Abstract
Reporter genes produce a protein product in transfected cells that can be easily measured in intact or lysed cells and they have been extensively used in numerous basic and applied research applications. Over the past 10 years, reporter gene assays have been widely accepted and used for analysis of 2,3,7,8-tetrachlorodibenzo-p-dioxin and related dioxin-like compounds in various types of matrices, such as biological, environmental, food and feed samples, given that high-resolution instrumental analysis techniques are impractical for large-scale screening analysis. The most sensitive cell-based reporter gene bioassay systems developed are the mechanism-based CALUX (Chemically Activated Luciferase Expression) and CAFLUX (Chemically Activated Fluorescent Expression) bioassays, which utilize recombinant cell lines containing stably transfected dioxin (AhR)-responsive firefly luciferase or enhanced green fluorescent protein (EGFP) reporter genes, respectively. While the current CALUX and CAFLUX bioassays are very sensitive, increasing their lower limit of sensitivity, magnitude of response and dynamic range for chemical detection would significantly increase their utility, particularly for those samples that contain low levels of dioxin-like HAHs (i.e., serum). In this study, we report that the addition of modulators of cell signaling pathways or modification of cell culture conditions results in significant improvement in the magnitude and overall responsiveness of the existing CALUX and CAFLUX cell bioassays.
Keywords: dioxin, reporter gene, bioassay, CALUX, CAFLUX
1 Introduction
Proper epidemiological, risk assessment and exposure analyses of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin) and related dioxin-like halogenated aromatic hydrocarbons (HAHs) require accurate measurements of these chemicals both in the species of interest and in various exposure matrices (i.e., biological, environmental, food and feed) [1–4]. While high-resolution instrumental analysis techniques are established for these chemicals, current procedures are impractical for large-scale screening analysis. Accordingly, numerous in vitro and cell-based bioanalytical methods have been developed for the detection and relative quantitation of these chemicals in extracts from a variety of matrices and the majority of these systems take the advantage of one or more aspects of the action mechanism of these chemicals (i.e., their ability to activate the Ah receptor (AhR) and/or AhR-dependent gene expression) [1–5]. The most sensitive cell-based bioassay systems developed are the CALUX (Chemically Activated Luciferase Expression) and CAFLUX (Chemically Activated Fluorescent Expression) bioassays, which utilize recombinant cell lines that contain stably transfected dioxin (AhR)-responsive firefly luciferase or enhanced green fluorescent protein (EGFP) reporter genes, respectively [2, 3]. Treatment of these cells with TCDD and related HAHs, other AhR ligands or extracts containing AhR agonists, results in induction of reporter gene expression in a time-, dose-, AhR-, and chemical-specific manner and the level of reporter gene expression directly correlates with the total concentration of TCDD-like AhR inducers (agonists) present in the sample [3, 4, 6, 7]. Although these systems use the same AhR-dependent mechanism to induce reporter gene expression, differences in the characteristics of the respective reporter gene products result in bioassay systems with distinct advantages and disadvantages [2, 3]. While the firefly luciferase reporter gene in the CALUX bioassay system has a very high degree of sensitivity and response, primarily due to enzymatic signal amplification, it also has limitations with respect to repeated measurement, cost and rapidity for high-throughput screening analysis. By contrast, measurement of EGFP reporter gene activity is more rapid, cost effective, and it is amenable to high throughput and repeated analysis and the induction response could be measured in “real time” [3]. However, as the EGFP output signal is directly proportional to the number of EGFP molecules (i.e., it is not an amplified response like luciferase) the reporter signal develops significantly slower than that of luciferase, but because EGFP is extremely stable (T1/2 > 23 h), much higher reporter gene signal output is obtained with extended time of incubation. While the current CALUX and CAFLUX bioassays are very sensitive, increasing their lower limit of sensitivity as well as magnitude of response and dynamic range for chemical detection would significantly increase their utility, particularly with samples that contain low levels of dioxin-like HAHs or where there is a limited sample size. Recent reports of enhancement of AhR and AhR-dependent signal transduction by chemical treatment as well as increased luciferase and EGFP reporter gene activity by modulating cell culture conditions provide several ways to improve the sensitivity, signal-to-noise ratio, detection limits and responsiveness of current CALUX and CAFLUX bioassays [6, 8–11]. Here we report the results of studies examining the effect of modulators of cell signaling pathways (i.e., dexamethasone (DEX) and phorbol-12-myristate-13-acetate (PMA)) as well as alterations in cell culture conditions on the magnitude and overall responsiveness of the CALUX and CAFLUX cell bioassays.
