Abstract
Background
The activity of transient receptor potential vanilloid subtype-1 (TRPV1) receptors, key nociceptive transducers in dorsal root ganglion sensory neurons, is enhanced by protein kinase C ε (PKCε) activation. The intravenous anesthetic propofol has been shown to activate PKCε. Our objectives were to examine whether propofol modulates TRPV1 function in dorsal root ganglion neurons via activation of PKCε.
Methods
Lumbar dorsal root ganglion neurons from wild-type and PKCε-null mice were isolated and cultured for 24 h. Intracellular free Ca2+ concentration was measured in neurons by using fura-2 acetoxymethyl ester. The duration of pain-associated behaviors was also assessed. Phosphorylation of PKCε and TRPV1 and the cellular translocation of PKCε from cytosol to membrane compartments were assessed by immunoblot analysis.
Results
In wild-type neurons, repeated stimulation with capsaicin (100 nM) progressively decreased the transient rise in intracellular free Ca2+ concentration. After desensitization, exposure to propofol rescued the Ca2+ response. The resensitizing effect of propofol was absent in neurons obtained from PKCε-null mice. Moreover, the capsaicin-induced desensitization of TRPV1 was markedly attenuated in the presence of propofol in neurons from wild-type mice but not in neurons from PKCε-null mice. Propofol also prolonged the duration of agonist-induced pain associated behaviors in wild-type mice. In addition, propofol increased phosphorylation of PKCε as well as TRPV1 and stimulated translocation of PKCε from cytosolic to membrane fraction.
Discussion
Our results indicate that propofol modulates TRPV1 sensitivity to capsaicin and that this most likely occurs through a PKCε-mediated phosphorylation of TRPV1.
Transient receptor potential vanilloid subtype-1 (TRPV1) receptors are expressed mainly by peripheral sensory neurons and serve as detectors of pain-producing chemical and thermal stimuli.1,2 They act as nonselective cation channels with high Ca2+ permeability and can be distinguished from other sensory transducers by their sensitivity to capsaicin.3 A variety of chemical agents acutely sensitize TRPV1 receptors to thermal stimuli and result in the sensation of pain,4–8 whereas repetitive stimulation of the receptor causes desensitization, which plays an important role in blocking pain transmission.5,6,9 The cellular mechanisms mediating sensitization, desensitization, and resensitization of the receptor are thought to involve changes in receptor phosphorylation.9–11 Specifically, activation of PKCε seems to mediate sensitization of TRPV1 to agonist stimulation via phosphorylation of TRPV1 at serine 800 (S800).5,12,13
Propofol is one of the most commonly used intravenous anesthetics for the induction and maintenance of general anesthesia and sedation. Apart from its anesthetic properties, propofol has several nonanesthetic effects, one of which is the modulation of pain. A recent study indicated that propofol increases intracellular free Ca2+ concentration ([Ca2+]i) in transfected human embryonic kidney 293 cells via a transient receptor potential ankrin subtype-1 (TRPA1)-dependent pathway.14 Moreover, recent evidence has suggested that functional regulation of the transient receptor potential family of receptors may be mediated by cross-talk between family members, particularly TRPV1 and TRPA1.15,16 In addition, our laboratory has previously shown that propofol activates PKCε in isolated cardiomyocytes17 as well as recombinant PKCε,18 suggesting that propofol may activate PKCε in dorsal root ganglion (DRG) neurons and potentially modulate TRPV1 sensitivity to agonist-stimulation.
In the current study, we tested the hypothesis that propofol not only restores the sensitivity of desensitized TRPV1 receptors, but also attenuates agonist-induced desensitization of TRPV1 in DRG neurons via a PKCε-dependent pathway. The major findings are that pretreatment with propofol or the PKCε activator peptide (ψεRACK) resensitizes TRPV1 receptors. Moreover, the PKCε selective inhibitor peptide (εV1-2) prevents the propofol-induced rescue of TRPV1 sensitivity, and propofol fails to exert any effect on restoration of TRPV1 in DRG neurons isolated from PKCε-null mice. Propofol attenuates agonist-induced desensitization of TRPV1 in DRG neurons isolated from wild-type (WT) mice but not in neurons isolated from PKCε-null mice. Moreover, propofol prolongs the duration of agonist-induced pain-associated behaviors in mice. Propofol also increases the membrane association of PKCε as well as the phosphorylation of PKCε at S729. Finally, propofol increases the PKCε-dependent phosphorylation of TRPV1 at S800. Our current findings indicate that propofol can resensitize TRPV1 receptors and attenuate agonist-induced desensitization of TRPV1 via a PKCε-dependent phosphorylation of TRPV1.
Materials and Methods
Animals
Twelve-week-old male C57BL/6 PKCε-null mice (PKCε−/−) and age/sex-matched PKCε WT mice (PKCε+/+) were used. PKCε+/− mice breeding pairs were kindly provided by Robert O. Messing, M.D. (Ernest Gallo Clinic and Research Center, University of California at San Francisco, San Francisco, California). All animals were housed at an animal care facility at Kent State University (Kent, Ohio) that is accredited by the American Association for Accreditation of Laboratory Animal Care.
DRG Cell Isolation and Culture
DRG neurons from WT and PKCε-null adult mice (30–40 g) were used in this study. The ganglia were dissected from the lumbar (L1–L6) segments of the spinal cord and incubated with collagenase (type IV, 0.15%) at 37°C for 50 min and dissociated by gentle trituration. Neurons were cultured on coverglasses precoated with poly-D-lysine and laminin at 37°C in Ham’s F-12 medium/Dulbecco’s modified Eagle’s medium (50/50) supplemented with 10% fetal bovine serum and antibiotics in a humidified atmosphere of 5% CO2 and 95% air. Proliferation of fibroblasts and Schwann cells was prevented by including cytosine arabinoside (5–10 μM) in the medium. Cells began to develop neurites within ~24 h, and studies were performed within 24 h from the time of isolation.
Intracellular Ca2+ Measurements
DRG neurons were incubated at room temperature (23°C) for 15 min with fura-2 acetoxymethyl ester (2 μM) in HEPES-buffered saline containing the following: 118 mM NaCl, 4.8 mM KCl, 1.2 mM MgCl2, 1.25 mM CaCl2, 11.0 mM dextrose, 5 mM pyruvate, and 25 mM HEPES, pH 7.35. Coverslips containing the fura-2 acetoxymethyl ester-loaded DRG neurons were placed in a temperature-regulated (30°C) chamber (Warner Instruments, Hamden, CT) mounted on the stage of an Olympus IX-81 inverted fluorescence microscope (Olympus America, Lake Success, NY). The cells were superfused continuously with HEPES-buffered saline at a flow rate of 2 ml/min. Drugs were delivered by switching from control buffer to drug-containing buffer for 20 s unless noted otherwise. [Ca2+]i measurements were simultaneously performed on multiple individual DRG neurons using a fluorescence imaging system (Easy Ratio Pro; Photon Technology International, Lawrenceville, NJ) equipped with a multi wavelength spectrofluorometer (DeltaRAM X) and a QuantEM 512SC electron multiplying charge-coupled device camera (Photometrics, Tuscan, AZ). Images and photometric data were acquired by alternating excitation wavelengths between 340 and 380 nm (20 Hz) and monitoring an emission wavelength of 510 nm. Because calibration procedures rely on a number of assumptions, the ratio of the light intensities at the two wavelengths was used to measure qualitative changes in [Ca2+]i. Just before data acquisition, background fluorescence was measured and automatically subtracted from the subsequent experimental measurement by using Easy Ratio Pro.
