Abstract
The role of chemokines in the pathogenesis of lung cancer has been increasingly appreciated. Monocyte chemoattractant protein–1 (MCP-1, also known as CCL2) is secreted from tumor cells and associated tumor stromal cells. The blockade of CCL2, as mediated by neutralizing antibodies, was shown to reduce tumorigenesis in several solid tumors, but the role of CCL2 in lung cancer remains controversial, with evidence of both protumorigenic and antitumorigenic effects. We evaluated the effects and mechanisms of CCL2 blockade in several animal models of non–small-cell lung cancer (NSCLC). Anti-murine–CCL2 monoclonal antibodies were administered in syngeneic flank and orthotopic models of NSCLC. CCL2 blockade significantly slowed the growth of primary tumors in all models studied, and inhibited lung metastases in a model of spontaneous lung metastases of NSCLC. In contrast to expectations, no significant effect of treatment was evident in the number of tumor-associated macrophages recruited into the tumor after CCL2 blockade. However, a change occurred in the polarization of tumor-associated macrophages to a more antitumor phenotype after CCL2 blockade. This was associated with the activation of cytotoxic CD8+ T lymphocytes (CTLs). The antitumor effects of CCL2 blockade were completely lost in CB-17 severe combined immunodeficient mice or after CD8 T-cell depletion. Our data from NSCLC models show that CCL2 blockade can inhibit the tumor growth of primary and metastatic disease. The mechanisms of CCL2 blockade include an alteration of the tumor macrophage phenotype and the activation of CTLs. Our work supports further evaluation of CCL2 blockade in thoracic malignancies.
Keywords: tumor immunology, CCL2, lung cancer, mesothelioma, tumor-associated macrophages
CLINICAL RELEVANCE.
Our data from lung cancer models show that monocyte chemoattractant protein–1 (CCL2) blockade can inhibit tumor growth of primary and metastatic disease. Our work improves the understanding of the importance of chemokines in the pathogenesis of lung cancer, and suggests a possible immunologic mechanism by which blocking a specific chemokine, CCL2, reduces tumor growth and spontaneous metastases in lung cancer. Our work supports further evaluation of CCL2 blockade in thoracic malignancies.
Tumor-induced immunosuppression is one of the most important ways by which cancer cells alter their microenvironments and inhibit endogenous antitumor immune responses (1, 2). One important, immunologically active factor secreted from tumor cells and associated tumor stromal cells is monocyte chemoattractant protein–1 (MCP-1, also known as CCL2), a member of the CC (β) chemokine superfamily. Although first identified as a chemokine produced by tumors and macrophages that could induce the migration of monocytes (3), CCL2 has a number of immunosuppressive properties, such as the attraction of T-regulatory cells and endothelial cells to sites of inflammation (3–5), and the direct inhibition of CD8+ T-cell effector functions (6, 7).
CCL2 was shown to play a role in tumorigenesis and metastases in several solid tumors, including breast cancer, melanoma, and prostate cancer (8, 9). The role of CCL2 in non–small-cell lung cancer (NSCLC) remains controversial, with evidence of both protumorigenic and antitumorigenic effects. Human NSCLC tumors secrete CCL2 at higher levels than normal lung tissue, and in some studies, high concentrations were associated with metastatic disease (10, 11). In contrast, the transfection of NSCLC lines with CCL2 does not alter the metastatic potential of these lines (12) or suppress lung metastases after an intravenous injection of Lewis lung carcinoma (LLC) cells (13). We therefore sought to evaluate the effects of CCL2 blockade on tumor growth and metastases in several animal models of NSCLC, using neutralizing monoclonal antibodies as therapy.
In the mouse, two human CCL2 orthologues bind to the CCR2 receptor: CCL2 (MCP-1) and CCL12 (MCP-5) (14). We evaluated the effects of blocking both murine CCL2 and murine CCL12, using a mixture of monoclonal antibodies (termed α-CCL2 mAbs) that specifically neutralize these chemokines (4, 15). We found that CCL2 blockade exerted a modest but statistically significant effect in reducing tumor growth in five syngeneic flank tumor models of NSCLC and mesothelioma, demonstrated a trend toward improving survival in two orthotopic models of NSCLC, and reduced the extent of spontaneous lung metastases in immunocompetent mice in a new model of spontaneous lung metastases. Our data suggest that that these effects are attributable to an inhibition of the endogenous antitumor immune response. CCL2 blockade thus offers a promising approach to the treatment of NSCLC alone, or combined with other modalities of therapy.
MATERIALS AND METHODS
Animals
Female C57BL/6 and Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA). Female C57BL/6J X 129P3/J hybrids (B6-129/J1) were purchased from Jackson Labs (Bar Harbor, ME). CB-17 severe combined immunodeficient (SCID) mice (6–8 weeks old, weighing 19–24 g) were bred at the Wistar Institute (Philadelphia, PA). The Animal Use Committee of the University of Pennsylvania approved all protocols, in compliance with the Guide for the Care and Use of Laboratory Animals, from the Institution for Laboratory Animal Research (ILAR).
Cell Lines
Tissue culture 1 (TC1) cells were derived from mouse lung epithelial cells of a C57BL/6 mouse, immortalized with the Human Papilloma Virus 16 (HPV16) antigens E6 and E7, and transformed with the c-Ha-ras oncogene (16). Lewis Lung Carcinoma (LLC) cells (syngeneic to C57BL/6 mice) were purchased from the American Type Culture Collection (Manassas, VA). The murine lung cancer line Lung cancer K-ras (LKR) was derived from an explant of a pulmonary tumor from an activated Kras G12D mutant mouse that had been induced in an F1 hybrid of 129Sv.J and C57BL/6 (17). The murine malignant mesothelioma cell line AB12 was derived from an asbestos-induced tumor in a Balb/C mouse (18). The murine malignant mesothelioma cell line AE17 was derived from the peritoneal cavity of C57BL/6J mice injected with asbestos (crocidolite) fibers, and was provided by Dr. Delia Nelson (University of Western Australia, Perth, Australia) (19).