2 Materials and methods
2.1 Chemicals
TCDD was from Dr. Steven Safe (Texas A&M University, USA), dexamethasone from Sigma Chemical Company (St. Louis, MO, USA) and phorbol-12-myristate-13-acetate from CalbioChem (LaJolla, CA, USA).
2.2 Preparation of sediment sample extracts for CALUX and CAFLUX bioassays
Dry, crushed sediment samples (2 g) were extracted three times with 10 mL of toluene each. All extracts were filtered and composited with a 10 mL toluene rinse of the filter column. The extract was reduced to near dryness and cleaned up and fractionated on a combination of an acid silica column and 1% carbon/celite fractionation column [12], with the PCB fraction eluted first followed by the Dioxin/Furan fraction. Both fractions were reduced to near dryness and resuspended in 4 mL of hexane and stored in the dark until analyzed. For bioassay tested in this study, aliquots of each sample extract (Dioxin/Furan fraction) were dried and resuspended in a small volume of DMSO and tested in the CALUX/CAFLUX bioassays as described.
2.3 Cell culture, chemical treatment, and CALUX/CAFLUX cell bioassays
Recombinant rat (H4L1.1c4, H4G1.1c2), mouse (H1L1.1c2, H1L6.1c2 and H1G1.1c3), human (HG2L6.1c3) hepatoma cells and guinea pig intestinal adenocarcinoma (G16L1.1c8) were grown and maintained as previously described [2, 13, 14]. H4L1.1c4, H1L1.1c2 and G16L1.1c8 cells contain the stably integrated DRE-driven firefly luciferase reporter plasmid pGudLuc1.1 [13] and H1L6.1c2 and HG2L6.1c3 cells contain pGudLuc6.1 [14], while H4G1.1c2 and H1G1.1c3 contain the stably integrated DRE-driven EGFP reporter plasmid pGreen1.1 [6]. Each of these recombinant cell lines responds to TCDD and related AhR agonists with the induction of luciferase/EGFP reporter gene activity in a time-, dose-, ligand-dependent manner as previously described [3, 4, 6, 7]. Cells were plated into white, clear-bottomed 96-well tissue culture dishes (for CALUX) or black, clear-bottomed 96-well tissue culture dishes (for CAFLUX) at 75000 cells per well and allowed to attach for 24 h. Cells were incubated with the carrier solvent DMSO (1% final solvent concentration), TCDD at indicated concentrations or the indicated treatment (e.g., sediment extracts) for either 4 h (H4L1.1c4, H1L1.1c2 and G16L1.1c8 cells) or 24 h (H1L6.1c2, HG2L6.1c3, H4G1.1c2 and H1G1.1c3 cells) at the specified temperature. For luciferase measurement, sample wells were washed twice with phosphate-buffered saline, followed by addition of cell lysis buffer (Promega) and shaking of the plates for 20 min at room temperature to allow complete cell lysis. Measurement of luciferase activity in each well was carried out using an Anthos Lucy2 (Durham, NC) microplate luminometer with automatic injection of Promega stabilized luciferase reagent. EGFP fluorescence in intact cells was measured directly in 96 well plates without media removal using a Tecan GENios microplate fluorometer with excitation and emission wavelengths of 485 and 515 nm, respectively. Reporter gene activity in each well was expressed either as the original relative light units (RLUs) for the CALUX bioassay, relative fluorescence units (RFUs) for the CAFLUX bioassay or values were normalized to the maximal activity induced by 1 nM TCDD.