Behavioral Experiments
In vivo assessment of pain-associated behaviors in WT mice were assessed as described by Matta et al.14 with slight modifications. Capsaicin and/or propofol were applied to the nasal epithelium of WT mice via a cotton applicator, and the duration (in seconds) of pain-associated behaviors (nose wiping in and out of sawdust bedding) was assessed and measured by a blinded observer.
Subcellular Fractionation of DRG Neurons
Fractionation of mouse DRG neurons was performed as previously reported for cardiomyocytes with slight modifications.17 After treatment with propofol, DRG neurons were pelleted by centrifugation (45 s at 800g) and washed with ice cold HEPES-buffered saline. Neurons were resuspended in lysis buffer (4 mM MgATP, 100 mM KCl, 10 mM imidazole, 2mM EGTA, 1 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 10 mM benzamidine, and 0.01 mM leupeptin) containing 20% glycerol and 0.05% Triton X-100. Cell permeabilization and membrane solubilization were facilitated by sonication for 5 min on ice. After centrifugation at 800g for 2 min, a nuclear, mitochondrial, and undigested cellular pellet was isolated. The supernatant was collected and labeled as supernatant 1. The pellet was resuspended in lysis buffer containing 20% glycerol and 0.05% Triton X-100 and sonicated for 5 min on ice. After another round of centrifugation, the supernatant was collected and added to supernatant 1. Supernatant 1 was centrifuged at 100,000g for 30 min at 4°C, producing a pellet (designated as the total cellular membrane fraction) and a supernatant (designated as the cytosolic fraction).
Immunoblot Analysis of PKCε and TRPV1 (Total and Phosphorylated)
Immunoblot analysis was carried out on whole DRG cell lysates as well as cytosolic and membrane fractions as described previously in cardiomyocytes with slight modifications.17 Protein concentration was assessed using the Bradford method.19 All samples were adjusted to a protein concentration of 1–2 mg/ml in sample buffer, boiled for 5 min, and then kept at −20°C until use. Equal amounts of protein (50 μg) from each fraction were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% polyacrylamide gels and transferred to nitrocellulose membranes. Nonspecific binding was blocked with Tris-buffered saline solution (0.1% [v/v] Tween 20 in 20 mM Tris base, 137 mM NaCl adjusted to pH 7.6 with HCl), containing 3% (w/v) bovine serum albumin for 1 h at room temperature. Monoclonal antibodies against total PKCε and polyclonal antibodies against S729-phosphorylated PKCε (PKCεpS729), total TRPV1, and S800-phosphorylated TRPV1 (TRPV1pS800) were diluted 1:1,000 in Trisbuffered saline containing 1% bovine serum albumin for immunoblotting (2 h). After washing three times for 10 min each in Tris-buffered saline, membranes were incubated for 1 h at room temperature with horseradish-peroxidase-linked secondary antibody (goat antimouse and goat antirabbit; 1:5,000 dilution in Tris-buffered saline containing 1% bovine serum albumin). Membranes were again washed, and bound antibody was detected by enhanced chemiluminescence. Immunoreactivity was quantified by scanning densitometry and analyzed using ImageJ software (National Institutes of Health, Washington, DC).
Immunocytochemistry of TRPV1
Mouse DRG neurons were cultured for 2 days on glass coverslips before fixation in 4% formaldehyde for 25 min. Fixed cells were blocked and permeabilized in phosphate-buffered saline containing 3% normal goat serum and 0.3% Triton X-100 and were incubated overnight at 4°C with anti-TRPV1 polyclonal antibody. Nonspecific binding was blocked using 5% milk in a phosphate-buffered saline solution for 1 h at room temperature. Coverslips were washed four times (at 5, 10, 10, and 10 min, respectively) with phosphate-buffered saline before being placed into a light-insulated container. Fluorescein-5-isothiocyanate (1:200) secondary antibody was incubated for 1 h at room temperature. After washing as described above, the coverslips were placed on slides with an 80% Vectashield (Vector Laboratories, Burlingame, CA) solution. Images were acquired using a laser-scanning spectral confocal microscope (TCS AOBS SP2; Leica, Heidelberg, Germany) with a 40× oil immersion objective (numerical aperture = 1.4) at zoom 2. An Argon laser was used to excite fluorescein-5-isothiocyanate-labeled TRPV1 at 488 nm. For background removal, a photomultiplier tube offset value was chosen and kept constant, eliminating any issues of image variability. Emission was collected between 500 and 550 nm for fluorescein-5-isothiocyanate.
Statistical Analysis
All experimental protocols were repeated in a minimum of five DRG neurons obtained from at least five different mice. Results obtained from each animal were averaged so that all animals were weighted equally. Within-group comparisons were made using repeated measures one-way analysis of variance and the Bonferroni post hoc test. Comparisons between groups were made using two-way analysis of variance. Differences were considered statistically significant at P < 0.05. All results are expressed as mean ± SD. Statistical analysis was conducted using NCSS software (Kaysville, UT).
Experimental Protocols
DRG neurons were chosen for study by distinguishing small (less than 30 μm) nociceptive neurons (capsaicin-sensitive) from large (more than 40 μm) mechanosensitive neurons (capsaicin-insensitive) using a precalibrated eyepiece reticle to measure the diameter of the neuronal cell body. The Diprivan® lipid emulsion form of propofol (56 mM [10 mg/ml] propofol, 10% soybean oil, 2.25% glycerol, 1.2% purified phospholipid) was used in all protocols and will be referred to as propofol throughout the article. Intralipid vehicle was used as a control. The effect of Intralipid was examined at amounts equivalent to those used for the propofol-Intralipid mixture (Diprivan®).
Protocol 1: Effect of Propofol on Restoration of TRPV1 Sensitivity in Mouse DRG Neurons
To determine the extent to which propofol is capable of resensitizing TRPV1 receptors, [Ca2+]i was monitored in DRG neurons that were repeatedly exposed to capsaicin (100 nM) every 30 s to induce desensitization of the channel. After desensitization, neurons were subsequently exposed to propofol (1, 5, and 10 μM; 10 min) or intralipid (10 μM; 10 min) vehicle before restimulation of TRPV1 receptors with capsaicin. Summarized results are expressed as a percentage of the response to the final application of capsaicin in the untreated control.
Protocol 2: Effect of PKCε Inhibition on Propofol-induced Restoration of TRPV1 Sensitivity in Mouse DRG Neurons
To determine the extent to which PKCε is involved in thepropofol-induced resensitization of TRPV1 receptors, DRG neurons were repeatedly exposed to capsaicin as described in protocol 1. After desensitization, neurons were pretreated with εV1-2 (0.5 μM) and then exposed to propofol (10 μM) in the continued presence of εV1-2. Parallel experiments demonstrating the effect of ψεRACK (0.5 μM) on resensitization of TRPV1 receptors in the absence of propofol were also performed. Summarized results are expressed as a percentage of the response to the final application of capsaicin in the untreated control.
Protocol 3: Effect of Propofol on Restoration of TRPV1 Sensitivity in DRG Neurons Isolated from PKCε-null Mice
To support our pharmacological findings of a role for PKCε in the propofol-induced resensitization of TRPV1 (protocols 1 and 2), protocol 1 was repeated in PKCε-null mice using 10 μM propofol. Summarized results are expressed as a percentage of the final application of capsaicin in the presence of propofol in DRG neurons from WT mice (control).