Anti-CCL2/CCL12 Monoclonal
We used a combination of monoclonal antibody (mAb) C1142, a rat/mouse chimeric antibody that neutralizes mouse CCL2 (MCP-1), and mAb C1450, a human/mouse chimeric antibody that neutralizes the second mouse homologue, CCL12 (MCP-5) (4, 9, 15, 20). Both mAbs were produced at Centocor, Inc. (Malvern, PA). Mice were injected intraperitoneally twice weekly with a mixture of mAbs, each at a concentration of 250 μg per mouse, in a total volume of 200 μl normal saline. Control mice were treated with an equal volume of normal saline.
Blocking with either mAb alone induced a nonsignificant trend toward slower tumor growth. However, the combination of both mAbs consistently inhibited tumor growth by 30–50% (P < 0.05) in the TC1 NSCLC cell line (20). Thus, for all remaining experiments, we used the combination of CCL2 and CCL12, referred to as either α-CCL2 or CCL2 blockade.
Animal Flank Tumor Models
Appropriate syngeneic host mice or SCID mice were injected in the right flank with 1–2 × 106 cells. Flank tumors were allowed to reach an average size of 200–250 mm3 (after ∼ 12–15 days). Mice were then randomized to the saline control group or to the α-CCL2 treatment group. All experiments contained at least five mice per group, and were repeated at least once. When needed (e.g., for FACS or RNA), flank tumors were harvested from the mice, minced, and digested with 2 mg/ml DNase I (Sigma, St. Louis, MO) and 4 mg/mL collagenase Type IV (Sigma) at 37°C for 1 hour. Tumors were harvested 2 days after a booster vaccination with Ad.E7, a time point at which no significant change in tumor size was occurring.
Orthotopic Tumor Models
The orthotopic lung cancer model in transgenic K-ras mice was previously described (17). Briefly, to activate the conditional K-ras oncogene and induce tumors, 100 μl of saline containing 3 × 1010 particles of adenovirus containing Cre recombinase (Ad.Cre) were administered to Lox Stop Lox (LSL) KrasG12D mice intranasally. When the animals appeared lethargic, had ruffled fur, or increased breathing rates, they were killed. A study with similar endpoints was conducted after an intravenous injection of 1 × 106 LLC cells.
LKR-M: A Variant NSCLC Line with Reproducible Spontaneous Lung Metastases
The LKR-M cell line was established from an observed lung metastasis that arose after subcutaneous flank injection of the LKR cell line. A cell line was produced from one such metastasis, and after two in vivo passage cycles, a stable line was established with a reproducible pattern of spontaneous lung metastatic induction (> 85% of untreated mice in 5 weeks).
B6X129/J1 or SCID mice were injected on the right flank with 2 × 106 LKR-M cells. After flank tumors reached an average size of 200–250 mm3, mice were randomized to the saline control group or to the α-CCL2 treatment group, and were dosed using the treatment schedule already described. After 5 and 6 weeks of treatment, the mice were killed and their lungs were excised, separated into discrete lobes, and weighed. Sections were cut from each of the five lobes and stained with hematoxylin and eosin. In each lung, the total number of nodules was counted, and the total tumor area measured and divided by the total area of the lung section, using Image J (National Institutes of Health, Bethesda, MD) software. These evaluations were performed by a technician blinded to the group origin of the lung.
Flow Cytometric Analysis of Tumors and Spleens
Splenocytes and tumor cells were studied by FACS analysis, as previously described (20, 21). All fluorescently labeled antibodies were purchased from BD Biosciences (San Jose, CA), except for CD206-PE from Serotec (Oxford, UK), GR-1-FITC from eBioscience (San Diego, CA), and 4-1BB (CD137)-PE from Abcam (Cambridge, UK). Flow cytometry was performed using a FACS Calibur flow cytometer (Becton-Dickinson, San Jose, CA). Data analysis was performed using FlowJo software (Ashland, OR).
RNA Isolation and Real-Time RT-PCR
Mice with tumors were either treated with saline (control) or with α-CCL2 mAbs, as already detailed (n = 5 in each group). Tumors were removed when the tumor volume curves started to diverge. The tumors were flash-frozen, and the RNA from each tumor was isolated. The quantification of tumor mRNA levels was performed as previously described (22). Relative levels of expression of each of the selected genes (fold change in each treatment versus control) in whole tumors were determined. Each sample was run in quadruplicate, and each experiment was repeated at least once.
Immunohistochemical Staining of Tumors
Animals bearing flank tumors, treated with saline or with α-CCL2 mAbs, were killed when the tumor volume curves started to diverge. Tumors were immediately placed in Tissue-Tek OCT compound (Sakura Finetek USA, Inc., Torrance, CA) and stored at −80°C. Immunostaining was performed as previously described (21).
In Vivo Depletion of CD8+ T Cells
To deplete CD8+ T cells systemically, mice received intraperitoneal injections of 200 μg of mAb purified from the anti-CD8 hybridoma 53-6.72 (Harlan Bioproducts, Indianapolis, Indiana). Injections were administered 3 and 2 days before inoculation with tumor cells. Thereafter, a maintenance dose of antibody was injected intraperitoneally twice weekly. CD8 T-cell depletion was confirmed by the flow cytometry of splenic suspensions at different times (data not shown).