3 Results and discussion
3.1 The effect of bioassay incubation temperature on the inducibility and responsiveness of the CALUX and CAFLUX cell bioassays
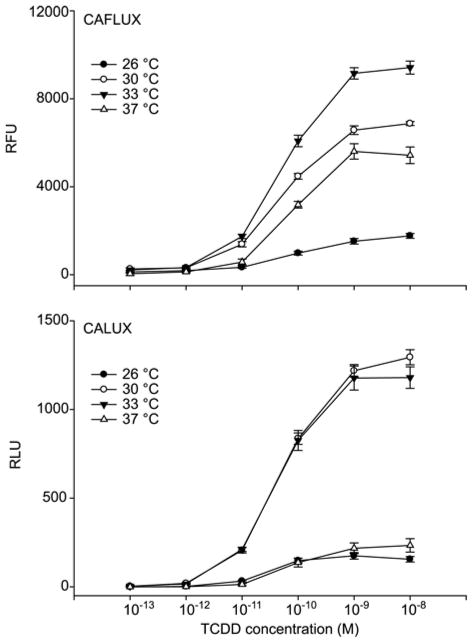
In previous studies using the mouse hepatoma CAFLUX bioassay we demonstrated that the tissue culture incubation temperature during the induction period was critical in maximizing the overall EGFP activity obtained by a defined amount of AhR agonist (TCDD) at 33 °C, resulting in several fold greater EGFP activity than cells grown at 37 °C [6]. This increase was presumed to be due to an increase in EGFP activity than from an increase in gene expression. Although the firefly luciferase used in reporter gene studies has been reported to be thermo labile at 37 °C, its relative activity in cells grown at other temperatures has not been determined [15]. To determine the effect of incubation temperature on the overall activity of EGFP and luciferase, DMSO- and TCDD-treated mouse hepatoma CALUX and CAFLUX cell lines (H1G1.1c3 and H1L6.1c2 cells, respectively) were incubated for 24 h at various temperatures (26 °C, 30 °C, 33 °C or 37 °C) followed by analysis of reporter gene activity. The results of dose response studies for each reporter gene at different temperatures are shown in Figure 1 (upper panel). As is readily apparent, cells incubated at 33 °C exhibited significantly more EGFP activity than those at 37 °C, 30 °C or 26 °C. The fluorescence of EGFP at 33 °C was 2–3 fold greater than that observed in cells incubated at 37 °C at all TCDD concentrations, and it improved the level of reporter gene activity at the minimal detection limit of 1 pM (compare 323 ± 31 RFUs at 33 °C to 126 ± 42 RFUs at 37 °C). Similarly, a 5–10 fold greater amount of luciferase activity was obtained from cells that had been incubated for 24 h at 30 °C or 33 °C than that obtained from cells incubated for 24 h at 37 °C or 26 °C (Figure 1, lower panel).
Figure 1.
Temperature-dependent enhancement of TCDD-inducible reporter gene expression in mouse CAFLUX (H1G1.1c3) and CALUX (H1L6.1c2) cell lines. H1L6.1c2 and H1G1.1c3 cells were incubated at the indicated temperature with increasing concentrations of TCDD for 24 h. EGFP and luciferase activity were determined as described. Values are expressed as the relative light or fluorescence units (RLUs and RFUs, respectively) and represent the mean ± SD of triplicate determinations.
Luciferase activity in cells treated with 1 pM TCDD for 24 h at 33 °C was significantly higher than that observed from cells incubated at 37 °C. In additional studies, we showed that the observed temperature-dependence of reporter gene activity results from an effect on the overall activity of the reporter gene itself, rather than an effect on the AhR signaling pathway (data not shown). Although the underlying cause of the temperature dependent enhancement effect remains to be established, given previous studies on GFP and luciferase reporter genes and the expected body temperature of the organism from which the reporter genes were obtained, it is likely due to the differences in the folding and/or stability of these proteins.
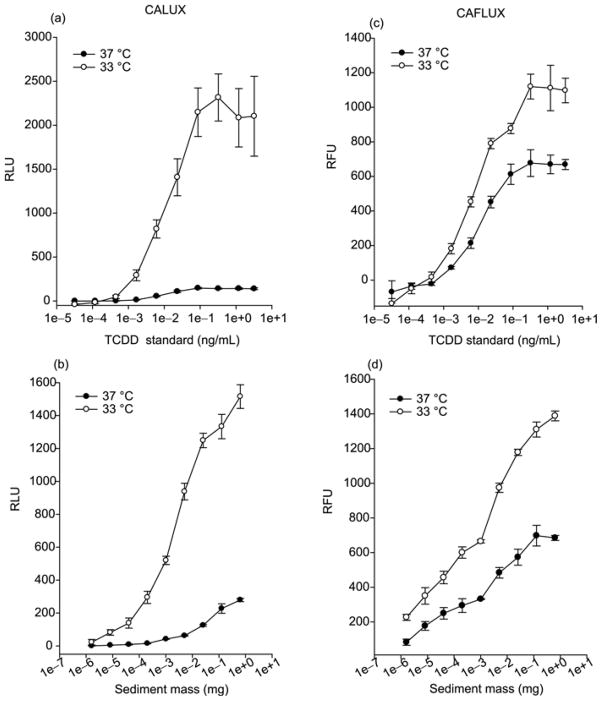
We extended the above studies to investigate whether reduced temperature (33 °C) would improve CALUX and CAFLUX analysis when applied to environmental sample extracts. Sediment samples were collected and cleaned up in the lab following the standard procedures to remove PAHs and other AhR agonists from the desired HAHs (i.e., dioxin-like PCDDs, PCDFs and PCBs). The TCDD standard was run in parallel with environmental samples as the positive control and for comparative purpose. The results of TCDD dose response studies for luciferase (CALUX assay) at 37 °C and 33 °C are shown in Figure 