vitro, we took advantage of our well-characterized model of the effects of chronic prenatal nicotine exposure on monkey lung development (2, 14, 26), in which pregnant monkeys are exposed to nicotine from days 30 to 160 of gestation (term, 165 days) to give exposures similar to those of human smokers. This model accurately reproduces the effects of human maternal smoking on lung development and newborn pulmonary function. Previous studies from our laboratory showed that in this model, nicotine exposure activates and upregulates nAChRs in offspring and leads to decreased pulmonary function (2, 3, 26). As shown by this model (Figure 3), prolonged exposure to nicotine increased concentrations of mRNA for GAD and GABAA receptors in developing monkey lungs, as also occurred in vitro.
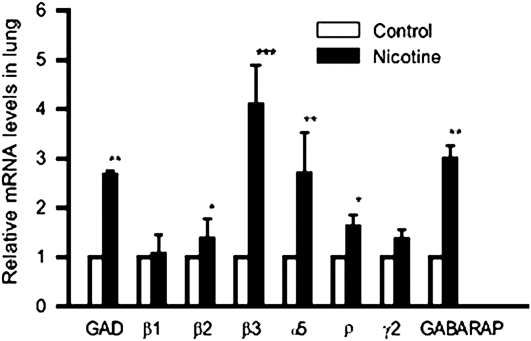
Figure 3.
Effect of chronic nicotine on GABA-signaling mRNA. Real-time PCR analysis of GAD and GABAA receptors β1, β2, β3, α5, ρ, and γ2 and GABARAP mRNA concentrations in lungs of control and nicotine-exposed animals. Relative mRNA levels were normalized to each control level. *P < 0.05, **P < 0.01, and ***P < 0.005 for nicotine exposure compared with controls.
As for the in vitro exposure of BECs to nicotine, increases in protein concentrations were derived from the increased RNA concentrations (Figure 4). As shown in Figure 4, concentrations of GAD65/67 and GABAA receptor β3 were markedly increased in the bronchial epithelia of lungs from newborn monkeys exposed to nicotine in utero (Figure 4). In control lungs, GAD65/67 was barely detectable, but exposure to nicotine significantly increased levels of GAD expression (Figures 4A and 4C). The expression of GAD occurred primarily in the apical aspect of bronchial epithelia, and colocalized with nAChR α7 (Figure 4A). GAD65/67 and nAChR α7 also colocalized in neuroepithelial bodies (Figure 4A). Similar to the increases in GAD, chronic exposure to nicotine also increased concentrations of GABAA receptor β3 in bronchial epithelium (Figures 4B and 4D). As for GAD, GABAA receptor β3 and α7 nAChR colocalized in bronchial epithelium (Figure 4B). Colocalization studies showed the proximity of these systems and the potential for direct interaction. Thus, just as for cultured BECs in vitro, chronic exposure to nicotine increased the expression of GAD, GABA receptors, and GABA transporters in vivo.
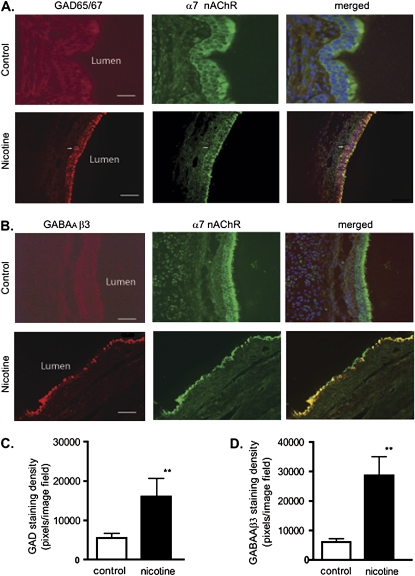
Figure 4.
Chronic nicotine exposure increased GAD and GABAA receptor β3 expression in monkey BECs. (A) Double-labeling with GAD65/67 (red) and α7 nAChR (green) was performed in lung sections of control monkey and nicotine-exposed lungs. Calibration bars represent 45 μM in control lung sections, and 35 μM in nicotine lung sections. (B) Double-labeling with GABAA receptor β3 (red) and α7 nAChR (green) was evident in lung sections of control and nicotine-exposed lung. Arrow indicates a neuroepithelial cell cluster. Calibration bars represent 35 μM. (C and D) Quantitative analysis of immunofluorescence staining, expressed as fluorescent pixels per image field. (C) Immunofluorescence showed an increase in intensity of GAD65/67 in the lung epithelium (control, 5,500 ± 2,000 pixels2; nicotine-exposed lung, 16,013 ± 8,000 pixels2). (D) Immunofluorescence showed an increase in intensity of GABAA receptor β3 in the lung epithelium (control, 6,000 ± 2,000 pixels2; nicotine-exposed lung, 28,630 ± 11,000 pixels2). n = 8 image fields of four lung slices from four monkeys. **P < 0.001.