Protocol 4: Effect of Propofol on Agonist-induced Desensitization of TRPV1 in Mouse DRG Neurons
To determine the extent to which propofol may alter agonist-induced desensitization of TRPV1, [Ca2+]i was monitored in DRG neurons that were repeatedly exposed to capsaicin (100 nM) every 30 s to induce desensitization of the channel. After desensitization, neurons were subsequently exposed to propofol (1, 5, and 10 μM; 30 min) or Intralipid (10 μM;30 min), and DRG neurons were again repeatedly exposed to capsaicin every 30 s. The percentage reduction in the capsaicin response (desensitization) in untreated DRG neurons was compared with the capsaicin response obtained in propofol-treated DRG neurons.
Protocol 5: Effect of Propofol on Agonist-induced TRPV1 Desensitization in DRG Neurons Isolated from PKCε-null Mice
To determine the extent to which PKCε is involved in the propofol-induced attenuation of agonist-induced desensitization of TRPV1 receptors, Protocol 4 was repeated in PKCε-null mice using 10 μM propofol. The percentage reduction in the capsaicin response in propofol-treated DRG neurons from WT mice was compared with the capsaicin response obtained in propofol-treated DRG neurons from PKCε-null mice.
Protocol 6: Effect of Propofol on Agonist-induced Pain-associated Behaviors in Mice
To further characterize the extent to which propofol may alter TRPV1 sensitivity, pain-associated behaviors in response to topical application of cap-saicin were assessed in WT mice. Specifically, capsaicin (500 μM, 20 μl) was applied to the nasal epithelium, and the duration of pain-associated behaviors was assessed. After the initial exposure to capsaicin, mice were treated with and without propofol (50% in mineral oil) for 10 min, and the duration of pain-associated behaviors was assessed. Capsaicin (500 μM, 20 μl) was then reapplied, and the duration of pain-associated behavior was reassessed.
Protocol 7: Effect of Propofol on PKCε Phosphorylation at S729 in Mouse DRG Neurons
To determine the extent to which propofol stimulates the phosphorylation of PKCε at S729, a key mechanism in PKCε activation, immunoblot analysis of S729 phosphorylated PKCε (PKCεpS729) in DRG neuronal whole cell fractions was performed before and after treatment with propofol (10 μM; 10 min) in the presence or absence of εV1-2 (0.5 μM). Parallel experiments demonstrating the effect of ψεRACK (0.5 μM) and Intralipid vehicle (10 μM) on PKCε S729 phosphorylation were also performed. PKCεpS729 levels were normalized to total PKCε protein levels detected in the DRG lysates. Summarized results are expressed as a percentage of the untreated control.
Protocol 8: Effect of Propofol on PKCε Translocation from Cytosolic to Membrane Fractions and PKCε Intracellular Localization in Mouse DRG Neurons
To determine the extent to which propofol stimulates the cellular redistribution of PKCε in DRG neurons, immunoblot analysis of PKCε in DRG neuronal subcellular fractions and immunocytochemical localization of PKCε in intact DRG neurons was performed before and after treatment with propofol (10 μM; 10 min) in the presence or absence of εV1-2 (0.5 μM). Parallel experiments demonstrating the effect ψεRACK (0.5 μM) and Intralipid vehicle (10 μM) on PKCε translocation were also performed. Summarized results for immunoblot analysis of PKCε are expressed as a percentage change in PKCε redistribution from the cytosolic to membrane subfraction. Membrane content of PKCε was normalized to membrane content of porin, a membrane-associated protein.
Protocol 9: Effect of Propofol on TRPV1 Phosphorylation at S800 in Mouse DRG Neurons
To determine the extent to which propofol stimulates phosphorylation of TRPV1 at S800 in DRG neurons, protocol 2 was repeated in WT and PKCε-null DRG neurons, and then immunoblot analysis of S800 phosphorylated TRPV1 (TRPV1pS800) was performed. In addition, TRPV1pS800 levels in the presence of propofol were also measured in parallel using DRG neurons isolated from PKCε-null mice. TRPV1pS800 levels are normalized to total TRPV1 protein levels detected in the lysate. Summarized results are expressed as arbitrary units.
Materials
Anti-PKCε and anti-PKCεpS729 antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-TRPV1pS800 antibody was kindly provided by Makoto Tominaga, Ph.D. (National Institute of Natural Sciences, Okazaki, Japan). Capsaicin was purchased from Sigma Chemical Co. (St. Louis, MO). εV1-2 and ψεRACK were synthesized and purified by high-performance liquid chromatography by the Molecular Biotechnology Core facility (Lerner Research Institute at Cleveland Clinic, Cleveland, OH). Propofol (Diprivan formulation) and Intralipid were obtained from Cleveland Clinic Pharmacy (Cleveland, OH). Collagenase was obtained from Worthington Biochemical Corp. (Lakewood, NJ).
Results
Effect of Propofol on Restoration of TRPV1 Sensitivity in Mouse DRG Neurons
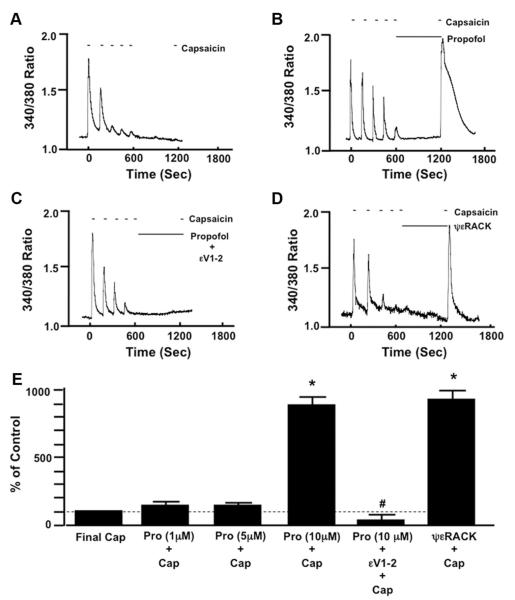
Repetitive stimulation of DRG neurons with capsaicin resulted in a progressive decrease (desen-sitization) in peak [Ca2+]i that was maintained after a 10-min pause in capsaicin stimulation as indicated by the lack of any response to capsaicin after reapplication of capsaicin to the bath (fig. 1A). In contrast, when propofol (10 μM) was added to the bath during the 10-min pause in stimulation, subsequent reapplication of capsaicin resulted in a robust transient increase in [Ca2+]i (resensitization) (fig. 1B). Intralipid vehicle alone (amount equivalent to that added with 10 μM propofol) was unable to restore TRPV1 sensitivity to capsaicin (98.3 ± 7% of control).
Fig. 1.
Representative traces depicting the effect of time (A), propofol (10 μM; B), propofol in the presence of the specific protein kinase C epsilon (PKCε) inhibitor peptide (εV1-2; 0.5 μM; C), and the specific PKCε activator peptide (ψεRACK; 0.5 μM; D) after capsaicin-induced (100 nM) desensitization on restoration of transient receptor potential vanilloid receptor type 1 sensitivity in mouse dorsal root ganglion neurons. Summarized data for A–D, including 1 and 5 μM propofol, are depicted in E. Data are expressed as a percentage of the response to the final application of capsaicin (Cap) in the untreated control (% of Control). * P < 0.05 compared with final Cap in the untreated control. # P < 0.05 compared with propofol (Pro) plus Cap. n = DRG neurons from six different mice.
Effect of PKCε Inhibition on Propofol-induced Restoration of TRPV1 Sensitivity in Mouse DRG Neurons
Pretreatment with εV1-2 (0.5 μM) inhibited the propofol-induced restoration of TRPV1 sensitivity compared with DRGs treated only with propofol (fig. 1C). Likewise, pretreatment with ψεRACK (0.5 μM) restored TRPV1 sensitivity (fig. 1D). Summarized data depicting the effect of propofol alone (1, 5, and 10 μM), propofol (10 μM) in the presence of εV1-2 (0.5 μM) or ψεRACK (0.5 μM) on TRPV1 resensitization are depicted in figure 1E.
