Heart failure and hypertrophic cardiomyopathy (HCM)
Heart failure, a pathophysiologic state in which blood delivery is inadequate for tissue requirements, arises in the context of antecedent cardiovascular diseases such as coronary artery disease, hypertensive heart disease, valvular heart disease, congenital malformations, rhythm disturbances, or cardiomyopathy. Despite the technological advances in diagnosis and aggressive therapeutic interventions for cardiovascular diseases, reaching almost epidemic proportions, heart failure currently affects 5.7 million Americans, and each year over 670,000 new cases are diagnosed. These statistics are associated with social and economic costs. In 2006, heart failure contributed to 1.1 million hospital discharges and over 300,000 deaths, accounting for the estimated direct and indirect annual cost of 37.2 billion dollars [Lloyd-Jones D Circulation 2009]. In Japan, regardless of the lack of formal statistics, the number of the patients affected of heart failure is estimated to exceed 1.0 million.
Cardiac hypertrophy is a well-established risk factor for heart failure. In an epidemiological study in elderly, eccentric and concentric left ventricular hypertrophy conferred adjusted hazards ratios, compared with normal left ventricular geometry, of 2.95 and 3.32 for incident congestive heart failure [Gardin JM Am J Cardiol 2001]. So as to understand the genetic background of heart failure, to know the genetic causes of cardiac hypertrophy would be very helpful. Indeed, cardiac hypertrophy is caused by the combination of common variants (e.g. single nucleotide polymorphism) and rare variants (e.g. single gene mutation). Understanding the rare variant and its consequence to cardiac hypertrophy and heart failure would provide the potential rationale for the clinical usefulness of genetic testing. In this review, therefore, we focus on the hypertrophic cardiomyopathy (HCM) known to be caused by a single gene mutation, one of the major parts of cardiomyopathy which leads to cardiovascular deaths and progressive heart failure-related disability. Cardiomyopathy is now defined as the a heterozygous group of the diseases of the myocardium associated with mechanical and/or electrical dysfunction that usually exhibit inappropriate ventricular hypertrophy or dilation and are due to a variety of causes that frequently are genetic [Maron BJ Circulation 2006].
HCM should be potentially paid attention as the most common cause of sudden death in the young including trained athletes [Maron BJ N Engl J Med 2003], but is also known to be an important substrate for heart failure disability at any age [Maron BJ JAMA 2002]. In HCM, systolic left ventricular contractile function is vigorous and often appears supra normal, but the thickened muscle is stiff, resulting in impaired ventricular relaxation and high diastolic pressures. Vigorous contraction of the hypertrophied left atrium contributes importantly to the filling of the stiff left ventricule. Symptoms such as exertional dyspnea, orthopnea, paroxysmal nocturnal dyspnea and fatigue are common. The loss of the atrial systolic contribution to ventricular filling, i.e. of the atrial kick, during paroxysms of atrial fibrillation is particularly troublesome to patients with HCM, causing a sudden reduction of cardiac output and elevation of left atrial pressure. Cardiac collapse and/or acute pulmonary edema may result, especially when hypertrophy is marked and ventricular rate is very rapid. Patients who have asymmetric hypertrophy of the proximal interventricular septum may display additional findings related to transient obstruction of left ventricular outflow during systole. Of particular note, a small subset (10–15%) of HCM develop severe heart failure, this end-stage (burnt-out) phenotype resembling DCM has a markedly poor prognosis and often necessitates cardiac transplantation. Taken together with the high frequency of HCM in the general population (1 out of 500) [Maron BJ Circulation 1995], HCM is one of the leading causes of heart failure in the cardiac diseases originating primarily from myocardium.