GABAA Receptors Mediate Nicotine-Induced Overproduction of Mucus
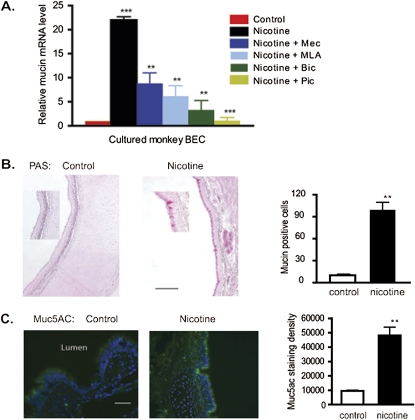
The overproduction of mucus characterizes most smoking-associated lung diseases, including COPD and severe asthma. Xiang and colleagues (6) found that increased GABA signaling leads to increased mucin production, which suggests that the ability of nAChR to activate GABA signaling may provide a new mechanism linking smoking to excess mucin expression. This finding was tested both in vitro in cultured BECs and in vivo in the monkey model already described, and by the PAS staining and measurement of mucin 5AC (MUC5AC) mRNA and protein. The incubation of BECs with 1 μM nicotine for 48 hours significantly increased mucin mRNA concentrations compared with controls (Figure 5A). Critically, the effect of nicotine was blocked by the nicotinic antagonists mecamylamine (25 μM) and MLA (30 nM) and by the GABAA receptor antagonists picrotoxin (30 μM) and bicuculline (20 μM) (Figure 5C). This suggests that the sequential activation of nicotinic signaling, followed by GABAergic signaling, is necessary for nicotine to stimulate the production of bronchial epithelial mucus, and that the nicotine-induced overproduction of mucin is in part dependent on GABAA receptor signaling in BECs.
Figure 5.
Interactions of nicotine and GABA signaling affect the expression and secretion of mucin. (A) Real-time PCR analysis of mucin 5A expression in cultured BECs treated with nicotine (1 μM for 48 hours) plus the nAChR antagonists mecamylamine (Mec; 25 μM,) and methyllycaconitine (MLA; 30 nM), and the GABAA receptor antagonists, picrotoxin (Pic; 30 μM) and bicuculline (Bic; 30 μM). n = 18 from three experiments. *P < 0.05 for nicotine + antagonist groups compared with nicotine alone group. **P < 0.01 for nicotine-treated group compared with control group. (B) Periodic acid–Schiff (PAS) staining shows increased expression of mucin in nicotine-exposed lung compared with control lung. Quantitation of PAS-positive cells from control and nicotine-exposed animals is shown at right (three animals per group, four sections per animals). **P < 0.01. (C) Immunohistochemistry shows increased expression of mucin-5AC in nicotine-exposed animals. Quantification of immunostained pixels per image field involved three animals per group, and four sections per animal. **P < 0.01.
Prenatal exposure to nicotine similarly increased mucin expression in vivo. In lungs from control animals, as determined by PAS staining, mucus-positive cells were relatively rare in large airway epithelia, but prenatal exposure to nicotine greatly increased their frequency (Figure 5B). Similarly, as determined by immunohistochemistry, prenatal exposure to nicotine significantly increased MUC5AC concentrations in bronchial epithelium (Figure 5C). These results show that chronic exposure to nicotine increases mucin and mucus production by bronchial epithelium.