Effect of Propofol on Restoration of TRPV1 Sensitivity in DRG Neurons Isolated from PKCε-null Mice
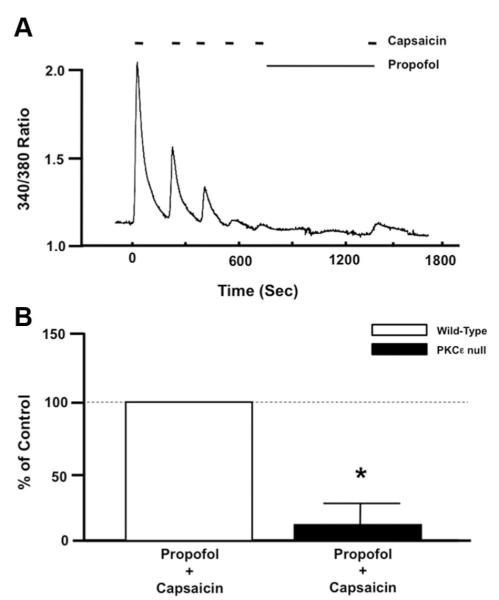
In DRG neurons obtained from PKCε-null mice, the propofol (10 μM) failed to restore TRPV1 sensitivity to capsaicin (fig. 2A). Summarized data depicting the effect of propofol (10 μM) on TRPV1 resensitization in DRG neurons obtained from WT and PKCε-null mice are depicted in figure 2B.
Fig. 2.
(A) Representative trace depicting the effect of propofol (10 μM) after capsaicin-induced (100 nM) desensitization on restoration of transient receptor potential vanilloid receptor type 1 (TRPV1) sensitivity in dorsal root ganglion (DRG) neurons isolated from protein kinase C ε (PKCε)-null mice. (B) Summarized data depicting the effect of propofol after capsaicin-induced desensitization on restoration of TRPV1 sensitivity in DRG neurons isolated from wild-type (open bar) and PKCε-null (closed bar) mice. Data is expressed as a percentage of the response to the final application of capsaicin in the untreated control (% of Control). * P < 0.05 compared with propofol plus capsaicin treated wild-type DRG neurons. n = DRG neurons from six different PKCε-null mice.
Effect of Propofol on Agonist-induced Desensitization of TRPV1 in Mouse DRG Neurons
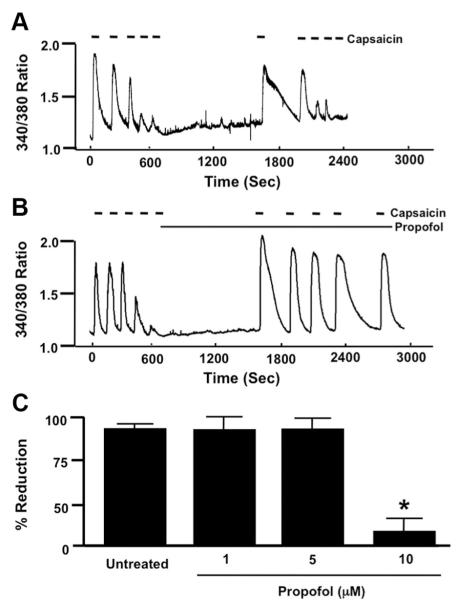
After successive applications of capsaicin to induce TRPV1 desensitization, a 30-min pause in capsaicin stimulation was able to restore TRPV1 responsiveness to capsaicin as indicated by the robust transient increase in [Ca2+]i upon reapplication of capsaicin (fig. 3A). Subsequent successive reapplications of capsaicin resulted in a progressive decrease (desensitization) in peak [Ca2+]I (fig. 3A). In contrast, when propofol (10 μM) was added to the bath during the 30-min pause in stimulation, successive applications of capsaicin did not result in desensitization in peak [Ca2+]i (fig. 3B). Moreover, when Intralipid (amount of Intralipid equivalent to that added with 10 μM propofol) was added to the bath during the 30-min pause in stimulation, successive applications of capsaicin resulted in desensitization in peak [Ca2+]i (92.3 ± 3% reduction). Summarized data depicting the effect of propofol (1, 5, and 10 μM) on capsaicin-induced desensitization of TRPV1 receptors are depicted in figure 3C.
Fig. 3.
(A) Representative control trace depicting the effect of a 30-min rest period after capsaicin-induced (100 nM) desensitization on restoration of transient receptor potential vanilloid receptor type 1 (TRPV1) sensitivity and subsequent capsaicin-induced desensitization of TRPV1 in mouse dorsal root ganglion (DRG) neurons. (B) Same as in A with the addition of propofol (10 μM) after initial capsaicin-induced desensitization of TRPV1. (C) Summarized data for A and B including 1 and 5 μM propofol. Data are expressed as a percentage reduction in peak [Ca2+]i response to capsaicin before and after desensitization (measure of desensitization) as indicated by brackets in untreated and propofol-treated DRG neurons. * P < 0.05 compared with percentage reduction in untreated wild-type DRG neurons.
Effect of Propofol on Agonist-induced Desensitization of TRPV1 in Mouse DRG Neurons Isolated from PKCε-null Mice
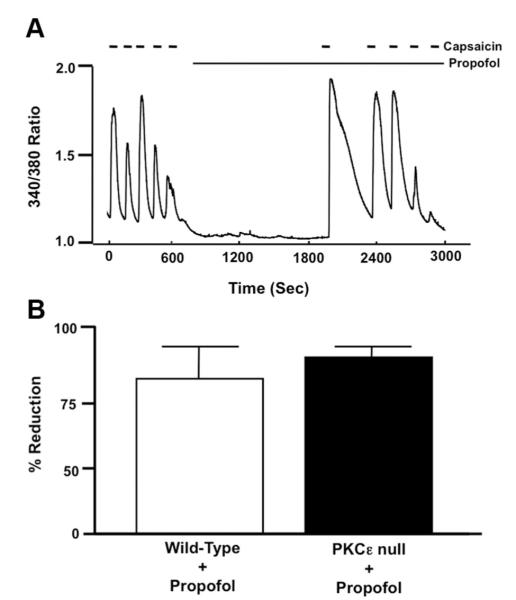
In DRG neurons obtained from PKCε-null mice, successive applications of capsaicin in the presence of propofol resulted in a progressive decrease (desensitization) in peak [Ca2+]I (fig. 4A). Summarized data depicting the effect of propofol (1, 5, and 10 μM) on capsaicin-induced desensitization of TRPV1 receptors in DRG neurons obtained from PKCε-null mice is depicted in figure 4B.
Fig. 4.
(A) Representative trace depicting the effect of propofol (10 μM) after initial capsaicin-induced desensitization of transient receptor potential vanilloid receptor type 1 (TRPV1) on restoration of TRPV1 sensitivity and subsequent capsaicin-induced desensitization in mouse dorsal root ganglion (DRG) neurons isolated from protein kinase Cε (PKCε)-null mice. (B) Summarized data for A. Data are expressed as a percentage reduction in peak [Ca2+]i response to capsaicin before and after desensitization (measure of desensitization) as indicated by brackets in propofol-treated DRG neurons from wild-type (open bar) and PKCε-null mice (closed bar).
