Abstract
Sialic acid–binding immunoglobulin-like lectin (Siglec)–F, an inhibitory receptor on mouse eosinophils, preferentially recognizes the glycan ligand 6′-sulfated sialyl Lewis X, but little is known about the requirements for its lung expression. RT-PCR and immunohistochemistry were used to detect and localize the sulfotransferase keratin sulfate galactose 6-O sulfotransferase (KSGal6ST, also known as carbohydrate sulfotransferase 1; gene name, Chst1) that is putatively required for 6′-sulfated Sialyl Lewis X synthesis. RT-PCR detected the greatest constitutive expression of Chst1 in lung, liver, and spleen tissue. Immunohistochemistry localized the expression of KSGal6ST in lung tissue primarily to airway epithelium. Siglec-F–Ig fusion protein selectively bound in a similar pattern, and was unaffected in lung tissue treated with methanol or deficient in Type 2 α2,3 sialyltransferase (St3gal2), but was eliminated by proteinase K or sialidase, and was absent in tissue deficient in the Type 3 α2,3 sialyltransferase (St3gal3). Binding of the Siglec-F–Ig fusion protein was similar in pattern to, and completely blocked by, a plant lectin recognizing α2,3-linked sialic acid. Thus, α2,3-linked sialic acid–containing glycoprotein Siglec-F ligands and the enzymes required for their synthesis are constitutively expressed in murine lungs, especially by airway epithelium. St3gal3, but not St3gal2, is required for constitutive Siglec-F ligand synthesis. The survival of eosinophils entering the lung may be shortened by encountering these Siglec-F sialoside ligands.
Keywords: eosinophil, sialyltransferase, lung epithelium, sialic acid, glycan ligand
CLINICAL RELEVANCE.
The epithelial glycoprotein ligand studied here is believed to engage Siglec–F selectively on mouse eosinophils to induce their apoptosis. Therefore, understanding the pathways that control the expression of this sialylated structure, including its posttranslational modifications, may be critical to our understanding of asthma and other disorders associated with lung eosinophilia.
Sialic acid–binding immunoglobulin-like lectin (Siglec)–8, simultaneously discovered by two separate laboratories (1, 2), is a member of the CD33 subset of the Siglec family (3–6). It is a single-pass cell surface receptor found only on human mast cells, eosinophils, and basophils key effector cells of allergic inflammation (1, 6). This transmembrane protein has an extracellular binding domain that recognizes specific complex glycans, and an intracellular immunoreceptor tyrosine–based inhibition motif (ITIM) that putatively transduces receptor binding into immune suppression (1, 2). Although no exact murine orthologue exists, strong data support the conclusion that murine Siglec-F and human Siglec-8 are close, functionally convergent paralogues (6–8). Mouse Siglec-F is also a CD33-related siglec that contains multiple immunoglobulin-like extracellular domains, including an N-terminal carbohydrate-binding domain, a transmembrane domain, and a cytoplasmic tail that, like Siglec-8, contains a putative ITIM (8, 9). Siglec-F is expressed predominantly in murine eosinophils, but unlike Siglec-8, it is found on other cells such as alveolar macrophages (7, 8, 10, 11).
Until recently, the functions of Siglec-8 and Siglec-F were unknown. The incubation of human eosinophils with specific Siglec-8 monoclonal antibodies (mAbs) or polyclonal anti–Siglec-8 antibodies was reported to cause rapid caspase-dependent and reactive oxygen species (ROS)-dependent apoptosis (12–15). Additional studies revealed that Siglec-F engagement on murine eosinophils by antibodies also induced their apoptosis in vitro, and the administration of Siglec-F antibodies to eosinophilic mice reduced their circulating and tissue eosinophil concentrations (16–18). Studies of Siglec-F–deficient mice show that in the baseline state, normal eosinophil levels occur in the blood, bone marrow, and lungs. After sensitization and challenge with ovalbumin, these mice manifest a twofold elevation of eosinophils in their lungs, peripheral blood, and bone marrow, along with a delay in resolution of this eosinophilic inflammation (17). Song and colleagues demonstrated that the administration of Siglec-F antibody in a chronic mouse asthma model caused improvements in airway remodeling (19). These data suggest that Siglec-F is involved in regulating eosinophilic inflammation in the lung. Although the natural Siglec-F ligand is not known, using a Siglec-F–Ig fusion protein, predominant airway epithelial and mononuclear cell staining of lung sections was evident (17, 19). These findings are consistent with the hypothesis that epithelial-derived glycans help limit lung eosinophilic inflammation (6).
