Abstract
Objective
We previously reported that inhibition or loss of CD26 (DPPIV/dipeptidylpeptidase IV) results in a defect in normal mobilization of hematopoietic stem and progenitor cells induced by granulocyte-colony stimulating factor (G-CSF). This suggests that CD26 is a necessary component of the mobilization pathway. Our goal in this study was to determine whether mobilization can be induced by the CXCR4 antagonist AMD3100 in mice lacking CD26 (CD26-/-).
Materials and Methods
Ten week old CD26-/- and C57BL/6 mice received a subcutaneous injection of AMD3100. One hour post-injection the mice were euthanized and peripheral blood and bone marrow were collected and evaluated.
Results
AMD3100 mobilizes hematopoietic progenitors into the peripheral blood of CD26-/- and mice.
Conclusions
Our finding that AMD3100 rapidly mobilizes hematopoietic progenitor cells from the bone marrow into the periphery in CD26 deficient transgenic mice that otherwise exhibit a mobilization defect in response to G-CSF suggests that: (1) CD26 is downstream of G-CSF but upstream of the CXCL12-CXCR4 axis and (2) AMD3100 can be used as a single agent to mobilize hematopoietic stem and progenitor cells in normal donors or patients that have an intrinsic defect in their response to G-CSF treatment. Stem cell transplants are often the only curative treatment in some cancer patients. The ability to perform the transplant and its success is dependent on the ability to mobilize adequate numbers of hematopoietic progenitor cells. The use of AMD3100 as a single agent would give patients or donors an additional option for a successful stem cell transplant.
Keywords: peripheral blood stem cell mobilization, hematopoietic stem cell transplant, G-CSF, AMD3100, CD26, CXCR4
Introduction
Granulocyte-colony stimulating factor (G-CSF) is used routinely in the clinic to mobilize hematopoietic stem and progenitor cells (HSC/HPC) from the bone marrow into the peripheral blood. Once in the circulation, apheresis procedures are effective at collecting cell isolates that are enriched for peripheral blood stem cells (PBSC), which can then be transplanted into patients who are candidates for autologous stem cell transplantation or cryopreserved for later usage. In addition to G-CSF [1] and granulocyte macrophage colony-stimulating factor (GM-CSF) [2], several other growth factors have been identified as mobilizing agents but are not ready for clinical use. For example, mobilization can be achieved by treatment with several different cytokines and/or growth factors individually or in combination: stem cell factor (SCF) [3], flt-3 ligand (FL) [4], interleukin-3 (IL-3) [5], and interleukin-8 (IL-8) [6] and others.
Although many of the key components of HSC/HPC trafficking in and out of the bone marrow have been identified, the mechanism by which G-CSF mobilizes HSC/HPC into the peripheral blood is still heavily debated. Normally there are interactions between HSC/HPC and the components of the bone marrow such as chemokines, selectins, and integrins. HSC/HPC express a wide range of adhesion molecules [CXCR4, very late antigen-4 (VLA-4), c-kit (CD117), L-Selectin (CD62L), and CD44]. The marrow stroma express the ligands corresponding to these adhesion molecules: CXCL12/stromal cell derived factor-1 (SDF-1), vascular cell adhesion molecule-1 (VCAM-1), kit ligand (KL), P-selectin glycoprotein ligand-1 (PSGL), and hyaluronic acid (HA). Mobilization is believed to result from cytokine-induced changes in the profile of adhesion molecules expressed on HSC/HPC and/or their relationship to the corresponding ligands on the bone marrow cells [7]. G-CSF is thought to induce, through an unknown cell type, the release of a number of proteases into the bone marrow (BM), including neutrophil elastase (NE), Cathepsin G (CG), and matrix metalloproteinase-9 (MMP-9). IL-8 and Groβ are believed to release the same enzymes via neutrophils and monocytes. These proteases have the ability to cleave several adhesion molecules thought to play an important role in HSC trafficking and mobilization, including c-kit, VCAM-1, CXCR4, and SDF-1. However, mice lacking NE and CG display normal G-CSF-induced HSC/HPC mobilization [8], and mice lacking MMP-9 exhibit normal G-CSF-induced [8-9] and IL-8-induced [10] HSC/HPC mobilization. Therefore, the overall importance of these enzymes in HSC/HPC mobilization remains unresolved.