2(a) and consistent with previous results. Cells incubated at 33 °C exhibited significantly more luciferase activity than that at 37 °C. The luciferase activity at 33 °C was 5–15 fold greater than that observed in cells incubated at 37 °C at all TCDD concentrations, and it increased the amount of luciferase activity at the minimal detection limit of 0.45 pg TCDD/mL (compare 46 ± 19 relative light units (RLUs) at 33 °C to 1 ± 1 RLUs at 37 °C). The luciferase activity at 33 °C was similarly 5–15 fold greater than that observed in cells incubated at 37 °C when incubated with HAH containing sediment extracts (Figure 2(b)). The amount of dioxin or dioxin-like compounds contained in extracts from less than 1 μg of the sediment was not detectable (i.e., producing less than 10 RLU over the solvent control) in the standard CALUX assay temperature (37 °C), while they were significantly above the background activity when incubated at 33 °C given the significantly higher luciferase activity (up to 300 RLU). This small temperature change (from 37 °C to 33 °C) could significantly increase the bioassay’s detection of TCDD-like compounds in environmental samples using the CALUX bioassay by up to 2 orders of magnitude. Similar results were obtained using the CAFLUX assay, where about a 2-fold greater amount of fluorescence activity from GFP was obtained from cells that had been incubated at 33 °C compared to those incubated at 37 °C with either TCDD standard or environment extract (Figure 2(c) and (d)). GFP fluorescence activity in cells treated with 0.45 pg TCDD/ml for 24 h at 33 °C was significantly higher than that observed from cells incubated at 37 °C, and the overall activity at 33 °C was significantly above the background, while GFP activity at 37 °C was indistinguishable from the background (Figure 2(c)). The GFP fluorescence activity induced by TCDD at 33 °C was ~2 fold greater than that observed in cells incubated at 37 °C at all concentrations of sediment extracts (Figure 2(d)). We observed similar temperature-dependent differences in reporter gene activity (with optimal activity at 33 °C) using rat and human liver and guinea pig intestinal cell lines (H4L1.1c4, H4G1.1c2; HG2L6.1c3; G16L1.1c8 cells) containing a stably transfected AhR- responsive luciferase or EGFP reporter gene (Table 1).
Figure 2.
Temperature-dependent enhancement of reporter gene expression in mouse CALUX (H1L6.1c2) and CAFLUX (H1G1.1c3) cell lines by sediment extracts. H1L6.1c2 and H1G1.1c3 cells were incubated at the indicated temperature with either increasing concentrations of TCDD standard ((a) and (c)) or a sediment sample extract ((b) and (d)) for 24 h. Luciferase and EGFP activity were determined as described. Values are expressed as the relative light or fluorescence units (RLUs and RFUs, respectively) and represent the mean ± SD of triplicate determinations.
Table 1.
Summary of temperature-dependent reporter gene expression in a number of CALUX or CAFLUX cell lines
| Reporter | Cell linea) | Reporter gene activity (RLU/RFU) |
|||
|---|---|---|---|---|---|
| 33 °C DMSO | TCDD | 37 °C DMSO | TCDD | ||
| AhR luciferase | |||||
| H4L1.1c4 | 86 ± 1 | 3187 ± 100 | 12 ± 1 | 1252 ± 62 | |
| G16L1.1c8 | 44 ± 3 | 310 ± 8 | 6 ± 1 | 64 ± 2 | |
| H1L6.1c2 | 100 ± 7 | 1080 ± 97 | 6 ± 1 | 139 ± 6 | |
| H1L1.1c2 | 1 ± 1 | 163 ± 27 | 1 ± 1 | 33 ± 11 | |
| HG2L6.1c3 | 19 ± 1 | 245 ± 24 | 2 ± 1 | 178 ± 1 | |
| EGFP | H1G1.1c2 | 3409 ± 86 | 12477 ± 365 | 2698 ± 73 | 8188 ± 485 |
| H4G1.1c2 | 1350 ± 18 | 3309 ± 15 | 1266 ± 21 | 2123 ± 40 | |
| ER luciferase | ETOH | estradiol | ETOH | estradiol | |
| BG1Luc4E2 | 1 ± 1 | 438 ± 32 | 1 ± 1 | 59 ± 2 | |
Rat (H4L1.1c4, H4G1.1c2), guinea pig (G16L1.1c8), mouse (H1L6.1c2, H1L1.1c2, H1G1.1c2) and human (HG2L6.1c3, BG1Luc4E2) cell lines.
