Abstract
Muc1 is a heterodimeric mucin that is expressed on the apical surface of airway epithelial cells as well as hematopoietic cells. Both in vivo and in vitro studies revealed that Muc1 suppresses inflammatory responses induced by Pseudomonas aeruginosa (PA). In this study, we sought to determine, using intact animals (C57BL/6 mice), whether the expression of Muc1 is important during airway PA infection, and how Muc1 levels are controlled during inflammation. Our results showed that: (1) Muc1 levels in the wild-type (WT) mice were initially low, but gradually increased after PA inhalation, reaching a peak on Day 2, remaining elevated until Day 4, and then gradually decreasing to basal levels on Day 7; (2) TNF receptor 1−/− mice failed to increase Muc1 levels after PA infection; (3) after PA inhalation, more inflammatory cells were present in the bronchoalveolar lavage fluid from either Muc1−/− or TNF receptor−/− mice compared with their WT control animals; (4) more apoptotic neutrophils were present in bronchoalveolar lavage fluid from WT mice compared with Muc1−/− mice. We conclude that Muc1−/− mice are more inflammatory than WT mice during airway PA infection as a result of both an increase in neutrophil influx and a decrease in neutrophil apoptosis. These results suggest that the up-regulation of Muc1 during airway PA infection might be crucial for suppressing excessive and prolonged inflammatory responses, and is induced mainly by TNF-α, the key proinflammatory mediator.
Keywords: mucin, resolution, TNF receptor, chronic obstructive pulmonary disease
CLINICAL RELEVANCE.
Failure to control airway inflammation can lead to the development of chronic inflammatory lung diseases. This article describes that TNF-α is the key regulator of MUC1, a recently discovered anti-inflammatory molecule in the lung, and suggests a critical role of MUC1 during the resolution stage of airway inflammation, and the possibility of its clinical application in the treatment of hyperinflammatory lung diseases such as chronic obstructive pulmonary disease.
Pseudomonas aeruginosa (PA) is an opportunistic gram-negative bacterium that is associated with serious respiratory diseases, such as pneumonia and cystic fibrosis (1). Normally, inhaled PA is rapidly removed by mucociliary clearance as well as inflammatory cells, such as neutrophils and macrophages. The mechanism of innate immunity during airway PA infection is well established and involves the interactions between pathogen-associated molecular patterns and their corresponding pattern recognition receptors (2).
MUC1 (MUC in human and Muc in nonhuman species) is a transmembrane glycoprotein expressed in mucosal epithelial cells as well as hematopoietic cells (3), and has been postulated to be involved in the regulation of cell growth (4), differentiation (5), apoptosis (6), and inflammation (7). Particularly, its aberrant overexpression in various carcinomas has been implicated in the pathogenesis of cancer (5).
Recently, we have shown that Muc1 is an adhesion site of PA (8, 9), and the binding of PA or its flagellin to Muc1 results in phosphorylation of the cytoplasmic tail of Muc1, followed by activation of mitogen-activated protein kinase (10), suggesting the possible role of MUC1/Muc1 as a receptor for PA. Overexpression of MUC1 in cultured epithelial cells suppressed flagellin-induced increases in the production of TNF-α, as well as IL-8, suggesting the anti-inflammatory role of MUC1 during bacterial infection (7). Our subsequent studies using Muc1−/− mice revealed that these mice are hyperinflammatory during airway PA infection, at least during the initial stage of infection (e.g., 4 h)—in other words, higher levels of inflammatory mediators as well as neutrophils in bronchoalveolar lavage fluid (BALF) and significantly reduced levels of live PA in the lung (7). Given the importance of timely inflammatory responses during bacterial infection, these results raised an important question as to the levels of MUC1/Muc1 during airway PA infection in the context of airway inflammation. Subsequent studies with cultured epithelial cells revealed that MUC1 is up-regulated by inflammatory mediators, such as neutrophil elastase (NE) (11) and TNF-α (12), which has led to an interesting hypothesis that Muc1 levels increase during airway inflammation by inflammatory mediators to control excessive inflammation.
In the present study, we tested this hypothesis using intact animals, and also determined the degree of contribution by TNF-α in the regulation of Muc1 during airway PA infection.
MATERIALS AND METHODS
Materials
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated.