The enzyme CD26 (also known as DPPIV/dipeptidylpeptidase IV) cleaves dipeptides from the N-terminus of proteins that contain the required X-Pro or X-Ala motif. Inhibition or loss of CD26 activity in mice results in a deficiency in normal G-CSF induced HSC/HPC mobilization. This suggests that CD26 is an important component of G-CSF induced mobilization [11-12]. Loss of CD26 on donor cells results in enhanced homing and engraftment into congenic mouse recipients [13]. In addition, the chemokine CXCL12 (SDF-1, stromal cell derived factor-1) is one of several chemokines that contains the appropriate recognition sequence necessary for CD26-mediated cleavage [14]. This information, combined with the known role of CXCL12 in directing the trafficking of HSC/HPC suggests that CXCL12 is a likely target of CD26 during G-CSF-induced mobilization of HSC/HPC.
AMD3100, an antagonist of CXCR4, blocks binding of the chemokine CXCL12 to its receptor, CXCR4, and induces rapid mobilization of HSC/HPC in humans and mice [15-16]. Cells mobilized by AMD3100 are capable of multi-lineage engraftment into primary and secondary recipient mice [16]. Additionally, AMD3100 mobilized CD34+ cells from humans engraft into non-obese diabetic-severe combined immunodeficient (NOD-SCID) mice [16]. For these reasons, as well as the recent introduction into the clinic of AMD3100 in combination with G-CSF, we decided to determine whether single agent AMD3100 mobilizes HSC/HPC in CD26-deficient mice (CD26-/-).
Materials and methods
AMD3100 treatment of mice
C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). CD26/DPPIV deficient (CD26-/-) mice [17] (also on a C57BL/6 background) were maintained as a breeding colony. All animal studies were done under an IACUC approved protocol. A single subcutaneous injection of AMD3100 (5 mg/kg) was given to ten week old C57BL/6 and CD26-/- mice. Age-matched untreated control mice were given a subcutaneous injection of an equivalent volume (100 μl) of saline. The mobilization kinetics in response to a 5mg/kg AMD3100 bolus injection in mice has been well described historically [16] and more recently revisited [18]. In the more recent study, mobilization into the peripheral blood peaked after 1 hour and returned to normal within 4 hours [18]. Consequently, for our studies mice were euthanized one hour post-injection by CO2 inhalation to obtain cells for analysis.
Isolation of Cells
Peripheral blood (PB) was obtained from euthanized mice by cardiac puncture using a 25 gauge needle and a 1.2 mL syringe containing EDTA as an anticoagulant (Sarstedt, Germany). A complete blood count and blood differential analysis was done for each peripheral blood sample using a HemaVet® 950FS Cell Counter (Drew Scientific, Waterbury, CT). Comparisons of blood differentials between groups were made using two-tailed students t-test (SigmaPlot 10 / SigmaStat 3.5, Systat, Richmond, CA). Three to five-hundred μL of PB from each sample was diluted to 10 mL with Dulbecco's phosphate-buffered saline (DPBS) and centrifuged at 1000 g for 20 minutes. Red blood cell lysis was done with hypotonic ACK lysis buffer (155 mM NH4CL, 10 mM KHCO3, and 0.1 mM EDTA, pH 7.2). Samples were centrifuged at 800 g for 10 minutes and the cells resuspended in IMDM media (Iscove's Modified Dulbecco's Medium, HyClone, Logan, UT) supplemented with 10% Fetal Bovine Serum (FBS) (HyClone, Logan, UT), 2 mM L-Glutamine (HyClone, Logan, UT), 100 U/mL penicillin, 100 U/mL streptomycin, and 0.025μg/mL amphotericin B (HyClone, Logan, UT).
Bone marrow from each femur was collected by flushing the femur with 1 mL DPBS passed through a 25 gauge needle. Cells were washed by diluting to 10 mL with DPBS followed by centrifugation at 1000 g for 20 minutes. The supernatant was removed, the cells were washed again and centrifuged at 800 g for 10 minutes, and the cells were resuspended in supplemented IMDM media.