Statistical Analyses
For the RT-PCR, FACS, and flank tumor studies that compared differences between two groups, we used unpaired Student t tests. Differences in survival were analyzed using the log-rank test. Differences were considered significant when P < 0.05. Data are presented as mean ± SEM in all tables and figures.
RESULTS
CCL2 Blockade Inhibits Tumor Growth in Syngeneic Flank and Orthotopic Murine Models of NSCLC and Mesothelioma
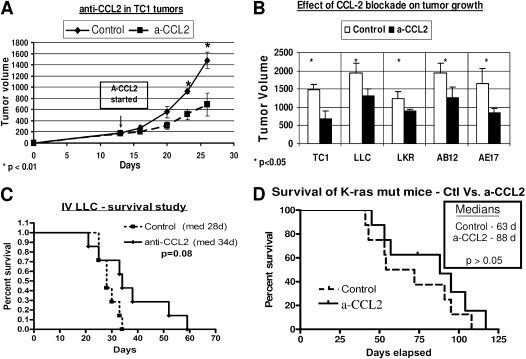
Anti-CCL2 mAbs significantly inhibited tumor growth by 29–53% in three NSCLCs (TC1, LLC, and LKR) and two mesotheliomas (AB12 and AE17) in our syngeneic flank tumor models. Figure 1A shows typical growth curves in the TC1 model. Figure 1B compares tumor volumes in control mice treated with saline versus mice treated with CCL2 blockade, and indicates significant inhibition of growth in all five lines (P < 0.05 versus control mice for each line).
Figure 1.
Monocyte chemoattractant protein–1 (CCL2) blockade inhibits the growth of thoracic malignancies. (A and B) Mice (n = 5–8 for each group) bearing 200-mm3 flank tumors were treated with either saline (Control) or intraperitoneal α-CCL2/CCL12 monoclonal antibodies (mAbs) twice per week (a-CCL2). (A) Growth curves of tumors with these treatments in the non–small-cell lung cancer (NSCLC) cell line tissue culture 1 (TC1) (*P < 0.01). (B) Differences in tumor volume between control and α-CCL2–treated mice, at the time that the size of tumors in control mice was above 10% of their weight, in three NSCLC cell lines (TC1 and Lewis lung carcinoma [LLC] at 25 days, and lung cancer K-ras (LKR) at 40 days), and two mesothelioma cell lines (AB12 and AE17 at 35 days) (*P < 0.05 versus control for each). (C and D) Effects of α-CCL2 blockade were evaluated in two orthotopic NSCLC tumors: intravenous (IV) LLC (C) or a k-ras mutation model (D). Mice (n = 7–9 for each group) were treated with either saline (control) or intraperitoneal α-CCL2 mAb twice per week (a-CCL2). A trend toward increased survival was evident with CCL2 blockade.
We next evaluated the effects of α-CCL2 mAbs on survival in two different NSCLC orthotopic models (mutated K-ras and intravenously injected LLC). In both models, a trend toward improved survival was evident in the CCL2 blockade–treated mice, but it did not quite reach statistical significance (Figures 1C and 1D).
Blocking CCL2 Markedly Reduces Spontaneous Lung Metastases in a Model of NSCLC with Spontaneous Lung Metastases (LKR-M)
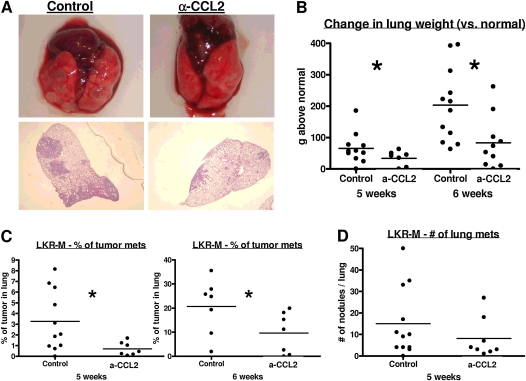
We developed a subline called LKR-M (derived from the LKR cell line) (23) that spontaneously and consistently metastasizes to the lung, starting at 4 weeks after subcutaneous (SQ) injection (see Materials and Methods). To evaluate the effects of CCL2 blockade on spontaneous lung metastases, we injected 2 × 106 cells into the flank of each mouse. After 10 days, treatment with α-CCL2 mAbs was initiated. Five and 6 weeks after tumor injection, the extent of lung metastatic disease was evaluated (Figure 2). Figure 2A shows the gross and microscopic appearance of representative lungs from control (Figure 2A, left) or α-CCL2–treated (Figure 2A, right) mice. Tumor weight, defined as the weight of the lungs above the average lung weight of control naive mice of the same age (120 mg), was approximately twofold higher in control mice compared with mice treated with α-CCL2 mAbs (Figure 2B). The percentage of the lung occupied by tumor was 2–4-fold greater in control mice compared with mice treated with the antibodies (Figure 2C, right). Interestingly, the differences were more prominent in the size of the nodules versus the number of nodules (Figure 2D).
Figure 2.
CCL2 blockade inhibited spontaneous metastases in a model of NSCLC. LKR-M flank tumors were allowed to reach an average size of 200–250 mm3. Mice were then treated with either saline (Control) or intraperitoneal α-CCL2/CCL12 mAbs twice weekly (a-CCL2). After 5 or 6 weeks of treatment, mice were evaluated for lung metastases. (A) Macroscopic (top) and hematoxylin-and-eosin–stained section (bottom) of lungs from control or a-CCL2–treated mice. (B and C) Changes in lung weight compared with lungs from naive mice (B), and the percentages of lung occupied by metastases (C) in each group, showed a significant reduction in tumor burden in mice treated with a-CCL2. (D) Number of nodules in each of the two groups at 6 weeks. No significant difference was evident in the number of nodules. (B–D) Each dot represents one mouse. *P < 0.05.