Animals
C57BL/6 Muc1−/− (Muc1 knockout [KO]), C57 BL/6 TNF receptor (TNFR) 1−/− (p55 KO, TNFR1 KO), and wild-type (WT) C57BL/6 mice of 10–12 weeks of age (27–28 g of body weight) were used for this study. TNF-α exhibits its effect through two cell surface receptors, TNFR1 (p55) and TNFR2 (p75). Proinflammatory effect of TNF-α is mediated mainly through TNFR1 (12). Therefore, TNFR1 KO mice were used for the present experiment. Muc1 KO mice (13) were originally developed by Dr. Sandra Gendler (Mayo Clinic, Scottsdale, AZ) and backcrossed every five generations in our animal facility, and both WT and TNFR1 KO mice were purchased from Jackson Laboratories (Bar Harbor, ME). Offspring from Muc1 KO mice were genotyped by PCR analysis of tail DNA using two sets of oligonucleotides specific for the Muc1 gene (13) (forward, 5′-ACCTCACACACGGAGCGCCAG-3′ [corresponding to base pair (bp) 7–27, sense strand], and reverse, 5′-TCCCCCCTGGCACATACTGGG-3′ [corresponding to bp 268–248, antisense strand]) or the LacZ gene (5′-TTCTGGTGCCGGAAACCAGGC-3′ [corresponding to bp 201–181, antisense strand]). Mice were housed within an air-filtered, temperature-controlled (24°C), and pathogen-free barrier, with free access to food and water. All animal experiments were conducted in accordance with the guidelines provided by the Institutional Animal Care and Use Committees of the Lovelace Respiratory Research Institute and the Temple University School of Medicine.
Lung Infection with PA and Surgery
PA K strain (PAK) was cultured in Luria broth at 37°C for 16 hours, and an aliquot of the bacterial culture was cultured for another 2 hours to produce bacteria at the log phase. The PAK culture was centrifuged for 10 minutes at 600 × g and resuspended in sterile PBS to make 1 × 107 CFU/40 μl. Mice were anesthetized for 1 minute by inhalation of Isoflurane (Vedco Inc., St. Joseph, MO), and instilled with 1 × 107 CFU/40 μl intranasally. Immediately after CO2 asphyxia at the indicated times after infection, the left lung was tied with surgical suture, and BALF was collected from the right lung using 3 × 0.6 ml of saline containing 0.6 mM EDTA, and assayed for cytokines and differential cells as described subsequently here. The left lung lobe was removed for the measurement of the numbers of PAK, as well as Muc1 levels. The remaining lung lobes were perfused first with 5 ml PBS, and then with 10% paraformaldehyde via the right ventricle of the heart. The right caudal was then removed from the chest cavity and fixed overnight in 10% paraformaldehyde under vacuum. The fixed lobe was embedded in paraffin and sectioned at 3 μm. The tissue sections were stained with hematoxylin and eosin (H&E).
CFU Assay and Differential Cell Counting
The left lung lobe, excised as described previously here, was homogenized with 0.4 ml PBS. Aliquots (100 μl) of the serially diluted homogenates were plated onto LB agar, incubated at 37°C overnight, and CFU of PAK was counted and calculated for the whole lung. The remaining homogenate was further centrifuged at 600 × g for 5 minutes at 4°C, the supernatant removed, and the pellet resuspended in a lysis buffer (40 mM PBS [pH] 7.2, 1% Triton X-100, 1% deoxycholate, 50 mM NaF, 150 mM NaCl, and 1 mM EDTA) with protease inhibitors on ice. The lysate was stored at −80°C for Muc1 assay.
Total cell numbers in BALF were measured using a hemocytometer. The BALF was centrifuged at 600 × g for 5 minutes, and the resulting supernatant was stored at −80°C for cytokine analysis. The cell pellets were resuspended in 500 μl of saline containing 0.6 mM EDTA, and 200-μl aliquots were cytospun for differential cell counting. Cells on the slides were stained using Diff-Quik Stain Set (Dade Behring, Newark, DE) and subjected to differential cell counting. Apototic cells on the slides were identified and quantified based on apoptotic morphological changes (H&E stain) (14) and confirmed by labeling DNA strand breaks with terminal deoxynucleotidyl transferase–mediated fluorescein-dUTP nick end labeling using an in situ cell death detection kit (Roche, Indianapolis, IN) according to the manufacturer's protocol.
Cytokine ELISA
Anti–TNF-α antibody (eBioscience, San Diego, CA) was diluted with Tris-buffered saline (pH 7.0) containing 0.3% Triton X-100, 0.2% Tween-80, and 1% BSA (buffer A), whereas all the other antibodies were diluted with PBS (pH 7.0) containing 0.3% Triton X-100, 0.2% Tween-80, and 1% BSA (buffer B). Anti-mouse TNF-α capture antibody (100 μl) at a dilution of 1 μg/ml or anti-mouse keratinocyte-derived chemokine (KC) capture antibody (R&D Systems, Minneapolis, MN) diluted at 1:500 was added to the wells of a 96-well Maxisorp plate (Nunc, Rochester, NY) and incubated overnight at 4°C. The plate was washed once with PBS containing 0.05% Tween-20, followed by the addition of 300 μl of blocking buffers (blocking buffer A: PBS [pH 7.0], 10 mg/ml BSA, 50 mg/ml sucrose, and 0.02 NaN3 for KC; blocking buffer B: TBS [pH 7.0], 10 mg/ml BSA, 50 mg/ml sucrose, and 0.02 NaN3 for TNF-α) and incubated for 2 hours at room temperature. After washing the plate, 100 μl of BALF was added to each well in triplicates and incubated for 2 hours at room temperature. After washing the plate three times, 100 μl of biotin-conjugated anti-mouse KC (1:500 dilution; Cell Sciences, Canton, MA) or biotin-conjugated anti-mouse TNF-α antibody was added to each well and incubated at room temperature for 2 hours. The plate was washed six times before adding 100 μl of tetramethylbenzidine substrate (SureBlue; KPL, Gaithersburg, MD) to each well and incubating at room temperature for 15 minutes. Colorization reaction was stopped by adding 100 μl 1 N HCl, and the intensity of the color was measured at 450 nm using a SpectraMax Plus 384 spectrophotometer (Molecular Devices, Sunnyvale, CA).