Flow Cytometry
Multivariate flow cytometric analysis was done for freshly isolated cells from mouse peripheral blood and bone marrow. Cells were stained with (i) APC mouse lineage cocktail (Gr-1 (Ly-6G and Ly-6C), Mac-1(CD11b), B220(CD45R), CD3ε, and Ter119), (ii) anti mouse Sca-1 v500, (iii) anti-mouse c-kit v450, (iv) anti-mouse CD34 FITC, (v) anti-mouse CD48 PE-Cy7, (vi) anti-mouse CD90.2 (thy1.2) APC-efluor780, (vii) anti-mouse CD135 (Flt3/Flk2) PE and (viii) anti-mouse CD150 (SLAM) PerCP-Cy5.5 (BD Biosciences, San Diego, CA or eBioscience, San Diego, CA or BioLegend, San Diego, CA). Cells were labeled using a staining protocol described previously [11-13]. Approximately 2,000,000 events were collected on an FACSCanto II flow cytometer (BD Biosciences) for each sample analysis. Analysis was done using BD FACSDiva software. Comparisons were made using non-parametric Mann Whitney U test (SigmaPlot 10 / SigmaStat 3.5, Systat, Richmond, CA).
Myeloid colony assay
Colony assays were done by adding the equivalent of 1.25% of the total volume of bone marrow mononuclear cells (MNC) (approximately 5×104 cells) and the equivalent of 75 ul of peripheral blood (approximately 4-5×105 cells) to 1 mL of 1% methyl-cellulose supplemented with 15% FBS, 1% Bovine Serum Albumin, 10 μg/mL recombinant human (rh) Insulin, 200 μg/mL Iron Saturated Human Transferrin, 10-4 M 2-Mercaptoethanol, 2 mM L-glutamine, 50 ng/mL recombinant mouse (rm) stem cell factor, 10 ng/mL rm Interleukin-3, 10 ng/mL rh interleukin-6, and 3 U/mL rh erythropoietin. These suspensions were incubated under 5% O2, 5% CO2, and 100% humidity at 37°C. Plates were scored for colony-forming units of granulocyte-macrophage (CFU-GM), burst-forming units-erythroid (BFU-E), and colony-forming units of granulocytes, erythroblasts, macrophages and megakaryocytes (CFU-GEMM) after 7 days of incubation. Comparisons were made using two-tailed students t-test (SigmaPlot 10 / SigmaStat 3.5, Systat, Richmond, CA).
Results
Complete blood counts (CBC) were obtained for the peripheral blood of mice following treatment with AMD3100. White blood cell (WBC) counts were elevated in the peripheral blood of C57BL/6 mice that received AMD3100. Specifically, WBC counts increased from 5.17±0.37×106/mL in untreated mice to 13.26±0.89×106/mL in AMD3100-treated C57BL/6 mice (p≤0.01, n=14). This represents a 2.6 fold increase in the number of circulating WBC. An elevation in the WBC count was also observed following AMD3100 treatment of CD26-/- mice – from 5.87±0.68×106/mL in untreated mice to 17.33±1.27×106/mL in treated mice (p<0.01, n=14). This is a 3.0 fold increase in circulating WBC counts. When blood differentials were examined, it was noted that the overall elevation in WBC had resulted from increases in neutrophils, lymphocytes, and monocytes for both C57BL/6 and CD26-/- treated mice (Table 1). No significant difference in WBC counts were noted in untreated C57BL/6 mice as compared to CD26-/- mice but a significantly higher number of circulating WBC was observed in the PB of AMD3100 CD26-/- mice as compared to AMD3100 treated C57BL/6 mice (p=0.015, n=14) which is explained primarily by an increase in lymphocyte counts (p=0.036, n=14).
Table 1.