CCL2 Blockade Does Not Change Recruitment of Immunocytes to NSCLC Tumors, but Skews the Polarization of Tumor-Associated Macrophages toward a Non-M2 Phenotype
CCL2 was shown to be important in the chemotaxis of monocytes in models of infection (23), and α-CCL2 mAbs were shown to inhibit tumor infiltration of macrophages in a prostate cancer model (4). We therefore evaluated the intratumoral percentage of different leukocytes in the TC1 model of NSCLC (n = 7–15; Table 1). We evaluated the tumors 2 days after the booster vaccination with Ad.E7, a time at which no significant change in tumor size was evident. Surprisingly, no significant changes occurred in the percentage of total intratumoral leukocytes (CD45+), macrophages, lymphocytes, or dendritic cells. A small difference was evident in the percentage of intratumor neutrophils in mice treated with α-CCL2 mAb (P < 0.05).
TABLE 1.
MONOCYTE CHEMOATTRACTANT PROTEIN–1(CCL2) BLOCKADE DOES NOT CHANGE RECRUITMENT OF IMMUNOCYTES INTO THE TUMOR
| Cell Type | Flow Marker | Control* | α-CCL2* | P Value |
|---|---|---|---|---|
| Leukocytes | CD45+ | 6.4 ± 0.4% | 6.5% ± 0.7% | NS |
| Tumor-associated macrophages | CD11b+ Ly6G− | 4.7 ± 0.3% | 4.7% ± 0.4% | NS |
| Neutrophils (TAN) | CD11b+ Ly6G+ | 0.16 ± 0.03% | 0.39% ± 0.1% | <0.05 |
| T-cytotoxic | CD8+ | 0.52 ± 0.07% | 0.45% ± 0.04% | NS |
| T-helper | CD4+ | 0.38 ± 0.18% | 0.39% ± 0.19% | NS |
| Dendritic cells | CD11c+ | 1.4 ± 0.4% | 1.1% ± 0.1% | NS |
Definition of abbreviation: NS, no significance.
Data are expressed as percentages of total tumor cells.
Mice (n = 7–15 for each marker) bearing large tissue culture 1 tumors (approximately 200 mm3) were treated with either saline (control) or intraperitoneal α-CCL2 monoclonal antibody. When tumor volume curves started to diverge, tumors were harvested, digested, and subjected to flow cytometry. The percentage of different cells in each group was calculated. No significant differences were evident in the percentage of different immune cells in the tumors, except for a small but significant change in the percentage of intratumoral neutrophils.
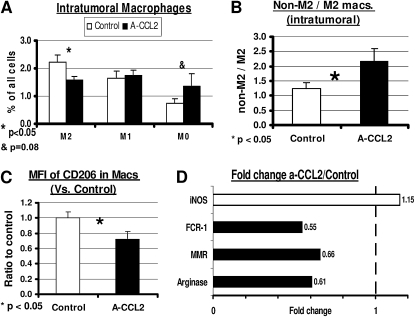
Given this finding, we sought to evaluate the effects of α-CCL2 mAbs on the phenotype of tumor-associated macrophages (TAMs). Although the identification of TAM phenotype by FACS is not standardized, we followed the definitions proposed by Luo and colleagues (24) and Nonaka and colleagues (25), using antibodies to CD11b to identify myeloid cells, Ly6G to identify neutrophils, F4/80 to identify mature macrophages, and CD206 (the mannose receptor), as the primary M2 differentiation marker. We thus defined M0 TAM (undifferentiated TAM) as CD11b+/Ly6G−/F4/80−, M1 TAM as CD11b+/Ly6G−/F4/80+/CD206−, and M2 TAM as CD11b+/Ly6G−/F4/80+/CD206+, and evaluated the percentage of each of these cells in all tumor cells (Figure 3A). The percentage of undifferentiated M0 TAM had doubled in mice after CCL2 blockade (P = 0.08). No difference was evident in the total percentage of M1 TAM. However, a significant reduction was evident in M2 macrophages, from 2.2% of tumor cells in control mice to 1.6% of tumor cells in α-CCL2–treated mice (P < 0.05). These values changed the ratio of nontumorigenic TAM (M0 and M1) to protumorigenic TAM (M2), from approximately 1:1 to a ratio of more than 2:1 after CCL2 blockade (P < 0.05; Figure 3B). Consistent with this change, the mean fluorescent intensity (MFI) of the M2 marker CD206 in all CD11b+/Ly6G− cells (TAM) was reduced by about a third after treatment with α-CCL2 (P < 0.05; Figure 3C).
Figure 3.
CCL2 blockade skews the ratio of M1/M2 tumor-associated macrophage (TAM) phenotype. Mice bearing large TC1 tumors were treated with either saline (Control) or intraperitoneal α-CCL2 mAb (A-CCL2). Tumors were analyzed when tumor volume curves started to diverge. (A) Percentages for each of three phenotypes: M0 (CD11b+/Ly6G−/F4/80−), M1 TAMs (CD11b+/Ly6G−/F4/80+/CD206−), and M2 TAMs (CD11b+/Ly6G−/F4/80+/CD206+) out of all tumor cells. The percentage of M2 TAMs was significantly reduced with CCL2 blockade, with a trend toward increased M0 TAMs. (B) Change in ratio of non-M2 to M2 macrophages showed an increased ratio in mice treated with a-CCL2. (C) Reduction was evident in mean fluorescence intensity (MFI) of the M2 TAM marker (the mannose receptor CD206) in mice treated with α-CCL2. (D) Fold changes were evident in the expression of mRNA of several known markers of M1/M2 TAMs in mice treated with α-CCL2, compared with control mice. Three markers of M2 TAMs (solid bars) were reduced to 55–66% of control levels in mice treated with α-CCL2, whereas the M1 marker inducible nitric oxide synthase (iNOS) (open bar) was increased by 15%.