In collaboration with the Consortium for Functional Glycomics (www.functionalglycomics.org), we previously screened for ligands using Siglec-8–Ig fusion protein, and identified a single, unique candidate glycan ligand, namely, NeuAcα2–3(6-O-sulfo)Galβ1–4[Fucα1–3]GlcNAc, also referred to as 6′-sulfated Sialyl Lewis X or 6′-su-sLex (20). This ligand was also found to bind Siglec-F and cause its internalization (7, 21). Therefore, the engagement of Siglec-8 (or Siglec-F) through its natural glycan ligand, or through mAbs or glycomimetic agonists, was proposed as a novel means toward the specific inhibition or depletion of eosinophils and mast cells (6, 22, 23).
The discovery that Siglec-F and Siglec-8 recognize the same unique glycan ligand, 6′-su-sLex, raises questions about its biosynthesis, which is essentially unknown. The synthesis of 6′-su-sLex is predicted to require the activity of specific sialyltransferases and sulfotransferases, most likely found in the Golgi complex of cells capable of synthesizing this glycan. Based on the known family of human Golgi complex–associated sulfotransferases (GSTs) (24), KSGal6ST (also known as carbohydrate sulfotransferase 1; gene name, Chst1), an enzyme known to sulfate galactose residues linked to N-acetyl glucosamine, is predicted to be involved. In addition, sialyltransferases, which are responsible for sialylating glycolipid and glycoprotein carbohydrate groups, are classified into four families according to the carbohydrate linkages they synthesize. At least 15 different sialyltransferases exist in mice, including those that add sialic acids in terminal positions at an α2–3, α2–6, or α2–8 linkage. Among the α2−3 sialyltransferases potentially involved in generating 6′-su-sLex, St3gal2, St3gal3, St3gal4, and St3gal5 were found in the lungs of mice (25).
Here, the tissue expression of Siglec-F ligand, and of putatively required biosynthetic enzymes including sialyltransferases and KSGal6ST, was explored at the mRNA and protein levels under basal conditions, using normal murine tissue. Binding patterns were compared with those seen with plant lectins known to recognize differing sialic acid confirmations. The specificity of Siglec-F binding was explored using competing plant lectins, sialidase, and other tissue treatments, whereas mice lacking specific sialyltransferases were used to determine their requirement for constitutive Siglec-F ligand expression.
MATERIALS AND METHODS
Animal Protocols
All mice were of a normal C57BL/6 strain, and were housed in cages with microfilters in a specific pathogen–free environment. All procedures performed on mice were in accordance with the National Institutes of Health guidelines for the humane treatment of animals, and were approved by our local Institutional Animal Use and Care Committees. Breeding sets of mice deficient in St3 gal2 or St3 gal3, generated as previously described (26), were kindly provided by Dr. Jamey Marth of the University of California at San Diego.
Nonquantitative RT-PCR
Total cellular RNA from lungs or other tissues was obtained using Trizol reagent, as described elsewhere (27). In RT-PCR assays, RNA samples were reverse-transcribed, and gene-specific primers were used to amplify selected regions of each target moiety. For each gene, the optimal numbers of cycles that produce a quantity of cytokine product that is directly proportional to the quantity of input mRNA were determined experimentally. To verify that equal amounts of undegraded RNA were added in each RT-PCR reaction, β-actin was used as an internal standard. PCR of actin: 27 cycles, with annealing at 60°C; PCR of CHST1: 27 cycles, with annealing at 59°C. Primers included CCT GCT GAG GAG CCT TTA TG for KSGal6ST (forward), and GGC CAG GTC CTC ATA ACG TA for KSGal6ST (backward).
Immunohistochemistry and Western Blotting
Mice were killed with injections of ketamine (2 mg/ml) and xylazine (0.4 mg/ml), a median sternotomy was performed, and pulmonary vasculature perfusion was achieved with calcium and magnesium-free PBS to clear the intravascular space. Lungs were inflated with 4% formaldehyde (Sigma-Aldrich, St. Louis, MO), removed, and fixed overnight in 4% formaldehyde, rinsed with PBS, cryoprotected in 18% sucrose in PBS, embedded in OCT medium (VWR Scientific, West Chester, PA), frozen, and sectioned at 10 μm.