Effect of Propofol on Agonist-induced Pain-associated Behaviors in Mice
Initial exposure of capsaicin (500 μM, 20 μl) to the nasal epithelium of untreated control animals resulted in pain-associated behaviors that lasted 154 ± 13 s (fig. 5A). After the 10-min rest period, the subsequent reapplication of capsaicin resulted in pain-associated behaviors that lasted 98 ± 11 s (36 ± 4% reduction) (fig. 5A). However, when propofol was applied during the 10-min rest period and capsaicin was then reapplied, the duration of pain-associated behaviors was 219 ± 19 s compared with 142 ± 14 s for the initial application of capsaicin (54.4 ± 10.5% increase) (fig. 5A). In addition, application of propofol during the rest period resulted in pain-associated behaviors lasting 147 ± 21 s. Summarized data depicting the effect of propofol on agonist-induced pain-associated behaviors is depicted in figure 5B.
Fig. 5.
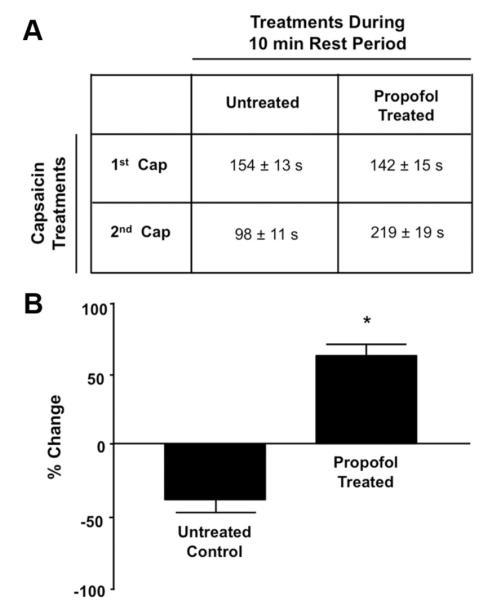
(A) Duration (in seconds [s]) of pain-associated behaviors (nose-wiping in and out of bedding) induced by two topical applications of capsaicin (Cap; 500 μM) separated by a 10-min rest period (untreated group). In the propofol-treated group, propofol (50% in mineral oil) was applied topically during the 10-min rest period. (B) Summarized data for A. Data are expressed as a percentage change in duration of capsaicin-induced pain-associated behaviors in untreated mice compared with the duration observed in propofol-treated mice. * P < 0.05 compared with percentage change in untreated control.
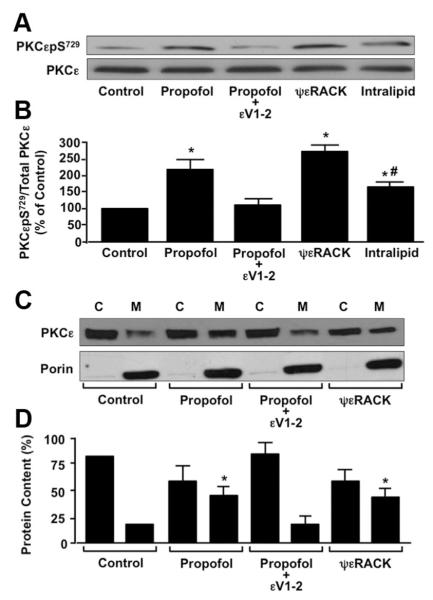
Effect of Propofol on PKCε Phosphorylation at S729 in Mouse DRG Neurons
Upon exposure of DRG neurons to propofol (10 μM), PKCεpS729 levels increased by 1.5 fold compared with untreated control (fig. 6A). The propofol-induced increase in PKCεpS729 was attenuated in DRG neurons treated with propofol in the presence of εV1-2 (0.5 μM) (fig. 6A). Moreover, pretreatment of DRG neurons with ψεRACK (0.5 μM), and Intralipid increased PKCεpS729 levels compared with control (fig. 6A). Summarized data depicting the effect of propofol (10 μM) alone, propofol in the presence of εV1-2 (0.5 μM), ψεRACK (0.5 μM) alone, and Intralipid (10 μM) alone on PKCεpS729 levels in mouse DRG neurons are depicted in figure 6B.
Fig. 6.
(A) Representative immunoblot depicting the effect of propofol (10 μM) alone, propofol in the presence of the protein kinase C epsilon (PKCε) inhibitor peptide εV1-2 (0.5 μM), PKCε activator peptide ψεRACK (0.5 μM), or Intralipid (amount equivalent to 10 μM propofol) alone on PKCε serine 729 phosphorylation (PKCεpS729) in mouse dorsal root ganglion (DRG) neurons. Total PKCε was used as a loading control. (B) Summarized data for A. Data are expressed as a percentage of the untreated control. *P < 0.05 compared with control. #P < 0.05 compared with propofol. n = DRG cell lysates from five different mice. (C) Representative immunoblot depicting the effect of propofol (10 μM) alone, propofol in the presence of εV1-2 (0.5 μM) or ψεRACK (0.5 μM) alone on PKCε membrane association in mouse DRG neurons. Porin was used a membrane fraction loading control. C = cytosolic fraction; M = membrane fraction. (D) Summarized data for C. Data are expressed as a percentage change in PKCε redistribution from the cytosolic to membrane subfraction. * P < 0.05 compared with control membrane fraction. n = DRG cell lysates from five different mice.
Effect of Propofol on PKCε Translocation from Cytosolic to Membrane Fractions in Mouse DRG Neurons
Immunoblot analysis revealed that PKCε was primarily associated with the cytosolic fraction but had some association with the membrane fraction in untreated DRG neurons (fig. 6C). After treatment with propofol (10 μM), the amount of immunodetectable PKCε in the membrane fraction was increased (fig. 6C). Pretreatment with εV1-2 (0.5 μM) attenuated the propofol-induced increase of PKCε in the membrane fraction, whereas treatment with ψεRACK (0.5 μM) stimulated translocation of PKCε from cytosolic to membrane fraction (fig. 6C). Intralipid vehicle did not have any effect on the membrane association of PKCε (99 ± 4% of control). Summarized data depicting the relative association of PKCε with the cytosolic and membrane fractions, before and after treatment with propofol alone, propofol in the presence of εV1-2, ψεRACK alone, or Intralipid alone are depicted in figure 6D.
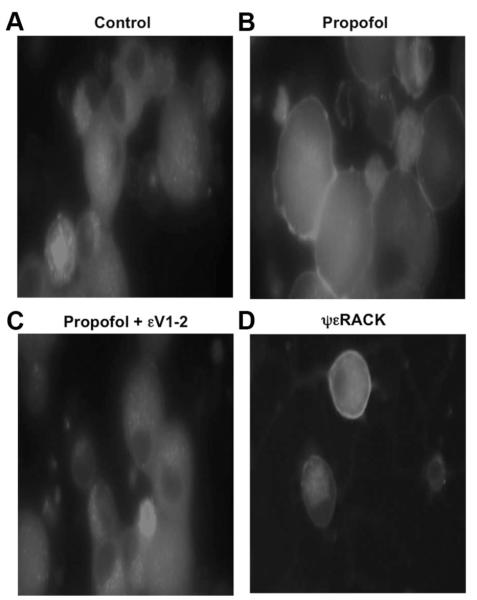
Effect of Propofol on PKCε Intracellular Localization in Mouse DRG Neurons
Immunocytochemical analysis revealed a diffuse deposition of PKCε in the cytoplasm of untreated control DRG neurons (fig. 7A). Propofol (10 μM) stimulated a greater association of PKCε to the periphery of the DRG neurons (fig. 7B). The propofol-induced peripheral association of PKCε was inhibited by εV1-2 (0.5 μM) (fig. 7C) and mimicked by ψεRACK (0.5 μM) (fig. 7D). Intralipid vehicle had no effect on PKCε intracellular localization.