A shift in macrophage phenotype was further supported by data comparing relative mRNA expression levels of macrophage markers in control tumors versus tumors from α-CCL2–treated mice (Figure 3D). The mRNA levels of several M2 markers (Fc receptor 1 [FcR1], MMR [CD206], and arginase) were reduced to approximately 60% of control levels after treatment. In contrast, the level of the M1 marker, inducible nitric oxide synthase (iNOS), mildly increased by 1.15-fold in α-CCL2–treated mice compared with control mice.
CCL2 was previously shown to exhibit chemoattractant properties for T-regulatory cells (T-regs) (5). However, we found no significant difference in the percentage of FoxP3+ T-regs out of all CD4+ cells in the tumor after CCL2 blockade. However, the number of T-regs within TC1 tumors was very low (data not shown). We also found no significant differences in the percentage of myeloid-derived suppressor cells (MDSCs) (CD11b+/GR-1+) from all splenic cells in mice bearing TC1 NSCLC tumors (data not shown).
CCL2 Blockade Is Associated with Activated Intratumoral CD8+ Cells
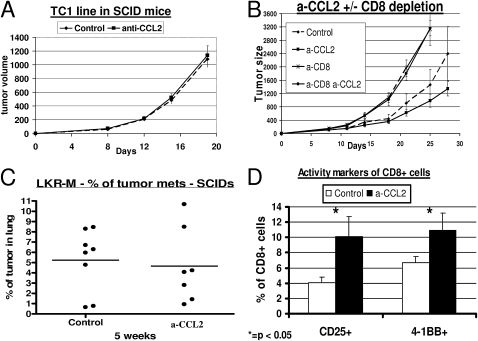
To assess the importance of the adaptive immune response, we evaluated the effect of α-CCL2 mAbs on TC1 tumor growth in SCID mice, where all of the antitumor effect was lost (compare Figure 1A with Figure 4A), and after specifically depleting CD8+ T cells. The depletion of CD8+ T cells resulted in the faster growth of TC1 NSCLC flank tumors. However, the antitumor effects of α-CCL2 in these CD8-depleted C57BL/6 mice versus intact C57BL/6 mice disappeared completely (Figure 4B). Similar results were found in the LKR-M lung metastasis model, where the percentage of lung occupied by tumor was no different between control and α-CCL2–treated mice in either SCID mice (Figure 4C) or after specific CD8 depletion (data not shown).
Figure 4.
Effect of CCL2 blockade on thoracic malignancies is dependent on intratumoral CD8+ T cells. (A) CB-17 severe combined immunodeficient (SCID) mice (n = 5–6 for each group) bearing 200-mm3 TC1 tumors were treated with saline (Control) or intraperitoneal α-CCL2 mAb (a-CCL2). No difference in the pattern of tumor growth was evident between the two groups. (B) Immunocompetent C57bL/6 mice (n = 5–6 for each group) bearing large TC1 tumors were treated with: (1) saline (diamonds, Control); (2) intraperitoneal α-CCL2 mAb (a-CCL2) (squares, a-CCL2); (3) saline, and injected with 300 μg of an anti-CD8 mAb intraperitoneal twice weekly (crosses, a-CD8); and (4) α-CCL2 mAB and depletion of CD8+ cells (circles, a-CD8 a-CCL2). Control and a-CCL2 groups were treated with an intraperitoneal control IgG antibody. The effect of CCL2 blockade on tumor growth disappeared completely in mice depleted of CD8+ T cells. (C) SCID mice (n = 7–8 for each group) were injected on the right flank with the NSCLC cell line LKR-M. After flank tumors reached an average size of 200–250 mm3, mice were treated with either saline (Control) or intraperitoneal α-CCL2/CCL12 mAb twice weekly (a-CCL2). After 5 weeks of treatment, lungs were evaluated for metastases. The percentage of lung occupied by metastases did not differ between the two groups. Each dot represents one mouse. (D) Percentages of intratumoral CD8+ T cells expressing the two activation markers, CD25 (left) and 4-1BB (right). The percentage of activated cells out of all CD8+ T cells was doubled in mice treated with a-CCL2 mAb (*P < 0.05).
No increase was evident in the number of intratumoral CD8+ T cells according to either immunohistochemistry (IHC) or flow cytometry (Table 1 and data otherwise not shown) after α-CCL2 treatment in wild-type mice. However, the percentage of activated intratumoral CD8+ T cells in mice with CCL2 blockade was doubled (P < 0.05), as assessed by the expression of two established surface activation markers, 4-1BB (CD137) and CD25 (Figure 4D). This finding suggests that increased CD8+ T-cell activity may account in part for the antitumor effect of CCL2 blockade.
DISCUSSION
The role of chemokines such as CCL5, CXCL8, and CXCL10 in the pathogenesis of lung cancer, and in other cancers, has been increasingly appreciated (27, 28). However, the role of other important chemokines in lung cancer, such as CCL2, remains unclear. The present experiments, using specific anti-murine CCL2 and CCL12 mAbs, show that CCL2 blockade can inhibit tumor growth and NSCLC metastatic disease via an immune-mediated mechanism that appears to affect innate and adaptive antitumor immune responses.