Muc1 ELISA
The lung tissue lysates described previously here were diluted 1:1 with 0.05 mM bicarbonate buffer (pH 9.5), and 50 μl aliquots of the lysate were incubated in 96-well Maxisorp plates overnight at 4°C. Plates were washed once with PBS containing 0.05% Tween-20 before adding 300 μl of blocking buffer A and incubating for 2 hours at room temperature. At the end of the incubation, the plate was washed once before adding a 50 μl aliquot of anti-Muc1 antibody CT33 (9) or F-19 (Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted at 1:5,000 in buffer A, and incubating 2 hours at room temperature. CT33 and F-19 recognize the cytoplasmic and extracellular N-terminal domains, respectively. At the end of the incubation, the plate was washed five times and an aliquot of 100 μl of horseradish peroxidase–conjugated anti-rabbit IgG antibody (for CT33) or anti-goat IgG antibody (for F-19) (Jackson Immunoresearch, West Grove, PA) that had been diluted at 1:10,000 was added to each well and incubated for 2 hours at room temperature. 3,3′,5,5′-Tetramethylbenzidine (TMB) was added for colorization, and the intensity of the color was measured as described previously here.
Statistical Analysis
Differences between groups were assessed using Student's t test for unpaired samples and a P value less than 0.05 was considered significant.
RESULTS
PA Clearance among WT, Muc1 KO, and TNFR1 KO Mice
Mice were given PA (107 CFU) intranasally, and the numbers of live PA present in the lung were measured on a time course. As can be seen in Table 1, the numbers of live PA present in the lung decreased rapidly in all groups, reaching less than 1% of the original inoculum at 24 hours, and becoming undetectable at 7 days. The numbers of PA present in the lung tissues of WT mice were significantly greater than those of either Muc1 KO or TNFR1 KO mice at both 8 and 24 hours. However, the differences between Muc1 KO and TNFR1 KO mice were not significant for either 8 or 24 hours. At Day 2 and thereafter, the numbers of PA present in the lung were not significantly different among these three groups. In summary, both Muc1 KO and TNFR1 KO mice cleared PA significantly better than did WT mice during the first 24 hours after instillation.
TABLE 1.
NUMBERS OF LIVE PSEUDOMONAS AERUGINOSA REMAINING IN THE WHOLE LUNG AT THE INDICATED TIMES AFTER INTRANASAL BACTERIAL INFECTION
| Genotype Time | WT (CFU/Lung) | Muc1 KO (CFU/Lung) | TNFR1 KO (CFU/Lung) |
|---|---|---|---|
| 8 h | 615,000 ± 98,000 | 325,000 ± 37,000* | 340,000 ± 69,000* |
| 1 d | 26,000 ± 2,700 | 1,700 ± 700* | 10,000 ± 2,700* |
| 2 d | 4,300 ± 1,500 | N.D. | 1,950 ± 900 |
| 7 d | 0 | 0 | 0 |
Definition of abbreviations: KO, knockout; N.D., not determined; TNFR, TNF receptor; WT, wild type.
Data represent means (±SEM) of four lung samples obtained from four different animals.
P < 0.05 compared with WT.
