Abstract
Background & Aims
Cholecystokinin (CCK) is a satiation peptide released during meals in response to lipid intake; it regulates pancreatic digestive enzymes that are required for absorption of nutrients. We proposed that mice with a disruption in the CCK gene (CCK-KO mice) that were fed a diet of 20% butter fat would have altered fat metabolism.
Methods
We used quantitative magnetic resonance imaging to determine body composition and monitored food intake of CCK-KO mice using an automated measurement system. Intestinal fat absorption and energy expenditure were determined using a noninvasive assessment of intestinal fat absorption and an open circuit calorimeter, respectively.
Results
After consuming a high-fat diet for 10 weeks, CCK-KO mice had reduced body weight gain and body fat mass and enlarged adipocytes, despite the same level of food intake as wild-type mice. CCK-KO mice also had defects in fat absorption, especially of long-chain saturated fatty acids, but pancreatic triglyceride lipase (PTL) did not appear to have a role in the fat malabsorption. Energy expenditure was higher in CCK-KO than wild-type mice and CCK-KO mice had greater oxidation of carbohydrates while on the high-fat diet. Plasma leptin levels in the CCK-KO mice fed the high-fat diet were markedly lower than in wild-type mice, although levels of insulin, gastric-inhibitory polypeptide, and glucagon-like peptide-1 were normal.
Conclusion
CCK is involved in regulating the metabolic rate and is important for lipid absorption and control of body weight in mice placed on a high-fat diet.
Keywords: fat absorption, CCK receptor, indirect calorimetry
Background & Aims
Obesity develops when energy intake exceeds energy expenditure over time, with the excess energy stored as fat (1). Gastrointestinal hormones stimulated by fatty meals, such as cholecystokinin (CCK), contribute to energy homeostasis, and some influence the brain by serving as satiation signals to inhibit further food intake (2). Peripheral CCK is secreted by intestinal I cells in response to fatty meals, and is involved in modulating intestinal motility, stimulating pancreatic enzyme secretion, and regulating meal size (3). Intraperitoneal or central administration of either purified CCK extracts or synthetic CCK-8 to fasted rats reduces meal size but not meal frequency (4; 5), classifying CCK as a satiation peptide. Two CCK receptors have been cloned: CCK1R and CCK2R. Selective CCK1R antagonists competitively block CCK's reduction of food intake, whereas selective CCK2R antagonists have no effect (6). CCK1R antagonists also block the suppression of food intake induced by gastric infusion of oleic acid (7). Thus, CCK's satiation action is mediated through interactions with CCK1R, but not with CCK2R.
Consumption of a high-fat diet (HFD) increases intestinal CCK secretion (8). Otsuka Long-Evans Tokushima Fatty (OLETF) rats have a CCK1R gene mutation and enhanced food intake and weight gain relative to lean controls, suggesting that loss of signaling through CCK1R attenuates inhibitory signals that normally limit weight gain on a HFD (9; 10). In contrast, CCK-KO and CCK1R-KO mice gain weight similarly as controls on a HFD, suggesting species differences in the effects of CCK (10; 11), possibly due to differing hypothalamic responses (12). In the present studies we examined the effect of HFD on food intake and metabolic parameters in mice lacking the gene for CCK, hypothesizing that impaired fat digestion or absorption would disturb energy balance.
Methods
Animals
The generation of the cholecystokinin-null (CCK-KO) mouse was described by Lacourse et al. (11). Based on a gene-targeting strategy which replaces part of the mouse CCK gene with the lacZ reporter gene, the process results in complete removal of the NH2 terminus of CCK, including the signal sequence (11). The CCK-KO mice were back-crossed for >10 generations onto a C57BL/6J genetic background and all mice were genotyped by PCR analysis of tail DNA (11). Male CCK-KO and wild-type (WT) mice (C57BL/6J background) were generated in an AAALAC-accredited facility under conditions of controlled illumination (12:12-h light-dark cycle, lights from 0600 to 1800 h). All animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Body weight and food consumption
After weaning, CCK-KO (n=8) and WT mice (n=10) were housed in groups of 3-4 and at 19 weeks of age housed individually. All animals received free access to semi-purified high-fat pellet or powdered diets (20% butter fat by weight; Research Diets, Inc, New Brunswick, NJ) and water starting at 20 weeks of age for 10 weeks. Body weights and food intake were recorded twice a week using a top-loading balance (± 0.01 g, Adenturer SL, Ohaus Corp, Pine Brook, NJ).
Fat mass/lean body mass and tissue collection
Fat and lean body masses were determined using an EchoMRI whole body composition analyzer (Houston, TX) (13). The EchoMRI is a QNMR instrument that provides precise measurements of whole-body composition parameters that include total body fat, lean mass, body fluids, and total body water in living rodents. Fat and lean mass were calculated as percent of total mass. After 10 weeks on a HFD, 5-h fasted animals were euthanized and nose-to-anus length was recorded. Pancreas, liver and fat pads were carefully dissected and weighed. Adipose tissue was embedded in paraffin and 4-μm sections were stained with hematoxylin and eosin. Adipocyte size was measured in 125 cells per mouse (n=4 mice) in five different fat pads and analyzed by NIH Image-J software.