HCM as a genetic disorder
HCM is an inherited primary heart muscle disease characterized by myocardial hypertrophy in the absence of secondary causes (e.g. systemic hypertension, obesity, aortic stenosis, and coronary artery disease) [Morita H J Clin Invest 2005]. With an incidence of about 1 of 500 in the general population, HCM is the most common genetic cardiovascular disease. Mutations causing HCM have been reported in at least 11 genes encoding proteins of the cardiac sarcomere which is the component of thick or thin filaments with contractile, structural, or regulatory functions. Over 400 dominant mutations in genes encoding cardiac β-myosin heavy chain (MYH7), cardiac myosin binding protein C (MYBPC3), cardiac troponin T (TNNT2), cardiac troponin I (TNNI3), cardiac troponin C (TNNC1), α-tropomyosin (TPM1), cardiac actin (ACTC), essential myosin light chain (MYL3), regulatory myosin light chain (MYL2), α-cardiac myosin heavy chain (MYH6) [Carniel E Circulation 2005] and titin (TTN) have been reported to cause HCM (http://cardiogenomics.med.harvard.edu/home). The other genes appear to account for far fewer cases of HCM and include MLP (CSRP3) [Geier C Circulation 2003], telethonin (TCAP) [Hayashi T J Am Coll Cardiol 2004], metavinculin (VCL) [Vasile VC Mol Genet Metab 2006], myozenin2 (MYOZ2) [Osio A Circ Res 2007], junctophilin-2 (JPH2) [Landstrom AP J Mol Cell Cardiol 2007], CARP (ANKRD1) [Arimura T J Am Coll Cardiol 2009] and so forth. Although some founding mutations have been identified [Jaaskelainen P J Mol Med 2002], the diversity of HCM-causing genes and mutations suggests that these genetic disorders occurred independently and recently in human evolution.
Comprehensive genetic analyses [Morita H Cold Spring Harb Symp Quant Biol 2002] [Richard P Circulation 2003] [Van Driest SL J Am Coll Cardiol 2004] clarified that approximately 60% of HCM could account for the dominant mutations of sarcomere protein genes, and among those MYH7 and MYBPC3 predominate in frequency. The pathophysiology and natural history of HCM are quite variable and appear related to particular mutations.
Pathophysiologocal consequences of HCM-causing mutation
Most HCM mutations encode defective polypeptide containing missense residues and small deletion, these poison peptides are likely to be stably incorporated into cardiac myofilaments and to induce hypertrophy because the sarcomere function is impaired [Seidman JG Cell 2001]. Uncoordinated contraction due to heterogeneity of mutant and normal sarcomere proteins, increased energy consumption due to the initial hyperdynamic contractile performance, and changes in Ca homeostasis could diminish myocyte survival and trigger replacement fibrosis. With insidious myocyte loss and increased fibrosis, the HCM heart transitions from hypertrophy to failure.
As for the truncated mutants, their consequences remain controversial. Due to the instability of truncated myosin-binding protein C, only small amounts of them could be incorporated into sarcomere, functioning as a poison peptide, leading to the disrupted myofibrillar architecture [Flavigny J J Mol Biol 1999]. The variable stability of altered proteins might influence clinical expression. Truncated protein is thought to be degraded by the ubiquitin-proteasome system. The decline in function of this degradation system with age might be consistent with the genetic findings that truncated mutations were quite infrequent in the pediatric LVH [Morita H N Engl J Med 2008]. Alternatively, haploinsufficiency is recently shown to be involved in the pathomechanism for the consequence of truncated mutants [van Dijk SJ Circulation 2009].
Genotype-phenotype relationship
The precise genetic mutation determines the age of onset, the extent and pattern of hypertrophy, and the person’s risk of developing symptomatic heart failure or sudden death. The targets for recent genetic screening involve even the asymptomatic patients with mild hypertrophy detected by 2-dimensional echocardiography, thus the prevalence and clinical manifestations of mutation-carriers is naturally different from those in the earlier studies which focused on the severe referral cases and/or large families with high penetrance. Although the descriptions of the genotype-phenotype correlations have been revised over and over, some findings remain unfaded. First, TNNT2 mutations are generally associated with a high incidence of sudden death despite only mild cardiac hypertrophy. Secondly, the correlation between MYBPC3 mutations and age of onset has been discussed [Niimura H N Engl J Med 1998]. Recognition of cardiac hypertrophy may be age related with any initial appearance well delayed into adulthood. In the analysis on the late-onset HCM, mutations in MYBPC3 are predominantly frequent both in Caucasian and Japanese [Niimura H Circulation 2002] [Anan R Am J Cardiol 2007]. However, to note, MYBPC3 mutations could not necessarily induce only the late-onset diseases. The recent genetic screening [Morita H N Engl J Med 2008] indicated that cardiac hypertrophy in the pediatric cases could be frequently caused by MYBPC3 mutation. For example, MYBPC3 Gly490Arg missense mutation, which was observed in an adult with mild LVH (max LV wall thickness was 14mm), was also detected in a pediatric case with max LV wall thickness of 17mm. Moreover, 5 weeks old baby with MYBPC3 Arg495Gly missense mutation showed the fulminating phenotype accompanied with the hypertrophied interventricular septum of 12mm (normal range; 3.5–5.0mm).