Frozen tissue sections were rehydrated and treated with PBS or sialidase (10 mU/ml, for 24 hours, at 37°C; Roche Biomedical Labs, Raritan, NJ). Paraffin-embedded sections were rehydrated and treated with either proteinase K (400 μg/ml; Dako Cytomation, Carpenteria, CA) for 6 minutes at room temperature to remove proteins, or with 100% methanol for 10 minutes at room temperature to remove lipids (28). Sections were washed three times with PBS and incubated as appropriate for 1 hour at 37°C with 1 μg/ml of Siglec-F–Ig fusion protein (human IgG1 Fc; R&D Systems, Minneapolis, MN), Siglec-10–Ig fusion protein (human IgG1 Fc, generated as previously described for Siglec-8–Ig fusion protein (1) and generously provided by John White at Glaxo SmithKline), or an isotype-matched, humanized IgG1 monoclonal antibody used as an IgG1 control (Omalizumab; Genentech, South San Francisco, CA); and 1 μg/ml of control preimmune sheep or rabbit polyclonal IgG, a commercially available polyclonal sheep IgG antibody (R&D Systems), or a newly generated and purified KSGal6ST rabbit polyclonal IgG antibody created for us (Invitrogen Corp., Carlsbad, CA), using a 15 amino-acid peptide unique to KSGal6ST and shared by both mouse and human proteins (CILASRSETFRDTYR). The specificity of binding of the two KSGal6ST polyclonal antibodies was compared in Western blot assays of whole normal mouse lung extracts. Whole mouse lungs were placed in 1% Triton lysis buffer plus a complete mini-protease inhibitor cocktail (Roche, Indianapolis, IN). A single-cell suspension was created using scissors and a mortar and pestle. Cells were lysed for 5 minutes on ice, centrifuged at 14,000 × g to remove debris, and protein concentrations were determined using the BCA protein assay reagent (Pierce Chemical Co., Rockford, IL). Samples were boiled for 6 minutes in 4 × SDS sample buffer (0.5 M Tris, pH 6.8, 16% glycerol, 3% SDS, 8% 2-mercaptoethanol, and 2 mg bromophenol blue), and 20 μg of protein were loaded onto a 12% tris-glycine SDS–polyacrylamide gel. After electrophoresis, proteins were transferred to Sequi-Blot PVDF membranes (Bio-Rad, Hercules, CA), using a mini two-gel device and mini tank electroblotter (Continential Laboratory Products, San Diego, CA). After transfer, membranes were stained with amido black stain (Bio-Rad), destained, washed in PBST (20 mM Tris, 137 mM sodium chloride, and 0.2% Tween-20; Bio-Rad), and incubated overnight at 4°C with 5% BSA (Fisher Scientific, Pittsburgh, PA) in PBST to block nonspecific binding sites. Membranes were then incubated 1 hour at room temperature with the novel polyclonal rabbit anti-KSGal6ST antibody at a 1:1,000 dilution, or a polyclonal sheep anti-KSGal6ST antibody (R&D Systems) at a 1:1,000 dilution. Membranes were subsequently washed twice for 5 minutes each with PBST and incubated with horseradish peroxidase–linked polyclonal donkey anti-rabbit IgG (GE Healthcare, Piscataway, NJ) or rabbit anti-sheep IgG (Pierce Chemical Co.), each at a 1:2,500 dilution for 45 minutes at room temperature. Blots were then washed four times for 10 minutes each with PBST. Bands were visualized using the ECL Western Blotting Detection System (GE Healthcare).