Meal patterns
Cohorts of CCK-KO and WT mice were acclimatized to individual metabolic cages (Accuscan Instruments, Inc, Columbus, OH) for 3 days prior to the start of data collection. Powdered HFD and water were freely available, and intake was recorded at single-minute intervals for 3 days using the manufacturer's software.
Plasma measurements
5-h fasting plasma was collected in heparin-treated microtubes with 1% dipeptidyl-peptidase-IV (DPP-IV) inhibitor (Millipore, Billerica, MA) to measure gastric-inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), and in microtubes without DPP-IV inhibitor for leptin and insulin assays.
Assays for leptin, insulin, GIP and GLP-1 were performed according to the manufacturer's specifications (Millipore, St. Charles, MO). The GIP ELISA measured active GIP(1-42) plus non-active GIP(3-42). The GLP-1 ELISA measured biologically active GLP-1(7-37) plus GLP-1-(7-36)NH2. Briefly, 10-μl plasma samples were added to a 96-well plate pre-coated with either anti-leptin, anti-insulin or anti-GIP monoclonal antibodies. After incubation, 100 μl of an streptavidin-horseradish peroxidase conjugate and 3,3′,5,5′-tetramethylbenzidine were added and the absorbance was read at 450 and 490 nm using a microplate reader (Synergy HT, BioTek Instruments, Inc, Richmond, VA). For GLP-1 determination, 100-μl plasma samples were added to a microtiter plate pre-coated with anti-GLP-1 antibodies, and 200 μl of anti-GLP-1 alkaline phosphatase conjugate and 4-methylumbelliferyl phosphate were added to each well. Phosphatase activity was measured using excitation/emission wavelengths of 335 nm/460 nm. Final concentrations were calculated using standards provided by Millipore ELISA kit.
Plasma triacylglycerols and cholesterol were assayed using Randox triglyceride kits (Antrim, UK) and Infinity cholesterol kits (Thermo Electron, Noble Park, Victoria, Australia). Samples (5-μl) of diluted plasma in mini Q water (ratio 1:4) were combined with 200 μl mixtures of enzyme reagent and buffer according to the manufacturer's protocol and the absorbance was read at 500 nm. Plasma glucose was determined using a Freestyle glucometer (Abbot Diabetes Care, Alameda, CA).
Locomotor activity
Home cages were placed in SmartFrame stainless steel cage rack frames (Hamilton-Kinder, Poway, CA). Infrared photobeam interruption sensors mounted in the frames detected each animal's movements. Activity counts (beam interruptions) were recorded for 4 days.
Fat absorption
Intestinal fat absorption was assessed by the noninvasive sucrose polybehenate (Olestra) method (14), which is based on measuring the ratio of absorbable fat (butter fat) to non-absorbable fat (Olestra) in the diet and in the feces. The powdered diet contained 20% fat, 45% nonfat dry milk, and 39% sucrose. The fat was a mixture of 95% butter fat without water plus 5% Olestra (Procter & Gamble, Cincinnati, OH) (Table 1). Animals consumed the powdered diet for 4 days and their fecal pellets were collected for analysis on the final day. Fecal pellets (∼15 mg) were extracted with methanolic sodium hydroxide and the fatty acids were methylated with borontrifluoride in methanol (15). The hexane fraction was injected into a gas chromatography system (Shimadzu GC 2010) equipped with a DB-23 Column (J & W Scientific, Folsom, CA). Analysis of fatty acid methyl esters was calculated using Schimadzu Class EZStart 7.4 software. The percentage of fat absorption was determined based on the ratio of total fatty acids to behenic acid in the diet and in the feces.
Table 1. Fat Composition in 20% Butter Fat Diets for Fat Absorption Test.
| Myristic acid (C14:0) | 10.5% |
| Palmitic acid (C16:0) | 30.8% |
| Stearic acid (C18:0) | 12.6% |
| Linoleic acid (C18:2) | 3.0% |
| Oleic acid (C18:1) | 25.2% |
| Olestra | 5.0% |
Olestra; sucrose polybehenate
Pancreatic triglyceride lipase activity
The pancreas (n=7 per group) was homogenized in a solution containing 10 mM sodium phosphate, pH 6.2, 0.1 M NaCl, 1 mM EDTA, 0.02% sodium azide, 1.5% glycerol, and 0.2% soybean trypsin inhibitor (Sigma, St. Louis, MO)(16). The homogenate was centrifuged at 14,000 × g for 10 min at 4 °C and the supernatant was harvested. Total pancreatic protein concentrations were determined by the method of Bradford (17). Triglyceride hydrolysis activity in the pancreatic extracts was determined as described previously (16). Briefly, 10 μCi of [3H]triolein (Amersham Biosciences) was dried and resuspended in 5 ml of assay buffer (30 mM Tris-HCl, pH 8.0, 1 mM CaCl2, 4 mM taurodeoxycholate) containing 1.56 μmol triolein. The mixture was sonicated for 3 min to generate an emulsion. Radiolabeled substrate emulsion (45 μl) and 5 μl of pancreatic extract from WT and CCK-KO mice were added to each tube. Reactions were carried out for 10 min and were stopped by adding 15 volumes of chloroform/ methanol/heptane (125:140:100; v/v/v), followed by 5 volumes of 50 mM sodium carbonate (pH 10.5). The mixture was centrifuged for phase separation and the amount of radiolabeled fatty acid liberated from [3H]triolein hydrolysis was determined by liquid scintillation counting of the aqueous phase.