Taken together, we have to carefully discuss the relationship among the mutation, clinical manifestation and prognosis. The mutation-specific – rather than gene-specific – consideration should be essential [Ehlermann P BMC Med Genet 2008]. As shown above, the HCM patients harboring the identical mutation display a striking phenotype heterogeneity. It should also be noted that the identical mutation, even within a family, could lead to the diverse clinical profiles. Moreover, the phenotype caused by sarcomere protein gene mutation is not limited to HCM. Dilated cardiomyopathy, restrictive cardiomyopathy and left ventricular non compaction [Klaassen S Circulation 2008] are also caused by sarcomere protein gene mutations. This way, in the genomic era, the hypertrophic-dilated-restrictive classification by itself might become old-fashioned.
Genetic modifiers
Genetic diversity is attributed to considerable inter- and intra-genic heterogeneity accompanied by the influence of genetic modifiers and environmental factors. The role of genetic modifiers has become the subject of recent investigations, and polymorphisms in the genes encoding rennin-angiotensin-aldosterone system [Perkins MJ Eur Heart J 2005] or sex hormone receptors [Lind JM J Mol Cell Cardiol 2008] are argued as candidates, which remain controversial. With the identical causing mutation, the correlation between causing mutation and clinical phenotype in one family is different from that in another family, thus the clarification of the “universal” genetic modifiers is practically challenging. There remains the possibility that each family has “private” genetic modifiers. The genome-wide mapping analysis [Daw EW Hum Mol Genet 2007] performed to identify the loci linked with the left ventricular weight in the 100 HCM patients with MYBPC3 mutation showed that the candidate locus is involved in 10p13 region. This region contains CARP and ITGA8, both of which are well-known to be functionally related to cardiac hypertrophy.
Common disease-common variant or Common disease-rare variant?
Re-sequencing studies are resolving the question regarding the relative contribution of rare variants to common diseases. Subjects with low HDL-cholesterol levels were significant likely to harbor protein-sequence-altering genetic variants in candidate genes (ABCA1, APOA1, LCAT) previously implicated in Mendelian forms of low HDL-cholesterol [Cohen JC Science 2004]. In another study [Cohen JC N Engl J Med 2006], nonsense sequence variants in PCSK9 were shown to lower LDL-cholesterol levels and protection from cardiovascular disease in the general population. Also in cardiology, the contribution of rare variant to the common disease such as cardiac hypertrophy and heart failure has been shown. In a community-based cohort (1862 Framingham Heart Study participants), about 3% of participants had echocardiographic LVH without any underlying causes, of whom 16% had sarcomere protein gene mutations, suggesting that even unexplained LVH in the general population could be partly explained by the rare variant in genes which are established as the causing-genes for HCM, an inherited disorder characterized by LVH [Morita H Circulation 2006]. This study could suggest the rare variants are related to the onset and/or progression of “common” LVH. Additionally, a sarcomere protein gene mutation was reported to be prevalent as a cause of heart failure in South Asia [Dhandapany PS Nat Genet 2009]. The 25bp-deletion variant in MYBPC3, previously known as an HCM-causing mutation [Waldmuller S J Mol Cell Cardiol 2003] was found to be a common variant in the Indian general population (4%) and highly associated with heritable cardiomyopathy and an increased risk for heart failure. Although the existence of founder effect should be considered, this report clearly demonstrated that the genetic analysis for heart failure should involve the genetic variants which are thought to be related to the rare inherited disorder.
Genetic testing in the diagnosis of HCM
Importantly, before a diagnosis of HCM, a full history-taking and physical examination should be carefully performed. Clues can be picked up to expose other causes of unexplained LVH. Aortic stenosis, hypertension, coronary artery disease, or the systemic disorder should be carefully ruled out. Moreover, taking the family history extending to at least 3 generations is essential for the diagnosis. Practitioners should record a pedigree to illustrate the family history data. In a clinical setting, diagnosis of HCM should be made by 2-dimensional echocardiography and genetic testing.