Biotinylated Maackia amurensis (MAA) lectin (specific for α2,3-linked sialic acid) and Sambucus nigra (SNA) lectin (specific for α2,6-linked sialic acid), each at 10 μg/ml (EY Laboratories, Inc., San Mateo, CA) were also used for histochemistry and in blocking experiments. Tissue sections were washed, and nonspecific binding sites were blocked with dual endogenous block (Dako) for 10 minutes at room temperature. Sections were washed with PBS and incubated as appropriate, with either a biotinylated goat anti-human IgG or biotinylated goat anti-rabbit IgG (1:100 dilution; Vector Laboratories, Burlingame, CA). Sections were again washed with PBS, and then streptavidin/alkaline phosphatase linker ABC (Vector Laboratories) was added, and staining was visualized using Vector Red Chromagen (Vector Laboratories). Sections were washed with deionized water, counterstained with hematoxylin (Fisher Scientific, Pittsburgh, PA), dehydrated, and coverslipped with a permanent mounting medium (Richard-Allan Scientific, Kalamazoo, MI). In some experiments, tissues were preincubated with the KSGal6ST immunogen peptide CILASRSETFRDTYR already mentioned (30 μg/ml for 18 hours at 4°C), and MAA or SNA (10 μg/ml, EY Laboratories, Inc.) for 1 hour at 37°C, and then incubated with Siglec-F–Ig fusion protein and other reagents as already described.
RESULTS
Localization of KSGal6ST Expression
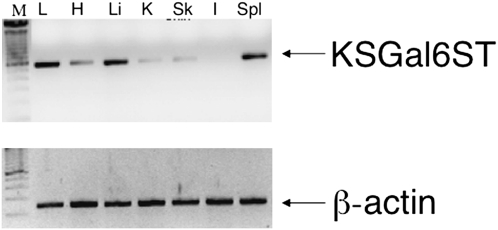
Based on the known family of GSTs, KSGal6ST (expressed by the Chst1 gene) is the only mammalian enzyme known to be capable of transferring sulfate to galactose residues linked to N-acetylglucosamine in the 6′ position. Given this unique property and its putative requirement for the synthesis of 6′-su-sLex, initial experiments were performed to determine which tissues expressed this enzyme. As shown in Figure 1, the RT-PCR analysis of normal mouse tissues readily detected Chst1 gene expression in lung, liver, and spleen, with less in heart, kidney, and skin, and none in intestine. This finding complements a previous report detecting Chst1 gene expression in brain tissue (29). Given our interest in allergic diseases, including asthma, subsequent experiments focused on murine lungs.
Figure 1.
Constitutive expression of keratin sulfate galactose 6-O-sulfotransferase (KSGal6ST) gene Chst1 was detected using RT-PCR in wild-type C57BL/6 mouse tissues. L, lung; H, heart; Li, liver; K, kidney; Sk, skin; I, intestine; Spl, spleen, with DNA ladder also shown at left. Results from one of two experiments with similar findings are shown. The amplified KSGal6ST fragment consisted of 553 base pairs, whereas the β-actin fragment consisted of 192 base pairs.
To localize the expression of KSGal6ST, a new polyclonal antibody was generated. This was ultimately compared with a commercial antibody that subsequently became available. Verification of specificity was confirmed by Western blotting, using normal whole mouse lung extracts. As shown in Figure 2A, both our new polyclonal rabbit antibody and the commercially available sheep antibody uniquely recognized an approximately 45-kD band, consistent with the predicted molecular weight of KSGal6ST. We next used our new rabbit antibody in immunohistochemistry. As seen in Figure 2B, these experiments revealed that prominent staining of the airway epithelium and some alveolar cells was completely blocked by preincubation with the KSGal6ST peptide used as the immunogen. Taken together, these data localize constitutive KSGal6ST protein expression primarily to the airway epithelium.
Figure 2.
Localization of KSGal6ST protein expression in normal mouse lung. (A) Two separate whole mouse lung extracts were run on gels in paired lanes (lanes 1–4) and probed with two different polyclonal antibodies recognizing KSGal6ST (Gel #1, probed with the rabbit antibody, lanes 1 and 2; Gel #2, probed with the sheep antibody, lanes 3 and 4). A single band occurred at approximately 45 kD. (B) Immunohistochemical localization of KSGal6ST protein in tissue sections of normal mouse lungs, using rabbit polyclonal antibody. Epithelial staining is particularly pronounced, but staining is also evident in some alveolar structural cells. Staining is blocked by preincubation with the KSGal6ST peptide used to create the polyclonal antibody. Results are shown from one of four experiments with identical findings. Original magnification, ×100. Original magnification, ×250 for insets.