Energy expenditure and respiratory quotient
A Physioscan open-circuit calorimeter (Accuscan Instruments Inc, Columbus, OH) monitored oxygen (O2) and carbon dioxide (CO2) gas fractions at the inlet and outlet ports to each of 8 test chambers. Airflow was 0.5 L/min and air from each chamber was sampled and analyzed every 10 sec. Animals (n=8/group) were monitored for 18 h with no food available after 5 weeks on HFD.
Statistical analysis
All values are expressed as mean ± SE. Parametric statistical analyses were performed for comparison of all groups using GraphPad™ Prism (version 5.0, San Diego, CA), and differences between groups were determined by Student's t test. Differences were considered significant if p < 0.05.
Results
Body weight and food intake
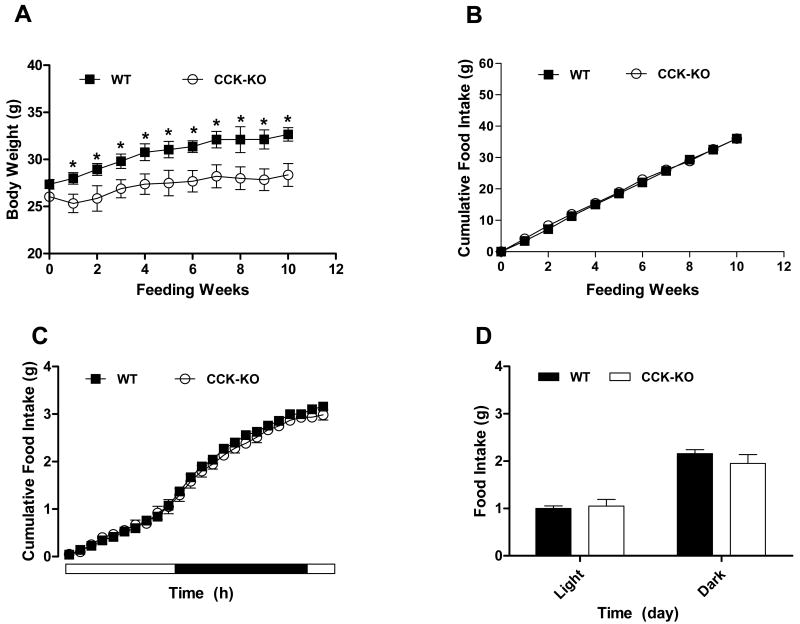
Prior to receiving the HFD, the body weight at 20 weeks of age in CCK-KO mice was not significantly different from that of WT mice (Figure 1A). After 10 weeks on the HFD, CCK-KO mice had significantly lower body weight (28.4 ± 1.2 g) than WT mice (32.7 ± 0.7 g). CCK-KO mice had gained 2.3 ± 0.8 g while the WT mice had gained 5.5 ± 0.6 g. The lower weight gain in CCK-KO mice was not attributable to reduced food intake; i.e., throughout the study, food intake of CCK-KO mice was comparable to that of WT mice (Figure 1B), and that was true during light and dark-phase feeding (Figure 1C& D).
Figure 1.
Mean body weight and food intake. (A) Mean body weight, and (B) food intake of CCK-KO and WT (n=8 per group) mice. (C) Cumulative food Intake, and (D) food intake during light and dark cycle feeding of CCK-KO and WT (n=6 per group) mice. During the food intake study, experimental mice were permitted free access to powdered high fat diet and food intake was recorded for each animal on day 4. Data are expressed as mean ± SEM and values with asterisks represent significant differences relative to age-matched WT mice (P < 0.05).