Human CCL2 has two murine orthologues: CCL2 (MCP-1) and CCL12 (MCP-5). Both bind to the CCR2 receptor, although CCL2 is a better agonist of murine CCR2 (14). Most functions described for CCL12 are similar to those found for CCL2 (15). We found that each of these mAbs exerted some effect on tumor growth, but significantly more growth inhibition was evident when the two mAbs were co-administered (20). To model the potential effects most accurately of neutralizing CCL2 in humans, we used a mixture of both mAbs for our experiments.
Some controversy has arisen about the role of CCL2 in tumor development. CCL2 can function as a T-cell chemoattractant and induce T-cell tumor tropism, including memory T cells (26, 27). Early work showed that the transfection of tumor cells with CCL2, which induced high levels of CCL2 secretion, resulted in massive monocyte/macrophage infiltration into the tumor mass, leading to its destruction (28). However, CCL2 was found at high concentrations in patients with multiple tumor types, including NSCLC (3, 11), and high concentrations usually correlated with poor clinical outcomes. The use of α-CCL2 mAb in vivo was recently shown to reduce tumorigenesis and metastases in prostate cancer xenograft models (9, 29).
These observations, along with the data from this study, fit a new paradigm suggesting that most of the effects of CCL2 in nontransduced tumors are actually protumorigenic (3). First, most monocytes recruited into tumors are recognized not to kill tumor cells, but to be subverted to an M2 phenotype, where they actually support tumor growth (30). Second, CCL2 appears to augment directly the growth and invasiveness of certain tumor cells that express the CCR2 receptor (3, 31). Third, CCR2 is expressed by endothelial cells, and CCL2 appears to promote angiogenesis (32). Fourth, CCL2 was observed to serve as a chemoattractant for T-regs (5). Finally, CCL2 is also recognized to exert direct immuno-inhibitory (protumorigenic) effects on T-cell function (6, 7), such as inhibiting T-cell effector functions and switching T-cell differentiation toward Th2-like cells (8).
In our tumor models, the antitumor effects of CCL2 blockade appear to be mostly immunologic. The antitumor activity of CCL2 blockade was lost after the depletion of CD8+ T cells, or when tumors were implanted in SCID mice lacking T cells (Figure 4). Thus our data suggest that the effect of blocking CCL2 in lung cancer is mediated mainly by CD8+ cytotoxic CD8+ T lymphocytes (CTLs). The role of macrophages in the activation of CD8+ lymphocytes in lung cancer was demonstrated previously (33, 34). Consistent with the present study, we recently found that α-CCL2 mAbs augment the effect of cancer vaccine immunotherapy by increasing the activity and antigen-specificity of CD8+ T cells (20).
In contrast to the work of Loberg and colleagues on prostate cancer (4), but similar to the paper by Valkovic and colleagues on breast cancer (35), we found no change in the number of infiltrating macrophages or other major immune cells in our mesothelioma or lung tumors (Table 1). Somewhat surprisingly, the neutralization of a monocyte-attracting chemokine did not reduce the total number of monocytes in the tumor. We have no definitive explanation for this observation, but we speculate that in tumors many other agents (such as CCL5 and M-CSF), some possibly induced by CD8+ T cell activity, may replace CCL2 in terms of the chemoattraction of monocytes (30).
Although the total numbers of macrophages did not change, our data showed a clear decrease in the protumorigenic M2 phenotype, with unchanged numbers of M1 TAMs and a mild increase in the more undifferentiated (M0) TAM (Figure 3). This datum was further supported by the reduction of the MFI of CD206 in TAM after CCL2 blockade, consistent with the notion that this change represents a polarization toward alternatively activated (M2) macrophages in the continuum of TAMs (36, 37). The net effect of these changes appeared to result in a more immunostimulatory tumor microenvironment. At least two possible explanations exist for this observation. First, CCL2 may function primarily to differentiate newly recruited monocytes (M0 TAMs) into an immunosuppresive M2 phenotype. It is widely accepted that TAMs can be regulated by their microenvironment (38). Merely recruiting increased numbers of macrophages, without changing their phenotype, may elicit very little antitumor effect (39). However, TAMs can be redirected from an M2 to a non-M2 phenotype, for example, by blocking NF-κB activity (40) or that of other macrophage-activating agents (34, 39), and enhanced antitumor activity may thus be achieved. Thus, one explanation for our data involves CCL2 inducing M2 differentiation, and the blocking of CCL2 prevents the conversion of M0 TAMs to M2 TAMs. Roca and colleagues recently showed that after CCL2 stimulation in vitro, human macrophages demonstrated a significant increase in the mannose receptor (CD206), suggesting a polarization of macrophages toward the CD206+ M2-type phenotype (37).
A second explanation posits that CCL2 mediates the differential recruitment of MDSCs (M2-like cells) versus blood monocytes (M0-like cells). MDSCs can enter tumors and differentiate to mature macrophages (TAMs) (41). Furthermore, Huang and colleagues showed that the recruitment of MDSCs into tumors is mediated by the CCL2/CCR2 axis (42). The phenotype of macrocytic MDSCs is similar to that of M2 TAMs. CCL2 blockade may thus reduce the influx of MDSCs into tumors, but not that of naive monocytes, a source of M0/M1 macrophages, hence changing the ratio of non-M2 to M2 macrophages (Figure 3B). Studies to test these two hypotheses are underway.