Differential Cell Counting
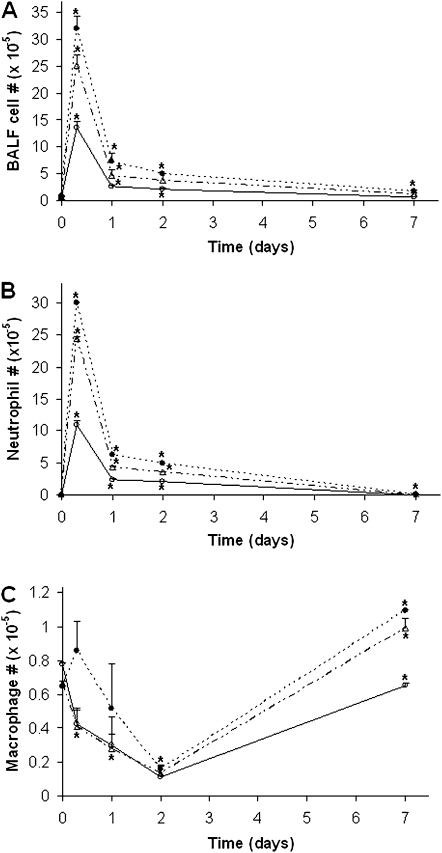
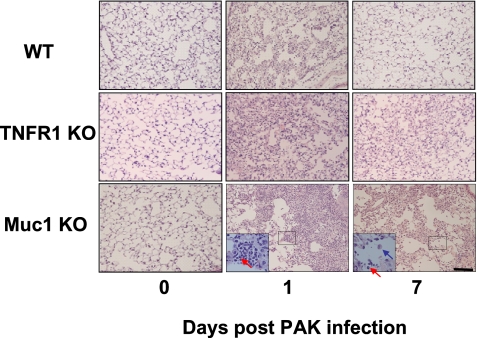
Inhaled PA is normally cleared from the lung by both mucociliary clearance and inflammatory cells. To compare the numbers of inflammatory cells recruited into the lung, mice were subjected to BAL at different time points after instillation with PA, and leukocytes in BALF were counted differentially. In this study, we focused on neutrophils and macrophages as other leukocytes were proven to be less important during PA infection (7). As can be seen in Figure 2A, the total numbers of cells in BALF were in the order of Muc1 KO, TNFR KO, and WT, with the numbers in both Muc1 KO and TNFR KO being significantly greater than those in WT. Figure 2B shows that the numbers (mean ± SEM; n = 4) of neutrophils in BALF at 8 hours after instillation were significantly different among the three groups—1,186,600 (±113,900) for WT, 2,437,933 (±31,416) for TNFR KO, and 2,997,333 (±35,555) for Muc1 KO. Although the numbers of neutrophils decreased drastically after 24 hours, both Muc1 KO and TNFR KO groups still had significantly greater numbers (mean ± SEM; n = 4) than WT: 239,708 (±18,638) for WT; 434,750 (±15,275) for TNFR KO; and 636,778 (±21,185) for Muc1 KO. Interestingly, the same pattern of difference was observed even at Day 7 (2,084 ± 634 for WT, 6,932 ± 2,698 for TNFR KO, and 17,961 ± 1,570 for Muc1 KO); that is, 3.3 times and 8.6 times greater numbers of neutrophils in TNFR KO and Muc1 KO, respectively, compared with WT. Thus, the numbers of neutrophils present in BALF were in the order of Muc1 KO > TNFR1 KO > WT. Therefore, it seems likely that the increased number of neutrophils in the lung was attributed to a greater PA clearance in both Muc1 KO and TNFR1 KO mice compared with WT mice, as shown in Table 1. The numbers of macrophages in BALF showed a similar pattern among these three groups (Figure 2C). The major type of leukocytes on Day 7 was macrophages, and their numbers were significantly greater in both TNFR1 KO and Muc1 KO compared with WT mice (mean ± SEM, n = 4: 65,523 ± 376 for WT, 98,975 ± 6,121 for TNFR KO, and 109,225 ± 992 for Muc1 KO). Indeed, our histological examination of the lung tissue revealed the presence of both mononuclear cells and neutrophils in Muc1 KO and TNFR1 KO mice, whereas these cells were hard to find in WT lung tissues at Day 7 after infection (Figure 1). Intranasal instillation of PBS showed no sign of inflammation, judging from the number of neutrophils in BALF, as we previously reported (7) (data not shown).
Figure 2.
Numbers of inflammatory cells in bronchoalveolar lavage fluid (BALF) after infection with PA. Different strains of mice were infected intranasally with 107 CFU of PA K strain (PAK). At the indicated times, animals were subjected to BAL, as described in Materials and Methods. Total cell number in BAL was counted under a microscope (A). Cells were stained using Diff-Quick dye and both neutrophils (B) and macrophages (C) were enumerated in WT, Muc1 KO, and TNFR1 KO mice. Open circles, WT; closed circles, Muc1 KO; open triangles, TNFR1 KO. Each data point represents a mean (±SEM) of four samples obtained from four different mice. The results are representative of two separate experiments. *P < 0.05.
Figure 1.
Lung histology of mice infected with Pseudomonas aeruginosa (PA). Wild-type (WT), TNF receptor (TNFR) 1 knockout (KO), and Muc1 KO mice were infected with PA intranasally and euthanized at different days after infection. The right caudal of the lung was excised, fixed, embedded in paraffin, and sectioned at 3 μm, as described in Materials and Methods. The tissue sections were stained with hematoxylin and eosin. The red and blue arrows in the insets point to a neutrophil and a macrophage, respectively. The bar represents 100 μm.