Comparison of complete blood counts (CBC) from untreated and AMD3100-treated C57BL/6 mice and CD26-/- mice (n=14). Comparisons of untreated groups to AMD3100-treated groups were done using a two-tailed student t-test. WBC: White Blood Cells; NE: Neutrophils; LY: Lymphocytes; MO: Monocytes: EO: Eosinophils; BA: Basophils; RBC: Red Blood Cells; Hb: Hemoglobin; HCT: Hematocrit; MCV: Mean Corpuscular Volume; MCH: Mean Corpuscular Hemoglobin; MCHC: Mean Corpuscular Hemoglobin Concentration; RDW: Red cell Distribution Width; PLT: Platelet; MPV: Mean Platelet Volume Table 1. Peripheral Blood Differential
| C57BL/6 | CD26-/- | |||||
|---|---|---|---|---|---|---|
| Untreated | AMD3100 | Untreated | AMD3100 | |||
| Mean ± SEM | Mean ± SEM | p | Mean ± SEM | Mean ± SEM | p | |
| Leukocytes | ||||||
| WBC (K/ul) | 5.17 ± 0.37 | 13.26 ± 0.89 | 0.00 | 5.87 ± 0.68 | 17.33 ± 1.27 | 0.00 |
| NE (K/ul) | 1.75 ± 0.33 | 5.40 ± 0.37 | 0.00 | 2.19 ± 0.43 | 6.41 ± 0.58 | 0.00 |
| LY (K/ul) | 3.08 ± 0.32 | 7.32 ± 0.82 | 0.00 | 3.31 ± 0.36 | 9.84 ± 0.79 | 0.00 |
| MO (K/ul) | 0.29 ± 0.02 | 0.47 ± 0.05 | 0.00 | 0.28 ± 0.05 | 0.67 ± 0.11 | 0.00 |
| EO (K/ul) | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.70 | 0.07 ± 0.02 | 0.38 ± 0.17 | 0.09 |
| BA (K/ul) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.41 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.25 |
| Erythrocytes | ||||||
| RBC (M/ul) | 7.22 ± 0.53 | 8.28 ± 0.27 | 0.09 | 9.17 ± 0.32 | 8.41 ± 0.34 | 0.11 |
| Hb (g/dl) | 10.09 ± 0.83 | 11.87 ± 0.42 | 0.07 | 13.08 ± 0.43 | 12.16 ± 0.51 | 0.18 |
| Hct (%) | 33.89 ± 2.66 | 39.22 ± 1.37 | 0.09 | 42.16 ± 1.33 | 66.27 ± 26.56 | 0.37 |
| MCV (fL) | 46.57 ± 0.44 | 47.33 ± 0.43 | 0.23 | 46.05 ± 0.45 | 47.42 ± 0.32 | 0.02 |
| MCH (pg) | 13.82 ± 0.23 | 14.35 ± 0.23 | 0.11 | 14.27 ± 0.15 | 14.41 ± 0.18 | 0.55 |
| MCHC (g/dL) | 29.65 ± 0.29 | 30.30 ± 0.26 | 0.11 | 31.03 ± 0.33 | 30.51 ± 0.37 | 0.30 |
| RDW (%) | 16.68 ± 0.24 | 16.96 ± 0.18 | 0.37 | 16.60 ± 0.19 | 16.31 ± 0.24 | 0.36 |
| Thrombocytes | ||||||
| PLT (K/ul) | 691.07 ± 59.23 | 832.71 ± 49.38 | 0.08 | 917.71 ± 53.62 | 744.50 ± 58.02 | 0.04 |
| MPV (fL) | 4.04 ± 0.12 | 4.31 ± 0.14 | 0.15 | 4.88 ± 0.16 | 4.52 ± 0.16 | 0.13 |
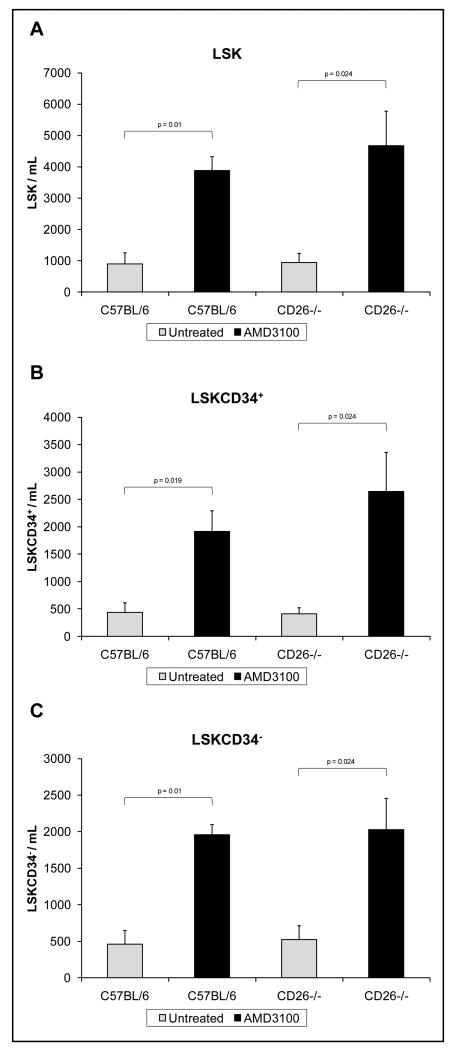