Multiple Other Neurotransmitters Are Expressed in BECs
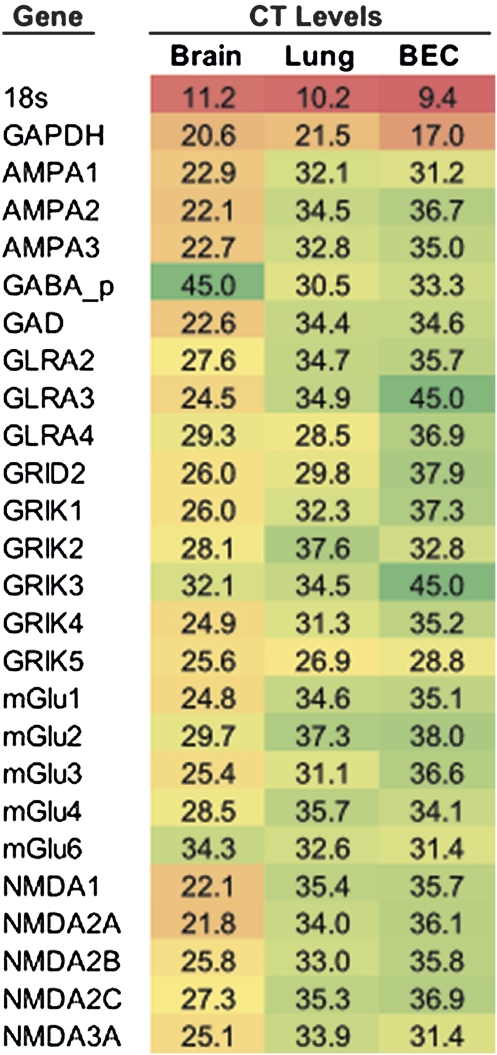
The interaction of nicotinic and GABAergic signaling in BECs is likely not the only interaction between classic neurotransmitter systems that occurs in BEC. As shown in Figure 6, BECs also express most glutamate and glycine receptor subtypes. The glycine receptor A2 and A4 subunits are present in BECs, as are N-methyl-D-aspartic acid (NMDA), alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), kainite, and metabotropic glutamate receptors, and several of their expression levels rivaled levels in the cortex. In addition, evidence exists that in BECs, the interactions of glycine and glutamate receptors are similar to those in the nicotinic and GABAergic systems, because we observed that nicotine also increased the concentrations of NMDA2A receptor mRNA and decreased the concentrations of metabotropic glutamate receptor 1 (mGlu1) and NMDA1 receptor mRNA in cultured BECs (data not shown). Thus, BECs express multiple neurotransmitter systems that interact with each other in a primitive form of neuron-like interaction.
Figure 6.
A network of neurotransmitters is expressed in bronchial epithelium. As well as nAChR and GABAA receptors, multiple other neurotransmitter systems are expressed in BECs. Real-time SYBR Green PCR was performed on monkey brain, lung, and BEC samples, 2–4 samples per group, and the critical threshold (CT) was determined. CT values are shown. Color scale: red, maximum; yellow, midpoint; green, minimum. BEC, cultured; AMPA, AMPA glutamate receptors; GAD, glutamate decarboxylase; GLRA, glycine A receptor; GLRB, glycine B receptor; GRIK, kainite glutamate receptors; mGlu, metabotropic glutamate receptors; N-methyl-D-aspartic acid (NMDA), NMDA glutamate receptors.
DISCUSSION
The present study reveals interactions between two classic transmitter systems (cholinergic and GABAergic) that are co-expressed in bronchial epithelium. Nicotine increased the mRNA expression in BECs of all elements of GABAergic signaling, including GAD, GABAA receptor subunits, GABA transporters, and GABARAP (Figure 1). The increases in GABA-related mRNAs resulted in increased protein and increased GABA-induced inward currents (Figures 2–4). The increase in GABA signaling was clearly mediated by nAChR, because nicotinic antagonists blocked the nicotine-induced increase in GAD, GABAA receptors, and GABA transporter mRNAs (Figure 1). The nicotine-induced increase in GABA signaling led, in turn, to increased expression of GABA.
The ability of nicotinic activation to increase GABA signaling provides a new mechanism underlying the smoking-induced expression of mucin and lung disease. Xiang and colleagues (6) reported that the GABAergic system in airway epithelium is essential for the overproduction of mucus in asthma. As shown in Figures 1 and 3, chronic exposure to nicotine increased the expression of mucin in BECs both in vitro and in vivo, leading to increased mucin expression in vivo. Confirming the sequential role of the nicotinic and GABA pathways, the nicotine-induced increase in mucin was blocked by both nicotinic and GABAergic antagonists (Figure 5). Thus, in smokers, the activation of nicotinic receptors by nicotine increases GABA signaling, which in turn increases the production of mucin. Although we only looked at the ability of nicotine to increase GAD in monkeys, microarray studies of human bronchial biopsies and brushings clearly indicate that concentrations of GAD mRNA are increased in smokers (27, 28). Importantly, in comparing bronchial biopsies from nonsmokers, smokers, and patients with COPD, Wang and colleagues (29) reported a fourfold increase in GAD mRNA in smokers compared with normal subjects, and an eightfold increase in patients with COPD. Thus our findings provide a mechanism for the increased GAD mRNA concentrations observed in smokers and patients with COPD, and provide a link between smoking and the production of mucin that characterizes COPD.