Neutrophil Apoptosis
Next we tried to understand the mechanism of the increased neutrophil numbers in BALF of Muc1 KO mice compared with those of WT. Two possible mechanisms were considered. First, the absence of the anti-inflammatory effect of Muc1 in Muc1 KO mice likely recruits more neutrophils compared with WT mice, as demonstrated in our earlier study (7). Alternatively, it also might be possible that neutrophils in BALF may live longer in Muc1 KO mice. To test the possibility, we compared the numbers of apoptotic neutrophils after PA infection using the criteria of both morphological changes (H&E staining) and labeled DNA strand breaks (terminal deoxynucleotidyl transferase–mediated fluorescein-dUTP nick end labeling assay). As shown in Table 2, although the total number of neutrophils on Day 2 after PA infection was signicantly greater in Muc1 KO (i.e., 252% of WT), the total number of apoptotic neutrophils was significantly smaller in Muc1 KO, constituting only 1.3% of the total number of neutrophils versus 9.3% in WT. Thus, these results seem to suggest that Muc1 promotes neutrophil apoptosis, which should contribute, at least in part, to the lower numbers of neutrophils in WT during PA infection. Together, the greater numbers of neutrophils in BALF of both Muc1 KO and TNFR KO mice appear to be a result of a combination of an increase in recruitment and a decrease in apoptosis of neutrophils.
TABLE 2.
NUMBERS OF APOPTOTIC CELLS IN BRONCHOALVEOLAR LAVAGE FLUID AFTER PSEUDOMONAS AERUGINOSA INFECTION
| Cell No./2 ml BALF/Lung | ||
|---|---|---|
| Days after PA Infection/Type of Leukocytes | WT | Muc1 KO |
| Day 0 | ||
| Neutrophil | ||
| Total | 1,750 ± 202 | 1,813 ± 464 |
| Apoptotic | Negligible | Negligible |
| Macrophage | ||
| Total | 68,250 ± 202 | 62,186 ± 464 |
| Apoptotic | Negligible | Negligible |
| Day 2 | ||
| Neutrophil | ||
| Total | 95,408 ± 943 | 240,130 ± 1,142* |
| Apoptotic | 8,917 ± 356 | 3,023 ± 431* |
| Macrophage | ||
| Total | 5,171 ± 356 | 15,979 ± 1,142* |
| Apoptotic | Negligible | Negligible |
| Day 7 | ||
| Neutrophil | ||
| Total | 1,246 ± 113 | 12,250 ± 471* |
| Apoptotic | Negligible | Negligible |
| Macrophage | ||
| Total | 61,540 ± 588 | 134,205 ± 2,880* |
| Apoptotic | Negligible | Negligible |
Definition of abbreviations: BALF, bronchoalveolar lavage fluid; KO, knockout; PA, Pseudomonas aeruginosa; WT, wild type.
Data represent means (±SEM) of four different animals.
P < 0.05 compared with WT.
Inflammatory Mediators in BALF
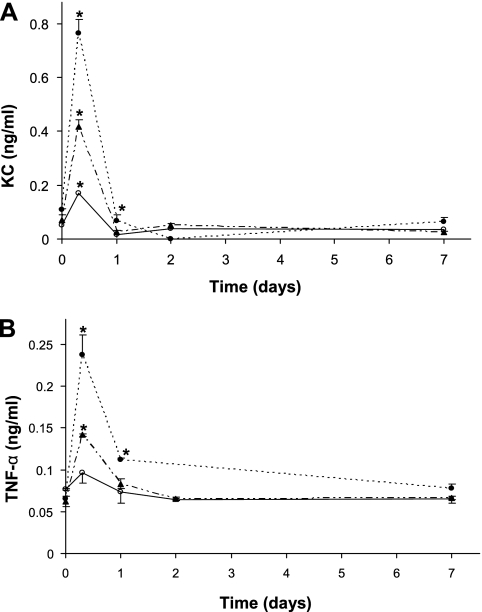
Because the numbers of inflammatory cells in BALF are determined mainly by the levels of inflammatory mediators produced in the lung, we measured the levels of two major inflammatory mediators in BALF: KC (mouse ortholog of human IL-8) and TNF-α. As seen in Figures 3A and 3B, both of these inflammatory mediators increased immediately after PA inoculation. At 8 hours, the levels (mean ± SEM; n = 4) of KC in WT, Muc1 KO, and TNFR1 KO were 0.172 (±0.005), 0.764 (±0.053), and 0.417 (±0.027) ng/ml, whereas the levels of TNF-α (mean ± SEM; n = 4) were 0.097 (±0.012), 0.237 (±0.024), and 0.142 (±0.002) ng/ml, respectively. Thus, the levels of these inflammatory mediators were significantly greater in both KO mice compared with the WT mice. The high levels of these chemoattractants seem to be responsible for the greater leukocyte recruitment into the lung of these KO mice, as shown in Figure 2. At 24 hours, the levels of KC were already near basal levels, whereas the levels of TNF-α were still higher than basal levels, although drastically lower than those at 8 hours (Figures 3A and 3B).
Figure 3.
Levels of inflammatory mediators in BALF after infection with PA. All the procedures were exactly the same as Figure 2, except for measuring keratinocyte-derived chemokine (KC) (A) and TNF-α (B) using ELISA, as described in Materials and Methods: Open circles, WT; closed circles, Muc1 KO; closed triangles, TNFR1 KO mice. Each data point represents a mean (±SEM) of four samples obtained from four different mice. The results are representative of two separate experiments. *P < 0.05.