Practically, knowledge of the genetic basis for HCM has led to the identification of the individuals harboring a disease-causing mutation but seemingly without evidence of LVH. Quantitative assessments of LV wall thickness represent a continuum, thus the individuals in the border zone exist. Virtually any LV wall thickening, even when within normal limits (max LV wall thickness < 13mm) might be consistent with the presence of an HCM-causing gene mutation. In the other word, there is no minimal LV wall thickness required for HCM. In addition, gene-based insights into pathophysiology may define more subtle clinical characteristics other than LVH. The recent progress in the diagnostic tools (i.e. Tissue Doppler echocardiography [Ho CY Circulation 2002], magnetic resonance imaging [Germans T J Am Coll Cardiol 2006]) could make the more subtle pre-clinical changes detectable. Consequently, in the genotype-positive patients, it should be tough to be diagnosed as normal. But, even if no clinical abnormality is detected, annual screening should be recommended for the genotype-positive patients.
Some diseases presenting chiefly with LVH and seemingly mimicking HCM turn out to have clearly distinct underlying pathophysiologies. Genetic mutations in PRKAG2 (encoding the γ2 regulatory subumit of the AMP-activated protein kinase), LAMP2 (encoding the lysosome-associated membrane protein-2) and GLA (encoding the α-galactosidase A) are known to cause the storage cardiomyopathy [Arad M N Engl J Med 2005] [Nakao S N Engl J Med 1995]. In particular, a specific enzyme supplementation therapy exists for the GLA-related storage cardiomyopathy which is well-known as the cardiac Fabry disease, thus the precise and timely differential diagnosis from HCM is of potent clinical value. In a viewpoint of differential diagnosis between HCM and the storage cardiomyopathy, genetic testing is potentially useful.
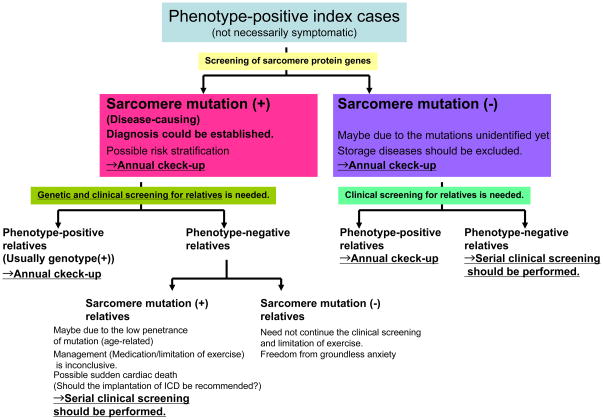
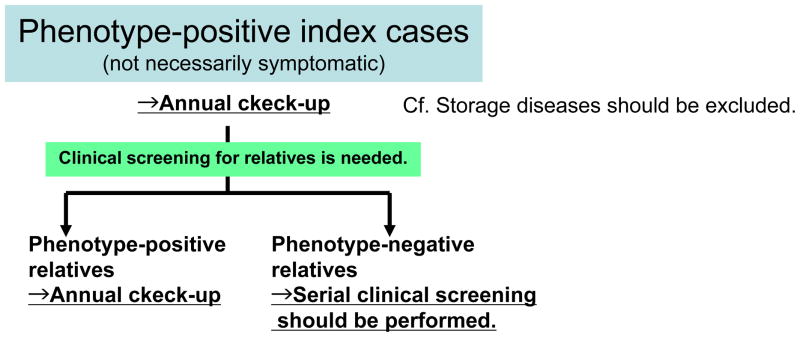
The potential impact of genetic testing (Figure 1 & 2)
Figure 1.
The diagnostic algorithm of HCM with genetic testing
Figure 2.