Localization and Characterization of Siglec-F Ligand Expression
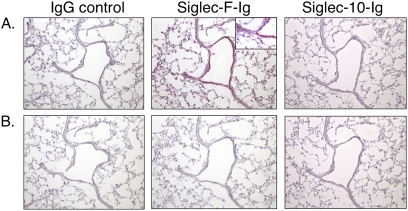
In subsequent experiments to localize lung ligands for Siglec-F, histochemical analyses were performed using various Siglec–Ig fusion proteins, as well as a human IgG isotype control. Figure 3 compares the binding characteristics in normal murine lung of Siglec-F–Ig fusion protein to that of two different controls, namely, Omalizumab (humanized anti-IgE antibody, IgG1, and IgG control) and Siglec-10-Ig, another Siglec-Ig fusion protein with different glycan-binding characteristics (Figure 3A). Distinct staining occurs with Siglec-F–Ig fusion protein in airway epithelium as well as alveolar structural cells. No staining is evident with the IgG control, nor is any staining evident using Siglec-10–Ig, a Siglec with very different glycan-binding characteristics that prefers α2,6-linked sialic acid and does not include recognition of 6′-su-sLex (3, 30) (see http://www.functionalglycomics.org/glycomics/publicdata/primaryscreen.jsp). After treatment with sialidase (Figure 3B), Siglec-F–Ig fusion protein staining was completely abolished. These data suggest that a sialic acid-containing ligand selective for Siglec-F is constitutively expressed in normal lungs by airway epithelium and other cells, and that this pattern of expression resembles that of KSGal6ST.
Figure 3.
Binding characteristics of control human IgG (Omalizumab), Siglec–F–Ig fusion protein, and Siglec-10–Ig fusion protein in normal murine lungs without (A) or with (B) sialidase treatment. Staining is predominantly epithelial, with some alveolar cell labeling, and is eliminated by treatment with sialidase. Results are shown from one of three experiments with identical findings. Original magnification, ×100. Original magnification, ×200 for inset in A for Siglec-F–Ig binding, to provide greater details of cellular staining patterns.
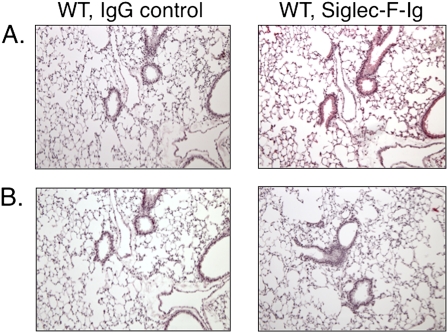
To determine if the Siglec-F ligand was a glycoprotein or glycolipid, lung sections were pretreated with either proteinase K or methanol to remove proteins or lipids, respectively, and then tested for Siglec-F-Ig fusion protein staining. As shown in Figure 4, constitutive Siglec-F ligand expression (Figure 4A) was completely eliminated by treatment with proteinase K (Figure 4B), whereas methanol treatment exerted no effect (data not shown).
Figure 4.
Binding of Siglec-F–Ig fusion protein to normal mouse lung without (A) or with (B) proteinase K treatment. Staining in both models was completely eliminated by protease treatment. Results are shown from one of three experiments with similar results. Original magnification, ×100.
Contribution of Sialyltransferases to Siglec-F Ligand Expression
To explore which ST3Gal enzymes were responsible for the constitutive expression of Siglec-F ligands, lungs were obtained from mice deficient in St3gal2 or St3gal3. Lungs from St3gal2−/− mice displayed staining patterns identical to those of wild-type mice, whereas the staining of lungs from St3gal3−/− mice was nearly devoid of Siglec-F–Ig fusion protein staining (Figure 5), demonstrating that St3gal3, but not St3gal2, is critically important for constitutive lung Siglec-F ligand expression. A contribution by St3gal4 or St3gal5 cannot be excluded.
Figure 5.
Contributions of Type 2 α2,3 sialyltransferase (St3gal2) and Type 3 α2,3 sialyltransferase (St3gal3) to the binding of Siglec-F–Ig fusion protein in murine lungs. C57BL/6 wild-type, St3gal2−/−, and St3gal3−/− murine lungs were examined as in Figure 4. (A) IgG control staining. (B) Siglec-F–Ig fusion protein staining. Staining intensity and pattern in wild-type and St3gal2−/− mice were identical, whereas St3gal3−/− murine lungs were nearly devoid of staining. Results are shown from one of three experiments with similar results. Original magnification, ×100.