To evaluate changes in the percentage of circulating HSC/HPC, multivariate flow cytometric analysis was performed on cells isolated from the peripheral blood of mice. Sub-populations of cells were defined using cell surface markers as lin-Sca-1+c-kit+ [LSK] mixed hematopoietic stem and progenitor cells, LSKCD34+ short term-repopulating hematopoietic stem cells [ST-HSC] and multipotent progenitors [MPP], and LSKCD34- long term-repopulating hematopoietic stem cells [LT-HSC]. Although the level of LSKCD34-CD48- and LSKCD34-CD48-Flt3-Thy1-/loCD150+ cells within the LSKCD34- cell population was evaluated, the percentage of these cell sub-types was too low in the peripheral blood to adequately quantify with statistical accuracy. For example, the LSKCD34-CD48- cell population represented less than 0.001% of the total cell population in the peripheral blood of both untreated and AMD3100 treated mice. This is the equivalent of less than 20 cells per 2×106 flow cytometric events. Treatment of C57BL/6 mice with AMD3100 resulted in a 4.33 fold increase in LSK cells,, a 4.43 fold increase in LSKCD34+ cells, and a 4.23 fold increase in LSKCD34- cells 1 hour following injection (Figure 1); For CD26-/- mice, there was a 5.01, 6.45, and 3.88 fold increase in HSC/HPC subsets respectively. In both C57BL/6 and CD26-/- mice, the increase in peripheral blood LSK, LSKCD34+, and LSKCD34-, cells resulting from treatment with AMD3100 was significant (p≤0.05, n=6).
Figure 1.
The numbers of circulating (A) lin-Sca+c-kit+ [LSK], (B) LSKCD34+, and (C) LSKCD34-, hematopoietic stem and progenitor cells per mL in the peripheral blood following AMD3100 mobilization (n=6). Comparisons of untreated groups to AMD3100 treated groups were done using a non-parametric Mann Whitney U test.
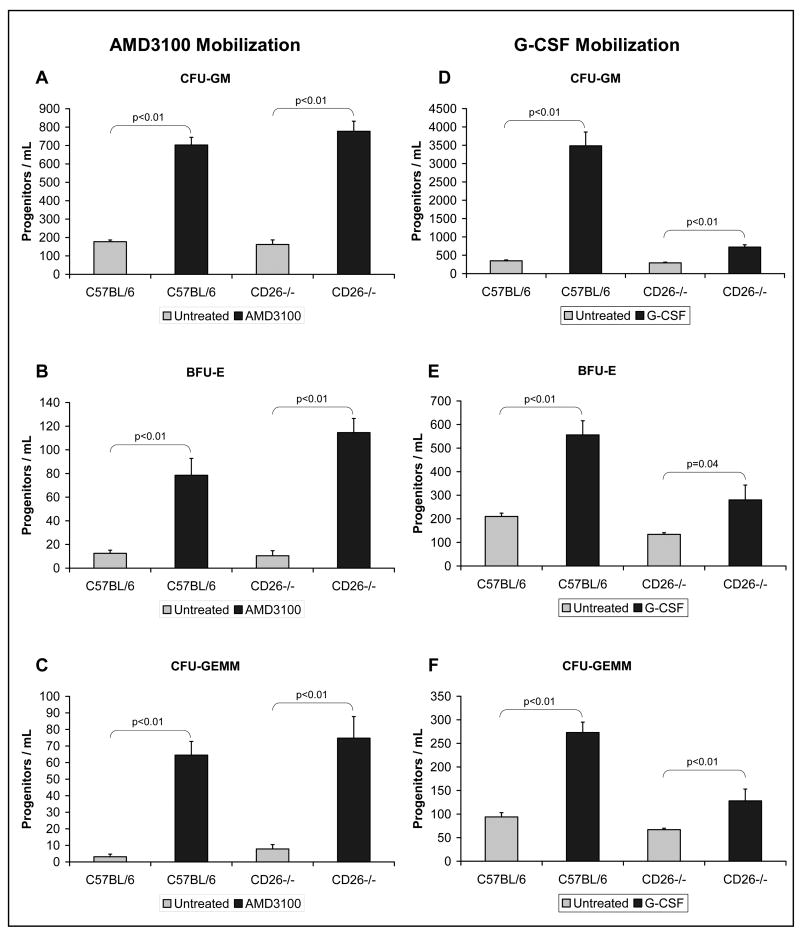
Methylcellulose-based myeloid colony assays were used to evaluate the numbers of myeloid progenitor cells present in the peripheral blood and bone marrow of mice. Increased numbers of circulating peripheral blood myeloid progenitors were observed in the peripheral blood of AMD3100-treated C57BL/6 and CD26-/- mice compared to control (untreated) mice (Figure 2, p≤0.01). Specifically, 4.0, 6.3, and 20.6 fold increases in, respectively, CFU-GM, BFU-E, and