Up-Regulation of Muc1 by PA
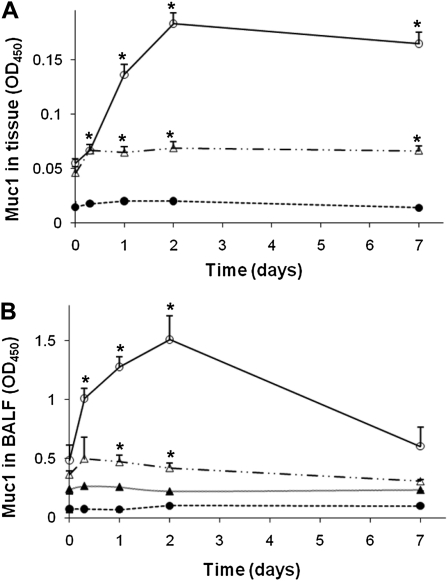
As predicted from our previous in vitro study (12), PA instillation resulted in a drastic increase in the TNF-α level in BALF (Figure 3B), followed by a steady increase in the whole lung Muc1 level in WT mice, reaching a maximum on Day 2 (Figure 4). The Muc1 levels 2 days after instillation were approximately 3.3-fold over basal levels. In contrast, the increase in Muc1 levels in TNFR1 KO mice on Day 2 after PA instillation was fairly small compared with that in WT mice (<20%), suggesting the critical role of TNFR1 in the regulation of Muc1 levels during PA infection. On the other hand, a small but significant increase in Muc1 levels in TNFR1 KO mice after PA inhalation seems to suggest the presence of an additional mechanism for Muc1 up-regulation that is independent of TNFR1. Figure 4B shows the presence of Muc1 in BALF based on its reactivity with F-19 antibody recognizing the extracellular domain. However, there was no reactivity with CT33 antibody recognizing the CT domain, indicating that these Muc1 molcules in BALF lack the CT domain, most likely as a result of shedding or cleavage. Shedding of Muc1 has been well documented in various cancer cells (15–18). As seen in Figure 4B, Muc1 levels in BALF samples from WT mice reached a maximum at 48 hours, whereas its contents in BALF samples from TNFR KO mice were barely detectable, suggesting an active production of Muc1 in WT mice, but not in TNFR KO mice after PA infection.
Figure 4.
Muc1 levels of the lung after PA infection. Different strains of mice were infected intranasally with 107 CFU of PAK. At the indicated times, animals were killed and the amounts of Muc1 in the whole lung (A) and the BALF samples (B) were measured by ELISA using anti-MUC1 antibody. CT33 antibody recognizing the cytoplasmic domain of Muc1 was used in (A), whereas F-19 recognizing the extracellular domain of Muc1 was used in (B), as described in Materials and Methods. Open circles, WT; closed circles, Muc1 KO; open triangles, TNFR1 KO; closed triangles, WT by CT33. Notice that Muc1 present in BALF reacts with F-19, but not CT33, indicating that it lacks the CT domain. Each data point represents a mean (± SEM of four samples obtained from four different mice. The results are representative of two separate experiments. *P < 0.05.
DISCUSSION
The present study demonstrates that the Muc1 level in the uninflamed lung is relatively low, but increases drastically after PA infection due to an increase in TNF-α, suggesting the critical role of the TNF-α/TNFR interaction in the regulation of Muc1 during airway infection. This study also suggests that the up-regulation of Muc1 during airway inflammation induced by bacterial infection is important to keep the lung free from the inflammatory cells at the end of inflammation.
The present results showing the anti-inflammatory role of Muc1 during the first 24 hours after infection with PA are consistent with those of our recent report obtained at 4 hours after infection (7). It is interesting, however, to see that almost all the PA in the lung was cleared within the first 2 days after PA infection in both WT and Muc1 KO mice. Muc1 KO mice cleared PA much better than did WT mice (Table 1), and retained a significant number of inflammatory cells even at the later stage of inflammation (i.e., 7 days after infection) (Table 2 and Figure 1). This might be a result of a spontaneous and “proportional” decrease of the significantly greater numbers of neutrophils at the early stage of infection (e.g., 8 h). However, given the “rapid” clearance of PA (2 d after inoculation) and a relatively short life of neutrophils in the lung (<2 d), the increased number of neutrophils at 7 days after PA infection seems to require additional explanations. The results from the present study suggest that the failure to control the number of inflammatory cells in the lung of Muc1 KO mice after PA infection could be due to the continuous influx of the inflammatory cells as a result of their inability to suppress Toll-like receptor (TLR) signaling during the course of inflammation (7), as well as a significant decrease in neutrophil apoptosis. We have previously shown, using various cell cultures, that MUC1/Muc1 can suppress TLR signaling (19, 20), and an increase in neutrophil apoptosis has been suggested to be responsible, at least in part, for the anti-inflammatory effect of IL-10 (21). How MUC1/Muc1 promotes neutrophil apoptosis remains to be answered. Given that the failure to control inflammation may lead to the development of chronic lung diseases (22), it is possible that MUC1 plays an important role in the resolution of airway inflammation, thus preventing the development of chronic lung diseases, such as chronic obstructive pulmonary disease.