Fat mass/ body weight
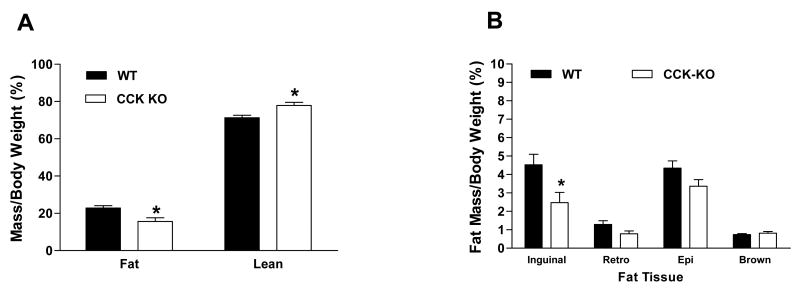
The percentages of fat mass and lean mass were comparable in CCK-KO and WT mice prior to HFD (data not shown). After 10 weeks on HFD, CCK-KO mice had significantly less fat mass (15.7%) than WT controls (22.9%), and consequently a greater percent of lean mass (Figure 2A). CCK-KO mice had significantly less inguinal (subcutaneous) adipose tissue per kg body weight (2.5 ± 0.6%) but a comparable retroperitoneal (0.8 ± 0.2%), epididymal (3.4± 0.4%) and brown fat (0.8 ± 0.1%) relative to WT mice (4.5 ± 0.6%; 1.3 ± 0.1%; 4.4 ± 0.4%; 0.7± 0.1%, respectively; Figure 2B). CCK-KO mice had larger adipocytes on average than WT mice as indicated by the cell-size distributions for inguinal and epididymal depots (Figures 2C-F). Thus, a relatively selective reduction of subcutaneous fat and overall enlarged adipocyte cell size contributes to the lower body weights of CCK-KO mice.
Figure 2.

Body composition of CCK-KO and WT mice. (A) Fat and lean mass of CCK-KO and WT (n=8 per group) mice were determined following a 10-week HFD. (B) Percentage of various fat tissues per body weight were collected after 5-h fasting at the end of 10-weeks HFD. (C) Distribution of fat cell size (in %) in inguinal fat tissue. (D) Morphology of inguinal fat adipocytes in WT and CCK-KO mice determined by H&E staining. (E-F) Distribution of fat cell size (in %) in retroperitoneal and epididymal fat tissue. Data are expressed as mean ± SEM and values with asterisks represent significant differences relative to the control group (P < 0.05).
Body length and plasma parameters
After 10 weeks on HFD, body length and liver weight were comparable for the two genotypes (Table 2). Plasma triacylglycerols and cholesterol levels in 5-h fasted mice were also comparable. In contrast, CCK-KO mice had significantly reduced plasma leptin, with no significant difference in plasma glucose, insulin, GIP or GLP-1 compared to WT controls.
Table 2. Tissues and plasma parameters in CCK-KO mice after a 10-week high fat diet.
| WT | CCK-KO | |
|---|---|---|
| Body Weight (g) | 32.7 ± 0.7 | 28.4 ± 1.2* |
| Liver (g) | 1.1 ± 0.2 | 1.0 ± 0.2 |
| Length (cm) | 10.0 ± 0.1 | 9.9 ± 0.1 |
| Triacylglycerols (mg/dl) | 64.5 ± 7.3 | 74.2 ± 9.9 |
| Cholesterol (mg/dl) | 152.1 ±16.6 | 132.8 ±17.9 |
| Glucose (mg/dl) | 171.4 ± 7.5 | 183.0 ±10.3 |
| Insulin (ng/ml) | 1.0 ± 0.2 | 1.2 ± 0.2 |
| Leptin (ng/ml) | 12.4 ± 1.6 | 5.7 ± 1.5* |
| GIP (pg/ml) | 120.2 ±11.9 | 163.9 ±35.2 |
| GLP-1(pg/ml) | 8.5 ± 2.1 | 4.7 ± 0.7 |
Tissues and plasma in CCK-KO and WT mice (n=4-8/ group) were collected after a 5 h-fasting after a 10-week high fat diet at 20 weeks of age. For GIP and GLP-1 determination, 1% DPPIV inhibitor was added in the plasma. Values represent mean ± SEM and asterisk indicates significant difference (P < 0.05) compared to WT mice.
Locomotor Activity
CCK-KO and WT mice had comparable diurnal patterns and total locomotor activity assessed over 4 continuous days (Figure 3).
Figure 3.
Locomotor activity in CCK-KO and WT mice. CCK-KO and WT mice (n=4 per group), 27-29 weeks of age, were individually housed in home cages which were then placed in a SmartFrame system for 4 continuous days. Values represent mean ± SEM.
Fat absorption
Total fat absorption in CCK-KO mice (81.0 ± 1.5%, Fig 4A) was significantly lower than that in WT mice (89.6 ± 1.2%). Relative to WT mice, CCK-KO mice could not absorb saturated fatty acids as well as unsaturated fatty acids. Specifically, CCK KO mice had lower absorption of myristic acid, palmitic acid and stearic acid relative to WT mice (Figure 4B). Stearic acid was the least absorbed fatty acid in CCK-KO mice, at 21% less than WT mice. Pancreatic PTL activity was significantly higher in CCK-KO than in WT mice (Figure 4C). Thus, whereas reduced absorption of longer-chain saturated fatty acids in CCK-KO mice might contribute to their lower body weight, fatty acid malabsorption is unlikely to be secondary to reduced lipid digestion by pancreatic enzymes.
Figure 4.
Fat absorption and pancreatic PTL activity in CCK-KO and WT mice. (A) Total fat absorption and (B) fatty acid profiles in fecal pellets were determined using gas chromatography. Fecal pellets were collected on the 4th day after animals started to receive a 20% fat diet mixture with 5% Olestra and again during the 9th week on the HFD. (C) Total pancreatic protein was analyzed for PTL activity. Data are expressed as mean ± SEM for 7 animals per group and values with asterisks represent significant differences relative to the WT mice (P < 0.05).