CFU-GEMM were observed in the peripheral blood of treated C57BL/6 mice. The numbers of myeloid progenitors observed in treated C57BL/6 mice were in the same range as seen in published literature [18-19] for both peripheral blood and bone marrow. Increases of 4.8, 11.0, and 9.5 fold in, respectively, CFU-GM, BFU-E, and CFU-GEMM were observed in the peripheral blood of CD26-/- mice after treatment. There were no significant differences in the numbers of circulating CFU-GM, BFU-E, and CFU-GEMM between C57BL/6 and CD26-/- mice either untreated or treated. This represents a major difference from what was seen when C57BL/6 and CD26-/- mice were treated with G-CSF. Previously published data, reproduced with permission (Christopherson et al., Experimental Hematology 31 (2003) 1126-1134), showed that C57BL/6 control mice exhibited a 13.3 fold increase in CFU-GM (Figure 2D), a 3.7 fold increase in BFU-E (Figure 2E), and a 6.6 fold increase in CFU-GEMM (Figure 2F) in response to treatment with G-CSF (2.5 μg 2×/day for 4 days). In contrast, CD26-/- mice exhibited smaller increases in response to G-CSF treatment when compared to untreated mice: a 3.2 fold increase in CFU-GM (Figure 2D), a 2.2 fold increase in BFU-E (Figure 2E), and a 2.7 fold increase in CFU-GEMM (Figure 2F). The data on G-CSF treatment of C57BL/6 mice is consistent with other published data for G-CSF. [19]
Figure 2.
The numbers of circulating myeloid progenitors, (A) granulocyte, macrophage [CFU-GM], (B) erythroid [BFU-E], and (C) granulocyte, erythroid, megakaryocyte, macrophage [CFU-GEMM]), in the peripheral blood following AMD3100-mobilization of C57BL/6 and CD26-/- mice as compared to untreated mice. Comparisons of untreated groups to AMD3100-treated groups were done using a two-tailed student t-test assuming equal variance, n=7-10 mice per group. Previously published data (Christopherson et al., Experimental Hematology 31 (2003) 1126-1134.) for G-CSF mobilization demonstrated that CD26-/- mice had poor mobilization after G-CSF treatment as compared to C57BL/6 mice. These data (D, E and F), reproduced with permission from Experimental Hematology, were also presented as the numbers of circulating myeloid progenitors including (D) CFU-GM, (E) BFU-E, and (F) CFU-GEMM.