The hypersecretion of mucus is a prominent feature of obstructive lung pathologies, including cystic fibrosis, COPD, and asthma. In asthma, mucus plugs occlude the small airways (9, 10, 30–32), and the overproduction of mucus is associated with mortality in severe attacks of asthma (32). The expression of mucin is also markedly increased in COPD, and the degree of mucin expression correlates with the degree of airflow obstruction (33). Thus, although mucin is a key component of the most common respiratory diseases, few effective and specific treatments exist for excess mucin production. Our finding that the nicotine-induced overproduction of mucin is significantly blocked by both nicotinic and GABAergic antagonists suggests new therapeutic approaches to blocking this major component of lung disease.
The effect of GABA on cultured monkey BECs was depolarizing and excitatory, with the reversal potential of IGABA above the resting potential, which is consistent with results previously reported for the human lung adenocarcinoma cell line A549 (6). During brain development, neurons expressing the GABAA receptor undergo a shift from the depolarizing responses to GABA observed in the fetus to hyperpolarizing responses in the adult as intracellular Cl− is lowered, coincident with the developmental upregulation of K+/Cl− cotransporter (KCC2) activity (8, 34, 35). Of particular interest, Liu and colleagues (17, 18) showed that nicotinic signaling modulates the switch of GABA from depolarizing to hyperpolarizing through its effect on KCC2 chloride channel gene expression. Whether chronic prenatal or postnatal nicotine might affect lung development or disease by modifying the expression of KCC2, thus modifying responses to GABA, needs further study. All the studies referenced here used BECs cultured from animals from 0–2 years of age, and thus the extent to which KCC2 expression changes in BECs during fetal development could not be studied.
In normal lungs of both monkeys and humans, chronic exposure to nicotine increases GAD and GABAergic signaling (27–29), whereas GAD and GABAergic signaling is decreased in lung cancer, as reported by Schuller and colleagues (36, 37). Schuller and colleagues (36, 37) showed that decreased GABA signaling promotes the growth of lung cancer, and thus the decrease in GABAergic signaling in lung cancer is similar to the increased nicotinic signaling that we reported in lung cancer (38), in which changes in BEC transmitter levels occur in the direction linked to growth, as opposed to inverse changes in normal tissue. Thus normal patterns of transmitter expression in BEC seem to be dysregulated in lung cancer.
Although the studies described here indicate the potential importance of nicotinic and GABAergic interactions to produce lung disease, the interactions of other BEC transmitter systems likely play a role in lung disease. As shown in Figure 6, BECs also expressed multiple other transmitters and receptors, including most glutamate receptor and glycine receptors, although the function of glutamate and glycine circuits in BECs is not yet known. Other investigators demonstrated the expression of serotonin receptors (39) and muscarinic receptors (40) in airway epithelium. Thus BECs express glutamate, glycine, acetylcholine, and GABA receptors, and their systems clearly interact with each other. This suggests that neurotransmitter expression in bronchial epithelium represents a primitive use of neurotransmitters before their segregation into neurons, as required for synaptic communication. This hypothesis is supported by the existence of similar networks of classic transmitters in the sea sponge, which lacks a nervous system and consists only of simple epithelial layers (15, 16).
Thus we show that the expression of multiple neurotransmitters in epithelium, which originated before the evolution of neurons, continues in respiratory epithelium. Because transmitters in the epithelium are synthesized and signal in a fashion similar to that of neurotransmitters in the brain, the rich pharmacology of neurotransmitters can likely be applied in novel ways to respiratory diseases.
Acknowledgments
The authors thank Yibing Jia and Randall Clark for technical assistance.
This work was supported by National Institutes of Health grants RR00163 and HL087710 (E.R.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0109OC on May 6, 2010
Author Disclosure: E.R.S. received a research grant from Boehringer–Ingelheim for over $100,001, and has a patent pending for the potential use of M3 antagonists in the treatment of lung cancer. K.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. X.W.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Proskocil BJ, Sekhon HS, Jia Y, Savchenko V, Blakely RD, Lindstrom J, Spindel ER. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology 2004;145:2498–2506. [DOI] [PubMed] [Google Scholar]
- 2.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 1999;103:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu XW, Lindstrom J, Spindel ER. Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells. Am J Respir Cell Mol Biol 2009;41:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol 2008;154:1558–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 2009;56:237–246. [DOI] [PubMed] [Google Scholar]
- 6.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, et al. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med 2007;13:862–867. [DOI] [PubMed] [Google Scholar]
- 7.Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit Rev Biochem Mol Biol 2007;42:3–14. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 2002;3:728–739. [DOI] [PubMed] [Google Scholar]
- 9.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 2009;15:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther 2009;121:332–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelkonen M. Smoking: relationship to chronic bronchitis, chronic obstructive pulmonary disease and mortality. Curr Opin Pulm Med 2008;14:105–109. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society/European Respiratory Society Task Force. Standards for the diagnosis and management of patients with COPD, version 1.2, 2004. Available from: http://www.thoracic.org/go/copd.