Contribution of α2,3-Linked Sialic Acids to Siglec-F Ligand Binding
To explore further the sialic acid dependency of Siglec-F binding, we used two different lectins, namely, MAA, recognizing α2,3-linked sialic acid, and SNA, whose binding is preferential for α2,6-linked sialic acid. Staining patterns for MAA were found to be sialidase-sensitive (Figure 6A), and were predominantly localized to the airway epitheium, similar to the staining observed with the Siglec-F–Ig fusion protein (compare with Figures 3–5). In contrast, staining with SNA occurred predominantly along the basal layer of the epithelium, and was also sialidase-sensitive (Figure 6A). Finally, preincubation of tissue sections with MAA completely blocked the binding of the Siglec-F–Ig fusion protein, whereas preincubation with SNA did not (Figure 6B). These data indicate that the ligands recognized by the Siglec-F–Ig fusion protein contain α2,3-linked terminal sialic acids.
Figure 6.
(A) Binding characteristics of Maackia amurensis (MAA) lectin and Sambucus nigra (SNA) lectin to normal murine lungs with or without sialidase treatment, as indicated. Staining patterns for MAA, but not SNA, are similar to Siglec-F–Ig fusion protein. Both MAA and SNA binding was completely eliminated with sialidase treatment. Results are shown from one of four experiments with similar findings. (B) Effect of pretreatment with MAA lectin or SNA lectin on binding of Siglec-F–Ig fusion protein (Siglec-F-Fc). Preincubation with MAA, but not SNA, blocked binding of the Siglec-F–Ig fusion protein. Results are shown from one of two experiments with identical findings. Original magnification, ×100.
DISCUSSION
Previous studies highlighted the importance of Siglec-F in regulating eosinophil accumulation and survival (16–19). Several studies also shed light on the presence of a potential endogenous, inducible, sialylated lung ligand for Siglec-F (17, 19). Additional studies identified a candidate ligand for Siglec-F, namely, 6′-su-sLex, which is the same glycan recognized by its human paralogue, Siglec-8 (7, 20). We therefore thought it important to explore the requirements for expression of such lung ligands, and as an initial effort, it was reasonable to assume that such a ligand would contain 6′-su-sLex.
The expression of 6′-su-sLex in tissues putatively requires the presence of galactose-specific sulfotransferases in the Golgi apparatus of cells. Based on the known family of human GSTs (24), KSGal6ST (gene name, Chst1) would most likely be involved in the synthesis of 6′-su-sLex. The gene is located on chromosome 11p11.1–11.2, and initial studies reported rather broad human tissue distribution (29, 31). Using RT-PCR rather than Northern blotting, as previously used by others (29), we detected constitutive Chst1 gene expression in mouse lung, liver, and spleen tissue, with less in heart, kidney, and skin. Using immunochemical staining with Siglec-F–Ig fusion protein and a novel rabbit polyclonal antibody to KSGal6ST, we demonstrated that a Siglec-F ligand and its putatively required synthetic enzyme KSGal6ST colocalize to airway epithelium and some alveolar structural cells. To prove formally that KSGal6ST is necessary for synthesis of the Siglec-F ligand, Chst1-null mice are under construction at the National Institutes of Health–funded Knockout Mouse Project (KOMP) repository, and studies are planned to examine this hypothesis after such mice become available. Without neutralization of its activity, the role of KSGal6ST in generating Siglec-F lung ligands remains speculative. Until such data are generated, it is also possible that additional sulfated and nonsulfated ligands exist for Siglec-F.
The expression of the Siglec-F ligand was lost after treatment with sialidase or protease. Further studies characterizing constitutive levels of Siglec-F ligand, using plant lectins and strains of mice deficient in various glycosyltransferases, verified the requirement for α2,3 sialic acids and St3gal3, but not St3gal2. The present work is limited because we still do not know exactly if, when, or how these various enzymes are regulated and therefore contribute to the constitutive or regulated synthesis of the Siglec-F ligand. Nevertheless, based on these findings, we speculate that St3 gal3−/− mice, because of a loss in ability to synthesize Siglec-F ligands, should exhibit exaggerated pulmonary eosinophilic inflammation, as seen with Siglec-F−/− mice (17). Unfortunately, because these mice develop a neurodegenerative disease at an early age, our attempts to study induced lung eosinophilic inflammation (which we predict will be exaggerated) have not yet been successful.