Skerrett and colleagues (23) reported that TNFR1 KO mice inhaled with PA also show enhanced inflammatory responses, judging from increased PA clearance, and increased levels of inflammatory mediators as well as inflammatory cells in the lung. These results are virtually identical to those from the current study. We have recently shown in A549 cells that NE up-regulated MUC1 through PKCδ → dual oxidase I → reactive oxygen species → TNF-α–converting enzyme → TNF-α→ TNFR1 → Sp1 (11, 24). In a separate publication (12), we elucidated the molecular mechanism for TNF-α–induced MUC1 up-regulation. Our present results (Figure 4) show that Muc1 levels increase drastically after PA infection in WT mice, whereas TNFR1 KO mice failed to show such a drastic increase, despite high levels of TNF-α in BALF (Figure 3B). A small but significant increase in Muc1 levels in TNFR1 KO mice after exposure to PA inoculum (Figure 4) suggests the presence of a TNFR-independent mechanism. Proinflammatory mediators, such as IFN-γ (25), IL-1β, and IL-6 (26, 27), have been shown to have ability to up-regulate MUC1 in some cancer cells, and therefore, might be responsible for the TNFR-independent mechanism. Thus, the present in vivo study not only supports the results of our previous in vitro study that TNF-α up-regulates Muc1, but also suggests that the TNF-α/TNFR1 interaction is critical for Muc1 up-regulation during PA infection. Taken together, these results suggest that the enhanced inflammatory response in TNFR1 KO mice after PA infection (23) is likely due to the inability to up-regulate Muc1 in these animals. This may also explain why the inflammatory responses in TNFR1 KO mice were close to, but less than, those of Muc1 KO mice. The failure to stimulate the secretion of IL-10 and/or to promote neutrophil apoptosis by TNFR1 activation (23) may also contribute additionally to the increased inflammation in TNFR KO mice.
In review of the published data, both in vitro and in vivo, the mechanism of anti-inflammatory action of MUC1/Muc1 during airway PA infection may be summarized as follows: (1) inhaled PA activates various TLRs present on both airway epithelial cells and macrophages, resulting in the production of inflammatory mediators, such as IL-8 (or KC) and TNF-α; (2) the released inflammatory mediators recruit neutrophils into the airway, initiating inflammation, resulting in the clearance of PA; (3) the inflammatory products, such as NE and TNF-α, up-regulate Muc1, mainly in airway epithelial cells; (4) the increased Muc1 suppresses TLR signaling and also promotes neutrophil apoptosis, thus gradually ending the inflammatory cycle.
Cystic fibrosis is considered a hyperinflammatory lung disease (28) in which chronic airway PA infection is almost invariably present. Given the anti-inflammatory role of MUC1/Muc1 and its up-regulation during airway inflammation, one might speculate that the hyperinflammatory state in cystic fibrosis might be associated with the functional deficiency of MUC1/Muc1 due to either its low level or a functional defect of MUC1/Muc1, the latter possibly due to mutations on the cytoplasmic tail, which has been shown to be responsible for its anti-inflammatory effect (20). These possibilities are currently under investigation in our laboratory.
One of the interesting questions that arise from the current study is why there are multiple anti-inflammatory molecules, and how they intereact with each other during PA infection. For example, it has been shown that MUC1 induces IL-10, an anti-inflammatory cytokine, in dendritic cells (29), suggesting the possible collaboration between the two during inflammation. The same question may be applied to other anti-inflammatory molecules in the lung that have been reported recently, including CD44 (30), aryl hydrocarbon receptor (31), and various lipid mediators (32, 33). Further studies in our laboratory are directed at elucidating the functional relationships between the known anti-inflammatory molecules during the resolution of airway inflammation.
Acknowledgments
The authors thank Dr. Thomas March (Experimental Toxicology Program, Lovelace Respiratory Research Institute, Albuquerque, NM) for his generous consultation on lung histology and Dr. Erik Lillehoj (University of Maryland School of Medicine, Baltimore, MD) for providing excellent editing and critical comments on this manuscript.
