Abstract
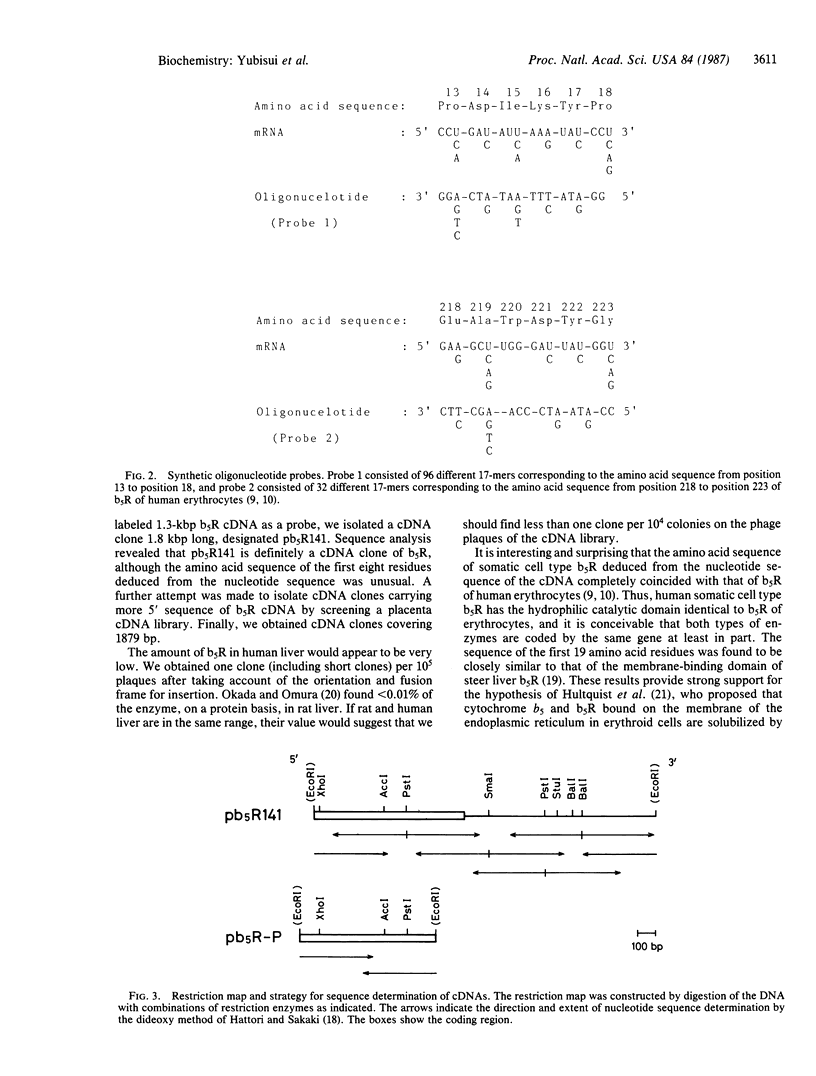
A cDNA coding for human liver NADH-cytochrome b5 reductase (cytochrome b5 reductase, EC 1.6.2.2) was cloned from a human liver cDNA library constructed in phage lambda gt11. The library was screened by using an affinity-purified rabbit antibody against NADH-cytochrome b5 reductase of human erythrocytes. A cDNA about 1.3 kilobase pairs long was isolated. By using the cDNA as a probe, another cDNA (pb5R141) of 1817 base pairs was isolated that hybridized with a synthetic oligonucleotide encoding Pro-Asp-Ile-Lys-Tyr-Pro, derived from the amino acid sequence at the amino-terminal region of the enzyme from human erythrocytes. Furthermore, by using the pb5R141 as a probe, cDNA clones having more 5' sequence were isolated from a human placenta cDNA library. The amino acid sequences deduced from the nucleotide sequences of these cDNA clones overlapped each other and consisted of a sequence that completely coincides with that of human erythrocytes and a sequence of 19 amino acid residues extended at the amino-terminal side. The latter sequence closely resembles that of the membrane-binding domain of steer liver microsomal enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A., Estabrook R. W. Evidence for the participation of cytochrome b 5 in hepatic microsomal mixed-function oxidation reactions. Arch Biochem Biophys. 1971 Mar;143(1):66–79. doi: 10.1016/0003-9861(71)90186-x. [DOI] [PubMed] [Google Scholar]
- Hultquist D. E., Passon P. G. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat New Biol. 1971 Feb 24;229(8):252–254. doi: 10.1038/newbio229252a0. [DOI] [PubMed] [Google Scholar]
- Hultquist D. E., Slaughter S. R., Douglas R. H., Sannes L. J., Sahagian G. G. Erythrocyte cytochrome b5; structure, role in methemoglobin reduction, and solubilization from endoplasmic reticulum. Prog Clin Biol Res. 1978;21:199–216. [PubMed] [Google Scholar]
- Keyes S. R., Cinti D. L. Biochemical properties of cytochrome b5-dependent microsomal fatty acid elongation and identification of products. J Biol Chem. 1980 Dec 10;255(23):11357–11364. [PubMed] [Google Scholar]
- Leroux A., Junien C., Kaplan J., Bamberger J. Generalised deficiency of cytochrome b5 reductase in congenital methaemoglobinaemia with mental retardation. Nature. 1975 Dec 18;258(5536):619–620. doi: 10.1038/258619a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Okada Y., Omura T. Synthesis and turnover of microsomal and mitochondrial NADH-cytochrome b5 reductases in rat liver. J Biochem. 1978 Apr;83(4):1039–1048. doi: 10.1093/oxfordjournals.jbchem.a131992. [DOI] [PubMed] [Google Scholar]
- Oshino N., Imai Y., Sato R. A function of cytochrome b5 in fatty acid desaturation by rat liver microsomes. J Biochem. 1971 Jan;69(1):155–167. doi: 10.1093/oxfordjournals.jbchem.a129444. [DOI] [PubMed] [Google Scholar]
- Ozols J., Korza G., Heinemann F. S., Hediger M. A., Strittmatter P. Complete amino acid sequence of steer liver microsomal NADH-cytochrome b5 reductase. J Biol Chem. 1985 Oct 5;260(22):11953–11961. [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Reddy V. V., Kupfer D., Caspi E. Mechanism of C-5 double bond introduction in the biosynthesis of cholesterol by rat liver microsomes. J Biol Chem. 1977 May 10;252(9):2797–2801. [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Yubisui T., Miyata T., Iwanaga S., Tamura M., Takeshita M. Complete amino acid sequence of NADH-cytochrome b5 reductase purified from human erythrocytes. J Biochem. 1986 Feb;99(2):407–422. doi: 10.1093/oxfordjournals.jbchem.a135495. [DOI] [PubMed] [Google Scholar]
- Yubisui T., Miyata T., Iwanaga S., Tamura M., Yoshida S., Takeshita M., Nakajima H. Amino acid sequence of NADH-cytochrome b5 reductase of human erythrocytes. J Biochem. 1984 Aug;96(2):579–582. doi: 10.1093/oxfordjournals.jbchem.a134871. [DOI] [PubMed] [Google Scholar]
- Yubisui T., Takeshita M. Purification and properties of soluble NADH-cytochrome b5 reductase of rabbit erythrocytes. J Biochem. 1982 May;91(5):1467–1477. doi: 10.1093/oxfordjournals.jbchem.a133838. [DOI] [PubMed] [Google Scholar]