Here, we describe a new cell line capable of spontaneous lung metastases in immunocompetent mice. The need for animal models with spontaneous metastatic disease, enabling a broader understanding of the biology of metastases, has been recognized (43). Most models of “metastases” use the injection of cancer cells directly into the systemic circulation. Depending on the tropism of the tumor cell, distant metastases may (or may not) develop in a target organ, in many cases the lung. The LLC cell line is commonly used in lung cancer (13, 44), as shown in Figure 2C. The main weakness of this approach involves the elimination of the early steps in the metastatic cascade (i.e., vascular or lymphatic invasion and extravasation), and therefore the metastases that form have different characteristics than those that develop spontaneously from primary tumors (45). Some sublines of LLC do develop spontaneous metastases and have been used (44). Here, we describe an additional cell line arising from ras-mutated NSCLC cells that can form spontaneous lung metastases when injected in the flank. We found that the spontaneous lung metastases are preceded by draining lymph node metastases (data not shown). This new model enabled us to evaluate the full cascade of metastatic disease, with specific tropism to the lungs, in a relatively rapid and fairly reproducible manner.
Using this model, we showed that the effects of CCL2 blockade on the development of spontaneous metastases are substantial, and even more substantial than the effects on local tumor growth. This finding is consistent with data showing that CCL2 plays an important role in the development of metastases in many human tumors, including prostate (29), breast (46), and lung cancer (10). Several possible mechanisms could account for the importance of CCL2 in the metastatic process, including the induction of angiogenesis (32), the generation of morphologic changes in the tumor facilitating cell proliferation and migration (47), and the improved homing and attachment of tumor cells to lymphatic endothelial cells (48). CCL2 appears to augment directly the growth and invasiveness of certain tumor cells that express the CCL2 receptor (CCR2), such as breast and prostate cancer (3, 31). Furthermore, some breast cancer cell lines expressing CCR2 were shown to respond chemotactically to CCL2 in vitro (49). Given our data in immunodeficient models and the fact that the LKR-M tumor exhibited no detectable expression of CCR2 (data not shown), immunologic mechanisms again seem most important.
Our observations in lung cancer suggest that the effects of CCL2 blockade are primarily attributable to alterations in the tumor microenvironment. Blocking CCL2 could thus exert an antitumor effect in thoracic malignancies, and specifically in the metastatic process, by polarizing the phenotype of intratumoral macrophages and activating CTLs.
Supplementary Material
Acknowledgments
We thank Luana Pereira and Aaron Blouin for technical assistance in the experiments.
This work was supported by a National Cancer Institution grant PO1 CA 66726 (S.M.A.), with partial support by grant 1574/09 from the Morasha Program of the Israel Science Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0080OC on April 15, 2010
Author Disclosure: Z.G.F. received lecture fees from Astra-Zeneca and GlaxoSmithKline (less than $1,000), and a sponsored research grant from the Israeli Academy of Sciences ($10,001–$50,000). S.M.A. received industry-sponsored grants from Biogen Idec Grant to conduct a clinical trial (more than $100,000), and from Centocor for a sponsored research agreement to evaluate the anti–MCP-1 antibody (more than $100,000). None of the other authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res 2006;12:4794–4803. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007;25:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol 2004;14:149–154. [DOI] [PubMed] [Google Scholar]
- 4.Loberg RD, Ying C, Craig MJ, Yan L, Snyder L, Pienta KJ. CCL2 is an important mediator of prostate cancer growth in vivo via regulation of macrophage infiltration. Neoplasia 2007;9:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan J, Sun W, Hussain S, DeAngulo G, Prabhu S, Heimberger A. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother 2008;57:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng L, Shu S, Krauss JC. Monocyte chemoattractant protein inhibits the generation of tumor-reactive T cells. Cancer Res 1997;57:4849–4854. [PubMed] [Google Scholar]
- 7.Terwey TH, Kim TD, Kochman AA, Hubbard VM, Lu S, Zakrzewski JL, Ramirez-Montagut T, Eng JM, Muriglan SJ, Heller G, et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood 2005;106:3322–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu K, Xiong J, Ji K, Sun H, Wang J, Liu H. Recombined CC chemokine ligand 2 into B16 cells induces production of Th2-dominanted cytokines and inhibits melanoma metastasis. Immunol Lett 2007;113:19–28. [DOI] [PubMed] [Google Scholar]
- 9.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wonjo K, Snyder LA, Yan L, Pienta KJ. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 2007;67:9417–9424. [DOI] [PubMed] [Google Scholar]
- 10.Cai Z, Chen Q, Chen J, Lu Y, Xiao G, Wu Z, Zhou Q, Zhang J. Monocyte chemotactic protein 1 promotes lung cancer–induced bone resorptive lesions in vivo. Neoplasia 2009;11:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arenberg D, Keane M, DiGiovine B, Kunkel SL, Strom SR, Burdick MD, Lannettoni MD, Strieter RM. Macrophage infiltration in human non–small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother 2000;49:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai E, Ogawa T, Kielian T, Ikubo A, Suzuki T. Irradiated tumor cells adenovirally engineered to secrete granulocyte/macrophage-colony–stimulating factor establish antitumor immunity and eliminate pre-existing tumors in syngeneic mice. Cancer Immunol Immunother 1998;47:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nokihara H, Yanagawa H, Nishioka Y, Yano S, Mukaida N, Matsushima K, Sone S. Natural killer cell–dependent suppression of systemic spread of human lung adenocarcinoma cells by monocyte chemoattractant protein–1 gene transfection in severe combined immunodeficient mice. Cancer Res 2000;60:7002–7007. [PubMed] [Google Scholar]