Energy expenditure
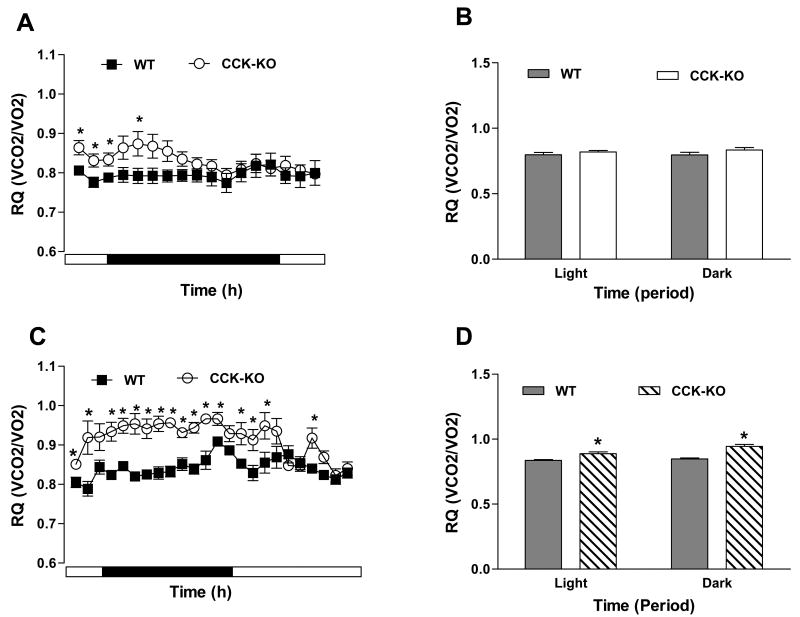
Overall energy expenditure did not differ between genotypes during an 18-h fast (Figure 5A-B), although it was higher in fed CCK-KO mice at some time points (Figure 5C-D). Fed CCK-KO mice also had a higher RQ than WT mice during both dark and light (Figure 6C-D), which was also observed in the first several hours during an 18-h fast (Figure 6A-B). Thus, CCK-KO mice oxidize more carbohydrate than WT mice, perhaps due to their fat malabsorption.
Figure 5.
Energy expenditure in CCK-KO and WT mice. (A) Hourly energy expenditure and (B) total energy expenditure during dark and light cycles during an 18-h fasting period. (C) Hourly energy expenditure and (D) total energy expenditure during dark and light cycles during a 24-h feeding period. Data are expressed as mean ± SEM and values with asterisks represent significant differences compared to the control group (P < 0.05).
Figure 6.
RQ value in CCK-KO and WT mice. (A) Hourly metabolic data (O2 consumption and CO2 production) and (B) RQ value during dark and light cycles during an 18-h fasting period. (C) Hourly metabolic rate and (D) RQ during dark and light cycles during a 24-h feeding period. RQ value was acquired in CCK-KO and WT mice during fasting and feeding. Data are expressed as mean ± SEM and values with asterisks represent significant differences relative to the WT mice (P < 0.05).
Discussion
CCK-KO mice fed low-fat chow (LFD) have normal body weight and food consumption (11; 19). Consistent with these observations, mice lacking CCK1R or CCK2R on LFD also have comparable body weights as WT mice (20; 21). When fed a HFD, CCK1R KO mice had comparable food intake and weight gain as WT mice (10). Short-term access of CCK-KO mice on a mixed genetic background to a HFD also resulted in similar body weight gain as in WT mice (11). Therefore, CCK activity is not required for normal homeostatic control of body weight. In the present experiments, we used a HFD to challenge CCK-KO mice, and in contrast to what might be predicted from the literature, they gained less weight than WT controls, although their food intake did not differ.
When fed LFD, CCK-KO mice had comparable body fat as WT controls, and the relative amounts of subcutaneous and visceral fat were also comparable (data not shown). Although maintenance on a HFD increased total fat mass in both genotypes, the increase in CCK-KO mice was less than in WT controls. Consuming a HFD by WT animals increases total body fat that is manifest as increased subcutaneous (inguinal) and visceral (retroperitoneal and epididymal) fat mass while enlarging fat-cell size (22-24). As mice gain weight, adipocyte size is enlarged before the number of adipocyte cells is increased (26). C57BL/6J mice are especially prone to this hypertrophic-hyperplastic obesity on a HFD (25). CCK-KO on the C57BL/6J background had a hypertrophic response to HFD that was manifest in every adipose depot assessed. Nonetheless, total body fat and the size of the inguinal depot were reduced, implying that CCK has an important role in regulating body fat accumulation and consequently body weight, and the effect is especially pronounced in subcutaneous fat.