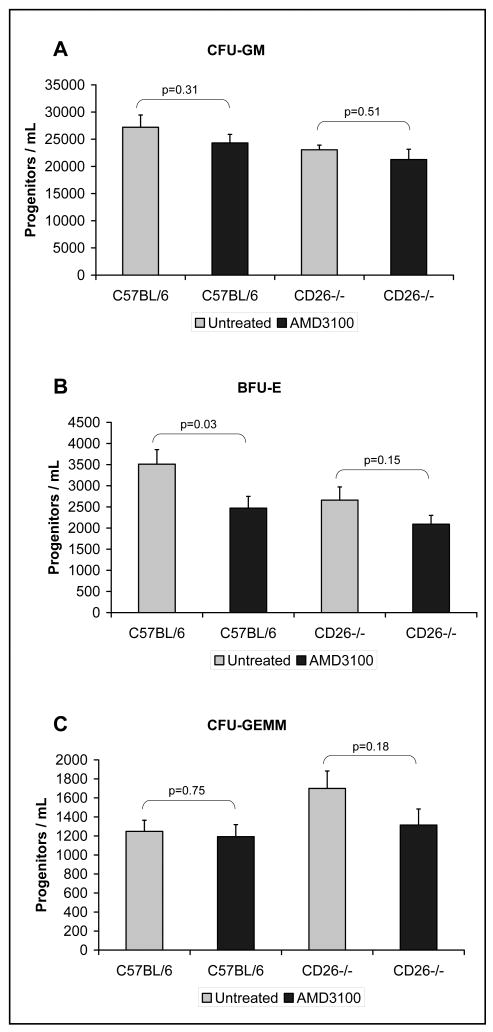
Some isolated changes in bone marrow progenitor content were noted in response to AMD3100, including a reduction in BFU-E and an increase in CFU-GEMM content in untreated CD26-/- mice compared to C57BL/6 mice (Figure 3). However, overall there were no differences in the number of myeloid progenitors across all lineages when untreated and AMD3100-treated bone marrow of C57BL/6 and CD26-/- mice were compared within its respective strain (Figure 3).
Figure 3.
The numbers of marrow derived myeloid progenitors, specifically (granulocyte, macrophage [CFU-GM], erythroid [BFU-E], and granulocyte, erythroid, megakaryocyte, macrophage [CFU-GEMM]), in the bone marrow of untreated and AMD3100-treated C57BL/6 control mice and CD26-/- mice. Comparisons of untreated groups to AMD3100-treated groups were done using a two-tailed student t-test assuming equal variance, n=7-10 mice per group.
Discussion
Pre-clinical and clinical data suggest that the CXCR4 antagonist AMD3100 rapidly mobilizes hematopoietic progenitor cells from the bone marrow into the periphery [15-16]. The results presented here provide pre-clinical evidence that AMD3100-induced inhibition of the binding of the chemokine CXCL12 to its receptor, CXCR4, mobilizes significant numbers of myeloid progenitors in mice in the absence of CD26. Whereas, G-CSF utilizes CD26 as part of the HSC/HPC mobilization mechanism. Although CD26-/- mice are not entirely deficient in G-CSF induced mobilization, G-CSF induced mobilization of HSC/HPC in CD26-/- mice is at a substantially reduced level when compared to wild type C57BL/6 mice following four days of G-CSF treatment. This suggests that G-CSF likely acts via multiple pathways, some of which are dependent on CD26, in order mobilize HSC/HPC.
These results support the idea that CD26 is downstream of G-CSF with respect to mobilization. These results also suggest the potential use of AMD3100 as a single-drug mobilizing agent to treat patients who have a decreased ability to respond to G-CSF treatment because of a reduction or loss of CD26 activity. An additional mobilizing regimen for poor G-CSF responders would increase the chances of a successful stem cell transplant. In addition, AMD3100 appears to have minimal side effects in donors. A clinical trial in which 25 HLA-matched sibling donors were mobilized with AMD3100 alone showed no toxicities in donors greater than grade one [20]. No alterations in CD26 expression or activity in normal donors or patients undergoing G-CSF-induced mobilization for transplant purposes have been reported. However, a recent study comparing AMD3100 and G-CSF in C57BL/6 mice showed that c-kit+ cells mobilized by AMD3100 retained their CD26 and CXCR4 expression, whereas c-kit+ cells mobilized by G-CSF did not [18]. These expression data indicate that G-CSF-induced mobilization occurs upstream of CD26 whereas, AMD3100-induced mobilization does not. Additionally, a previously reported chromosomal linkage analysis comparing mouse strains that were poor or good responders to G-CSF has suggested that the difference in mobilization response is due, at least in part, to genes located in a region on mouse chromosome 2 between genetic markers D2Mit83 [21] at 16.0 cM [22] and D2Mit229 [21] at 99.0 cM [23]. Interestingly, CD26 is located on chromosome 2 at 35.0 cM [24], which is positioned within this region and suggested by linkage analysis to be important for mobilization.[11] These published studies, in combination with our results, provides strong evidence that CD26 plays a pivotal role in mobilization by G-CSF. In addition, AMD3100 is able to by-pass the CD26 mechanism and successfully mobilize hematopoietic stem and progenitor cells. Future studies are warranted to evaluate potential variations in CD26 levels or activity in the general population, in different patient populations, and during different treatment regimens. Some individual patients or patient populations may benefit from the use of AMD3100 alone over G-CSF alone or AMD3100 in combination with G-CSF.