Fig. 7.
Confocal fluorescent images (40× magnification) of mouse dorsal root ganglion (DRG) neurons depicting the effect of no treatment (control; A) propofol (10 μM) alone (B), propofol in the presence of the protein kinase C epsilon (PKCε) inhibitor peptide εV1-2 (0.5 μM; C), or the PKCε activator peptide ψεRACK (0.5 μM) alone (D) on fluorescein isothiocyanate-labeled PKCε cellular localization. Similar results were obtained from DRG neurons from four different mice.
Effect of Propofol on TRPV1 Phosphorylation at S800 in Mouse DRG Neurons
Immunoblot analysis revealed that there was no detectable TRPV1pS800 in untreated DRG neurons obtained from WT mice. In the presence of propofol (10 μM), TRPV1pS800 levels were increased in WT DRG neurons but remained unchanged in DRG neurons isolated from PKCε-null mice (fig. 8A). ψεRACK also increased TRPV1pS800 levels, whereas Intralipid had no effect on TRPV1 phosphorylation at S800 (fig. 8A). Summarized data depicting the effect of propofol (10 μM) alone, ψεRACK (0.5 μM) alone, and Intralipid alone on TRPV1pS800 levels in WT mouse DRG neurons as well as the effect of propofol alone on TRPV1pS800 levels in DRG neurons obtained from PKCε-null mice are depicted in figure 8B.
Fig. 8.
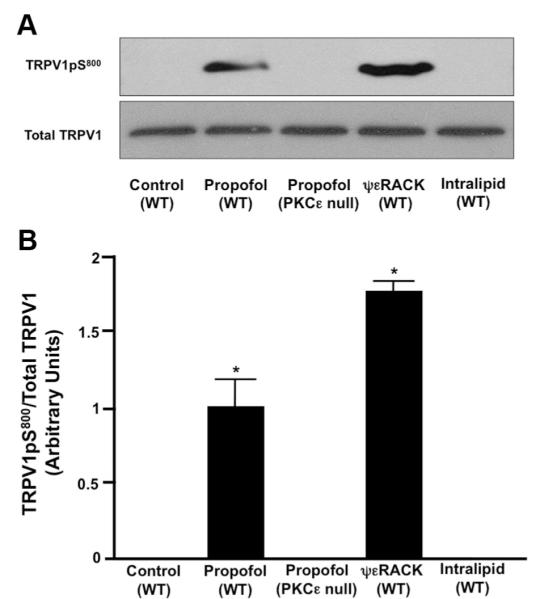
(A) Representative immunoblot depicting the effect of propofol (10 μM) alone, protein kinase C epsilon (PKCε) activator peptide ψεRACK (0.5 μM) alone, or Intralipid (10 μM) alone on transient receptor potential vanilloid receptor type 1 (TRPV1) serine 800 phosphorylation (TRPV1pS800) in wild-type (WT) mouse dorsal root ganglion (DRG) neurons as well as the effect of propofol (10 μM) on TRPV1pS800 levels in DRG neurons isolated from PKCε-null mice. Total TRPV1 was used to normalize TRPV1pS800.(B) Summarized data for A. Data are expressed as arbitrary units. n = DRG lysates from five different WT mice and four different PKCε-null mice. * P < 0.05 compared with control.
Discussion
This is the first study to assess the extent to which propofol is capable of modulating TRPV1 receptor sensitivity in nociceptive DRG sensory neurons. Recent studies have indicated that propofol does not activate TRPV1 in DRG neurons14 or in human embryonic kidney 293 cells transiently expressing the TRPV1 receptor.20 However, the extent to which propofol is capable of restoring TRPV1 sensitivity to agonist activation after agonist desensitization or modulating agonist-induced desensitization of TRPV1 was not assessed in these or any other studies. On the other hand, recent studies have demonstrated that PKCε can phosphorylate TRPV1 receptors at S800, restoring TRPV1 sensitivity to agonist stimulation.5,13 Moreover, our lab has previously shown that propofol can activate and allosterically modulate recombinant PKCε as well activate PKCε in freshly isolated adult rat ventricular cardiomyocytes.17,18,21,22 As a result, we tested the hypothesis that propofol can restore TRPV1 receptor sensitivity (resensitizes) of desensitized TRPV1 receptors via a PKCε-dependent pathway. We also tested the hypothesis that propofol attenuates agonist-induced desensitization of TRPV1 via a PKCε-dependent pathway. In addition, we tested the hypothesis that propofol prolongs agonist-induced pain-associated behaviors in mice. Our key finding is that the supraclinical concentration of propofol (10 μM) restores the sensitivity of TRPV1 receptors to capsaicin activation via a PKCε-dependent mechanism that is associated with an increase in phosphorylation of TRPV1 at S800 in mouse DRG neurons. In addition, propofol (10 μM) attenuates agonist-induced desensitization of TRPV1 in a PKCε-dependent manner and prolongs agonist-induced pain-associated behaviors. Moreover, propofol (10 μM) stimulates the phosphorylation of PKCε at S729 and induces the translocation of PKCε from cytosolic to membrane fractions, indicating PKCε activation
Effect of Propofol on Restoration of TRPV1 Sensitivity in Mouse DRG Neurons
PKCε has been shown to restore TRPV1 sensitivity to capsaicin through rephosphorylation of the channel at S800.5,13 In addition, a recent study from our laboratory indicated that propofol stimulates translocation of several PKC isoforms, including PKCε, to distinct intracellular locations in cardiomyocytes.17 Therefore, to examine whether propofol is capable of restoring TRPV1 receptor sensitivity to agonist stimulation, we treated DRG neurons with propofol after desensitization with capsaicin and then measured [Ca2+]i in response to another challenge with capsaicin. Our data indicate that after capsaicin-induced TRPV1 desensitization, addition of propofol itself resulted in no increase in [Ca2+]i. However, continued exposure of DRG neurons to propofol (10 μM) and reapplication of capsaicin restored TRPV1 sensitivity, resulting in a transient rise in [Ca2+]i. The restoration of TRPV1 sensitivity is not likely to be due to time-dependent recovery from desensitization, because incubation of DRG neurons in control buffer for an equivalent period of time did not restore TRPV1 sensitivity, as evidenced by the lack of any increase in [Ca2+]i. In addition, the Intralipid emulsion did not restore TRPV1, indicating that “pure” propofol (2,6 diisopropyl phenol), and not the lipid emulsion, can restore TRPV1 sensitivity.
Effect of PKCε Inhibition on Propofol-induced Restoration of TRPV1 Sensitivity in Mouse DRG Neurons
Although capsaicin acutely activates TRPV1 receptors in sensory neurons eliciting a painful response, repeated stimulation with capsaicin causes TRPV1 receptors to undergo desensitization, leading to a decrease in receptor activation.10,23 The process of receptor desensitization and resensitization is important for mediating TRPV1 receptor signal transduction in DRG neurons.5 Although the mechanisms of desensitization are not entirely clear, resensitization of the TRPV1 receptor seems to involve activation of a PKC-dependent pathway.5,12 Specifically, PKCε has been shown to reverse TRPV1 desensitization by capsaicin through rephosphorylation of the channel at S800.5,13 To examine whether propofol exerts a resensitizing effect on TRPV1 receptors via PKCε, we treated DRG neurons with propofol in the presence or absence of εV1-2 after desensitization and subsequently measured [Ca2+]i in response to another challenge with capsaicin. Our findings indicate that the propofol-induced resensitization of TRPV1 is almost fully attenuated in DRG neurons treated with εV1-2 and mimicked by ψεRACK. These results indicate that PKCε is needed for propofol to resensitize TRPV1. To further substantiate these results, we next assessed the effect of propofol on TRPV1 resensitization in DRG neurons isolated from PKCε-null mice. In PKCε-null mice, propofol was not able to restore TRPV1 sensitivity to capsaicin, further indicating that PKCε activation is critical for restoring the sensitivity of desensitized TRPV1. Moreover, the finding that the propofol-induced resensitization of TRPV1 was almost fully attenuated in PKCε-null mice indicates that other isoforms of PKC are most likely not involved.