The levels of several hormones are related to fat mass. Leptin secretion is highly related to adipocyte size and is increased when animals are fed a HFD (27). Although maintenance on a HFD increased plasma leptin in both genotypes relative to levels on a LFD (data not shown), CCK-KO mice had a smaller increase of plasma leptin than controls. The reduction of leptin apparently did not affect food intake in the CCK-KO animals since they had comparable food intake as WT controls. The CCK2R but not the CCK1R has been implicated in the regulation of plasma leptin (10; 21; 28). We therefore speculate that the changes in fat mass and leptin secretion in CCK-KO mice are likely mediated through CCK2R. Insulin is also related to body fat but was comparable between genotypes in the present experiment. GIP and GLP-1 were monitored in the present study since both have been implicated in the control of energy homeostasis. GLP-1 reduces food intake and body weight (2). Further, CCK is co-expressed with some GLP-1-positive cells in the small intestine (29). GIP has no effect on satiation (30), although it is involved in the control of adiposity (31). Mice lacking CCK had plasma GIP and GLP-1 levels comparable to those of WT mice.
CCK physiologically regulates pancreatic enzyme secretion and controls energy metabolism (3; 32). We assessed three mechanisms which might contribute to the reduced body weight of CCK-KO mice fed HFD: increased locomotor activity; reduced fat absorption; and increased energy expenditure. Blocking CCK2R in WT mice or the use of CCK2R-KO mice changes locomotor activity (33; 34). CCK-KO mice fed a HFD had a non-significant reduction of locomotor activity, suggesting that increased activity is probably not responsible for the reduced body weight gain in CCK-deficient mice. Pancreatic lipase hydrolyzes dietary triacylglycerols to monoacylglycerol and fatty acids in the intestinal lumen; these digestion products are subsequently absorbed to reform triacylglycerols in the mucosal cells (35). Exogenous CCK increases the secretion of pancreatic enzymes including lipase and amylase, and maintenance on a HFD increases pancreatic lipase secretion for several weeks although levels return to normal levels after prolonged exposure to a HFD (36; 37). In the present experiment, CCK-KO mice fed the HFD absorbed 8% less total fat than WT mice, and this was especially true for the absorption of long-chain saturated fatty acids such as stearic acid. In other studies we have found that CCK-KO mice absorb total polyunsaturated fat and monounsaturated fatty acids (linoleic acid and oleic acid) as well as WT controls (19). Our current findings are therefore consistent with studies indicating that stearic acid is absorbed less efficiently than either oleic acid or linoleic acid (38). These observations collectively imply that CCK-KO mice are unable to absorb saturated fatty acids as effectively as WT mice, but are able to absorb unsaturated fatty acids comparably well.
We found that fasted CCK-KO mice had up-regulated PTL activity on the HFD. CCK-KO mice fed a LFD have elevated pancreatic amylase synthesis and activity (11), indicating that there is a compensatory response in the pancreas of CCK-KO mice that enables elevated pancreatic enzyme activity. The human pancreas produces 3-to 10-fold more lipase than is required for lipid digestion (39), suggesting that altered lipid digestion due to up-regulated PTL activity in CCK-KO mice is not a primary factor for lipid malabsorption. It is more likely that CCK-KO mice have some other factor that reduces absorption of saturated fatty acids, but further studies on the process of lipid absorption and transport using a model such as the lymph fistula mouse will be necessary to ascertain this.
CCK2R KO mice expend more energy than WT mice on LFD (32). We observed that energy expenditure of CCK-KO mice on LFD is normal (19). Because consuming a HFD alters many parameters of energy homeostasis (40), we also assessed energy expenditure on the HFD. Maintenance on a HFD increased energy expenditure in CCK-KO mice considerably more than in WT at some time points, and this may have occurred via altered CCK2R signaling (32). Although HFD does not alter substrate oxidation in WT mice (C57BL/6J) under normal absorptive conditions (41), we found that CCK-KO mice fed HFD have increased RQ, indicating that they oxidize more carbohydrate and/or less fat than WT mice. The effect of fat malabsorption on RQ remains unclear. However, oleic acid could be converted from stearic acid catalyzed by stearoyl-CoA desaturase within cells, and oleic acid is an important energy substrate for lipid oxidation (42). Lower stearic acid absorption in CCK-KO mice results in less oxidation of oleic acid and, therefore, may result in more carbohydrates for use as an energy substrate. Consequently, reduced free fatty acids combine with decreased glycerol-3-phosphate, an intermediate product of glucose metabolism, to form triacylglycerol that is deposited within adipose cells (43). Although CCK-KO mice have altered carbohydrate metabolism, CCK-KO mice had comparable plasma glucose and insulin levels relative to WT animals. These findings suggest that mice lacking CCK utilize more energy and oxidize more carbohydrates to compensate for lower fat absorption when maintained on a HFD. Therefore, the combination of fat malabsorption and higher energy expenditure are likely key factors responsible for the lower adipose deposition and body weight gain exhibited in CCK-KO mice fed a HFD.