A similar enhancement of estrogen receptor-responsive luciferase reporter gene activity at 33 °C as compared to 37 °C (Table 1) confirms that the enhancing effect is not an AhR-specific response. These results demonstrate that our observed temperature-dependence of reporter gene activity is due to an effect on the reporter gene itself, rather than an effect on the AhR from an individual species and that the current CALUX and CAFLUX bioassays are carried out at suboptimal temperatures necessary for production of maximal reporter gene activity. In those situations where optimal reporter gene activity in transfected mammalian cells is desired, cells are incubated at lower temperatures (i.e., 33 °C) during the chemical incubation period. This simple modification of gene induction protocols could lead to substantial increase in measurable reporter gene activity and therefore could significantly increase the overall response, minimal detection limit and range of bioassay response for dioxin and dioxin-like compounds in sample extracts.
3.2 The effect of dexamethasone (DEX) on the inducibility and responsiveness of the CALUX cell bioassay
The ability of glucorticoid receptor (GR) agonists as DEX to enhance AhR-dependent induction in several cell lines and the rat hepatoma CALUX (pGudLuc1.1) cell line has been previously reported and is suggested to result from increased expression of the AhR [8, 9]. Therefore, we considered that the addition of DEX to the standard bioassay incubation might provide an approach to improve these bioassays by increasing their sensitivity and magnitude of response. Accordingly, the effect of DEX on the induction of reporter gene expression by TCDD was examined in rat, mouse and guinea pig CALUX cell lines (Figure 3). While exposure of the rat hepatoma and guinea pig intestinal adenocarcinoma CALUX cell bioassays to DEX resulted in an increase in the magnitude of TCDD induced luciferase activity, DEX had no effect on TCDD-dependent luciferase gene induction in the mouse CALUX cell lines. The ability of the GR antagonist RU486 to inhibit the DEX enhancement of TCDD induction but not affect TCDD induction in the absence of DEX in the rat cell lines indicates that the enhancement by DEX was independent of the AhR and involved the GR (data not shown). DEX alone induced luciferase/EGFP activity in the rat CALUX/CAFLUX cell lines and this induction was confirmed to be dependent on the GR as RU486 also inhibited this effect (data not shown). The inability of DEX to affect TCDD induction in the mouse liver cell lines, which are known to contain GRs, demonstrated the species-specificity of the response. The ability of DEX to enhance AhR-dependent reporter gene expression in the rat and guinea pig CALUX cell lines indicated it is a possible candidate for the enhancement of the bioassay system for detection and analysis of environmental samples. By contrast, an increased response in rat and guinea pig CALUX cell lines by complex mixtures of chemicals that contain both AhR and GR agonists could lead to overestimation of the potency and/or a false positive result. Accordingly, the mouse CALUX bioassay would be expected to result in a more accurate potency determination when GR agonists are present in an unknown sample extract.
Figure 3.
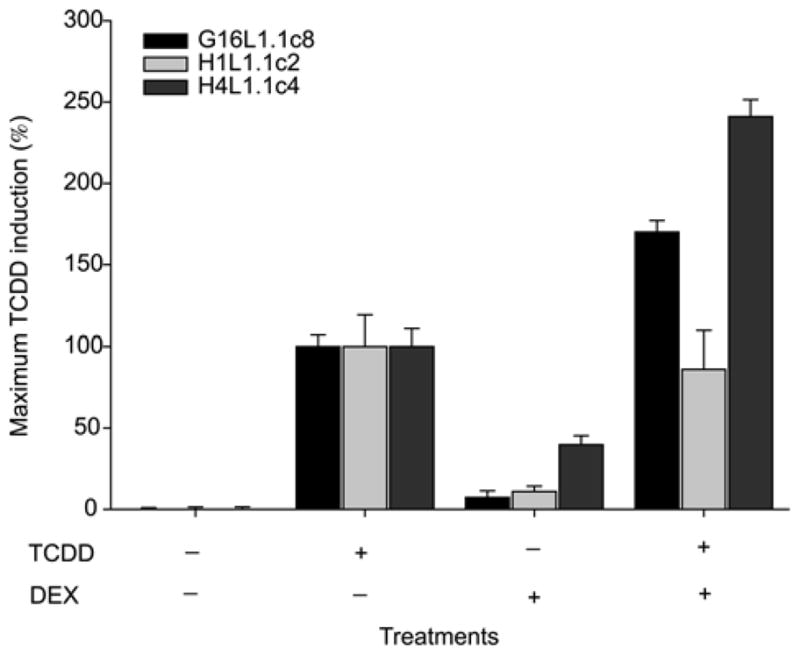
Addition of dexamethasone (DEX) synergistically increases TCDD-dependent induction of luciferase activity in the CALUX cell bioassay. H4L1.1c4, H1L1.1c2 and G16L1.1c8 cells were incubated with 1 nM TCDD, 10 μM DEX or TCDD plus DEX for 4 h as indicated. Luciferase activity was determined as described. Luciferase values are expressed as the percentage of that induced by 1 nM TCDD and represent the mean ± SD of triplicate determinations.