- 14.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med 1997;185:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsui P, Das A, Whitaker B, Tornetta M, Stowell N, Kesavan P, Kaiser E, Lacy ER, Yan L, Snyder LA, Sweet R. Generation, characterization and biological activity of CCL2 (MCP-1/JE) and CCL12 (MCP-5) specific antibodies. Hum Antibodies 2007;16:117–125. [PubMed] [Google Scholar]
- 16.Lin K-Y, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu T. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996;56:21–26. [PubMed] [Google Scholar]
- 17.Wilderman MJ, Sun J, Jassar AS, Kapoor V, Khan M, Vachani A, Suzuki E, Kinniry PA, Sterma DH, Kaiser LR, Albelda SM. Intrapulmonary IFN-beta gene therapy using an adenoviral vector is highly effective in a murine orthotopic model of bronchogenic adenocarcinoma of the lung. Cancer Res 2005;65:8379–8387. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick D, Bielefeldt-Ohman H, Himbeck R, Jarnicki A, Marzo A, Robinson B. Transforming growth factor–beta: antisense RNA–mediated inhibition affects anchorage-independent growth, tumorigenicity and tumor-infiltrating T-cells in malignant mesothelioma. Growth Factors 1994;11:29–44. [DOI] [PubMed] [Google Scholar]
- 19.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, vanHagen D, Robinson BWS, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol 2003;171:5051–5063. [DOI] [PubMed] [Google Scholar]
- 20.Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, Crisanti MC, Wang LS, Heitjan DF, Snyder LA, Albelda SM. CCL2 blockade augments cancer immunotherapy. Cancer Res 2010;70:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, Albelda SM. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res 2006;12:214–222. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005;11:6713–6721. [DOI] [PubMed] [Google Scholar]
- 23.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1–deficient mice. J Exp Med 1998;187:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest 2006;116:2132–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonaka K, Saio M, Suwa T, Frey AB, Umemura N, Imai H, Ouyang G, Osada S, Balazs M, Adany R, et al. Skewing the Th cell phenotype toward Th1 alters the maturation of tumor-infiltrating mononuclear phagocytes. J Leukoc Biol 2008;84:679–688. [DOI] [PubMed] [Google Scholar]
- 26.Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, Jensen MC. Tumor-derived chemokine MCP-1/CCL2 Is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol 2007;179:3332–3341. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Dai H, Wan N, Moore Y, Dai Z. The role for monocyte chemoattractant protein–1 in the generation and function of memory CD8+ T cells. J Immunol 2008;180:2886–2893. [DOI] [PubMed] [Google Scholar]
- 28.Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein–1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol 2001;166:6483–6490. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res 2009;69:1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allavena P, Sica A, Garlanda C, Mantovani A. The yin–yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 2008;222:155–161. [DOI] [PubMed] [Google Scholar]
- 31.Loberg RD, Day LL, Harwood J, Ying C, St. John LN, Giles R, Neeley CK, Pienta KJ. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia 2006;8:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salcedo R, Ponce M, Young H, Wasserman K, Ward JM, Kleinman HK, OPPenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 2000;96:34–40. [PubMed] [Google Scholar]
- 33.Kataki A, Scheid P, Piet M, Marie B, Martinet N, Martinet Y, Vingaud JM. Tumor infiltrating lymphocytes and macrophages have a potential dual role in lung cancer by supporting both host-defense and tumor progression. J Lab Clin Med 2002;140:320–328. [DOI] [PubMed] [Google Scholar]
- 34.Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, Albelda SM. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4–acetic acid induces an effective CD8+ T-cell–mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res 2005;65:11752–11761. [DOI] [PubMed] [Google Scholar]
- 35.Valkovic T, Fuckar D, Stifter S, Matusan K, Hasan M, Dobrila F, Jonjic N. Macrophage level is not affected by monocyte chemotactic protein–1 in invasive ductal breast carcinoma. J Cancer Res Clin Oncol 2005;131:453–458. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–555. [DOI] [PubMed] [Google Scholar]
- 37.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 2009;284:34342–34354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964. [DOI] [PubMed] [Google Scholar]
- 39.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 2005;65:3437–3446. [DOI] [PubMed] [Google Scholar]
- 40.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med 2008;205:1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol 2005;175:4583–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang G, Feng Z. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett 2007;252:86–92. [DOI] [PubMed] [Google Scholar]
- 43.Man S, Munoz R, Kerbel R. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev 2007;26:737–747. [DOI] [PubMed] [Google Scholar]
- 44.Yamaura T, Doki Y, Murakami K, Saiki I. Model for mediastinal lymph node metastasis produced by orthotopic intrapulmonary implantation of lung cancer cells in mice. Hum Cell 1999;12:197–204. [PubMed] [Google Scholar]
- 45.Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis 2005;26:513–523. [DOI] [PubMed] [Google Scholar]
- 46.Soria G, Yaal-Hahoshen N, Azenshtein E, Shina S, Leider-Trejo L, Ryvo L, Cohen-Hillel E, Shtabsky A, Ehrlich M, Meshel T, et al. Concomitant expression of the chemokines RANTES and MCP-1 in human breast cancer: a basis for tumor-promoting interactions. Cytokine 2008;44:191–200. [DOI] [PubMed] [Google Scholar]
- 47.van Golen KL, Ying C, Sequeira L, Dubyk CW, Reisenberger T, Chinnaiyan AM, Pienta KJ, Loberg RD. CCL2 induces prostate cancer transendothelial cell migration via activation of the small GTPase Rac. J Cell Biochem 2008;104:1587–1597. [DOI] [PubMed] [Google Scholar]
- 48.Kawai Y, Kaidoh M, Yokoyama Y, Sano K, Ohhashi T. Chemokine CCL2 facilitates ICAM-1–mediated interactions of cancer cells and lymphatic endothelial cells in sentinel lymph nodes. Cancer Sci 2009;100:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youngs SJ, Ali SA, Taub DD, Rees RC. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer 1997;71:257–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.