Conclusion
Cholecystokinin is thought to be important in several peripheral metabolic actions related to the digestion and absorption of food as well as meal size. We found that after a 10-week maintenance on a HFD, CCK-KO mice have lower body weight and reduced fat mass, with a marked reduction in inguinal (subcutaneous) adipose tissue, and enlarged adipocyte size, relative to WT mice despite normal food intake. CCK-KO mice have lipid malabsorbtion and higher energy expenditure and RQ than WT mice. On the HFD, mice lacking CCK had reduced plasma leptin, but normal GLP-1, GIP, glucose and insulin, and increased PTL. Based on these findings, we conclude that the functions of CCK related to energy homeostasis are manifested mainly when the diet is especially high in saturated fat.
Acknowledgments
This work was supported by National Institutes of Health Grants DK76928, DK59630, DK56863, DK83550 and DK17844. We thank Ms. Victoria Thoman for her excellent editorial assistance.
Footnotes
No conflicts of interest exist for any of the authors listed above.
C.M. Lo – principal author
A. King, T. Kindel, T. Rider – tech support
L. Samuelson – provide CCK KO
H. Raybould – collaborator & study design
R. Jandacek – fat absorption
S. Woods, P. Tso – collaborator, study design, & data interpretation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 2.Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–S50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh JH. Gastrointesinal hormones. In: Johnson LR, Christensen J, Jackson MJ, Jacobson ED, Walsh JH, editors. Physiology of the gastrointesinal tract. second. New York: Raven Press; 1986. pp. 181–253. [Google Scholar]
- 4.Muller K, Hsiao S. Specificity of cholecystokinin satiety effect: reduction of food but not water itnake. Pharmacol Biochem Behav. 1977;6:643–646. doi: 10.1016/0091-3057(77)90089-2. [DOI] [PubMed] [Google Scholar]
- 5.Kraly FS, Carty WJ, Resnick S, Smith GP. Effect of cholecystokinin on meal size and intermeal interval in the sham-feeding rat. J Comp Physiol Psychol. 1978;92:697–707. doi: 10.1037/h0077501. [DOI] [PubMed] [Google Scholar]
- 6.Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 7.Yox DP, Brenner L, Ritter RC. CCK-receptor antagonists attenuate suppression of sham feeding by intestinal nutrients. Am J Physiol. 1992;262:R554–R561. doi: 10.1152/ajpregu.1992.262.4.R554. [DOI] [PubMed] [Google Scholar]
- 8.Little TJ, Feltrin KL, Horowitz M, Meyer JH, Wishart J, Chapman IM, Feinle-Bisset C. A high-fat diet raises fasting plasma CCK but does not affect upper gut motility, PYY, and ghrelin, or energy intake during CCK-8 infusion in lean men. Am J Physiol Regul Integr Comp Physiol. 2008;294:R45–R51. doi: 10.1152/ajpregu.00597.2007. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Whitney A, Skoglund C, Castonguay TW, Moran TH. Decreased responsiveness to dietary fat in Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors. Am J Physiol. 1999;277:R1144–R1151. doi: 10.1152/ajpregu.1999.277.4.R1144. [DOI] [PubMed] [Google Scholar]
- 10.Bi S, Chen J, Behles RR, Hyun J, Kopin AS, Moran TH. Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R55–R63. doi: 10.1152/ajpregu.00002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am J Physiol. 1999;276:G1302–G1309. doi: 10.1152/ajpgi.1999.276.5.G1302. [DOI] [PubMed] [Google Scholar]
- 12.Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004;145:3873–3880. doi: 10.1210/en.2004-0284. [DOI] [PubMed] [Google Scholar]
- 13.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 14.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 2004;127:139–144. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- 16.Huggins KW, Camarota LM, Howles PN, Hui DY. Pancreatic triglyceride lipase deficiency minimally affects dietary fat absorption but dramatically decreases dietary cholesterol absorption in mice. J Biol Chem. 2003;278:42899–42905. doi: 10.1074/jbc.M303422200. [DOI] [PubMed] [Google Scholar]
- 17.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Jequier E, Schutz Y. Long-term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr. 1983;38:989–998. doi: 10.1093/ajcn/38.6.989. [DOI] [PubMed] [Google Scholar]
- 19.Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Woods SC, Tso P. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2008;294:R803–R810. doi: 10.1152/ajpregu.00682.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kopin AS, Mathes WF, McBride EW, Nguyen M, Al Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest. 1999;103:383–391. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Kent S, Morris MJ. Is the CCK2 receptor essential for normal regulation of body weight and adiposity? Eur J Neurosci. 2006;24:1427–1433. doi: 10.1111/j.1460-9568.2006.05016.x. [DOI] [PubMed] [Google Scholar]