- 13.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2001;163:429–436. [DOI] [PubMed] [Google Scholar]
- 14.Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung: association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 2002;26:31–41. [DOI] [PubMed] [Google Scholar]
- 15.Kosik KS. Exploring the early origins of the synapse by comparative genomics. Biol Lett 2009;5:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, Degnan BM, Oakley TH, Kosik KS. A post-synaptic scaffold at the origin of the animal kingdom. PLoS ONE 2007;2:e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Zhang J, Berg DK. Role of endogenous nicotinic signaling in guiding neuronal development. Biochem Pharmacol 2007;74:1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Sci 2006;314:1610–1613. [DOI] [PubMed] [Google Scholar]
- 19.McClure-Begley TD, King NM, Collins AC, Stitzel JA, Wehner JM, Butt CM. Acetylcholine-stimulated [3H]GABA release from mouse brain synaptosomes is modulated by {alpha}4{beta}2 and {alpha}4{alpha}5{beta}2 nicotinic receptor subtypes. Mol Pharmacol 2009;75:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu R, Martin WR, Robinson CB, St. George JA, Plopper CG, Kurland G, Last JA, Cross CE, McDonald RJ, Boucher R. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am J Respir Cell Mol Biol 1990;3:467–478. [DOI] [PubMed] [Google Scholar]
- 21.Robinson CB, Wu R. Culture of conducting airway epithelial cells in serum-free medium. J Tissue Cult Methods 1991;13:95–102. [Google Scholar]
- 22.Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Jia Y, Hull WM, Whitsett JA, Starcher BC, Spindel ER. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med 2005;171:1032–1039. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Clarke K, Zhong H, Vollmer C, Nurse CA. Postsynaptic action of GABA in modulating sensory transmission in co-cultures of rat carotid body via GABA(A) receptors. J Physiol 2009;587:329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol 1996;65:251–264. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci 1994;17:569–602. [DOI] [PubMed] [Google Scholar]
- 26.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med 2001;164:989–994. [DOI] [PubMed] [Google Scholar]
- 27.Harvey BG, Heguy A, Leopold PL, Carolan BJ, Ferris B, Crystal RG. Modification of gene expression of the small airway epithelium in response to cigarette smoking. J Mol Med 2007;85:39–53. [DOI] [PubMed] [Google Scholar]
- 28.Ammous Z, Hackett NR, Butler MW, Raman T, Dolgalev I, O'Connor TP, Harvey BG, Crystal RG. Variability in small airway epithelial gene expression among normal smokers. Chest 2008;133:1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Wang R, Hackett NR, Crystal RG. Enhanced expression of the glutamate decarboxylase 67 in human small airway epithelium in response to cigarette smoking. Am J Respir Crit Care Med 2009;179:A1904. (abstract). [Google Scholar]
- 30.Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med 2006;12:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709–721. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 2008;8:183–192. [DOI] [PubMed] [Google Scholar]
- 33.Caramori G, Casolari P, Di GC, Saetta M, Baraldo S, Boschetto P, Ito K, Fabbri LM, Barnes PJ, Adcock IM, et al. MUC5AC expression is increased in bronchial submucosal glands of stable COPD patients. Histopathology 2009;55:321–331. [DOI] [PubMed] [Google Scholar]
- 34.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. N 1999;397:251–255. [DOI] [PubMed] [Google Scholar]
- 35.Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci 2006;26:11720–11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuller HM, Al-Wadei HA, Majidi M. GABA B receptor is a novel drug target for pancreatic cancer. Can 2008;112:767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuller HM, Al-Wadei HA, Majidi M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis 2008;29:1979–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, Proskosil BJ, Gravett C, Lindstrom J, Mark GP, et al. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res 2008;68:4693–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kummer W, Wiegand S, Akinci S, Schinkel AH, Wess J, Koepsell H, Haberberger RV, Lips KS. Role of acetylcholine and muscarinic receptors in serotonin-induced bronchoconstriction in the mouse. J Mol Neurosci 2006;30:67–68. [DOI] [PubMed] [Google Scholar]
- 40.Hislop AA, Mak JC, Reader JA, Barnes PJ, Haworth SG. Muscarinic receptor subtypes in the porcine lung during postnatal development. Eur J Pharmacol 1998;359:211–221. [DOI] [PubMed] [Google Scholar]