3.3 The effect of phorbol-12-myristate-13-acetate (PMA) on the inducibility and responsiveness of the CALUX cell bioassay
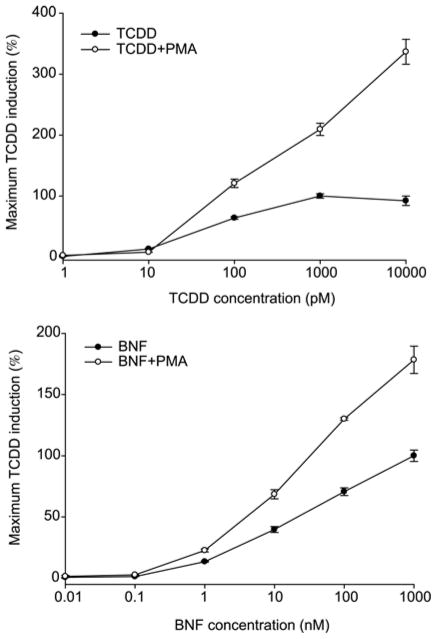
The AhR and ARNT have been shown to be phosphoproteins and the role of phosphorylation in the regulation of AhR signaling and species- and tissue-specific responsiveness to TCDD and other ligands is being examined. The role of protein kinase C (PKC) in the modulation of AhR responsiveness is of particular interest since activation of PKC by PMA was reported to augment TCDD-inducible AhR- and DRE-dependent gene expression by 2- to 3-fold in rats and mice [10, 11]. Thus, we tested whether the inclusion of PMA into the standard CALUX/CAFLUX bioassay incubation protocol might improve bioassay detection and response. In contrast to the DEX experiments, exposure of the mouse or rat hepatoma CALUX cells to PMA alone had no significant effect, but when added together with TCDD or BNF (another AhR agonist), it significantly enhanced dose-dependent luciferase gene induction by 3–4 folds (Figure 4). While these results demonstrated that the addition of PMA to the inducing solution can increase the magnitude of the induction response, comparisons, it did not increase the minimal detection limits of the assay. The PMA enhancement of AhR signaling is transient, occurring within eight hours after PMA treatment, and decreasing thereafter as PKC is feedback inhibited (data not shown). Thus, while PMA could be used to increase the responsiveness of the CALU bioassay, the transient nature of the enhancement restricts the window of time that PMA could be used to improve detection and quantitation of dioxin-like chemicals by the CALUX bioassay.
Figure 4.
Coincubation with PMA synergistically increases TCDD-dependent induction of luciferase activity in the CALUX cell bioassay. Mouse CALUX (H1L1.1c2) cells were incubated with increasing concentrations of TCDD or beta-naphthoflavone (BNF) in the absence or presence of PMA (81 nM) for 4 h. Luciferase activity was determined as described under Materials and methods. Luciferase values are expressed as the percentage of that induced by 1 nM TCDD and represent the mean ± SD of triplicate determinations.