- 22.Herberg L, Doppen W, Major E, Gries FA. Dietary-induced hypertrophic-hyperplastic obesity in mice. J Lipid Res. 1974;15:580–585. [PubMed] [Google Scholar]
- 23.Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism. 1993;42:1405–1409. doi: 10.1016/0026-0495(93)90190-y. [DOI] [PubMed] [Google Scholar]
- 24.Shillabeer G, Lau DC. Regulation of new fat cell formation in rats: the role of dietary fats. J Lipid Res. 1994;35:592–600. [PubMed] [Google Scholar]
- 25.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 26.Avram MM, Avram AS, James WD. Subcutaneous fat in normal and diseased states: 1. Introduction. J Am Acad Dermatol. 2005;53:663–670. doi: 10.1016/j.jaad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 28.Attoub S, Levasseur S, Buyse M, Goiot H, Laigneau JP, Moizo L, Hervatin F, Marchand-Brustel Y, Lewin JM, Bado A. Physiological role of cholecystokinin B/gastrin receptor in leptin secretion. Endocrinology. 1999;140:4406–4410. doi: 10.1210/endo.140.10.7079. [DOI] [PubMed] [Google Scholar]
- 29.Aiken KD, Kisslinger JA, Roth KA. Immunohistochemical studies indicate multiple enteroendocrine cell differentiation pathways in the mouse proximal small intestine. Dev Dyn. 1994;201:63–70. doi: 10.1002/aja.1002010107. [DOI] [PubMed] [Google Scholar]
- 30.Woods SC, West DB, Stein LJ, McKay LD, Lotter EC, Porte SG, Kenney NJ, Porte D., Jr Peptides and the control of meal size. Diabetologia. 1981;20(Suppl):305–313. [PubMed] [Google Scholar]
- 31.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 32.Miyasaka K, Ichikawa M, Ohta M, Kanai S, Yoshida Y, Masuda M, Nagata A, Matsui T, Noda T, Takiguchi S, Takata Y, Kawanami T, Funakoshi A. Energy metabolism and turnover are increased in mice lacking the cholecystokinin-B receptor. J Nutr. 2002;132:739–741. doi: 10.1093/jn/132.4.739. [DOI] [PubMed] [Google Scholar]
- 33.Vasar E, Harro J, Lang A, Pold A, Soosaar A. Differential involvement of CCK-A and CCK-B receptors in the regulation of locomotor activity in the mouse. Psychopharmacology (Berl) 1991;105:393–399. doi: 10.1007/BF02244435. [DOI] [PubMed] [Google Scholar]
- 34.Weiland TJ, Voudouris NJ, Kent S. The role of CCK2 receptors in energy homeostasis: insights from the CCK2 receptor-deficient mouse. Physiol Behav. 2004;82:471–476. doi: 10.1016/j.physbeh.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 35.Mayes P. Digestion & absorption. In: Murray RK, Mayes PA, Granner DK, Rodwell VW, editors. Harper's Biochemistry. Norwalk, Connecticut: Appleton & Lange; 1990. pp. 580–590. [Google Scholar]
- 36.Schmidt WE, Creutzfeldt W, Schleser A, Choudhury AR, Nustede R, Hocker M, Nitsche R, Sostmann H, Rovati LC, Folsch UR. Role of CCK in regulation of pancreaticobiliary functions and GI motility in humans: effects of loxiglumide. Am J Physiol. 1991;260:G197–G206. doi: 10.1152/ajpgi.1991.260.2.G197. [DOI] [PubMed] [Google Scholar]
- 37.Rippe C, Berger K, Mei J, Lowe ME, Erlanson-Albertsson C. Effect of long-term high-fat feeding on the expression of pancreatic lipases and adipose tissue uncoupling proteins in mice. Pancreas. 2003;26:e36–e42. doi: 10.1097/00006676-200303000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones PJ, Pencharz PB, Clandinin MT. Whole body oxidation of dietary fatty acids: implications for energy utilization. Am J Clin Nutr. 1985;42:769–777. doi: 10.1093/ajcn/42.5.769. [DOI] [PubMed] [Google Scholar]
- 39.Birk RZ, Brannon PM. Regulation of pancreatic lipase by dietary medium chain triglycerides in the weanling rat. Pediatr Res. 2004;55:921–926. doi: 10.1203/01.PDR.0000127430.04127.4F. [DOI] [PubMed] [Google Scholar]
- 40.Pagliassotti MJ, Gayles EC, Hill JO. Fat and energy balance. Ann N Y Acad Sci. 1997;827:431–448. doi: 10.1111/j.1749-6632.1997.tb51853.x. [DOI] [PubMed] [Google Scholar]
- 41.Funkat A, Massa CM, Jovanovska V, Proietto J, Andrikopoulos S. Metabolic adaptations of three inbred strains of mice (C57BL/6, DBA/2, and 129T2) in response to a high-fat diet. J Nutr. 2004;134:3264–3269. doi: 10.1093/jn/134.12.3264. [DOI] [PubMed] [Google Scholar]
- 42.Mead JF, Slaton WH, Jr, Decker AB. Metabolism of the essential fatty acids. II. The metabolism of stearate, oleate, and linoleate by fat-deficient and normal mice. J Biol Chem. 1956;218:401–407. [PubMed] [Google Scholar]
- 43.Avram AS, Avram MM, James WD. Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. J Am Acad Dermatol. 2005;53:671–683. doi: 10.1016/j.jaad.2005.05.015. [DOI] [PubMed] [Google Scholar]