Acknowledgments
KWC was supported during these projects by the American Association for Cancer Research (07-10-19-CHRI), the Leukemia & Lymphoma Society (6044-08), the National Blood Foundation / American Association of Blood Banks (031824), the NIH – National Institute of Diabetes and Digestive and Kidney Diseases award (DK074892), the Rubschlager Foundation, and The Coleman Foundation.
Footnotes
Conflict-of-interest disclosure: No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duhrsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988;72:2074–2081. [PubMed] [Google Scholar]
- 2.Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD. Granulocyte-macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet. 1988;1:1194–1198. doi: 10.1016/s0140-6736(88)92012-0. [DOI] [PubMed] [Google Scholar]
- 3.Molineux G, Migdalska A, Szmitkowski M, Zsebo K, Dexter TM. The effects on hematopoiesis of recombinant stem cell factor (ligand for c-kit) administered in vivo to mice either alone or in combination with granulocyte colony-stimulating factor. Blood. 1991;78:961–966. [PubMed] [Google Scholar]
- 4.Brasel K, McKenna HJ, Morrissey PJ, et al. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. [PubMed] [Google Scholar]
- 5.Orazi A, Cattoretti G, Schiro R, et al. Recombinant human interleukin-3 and recombinant human granulocyte-macrophage colony-stimulating factor administered in vivo after high-dose cyclophosphamide cancer chemotherapy: effect on hematopoiesis and microenvironment in human bone marrow. Blood. 1992;79:2610–2619. [PubMed] [Google Scholar]
- 6.Laterveer L, Lindley IJ, Hamilton MS, Willemze R, Fibbe WE. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85:2269–2275. [PubMed] [Google Scholar]
- 7.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 8.Levesque JP, Liu F, Simmons PJ, et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 9.Papayannopoulou T, Priestley GV, Bonig H, Nakamoto B. The role of G-protein signaling in hematopoietic stem/progenitor cell mobilization. Blood. 2003;101:4739–4747. doi: 10.1182/blood-2002-09-2741. [DOI] [PubMed] [Google Scholar]
- 10.Pruijt JF, Verzaal P, van Os R, et al. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci U S A. 2002;99:6228–6233. doi: 10.1073/pnas.092112999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christopherson KW, 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–4686. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- 12.Christopherson KW, Cooper S, Hangoc G, Broxmeyer HE. CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26-/- mice. Exp Hematol. 2003;31:1126–1134. doi: 10.1016/j.exphem.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 14.Lambeir AM, Proost P, Durinx C, et al. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276:29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- 15.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marguet D, Baggio L, Kobayashi T, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A. 2000;97:6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonig H, Chudziak D, Priestley G, Papayannopoulou T. Insights into the biology of mobilized hematopoietic stem/progenitor cells through innovative treatment schedules of the CXCR4 antagonist AMD3100. Exp Hematol. 2009;37:402–415. e401. doi: 10.1016/j.exphem.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HM, Wysoczynski M, Liu R, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2010;24:573–582. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112:990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa M, Baldwin TM, Metcalf D, Foote SJ. Progenitor cell mobilization by granulocyte colony-stimulating factor controlled by loci on chromosomes 2 and 11. Blood. 2000;95:1872–1874. [PubMed] [Google Scholar]
- 22.MGI:92220 Mouse Genome Database (MGD): Mouse Genome Informatics: The Jackson Laboratory. Bar Harbor Maine. 2010 [Google Scholar]
- 23.MGI:92096 Mouse Genome Database (MGD): Mouse Genome Informatics: The Jackson Laboratory. Bar Harbor Maine. 2010 [Google Scholar]
- 24.MGI:94919 Mouse Genome Database (MGD): Mouse Genome Informatics: The Jackson Laboratory. Bar Harbor Maine. 2010 [Google Scholar]