In conclusion, α2,3-linked sialic acid–containing Siglec-F glycoprotein ligands, and enzymes required for 6′-su-sLeX synthesis (Chst1 and St3gal3 gene products), are constitutively expressed in the murine lung, especially in the epithelium. Although the exact role and importance of each of these enzymes will require further exploration, our observations lead us to predict that Siglec-F ligation on the surface of eosinophils by its natural ligand is a mechanism for the tissue-based downregulation of allergic inflammatory responses. Presumably, natural ligands for Siglec-F expressed in tissues such as airway epithelium would engage Siglec-F on recruited eosinophils, and dampen their inflammatory potential by inducing apoptosis. Knowledge of the natural cellular or tissue ligands of Siglec-F and Siglec-8, as well as the regulation of their synthesis, will afford insights into the regulation of these allergic effector cells, and may provide novel therapeutic targets.
Acknowledgments
The authors thank Dr. Sun-Young Oh, Fan Wu, and Holly Rohde for expert technical assistance. Breeding sets of St3gal2-null and St3gal3-null mice were kindly provided by Dr. Jamey Marth of the University of California at San Diego. Dr. John White at GlaxoSmithKline generously provided the Siglec-10–Ig fusion protein.
Portions of this work were previously published as abstracts: Guo JP, Myers A, Choi O, Lee H-S, Zhu Z., Hudson SA, Brummet M, Bovin NV, Crocker PV, Bochner BS. Ligands for Siglec-8 and Siglec-F: binding characteristics and tissue distribution. J Allergy Clin Immunol 2007;119(Suppl):S299; and Na HJ, Guo JP, Brummet ME,. Myers AC, Kuperman DA, Rowland E, Schnaar RL, Zheng T, Zhu Z, Bochner BS. Partial characterization of glycan ligands in mouse lung for the eosinophil-selective surface protein Siglec-F. J Allergy Clin Immunol 2010;125:AB132.
This work was supported by National Institutes of Health grants AI41472 and AI72265. B.S.B. also received support as a Cosner Scholar in Translational Research from Johns Hopkins University School of Medicine.
Originally Published in Press as DOI: 10.1165/rcmb.2010-0007OC on April 15, 2010
Author Disclosure: B.S.B. received consultancy fees from Genentech ($1,001–$5,000), Amgen ($1,001–$5,000), Pharmacyclics ($1,001–$5,000), GlaxoSmithKline ($1,001–$5,000), Sanofi-Aventis ($50,001–$100,000), Bayhill Therapeutics, Inc. (up to $1,000), Hoffmann-La Roche, Inc. ($1,001–$5,000), and Bristol-Myers Squibb ($1,001–$5,000), and served on the advisory board for Glycomimetics, Inc. ($1,001–$5,000). B.S.B. received industry-sponsored grants from Enobia ($10,001–$50,000) and Sanofi-Aventis ($10,001–$50,000). As an employee of Johns Hopkins University, B.S.B. is co-inventor on four Siglec-8–related patents. Siglec-8 is a functional orthologue of Siglec-F, which is the main focus of this manuscript. B.S.B. receives textbook royalties from Elsevier ($10,001–$50,000) and Editorial Board royalties from Up-To-Date ($10,001–$50,000). B.S.B. owns stock options in Glycomimetics, Inc. (no current value), and received sponsored grants from the Dana Foundation (more than $100,001), Cosner Scholarship (more than $100,001), and National Institutes of Health (more than $100,001). A.C.M. is employed by Johns Hopkins University (more than $100,001) as a faculty member, and has received consultancy fees from Sanofi-Aventis ($1,001–$5,000) and a sponsored grant (more than $100,001) for department-wide research. A.C.M. received a sponsored grant from the National Institutes of Health (more than $100,001) for research. T.Z. received a sponsored research grant from the National Institutes of Health (more than $100,001). Z.Z. received a sponsored research grant from the National Institutes of Health (more than $100,001). None of the other authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kikly KK, Bochner BS, Freeman S, Tan KB, Gallagher KT, D'Alessio K, Holmes SD, Abrahamson J, Hopson CB, Fischer EI, et al. Identification of SAF-2, a novel Siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol 2000;105:1093–1100. [DOI] [PubMed] [Google Scholar]
- 2.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, Steel J, Crocker PR. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem 2000;275:861–866. [DOI] [PubMed] [Google Scholar]
- 3.Varki A, Angata T. Siglecs: the major sub-family of I-type lectins. Glycobiology 2006;16:1R–27R. [DOI] [PubMed] [Google Scholar]