3.4 Demonstration of superinduction of CALUX luciferase activity by environmental samples
Given the ability to enhance the CALUX response by addition of exogenous chemicals, we found sample extracts that produce a similar superinduction phenomenon. During our extensive analyses of sediment and soil samples by CALUX, we found numerous samples that induced CALUX luciferase gene expression to levels of 2–3 times greater than that induced by a maximal inducing concentration of TCDD. An example is shown in Figure 5. While one sediment sample extract induced CALUX luciferase activity in mouse H1L1.1c2 cells to the same maximal activity as that of TCDD (Figure 5, sample 1), another sample extract prepared at the same time resulted in superinduction of CALUX luciferase reporter gene activity, still producing a full concentration-response curve (Figure 5, sample 2). The superinduction phenomenon is not unique or an artifact of the AhR CALUX bioassay, as we have observed similar superinduction responses using a stably transfected estrogen receptor (ER)-responsive luciferase reporter gene in human ovarian carcinoma cells (BG1) following exposure to crude solvent extracts of plants (data not shown). It remains to be determined what accounts for the superinduction response to the sediment extracts in the CALUX cell line. It was envisioned that the presence of chemicals in this sample extract could activate cell signaling pathways to enhance AhR-dependent gene expression, CALUX plasmid promoter activity or luciferase reporter gene activity, similar to effects shown in Figures 3 and 4. While the exact mechanism of superinduction from the environmental sample is unknown, further investigation may allow identification of responsible mechanisms for the enhancement effect that could be incorporated into the standard CALUX bioassay protocol to significantly increase assay sensitivity and response.
Figure 5.
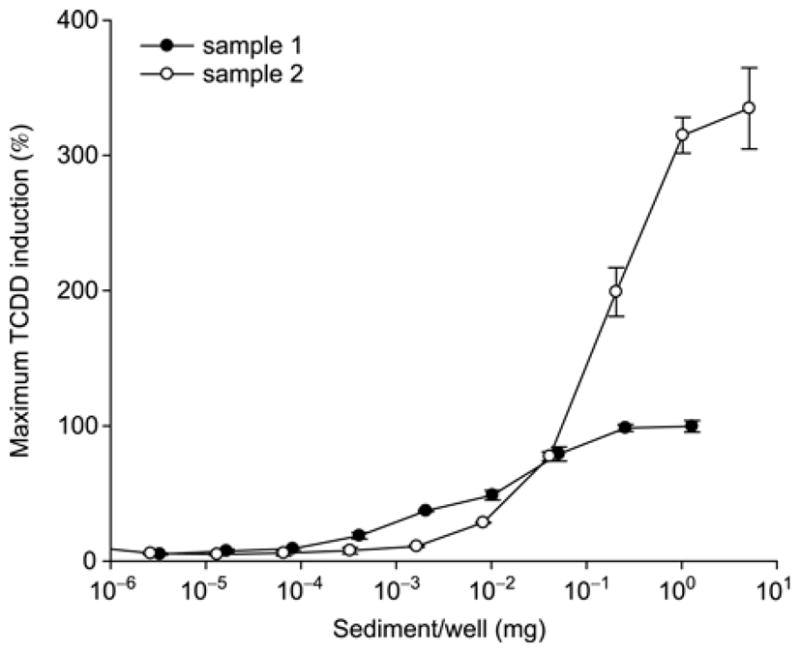
Dose dependent luciferase reporter gene expression induced by sediment sample extracts in mouse CALUX (H1L6.1c2) cells. H1L6.1c2 cells were incubated with the indicated equivalent concentration of sediment extract for 24 h. Luciferase activity was determined as described under Materials and methods. Values are expressed as the percentage of that induced by 1 nM TCDD and represent the mean ± SD of triplicate determinations.
4 Conclusions
While the current CALUX and CAFLUX bioassays are very sensitive, increasing their lower limit of sensitivity as well as magnitude of response and dynamic range for chemical detection would significantly increase their utility, particularly for those samples that contain low levels of dioxin-like HAHs or in which low sample volumes are available. In this study we demonstrated that by the application of modulators of cell signaling pathways (i.e., dexamethasone (DEX) and phorbol-12-myristate-13-acetate (PMA)) as well as alterations in cell culture conditions (bioassay incubation temperature), we could significantly improve the sensitivity, signal-to-noise ratio, detection limits and responsiveness of the CALUX and CAFLUX bioassays. Further studies of the biochemical and molecular mechanisms may facilitate the integration of these and other changes into protocols, which may improve the CALUX and CAFLUX bioassays for dioxins and related dioxin-like chemicals as well as other AhR agonists.
Acknowledgments
The authors express their great thanks for the jointly support from the National Institutes of Environmental Health Sciences Superfund Basic Research Grant (ES004699 (MSD)), the California Agricultural Experiment Station, the Startup Fund of 100 Talents Program of Chinese Academy of Sciences, the National Natural Science Foundation of China (20921063) and the National Basic Research Program (2010CB933500).