Effect of Propofol on Agonist-induced Desensitization of TRPV1 in Mouse DRG Neurons
The mechanisms governing desensitization of TRPV1 are complex but involve calciumdependent and -independent mechanisms.24 For instance, capsaicin-induced TRPV1 desensitization is mediated by calcineurin-dependent dephosphorylation of the receptor but is mitigated by PKC-dependent phosphorylation.24 Although our findings indicate that propofol can restore the sensitivity of TRPV1 that has been previously desensitized by capsaicin, we next wanted to determine whether propofol could modulate the ability of capsaicin to desensitize TRPV1 via a PKCε-dependent pathway. Our findings indicate that capsaicin-induced desensitization of TRPV1 is reduced in the presence of propofol, as indicated by the increased number of applications of capsaicin that are required to fully desensitize the receptor. Moreover, propofol also prolonged the decay of [Ca2+]i in both WT and PKCε-null mice, consistent with our previous finding that propofol prolongs the decay of [Ca2+]i during the restoration of TRPV1 sensitivity as previously mentioned. Although other agonists of TRPV1 (protons and heat) were not tested in these experiments, our findings point to a potential role of propofol in limiting the ability of the TRPV1 receptor to become desensitized, a property that may alter other signaling pathways regulating pain, including other members of the transient receptor potential family of receptors. For instance, the propofol-induced regulation of TRPV1 function may also affect TRPA1 receptor function due to the known interactions of the two receptors.16
Effect of Propofol on Agonist-induced Desensitization of TRPV1 in Mouse DRG Neurons Isolated from PKCε-null Mice
PKCε has been shown to regulate desensitization of TRPV1 by capsaicin.25 Based on our findings that propofol can restore the sensitivity of previously desensitized TRPV1 receptors, we wanted to determine whether or not propofol attenuates the ability of TRPV1 to become desensitized via activation of PKCε. Our findings indicate that PKCε is required, at least in part, for propofol to inhibit TRPV1 to desensitization by repeated agonist stimulation. Elucidation of the mechanisms by which propofol alters TRPV1 function will provide better mechanistic insight into how propofol may regulate other pain receptors.
Effect of Propofol on Agonist-induced Pain-associated Behaviors in Mice
To further characterize the extent to which propofol modifies TRPV1 sensitivity, the effect of propofol on pain-associated behaviors was assessed in WT mice. We assessed the effect of propofol on the duration of nose-wiping in and out of bedding in response to a topical application of capsaicin to the nasal epithelium. This allowed us to assess the effects of locally administered propofol without the potential confounding depressive effects of propofol on the central nervous system. Using this model, we determined that capsaicin induced a pain-associated behavior in mice, and that a subsequent second application of capsaicin (after a 10-min rest period) resulted in a marked reduction in the duration of the nocifensive behavior, indicating reduced TRPV1 sensitivity. In contrast, application of propofol during the 10-min rest period increased the duration of pain-associated behaviors induced by the second application of capsaicin, indicating an increase in TRPV1 sensitivity. These data provide compelling evidence further supporting our in vitro data of a propofol-induced restoration of TRPV1 sensitivity, as well as a propofol-induced attenuation of agonist-induced TRPV1 desensitization.
Effect of Propofol on PKCε Phosphorylation at S729 in Mouse DRG Neurons
To provide biochemical evidence to further support our finding of a propofol-induced restoration of TRPV1 sensitivity via PKCε, we next assessed the effect of propofol on PKCε activation. Activation of PKCε, as well as membrane targeting and substrate specificity, is regulated by several factors, including phosphorylation, diacylglycerol, phospholipids, and anchoring proteins. As part of the activation process, PKCε is auto-phosphorylated at S729 in the hydrophobic motif of the enzyme, rendering it catalytically competent. Our findings indicate that propofol increases PKCεpS729 levels in DRG neurons. These results are consistent with our previous finding that propofol can increase the S729 phosphorylation of recombinant PKCε.18 Moreover, Intralipid was also capable of stimulating S729 phosphorylation of PKCε. This is probably due to the presence of fatty acids (soy bean oil) and phospholipids (lecithin), which create the lipid emulsion facilitating solubility of propofol. Fatty acids are known to activate some isoforms of PKC, and phospholipids serve as cofactors for activation.26 However, the Intralipid-induced phosphorylation of PKCε is somewhat surprising, because Intralipid did not resensitize TRPV1. This may be because S729 phosphorylation of PKCε only renders the enzyme catalytically competent. Other events subsequent to S729 phosphorylation such as pseudosubstrate displacement must occur for full PKCε activation.
Effect of Propofol on PKCε Translocation from Cytosolic to Membrane Fractions in Mouse DRG Neurons
A subcellular translocation of PKC isoforms from the cytosolic to membrane fraction is associated with activation of PKC isoforms, and translocation is facilitated by receptors for activated C kinases located in cellular membranes.27,28 Binding of the PKC isoform to its specific receptors for activated C kinase is also critical to the phosphorylation of substrate proteins that are specific for that PKC isoform. This study is the first to examine the translocation of PKCε from cytosolic to membrane fractions in DRG neurons after exposure to propofol. Our findings indicate that propofol increases the translocation of PKCε from the cytosolic to membrane fraction in DRG neurons. This result is consistent with our previous finding that propofol stimulated the translocation of PKCε from cytosolic to membrane fraction in cardiomyocytes.17 Surprisingly, Intralipid did not induce translocation of PKCε. Again, as mentioned earlier, Intralipid may increase S729 phosphorylation of PKCε without inducing membrane translocation.
Effect of Propofol on PKCε Intracellular Localization in Mouse DRG Neurons
Translocation of PKCε from cytosolic to membrane fraction indicates movement of the protein from one intracellular domain to another. Previous studies examining the intracellular localization of individual PKC isoforms before and after an intervention have been useful for identifying the potential cellular target(s) of each isoform.29,30 Identifying the cellular targets of PKCε provides important information for predicting changes in cellular regulation and function that may occur. Our novel findings indicate that propofol increases the association of PKCε at the periphery of DRG neurons. Combined with the finding that propofol induces the translocation of PKCε to membrane fractions, this finding suggests that propofol induces the translocation of PKCε in DRG neurons to the plasma membrane, potentially interacting with and phosphorylating TRPV1 located in the plasma membrane. As a result, we next examined the extent to which propofol increased the PKCε-dependent phosphorylation of TRPV1 at S800.