The diagnostic algorithm of HCM without genetic testing
For index cases that clinically manifest the disease, the genetic testing is diagnostic [Maron BJ J Am Coll Cardiol 2004]. Identification of a sarcomere protein gene mutation in an individual with unexplained cardiac hypertrophy establishes the diagnosis of HCM. At present, no single gene mutation is capable of stratifying risk profile, and the genetic modifiers and environmental factors should be considered in combination. That is one of the reasons why genetic testing does not yet represent a clinically relevant strategy that routinely affects disease management. Studies on the natural history associated with a specific disease-causing mutation can provide additional information to aid in the risk stratification. The future identification of genotype-phenotype correlation will enable us to choose the most appropriate therapy based on the causing mutation. For example, the prophylactic implantation of implantable cardioverter defibrillator (ICD) should be recommended to the patient bearing the sudden cardiac death-liable mutation. Preemptive starting of the potent medical therapy, whose beneficial effects have to be testified, could be performed for the case with the mutation related to the progression to the dilated-pattern. When a specific therapy for HCM becomes clinically a reality, genetic testing antecedent to that specific therapy will be potentially recommended.
Genetic testing of the index case could provide the diagnostic gold standard for his/her offspring, siblings, parents and other relatives. Theoretically, genotype-positive individuals have a 50% probability of transmitting mutation and cardiomyopathy to each offspring. While the familial cases are just caused by the inheritance of mutation, the sporadic cases might reflect either inadequate clinical ascertainment or de novo mutation. De novo mutations as well as inherited ones could initiate new familial diseases, and the offspring of individuals with sporadic HCM are at risk of inheriting the mutation and disease. The index case should be surely informed of the familial nature and autosomal dominant transmission of HCM. In addition, detailed information should be given also to relatives on the pros and cons of genetic family screening. Of particular note, the possible setting that an asymptomatic relative without any phenotype of disease could be pronounced to be a mutation-carrier should be presumed.
However, in the absence of genetic testing, clinical pedigree screening has to be gropingly performed. Practically, any HCM family member of full adult maturity (18–21yrs of age) and with normal echocardiographic findings can harbor the potential to develop LVH and HCM at virtually any age. Based on that, the strategy for clinical screening with echocardiography in family members is recommended as follows; every 12–18 months at 12 to 18–21 yrs old, probably about every 5 yrs at >18–21 yrs old. Depending on the clinical manifestations (e.g. late-onset or malignant clinical course), screening at more frequent intervals should be recommended, anyway, serial clinical screening is needed over substantial periods of time [Hershberger RE J Card Fail 2009]. This phenotype-oriented strategy needs considerable motivation by physicians and at-risk family members over a long time. In approximately 50% of these cases, burden of medical expense, patient compliance and vague anxiety due to diagnostic uncertainty can be clearly alleviated by one-time genetic testing. In the other word, when a phenotype-negative family member proves to be genotype-negative for the index case’s mutation, serial “expensive and annoying” cardiac evaluations and limitation on athletic participation for that family member and his/her progeny might be no longer necessary.
Roughly estimated, 50–60% of HCM patients have any causing mutation in sarcomere protein genes, which could be theoretically identified in approximately 50% of their relatives. If 100,000 index cases exist and each index case has 4 relatives, 100,000 (=100,000 × 0.5 × 4 × 0.5) relatives are estimated to be free from serial clinical screening.
Here, we should consider the medical cost for clinical screening. In Japan, 12-lead ECG, cardiac echocardiography and cardiac magnetic resonance imaging cost 1,500 yen ($17), 8,800 yen ($98), and 16,000 yen ($178), respectively. Since 1961, it has been mandatory for all Japanese residents to utilize the public health insurance system, which provides for the healthcare needs of every Japanese resident. The system is financed by a combination of social insurance fees, tax subsidies and copayment. Thus, if needed, every Japanese resident from coast to coast can receive the cardiac check-up with the payment of 30% of amounts shown above. For example, each patient pays in hospitals 2640 yen (approximately $29) for cardiac echocardiography, and 4800 yen (approximately $53) for cardiac magnetic resonance imaging. In USA, however, the medical costs for clinical screening are awfully higher, and are not necessarily covered by every kind of medical insurances. (In USA, eco and MRI cost $.....)
Also on such economical grounds, unnecessary clinical screenings should be avoided. Genetic testing is helpful for saving money.