- 4.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol 2007;7:255–266. [DOI] [PubMed] [Google Scholar]
- 5.von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann N Y Acad Sci 2008;1143:61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy 2009;39:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-Sialyl Lewis X as a preferred glycan ligand. Glycobiology 2005;15:1125–1135. [DOI] [PubMed] [Google Scholar]
- 8.Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G). Genomics 2003;82:521–530. [DOI] [PubMed] [Google Scholar]
- 9.Angata T, Hingorani R, Varki NM, Varki A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3–related gene clusters. J Biol Chem 2001;276:45128–45136. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol 2004;34:1175–1184. [DOI] [PubMed] [Google Scholar]
- 11.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods 2007;327:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 2003;101:5014–5020. [DOI] [PubMed] [Google Scholar]
- 13.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8–induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun 2005;336:918–924. [DOI] [PubMed] [Google Scholar]
- 14.von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, Simon HU. Intravenous immunoglobulin preparations contain anti–Siglec-8 autoantibodies. J Allergy Clin Immunol 2007;119:1005–1011. [DOI] [PubMed] [Google Scholar]
- 15.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol 2008;38:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 2008;63:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 2007;109:4280–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, Broide DH. Anti–Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol 2009;131:157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti–Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol 2009;183:5333–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem 2005;280:4307–4312. [DOI] [PubMed] [Google Scholar]
- 21.Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, Paulson JC. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol 2007;27:5699–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Gunten S, Bochner BS. Expression and function of Siglec-8 in human eosinophils, basophils and mast cells. In: Allergy frontiers: classification and pathomechanisms. Pawankar R, Holgate S, Rosenwasser LJ, editors. Tokyo: Springer; 2009. pp. 297–313.
- 23.Hudson SA, Bovin N, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and pro-apoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated Sialyl Lewis X. J Pharmacol Exp Ther 2009;330:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda M, Hiraoka N, Akama TO, Fukuda MN. Carbohydrate-modifying sulfotransferases: structure, function, and pathophysiology. J Biol Chem 2001;276:47747–47750. [DOI] [PubMed] [Google Scholar]
- 25.Takashima S, Tachida Y, Nakagawa T, Hamamoto T, Tsuji S. Quantitative analysis of expression of mouse sialyltransferase genes by competitive PCR. Biochem Biophys Res Commun 1999;260:23–27. [DOI] [PubMed] [Google Scholar]
- 26.Ellies LG, Sperandio M, Underhill GH, Yousif J, Smith M, Priatel JJ, Kansas GS, Ley K, Marth JD. Sialyltransferase specificity in selectin ligand formation. Blood 2002;100:3618–3625. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004;304:1678–1682. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz A, Futerman AH. Determination of the localization of gangliosides using anti-ganglioside antibodies: comparison of fixation methods. J Histochem Cytochem 1997;45:611–618. [DOI] [PubMed] [Google Scholar]
- 29.Fukuta M, Inazawa J, Torii T, Tsuzuki K, Shimada E, Habuchi O. Molecular cloning and characterization of human keratan sulfate Gal-6–sulfotransferase. J Biol Chem 1997;272:32321–32328. [DOI] [PubMed] [Google Scholar]
- 30.Munday J, Kerr S, Ni J, Cornish AL, Zhang JQ, Nicoll G, Floyd H, Mattei MG, Moore P, Liu D, et al. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid–binding receptor. Biochem J 2001;355:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiro RG, Yasumoto Y, Bhoyroo V. Characterization of a rat liver Golgi sulphotransferase responsible for the 6-O-sulphation of N-acetylglucosamine residues in beta-linkage to mannose: role in assembly of Sialyl–galactosyl–N-acetylglucosamine 6-sulphate sequence of N-linked oligosaccharides. Biochem J 1996;319:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]