Effect of Propofol on TRPV1 Phosphorylation at S800 in Mouse DRG Neurons
The PKCε-dependent phosphorylation of TRPV1 at S800 has been shown to increase the sensitivity of TRPV1 to agonist stimulation.5,13 The propofol-induced activation of PKCε and the subsequent phosphorylation of TRPV1 at S800 may be the mechanism by which propofol not only restores the sensitivity of TRPV1 to capsaicin but also attenuates the ability of capsaicin to desensitize TRPV1. Our novel findings indicate that the propofol increased TRPV1pS800 levels in DRG neurons and that the propofol-induced increase in TRPV1 phosphorylation was completely blocked in DRG neurons isolated from PKCε-null mice. These results indicate that the propofol-induced phosphorylation of TRPV1 by PKCε may constitute the underlying mechanism by which propofol resensitizes TRPV1 to capsaicin stimulation and attenuates capsaicin-induced TRPV1 desensitization. Further studies incorporating cell lines transfected with mutant forms of TRPV1 in which the PKCε-dependent phosphorylation site (S800) has been removed will be needed to convincingly conclude that the propofol-induced phosphorylation of TRPV1 at S800 is the mechanism of the propofol-induced modulation of TRPV1 sensitivity.
The clinical relevance of the current findings regarding the effect of propofol on TRPV1 sensitivity is doubtful for the following reasons. First, propofol had no effect on TRPV1 sensitivity except at 10 μM, a propofol concentration outside the clinically relevant range. The peak plasma concentration of propofol after bolus administration has been estimated at 50 μM.31 In normal circumstances, the steady-state free plasma concentration of propofol would probably not exceed 1–2 μM, because 97–98% binds to serum protein.32 Therefore, 10 μM propofol is a supraclinical concentration. Second, propofol did not exert a concentration-dependent effect on TRPV1 sensitivity. Both pharmacologists and physiologists recognize the importance of observing concentration-dependent effects of drugs on physiologic function to establish any potential clinical significance of the drug. Unfortunately, we observed no concentration-dependent effect of propofol. This is a confusing issue that remains unresolved, but it may be due to some pharmacological threshold effect of propofol on TRPV1 receptor function. Although the data provide some insight into the potential pharmacological interactions between propofol and TRPV1 receptors, they provide little insight into the clinical relevance of their interaction.
What We Already Know about This Topic
 Capsaicin and heat stimulate a receptor on peripheral nerve fibers (TRPV1) that becomes desensitized with sustained presence of capsaicin.
Capsaicin and heat stimulate a receptor on peripheral nerve fibers (TRPV1) that becomes desensitized with sustained presence of capsaicin. Propofol also interacts with the TRPV1 channel.
Propofol also interacts with the TRPV1 channel.
What This Article Tells Us That Is New
 Propofol, in concentrations above those used clinically, prevents and reverses desensitization of TRPV1 channels and might be used to probe mechanisms of desensitization.
Propofol, in concentrations above those used clinically, prevents and reverses desensitization of TRPV1 channels and might be used to probe mechanisms of desensitization.
Acknowledgments
This study was supported by Grant HL65701 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland (to Dr. Damron).
References
- 1.Palazzo E, Rossi F, Maione S. Role of TRPV1 receptors in descending modulation of pain. Mol Cell Endocrinol. 2008;286:S79–83. doi: 10.1016/j.mce.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 3.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 4.Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol. 2006;577:169–90. doi: 10.1113/jphysiol.2006.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium. 2004;35:471–8. doi: 10.1016/j.ceca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Larrucea C, Castro P, Sepulveda FJ, Wandersleben G, Roa J, Aguayo LG. Sustained increase of Ca2+ oscillations after chronic TRPV1 receptor activation with capsaicin in cultured spinal neurons. Brain Res. 2008;1218:70–6. doi: 10.1016/j.brainres.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol. 2008;155:1145–62. doi: 10.1038/bjp.2008.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y. The functional regulation of TRPV1 and its role in pain sensitization. Neurochem Res. 2008;33:2008–12. doi: 10.1007/s11064-008-9750-5. [DOI] [PubMed] [Google Scholar]
- 9.Novakova-Tousova K, Vyklicky L, Susankova K, Benedikt J, Samad A, Teisinger J, Vlachova V. Functional changes in the vanilloid receptor subtype 1 channel during and after acute desensitization. Neuroscience. 2007;149:144–54. doi: 10.1016/j.neuroscience.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 10.Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–32. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 11.Vyklický L, Nováková-Tousová K, Benedikt J, Samad A, Touska F, Vlachová V. Calcium-dependent desensitization of vanilloid receptor TRPV1: A mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol Res. 2008;57:S59–68. doi: 10.33549/physiolres.931478. [DOI] [PubMed] [Google Scholar]
- 12.Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–16. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Zhou ZS, Zhao ZQ. PKC regulates capsaicin-induced currents of dorsal root ganglion neurons in rats. Neuropharmacology. 2001;41:601–8. doi: 10.1016/s0028-3908(01)00106-x. [DOI] [PubMed] [Google Scholar]
- 14.Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci. 2008;105:8784–9. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–78. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–93. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickley PJ, Ding X, Murray PA, Damron DS. Propofol-induced activation of protein kinase C isoforms in adult rat ventricular myocytes. ANESTHESIOLOGY. 2006;104:970–7. doi: 10.1097/00000542-200605000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Wickley PJ, Yuge R, Martin BA, Meyer JS, Damron DS. Propofol activates and allosterically modulates recombinant protein kinase C epsilon. ANESTHESIOLOGY. 2009;111:36–43. doi: 10.1097/ALN.0b013e3181a3274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Hirota K, Smart D, Lambert DG. The effects of local and intravenous anesthetics on recombinant rat VR1 vanilloid receptors. Anesth Analg. 2003;96:1656–60. doi: 10.1213/01.ANE.0000061580.89627.91. [DOI] [PubMed] [Google Scholar]
- 21.Kanaya N, Gable B, Murray PA, Damron DS. Propofol increases phosphorylation of troponin I and myosin light chain 2 via protein kinase C activation in cardiomyocytes. ANESTHESIOLOGY. 2003;98:1363–71. doi: 10.1097/00000542-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Kurokawa H, Murray PA, Damron DS. Propofol attenuates β-adrenoreceptor-mediated signal transduction via a protein kinase C-dependent pathway in cardiomyocytes. ANESTHESIOLOGY. 2002;96:688–98. doi: 10.1097/00000542-200203000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100:8002–6. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–50. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan R, Wolfe D, Goss J, Watkins S, de Groat WC, Sculptoreanu A, Glorioso JC. Protein kinase C epsilon contributes to basal and sensitizing responses of TRPV1 to capsaicin in rat dorsal root ganglion neurons. Eur J Neurosci. 2008;28:1241–54. doi: 10.1111/j.1460-9568.2008.06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pi Y, Walker JW. Diacylglycerol and fatty acids synergistically increase cardiomyocyte contraction via activation of PKC. Am J Physiol Heart Circ Physiol. 2000;279:H26–34. doi: 10.1152/ajpheart.2000.279.1.H26. [DOI] [PubMed] [Google Scholar]
- 27.Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci USA. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mochly-Rosen D. Localization of protein kinases by anchoring proteins: A theme in signal transduction. Science. 1995;268:247–51. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 29.Mackay K, Mochly-Rosen D. Localization, anchoring, and functions of protein kinase C isozymes in the heart. J Mol Cell Cardiol. 2001;33:1301–7. doi: 10.1006/jmcc.2001.1400. [DOI] [PubMed] [Google Scholar]
- 30.Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210:287–97. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 31.Cockshott ID. Propofol (‘Diprivan’) pharmacokinetics and metabolism: An overview. Postgrad Med J. 1985;61(suppl 3):45–50. [PubMed] [Google Scholar]
- 32.Morgan DJ, Campbell GA, Crankshaw DP. Pharmacokinetics of propofol when given by intravenous infusion. Br J Clin Pharmacol. 1990;30:144–8. doi: 10.1111/j.1365-2125.1990.tb03755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]