The management and economic of genetic testing
Genomic medicine has moved outside the research laboratory to clinical practice [Bos JM J Am Coll Cardiol 2009]. Technological advances will continue to improve testing method, thereby dramatically decreasing costs. More speedy and inexpensive analysis commercially available will emerge. In USA, some institutes such as Harvard Partners, GeneDX, and PGxHealth commercially offer genetic testing. Although clear distinctions according to Clinical Laboratory Improvement Amendment (http://www.cms.hhs.gov/clia/) standards should be made between testing for clinical purposes and that undertaken for research purposes, as the genetic knowledge of heart failure is evolutionally emerging from research laboratories, it would be ideal that physician scientists with a profound knowledge of medical genetics should facilitate clinical genetic testing as well as genetic research. In Japan, the genetic testing of HCM is usually performed still inside research laboratory, therefore necessary expenses are met by research funds, therefore, the time is not quite ripe for the argument on the cost-effectiveness and the coverage by medical insurance.
Regulation and guidelines about genetic testing
Genetic testing raises great ethical and legal concerns. Subjects participating in genetic testing must be assured of protected confidentiality. The public is wary that genetic test might induce the genetic discrimination in access to life and health insurance. National legislation prohibiting genetic discrimination has been adopted recently and is a major step forward in this area. In the United States, the Genetic Information Nondiscrimination Act (GINA) prohibits the improper use of genetic information in health insurance and employment (http://www.govtrack.us/congress/billtext.xpd?bill=h110-493&show-changes=0&page-command=print).
In Japan, there remains no law regulating the genetic testing, but based on the Guidelines for Genetic Testing (2003) made by 10 Japanese genetic medicine-related societies (http://jshg.jp/resources/data/10academies_e.pdf), the Japanese Circulation Society made Guidelines for Genetic Test and Genetic Counseling in Cardiovascular Disease in 2006. As described above, in Japan, genetic testing of HCM is usually performed as a sort of genetic research, thus has to be carried out with the approval of the institutional review board in accordance with the Guidelines for Research on the Human Genome and Genes -Three Ministry Guidelines-(2001).
Genetic testing and genetic counseling
When the genetic testing is performed, a genotype-positive individual might feel anxiety about the potential clinical dangers led by an HCM-causing mutation, whereas a genotype-negative individual gains the psychological freedom. Genetic counseling is the process of communicating relevant genetic information to patients and their relatives, so that they might understand the genetic information presented and use it to make decisions as to genetic testing or therapeutic intervention. The process also helps individuals adapt to the medical, psychological and familial implications of genetic contributions to disease. This is one of the essential components of the evaluation, diagnosis and management of cardiomyopathy. Essential activities are obtaining a careful and comprehensive family history, educating the patient and relatives regarding the disease transmission, family risks and need for genetic testing, counseling after any genetic testing regardless of its results-positive, negative, or uncertain-, their integration into the future therapeutic plan. Genetic counseling also involves the psychological issues of patients and their relatives. According to the Dutch study for the psychological outcomes and the efficacy of genetic counseling [Christiaans I Am J Med Genet A 2009], in 143 predictively tested HCM mutation carriers, only 4% of them regretted being tested afterwards. Almost all were satisfied with genetic counseling, but a quarter of them had never seen cardiologist after DNA testing or no follow-up appointments. The impact of genetic counseling on the regular cardiac follow-up should be more established in the patients as well as practitioners. The practitioners have to remain up to date with the accelerating development in the medical genetics, integrating genetic and clinical evaluations with genetic counseling.
The problems on genetic testing to be solved
In order to prompt the genetic testing in a clinical setting, genetic research should solve the following problems. First, in approximately 40% of HCM patients, the causing mutation remains unidentified. Secondly, neither mutation-specific clinical phenotype nor mutation-specific management/therapy is defined. The latter one might reflect the complexity of the relationship among causing mutation, genetic modifiers and environmental factors. The elaborate accumulation of the clinical genetic findings is needed to solve these enigmatic equations. Demonstration of the contribution of genetic testing to choosing the most appropriate therapeutic intervention in each patient will potentially motivate the clinical use of genetic testing.
The low penetrance is the immanent issue of genetics. Due to this nature, the case bearing sarcomere protein gene mutation sometimes has no cardiac abnormality. Indeed, how to manage such cases is the most tough question to be answered. Do they need the limitation of exercise? Should the implantation of ICD be recommended? At present, we cannot but mention that serial clinical screening should be performed to these cases.
The index cases should be well informed in advance that the results of the genetic testing have a great impact on the relatives as well as themselves. That might possibly include the identification of the mutation-carriers without any abnormal phenotype. Ethical issues should be settled through the genetic counseling. In addition, genetic testing should prompt the following serial cardiac screening of mutation-positive relatives-even without any cardiac abnormalities.