This work was supported by National Institutes of Health grants RO1 HL-47125 and HL-80825 (K.C.K.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0323OC on May 6, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bodey GP, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis 1983;5:279–313. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun 2005;73:7151–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 2001;6:339–353. [DOI] [PubMed] [Google Scholar]
- 4.Hirasawa Y, Kohno N, Yokoyama A, Inoue Y, Abe M, Hiwada K. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am J Respir Cell Mol Biol 1997;17:501–507. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder JA, Masri AA, Adriance MC, Tessier JC, Kotlarczyk KL, Thompson MC, Gendler SJ. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene 2004;23:5739–5747. [DOI] [PubMed] [Google Scholar]
- 6.Yin L, Li Y, Ren J, Kuwahara H, Kufe D. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem 2003;278:35458–35464. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 2006;176:3890–3894. [DOI] [PubMed] [Google Scholar]
- 8.Lillehoj EP, Hyun SW, Kim BT, Zhang XG, Lee DI, Rowland S, Kim KC. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2001;280:L181–L187. [DOI] [PubMed] [Google Scholar]
- 9.Kato K, Lillehoj EP, Kai H, Kim KC. MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front Biosci 2010;2:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lillehoj EP, Kim H, Chun EY, Kim KC. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am J Physiol Lung Cell Mol Physiol 2004;287:L809–L815. [DOI] [PubMed] [Google Scholar]
- 11.Kuwahara I, Lillehoj EP, Hisatsune A, Lu W, Isohama Y, Miyata T, Kim KC. Neutrophil elastase stimulates MUC1 gene expression through increased Sp1 binding to the MUC1 promoter. Am J Physiol Lung Cell Mol Physiol 2005;289:L355–L362. [DOI] [PubMed] [Google Scholar]
- 12.Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, Kim KC. TNF-α induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol 2007;293:L693–L701. [DOI] [PubMed] [Google Scholar]
- 13.Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem 1995;270:30093–30101. [DOI] [PubMed] [Google Scholar]
- 14.Matsuu M, Shichijo K, Okaichi K, Wen CY, Fukuda E, Nakashima M, Nakayama T, Shirahata S, Tokumaru S, Sekine I. The protective effect of fermented milk kefir on radiation-induced apoptosis in colonic crypt cells of rats. J Radiat Res (Tokyo) 2003;44:111–115. [DOI] [PubMed] [Google Scholar]
- 15.Boshell M, Lalani EN, Pemberton L, Burchell J, Gendler S, Taylor-Papadimitriou J. The product of the human MUC1 gene when secreted by mouse cells transfected with the full-length cDNA lacks the cytoplasmic tail. Biochem Biophys Res Commun 1992;185:1–8. [DOI] [PubMed] [Google Scholar]
- 16.Parry S, Silverman HS, McDermott K, Willis A, Hollingsworth MA, Harris A. Identification of MUC1 proteolytic cleavage sites in vivo. Biochem Biophys Res Commun 2001;283:715–720. [DOI] [PubMed] [Google Scholar]
- 17.Lillehoj EP, Han F, Kim KC. Mutagenesis of a Gly-Ser cleavage site in MUC1 inhibits ectodomain shedding. Biochem Biophys Res Commun 2003;307:743–749. [DOI] [PubMed] [Google Scholar]
- 18.Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor–alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem 2003;278:3386–3394. [DOI] [PubMed] [Google Scholar]
- 19.Kato K, Lu W, Kai H, Kim KC. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of Toll-like receptor 5 signaling. Am J Physiol Lung Cell Mol Physiol 2007;293:L686–L692. [DOI] [PubMed] [Google Scholar]
- 20.Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC. MUC1 mucin is a negative regulator of Toll-like receptor signaling. Am J Respir Cell Mol Biol 2008;38:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox G. IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am J Physiol Lung Cell Mol Physiol 1996;271:L566–L571. [DOI] [PubMed] [Google Scholar]
- 22.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 2009;135:173–180. [DOI] [PubMed] [Google Scholar]
- 23.Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 1999;276:L715–L727. [DOI] [PubMed] [Google Scholar]
- 24.Kuwahara I, Lillehoj EP, Koga T, Isohama Y, Miyata T, Kim KC. The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription. Am J Respir Cell Mol Biol 2007;37:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagow EL, Carson DD. Synergistic stimulation of MUC1 expression in normal breast epithelia and breast cancer cells by interferon-gamma and tumor necrosis factor-alpha. J Cell Biochem 2002;86:759–772. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Wang L, Nunes DP, Troxler RF, Offner GD. Pro-inflammatory cytokines up-regulate MUC1 gene expression in oral epithelial cells. J Dent Res 2003;82:883–887. [DOI] [PubMed] [Google Scholar]
- 27.Li YY, Hsieh LL, Tang RP, Liao SK, Yeh KY. Macrophage-derived interleukin-6 up-regulates MUC1, but down-regulates MUC2 expression in the human colon cancer HT-29 cell line. Cell Immunol 2009;256:19–26. [DOI] [PubMed] [Google Scholar]
- 28.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol 2006;291:C218–C230. [DOI] [PubMed] [Google Scholar]
- 29.Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, Pontillo M, Mercalli A, Di Carlo V, Allavena P, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol 2004;172:7341–7349. [DOI] [PubMed] [Google Scholar]
- 30.Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, Bucala R, Noble PW. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol 2007;178:2469–2475. [DOI] [PubMed] [Google Scholar]
- 31.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol 2007;170:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnans C, Levy BD. Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am J Respir Cell Mol Biol 2007;36:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W, Hashimoto K, Goleniewska K, O'Neal JF, Ji S, Blackwell TS, Fitzgerald GA, Egan KM, Geraci MW, Peebles RS Jr. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol 2007;178:702–710. [DOI] [PubMed] [Google Scholar]