References
- 1.Behnisch PA, Hosoe K, Sakai S. Bioanalytical screening methods for dioxins and dioxin-like compounds a review of bioassay/biomarker technology. Environ Int. 2001;27:413–439. doi: 10.1016/s0160-4120(01)00028-9. [DOI] [PubMed] [Google Scholar]
- 2.Hahn ME. Biomarkers and bioassays for detecting dioxin-like compounds in the marine environment. Sci Total Environ. 2002;289:49–69. doi: 10.1016/s0048-9697(01)01016-6. [DOI] [PubMed] [Google Scholar]
- 3.Denison MS, Zhao B, Baston DS, Clark GC, Murata H, Han DH. Recombinant cell bioassay systems for the detection and relative quantitation of halogenated dioxins and related chemicals. Talanta. 2004;63:1123–1133. doi: 10.1016/j.talanta.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 4.Whyte JJ, Schmitt CJ, Tillitt DE. The H4IIE cell bioassay as an indicator of dioxin-like chemicals in wildlife and the environment. Crit Rev Toxicol. 2004;34:1–83. doi: 10.1080/10408440490265193. [DOI] [PubMed] [Google Scholar]
- 5.Harrison RO, Eduliee GH. Immunochemical analysis for dioxins-progress and prospects. Sci Total Environ. 1999;239:1–18. doi: 10.1016/s0048-9697(99)00306-x. [DOI] [PubMed] [Google Scholar]
- 6.Nagy SR, Sanborn JR, Hammock BD, Denison MS. Development of a green fluorescent protein-based cell bioassay for the rapid and inexpensive detection and characterization of ah receptor agonists. Toxicol Sci. 2002;65:200–210. doi: 10.1093/toxsci/65.2.200. [DOI] [PubMed] [Google Scholar]
- 7.Ziccardi MH, Gardner IA, Denison MS. Development and modification of a recombinant cell bioassay to directly detect halogenated and polycyclic aromatic hydrocarbons in serum. Toxicol Sci. 2000;54:183–193. doi: 10.1093/toxsci/54.1.183. [DOI] [PubMed] [Google Scholar]
- 8.Hoogenboom LA, Hamers AR, Bovee TF. Bioassays for the detection of growth-promoting agents, veterinary drugs and environmental contaminants in food. Analyst. 1999;124:79–85. doi: 10.1039/a804950e. [DOI] [PubMed] [Google Scholar]
- 9.Lai KP, Wong MH, Wong CK. Modulation of AhR-mediated CYP1A1 mRNA and EROD activities by 17beta-estradiol and dexamethasone in TCDD-induced H411E cells. Toxicol Sci. 2004;78:41–49. doi: 10.1093/toxsci/kfh045. [DOI] [PubMed] [Google Scholar]
- 10.Chen YH, Tukey RH. Protein kinase C modulates regulation of the CYP1A1 gene by the aryl hydrocarbon receptor. J Biol Chem. 1996;271:26261–26266. doi: 10.1074/jbc.271.42.26261. [DOI] [PubMed] [Google Scholar]
- 11.Long WP, Pray-Grant M, Tsai JC, Perdew GH. Protein kinase C activity is required for aryl hydrocarbon receptor pathway-mediated signal transduction. Mol Pharmacol. 1998;53:691–700. doi: 10.1124/mol.53.4.691. [DOI] [PubMed] [Google Scholar]
- 12.Brown DJ, Orelien J, Gordon JD, Chu AC, Chu MD, Murata HJ, Kayama F, Denison MS, Clark GC. Mathematical model developed for environmental samples: prediction of GC/MS dioxin TEQ from XDS-CALUX bioassay data. Environ Toxicol Chem. 2007;41:4354–4360. doi: 10.1021/es062602+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrison PM, Tullis K, Aarts J, Brouwer A, Giesy JP, Denison MS. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam Appl Toxicol. 1996;30:194–203. doi: 10.1006/faat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 14.Rushing RS, Denison MS. The silencing mediator of retinoic acid and thyroid hormone receptors can interact with the aryl hydrocarbon (Ah) receptor but fails to repress Ah receptor-dependent gene expression. Arch Biochem Biophys. 2002;403:189–201. doi: 10.1016/s0003-9861(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 15.Baggett B, Roy R, Momen S, Morgan S, Tisi L, Morsev D, Gillies RJ. Thermostability of firefly luciferases affects efficiency of detection by in vivo bioluminescence. Mol Imaging. 2004;3:324–33. doi: 10.1162/15353500200403178. [DOI] [PubMed] [Google Scholar]