Genetic testing is expected to be speedy, accurate and inexpensive. The rapid evolution of analytical technique would allow the genetic testing speedy and informative. Genetic testing costs have dropped with the development of new sequencing methods. The $1,000 genome-era will come in near future [Drmanac R Science 2009]. Importantly, other than the technical development by itself, the integration with the outcomes from genetic research (e.g. new discovery of disease-causing mutations, the identifications of genotype-phenotype correlations) could make the genetic testing truly useful in a clinical setting.
Conclusion
In this review, we mainly discuss the potential impact of genetic testing on HCM. The rapid evolution and accumulation of genetic data on HCM enable us to utilize the genetic testing as the potent diagnostic tool. Therefore, additional new discoveries of human gene mutations that remodel the heart and cause heart failure, not limited to cardiomyopathy, can prompt the genetic testing in heart failure. Our ultimate aim is to formulate the most appropriate therapeutic interventions based on the close meshed risk stratification in every patient at risk due to a gene mutation. Although we emphasize the importance of genetic testing here, we never neglect the clinical screening. Integrated with the genetic data, the clinical screening will be more effective. The combination of genetic screening and clinical one will help us perform the overall case assessment and choose the most appropriate therapeutic strategy.
References
- Anan R, Niimura H, Takenaka T, Hamasaki S, Tei C. Mutations in the genes for sarcomeric proteins in Japanese patients with onset sporadic hypertrophic cardiomyopathy after age 40 years. Am J Cardiol. 2007;99(12):1750–1754. doi: 10.1016/j.amjcard.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Arad M, Maron BJ, Gorham JM, Johnson WH, Jr, Saul JP, Perez-Atayde AR, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352(4):362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- Arimura T, Bos JM, Sato A, Kubo T, Okamoto H, Nishi H, et al. Cardiac ankyrin repeat protein gene (ANKRD1) mutations in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54(4):334–342. doi: 10.1016/j.jacc.2008.12.082. [DOI] [PubMed] [Google Scholar]
- Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54(3):201–211. doi: 10.1016/j.jacc.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Carniel E, Taylor MR, Sinagra G, Di Lenarda A, Ku L, Fain PR, et al. Alpha-myosin heavy chain: a sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation. 2005;112(1):54–59. doi: 10.1161/CIRCULATIONAHA.104.507699. [DOI] [PubMed] [Google Scholar]
- Christiaans I, van Langen IM, Birnie E, Bonsel GJ, Wilde AA, Smets EM. Genetic counseling and cardiac care in predictively tested hypertrophic cardiomyopathy mutation carriers: the patients’ perspective. Am J Med Genet A. 2009;149A(7):1444–1451. doi: 10.1002/ajmg.a.32915. [DOI] [PubMed] [Google Scholar]
- Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, Hobbs HH. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science. 2004;305(5685):869–872. doi: 10.1126/science.1099870. [DOI] [PubMed] [Google Scholar]
- Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- Daw EW, Chen SN, Czernuszewicz G, Lombardi R, Lu Y, Ma J, et al. Genome-wide mapping of modifier chromosomal loci for human hypertrophic cardiomyopathy. Hum Mol Genet. 2007;16(20):2463–2471. doi: 10.1093/hmg/ddm202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhandapany PS, Sadayappan S, Xue Y, Powell GT, Rani DS, Nallari P, et al. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat Genet. 2009;41(2):187–191. doi: 10.1038/ng.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drmanac R, Sparks AB, Callow MJ, Halpern AL, Burns NL, Kermani BG, et al. Human Genome Sequencing Using Unchained Base Reads on Self-Assembling DNA Nanoarrays. Science. doi: 10.1126/science.1181498. [DOI] [PubMed] [Google Scholar]
- Ehlermann P, Weichenhan D, Zehelein J, Steen H, Pribe R, Zeller R, et al. Adverse events in families with hypertrophic or dilated cardiomyopathy and mutations in the MYBPC3 gene. BMC Med Genet. 2008;9:95. doi: 10.1186/1471-2350-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavigny J, Souchet M, Sébillon P, Berrebi-Bertrand I, Hainque B, Mallet A, et al. COOH-terminal truncated cardiac myosin-binding protein C mutants resulting from familial hypertrophic cardiomyopathy mutations exhibit altered expression and/or incorporation in fetal rat cardiomyocytes. J Mol Biol. 1999;294(2):443–456. doi: 10.1006/jmbi.1999.3276. [DOI] [PubMed] [Google Scholar]
- Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87(9):1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- Geier C, Perrot A, Ozcelik C, Binner P, Counsell D, Hoffmann K, et al. Mutations in the human muscle LIM protein gene in families with hypertrophic cardiomyopathy. Circulation. 2003;107(10):1390–1395. doi: 10.1161/01.cir.0000056522.82563.5f. [DOI] [PubMed] [Google Scholar]
- Germans T, Wilde AA, Dijkmans PA, Chai W, Kamp O, Pinto YM, et al. Structural abnormalities of the inferoseptal left ventricular wall detected by cardiac magnetic resonance imaging in carriers of hypertrophic cardiomyopathy mutations. J Am Coll Cardiol. 2006;48(12):2518–2523. doi: 10.1016/j.jacc.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Arimura T, Itoh-Satoh M, Ueda K, Hohda S, Inagaki N, et al. Tcap gene mutations in hypertrophic cardiomyopathy and dilated cardiomyopathy. J Am Coll Cardiol. 2004;44(11):2192–2201. doi: 10.1016/j.jacc.2004.08.058. [DOI] [PubMed] [Google Scholar]
- Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA Heart Failure Society of America. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J Card Fail. 2009;15(2):83–97. doi: 10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105(25):2992–2997. doi: 10.1161/01.cir.0000019070.70491.6d. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen P, Kuusisto J, Miettinen R, Kärkkäinen P, Kärkkäinen S, Heikkinen S, et al. Mutations in the cardiac myosin-binding protein C gene are the predominant cause of familial hypertrophic cardiomyopathy in eastern Finland. J Mol Med. 2002;80(7):412–422. doi: 10.1007/s00109-002-0323-9. [DOI] [PubMed] [Google Scholar]
- Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, Schuler P, et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation. 2008;117(22):2893–2901. doi: 10.1161/CIRCULATIONAHA.107.746164. [DOI] [PubMed] [Google Scholar]
- Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol. 2007;42(6):1026–1035. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind JM, Chiu C, Ingles J, Yeates L, Humphries SE, Heather AK, et al. Sex hormone receptor gene variation associated with phenotype in male hypertrophic cardiomyopathy patients. J Mol Cell Cardiol. 2008;45(2):217–222. doi: 10.1016/j.yjmcc.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92(4):785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287(10):1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349(11):1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Seidman JG, Seidman CE. Proposal for contemporary screening strategies in families with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44(11):2125–2132. doi: 10.1016/j.jacc.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- Morita H, DePalma SR, Arad M, McDonough B, Barr S, Duffy C, et al. Molecular epidemiology of hypertrophic cardiomyopathy. Cold Spring Harb Symp Quant Biol. 2002;67:383–388. doi: 10.1101/sqb.2002.67.383. [DOI] [PubMed] [Google Scholar]
- Morita H, Seidman J, Seidman CE. Genetic causes of human heart failure. J Clin Invest. 2005;115(3):518–526. doi: 10.1172/JCI200524351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Larson MG, Barr SC, Vasan RS, O’Donnell CJ, Hirschhorn JN, et al. Single-gene mutations and increased left ventricular wall thickness in the community: the Framingham Heart Study. Circulation. 2006;113(23):2697–2705. doi: 10.1161/CIRCULATIONAHA.105.593558. [DOI] [PubMed] [Google Scholar]
- Morita H, Rehm HL, Menesses A, McDonough B, Roberts AE, Kucherlapati R, et al. Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med. 2008;358(18):1899–1908. doi: 10.1056/NEJMoa075463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333(5):288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- Niimura H, Bachinski LL, Sangwatanaroj S, Watkins H, Chudley AE, McKenna W, et al. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N Engl J Med. 1998;338(18):1248–1257. doi: 10.1056/NEJM199804303381802. [DOI] [PubMed] [Google Scholar]
- Niimura H, Patton KK, McKenna WJ, Soults J, Maron BJ, Seidman JG, et al. Sarcomere protein gene mutations in hypertrophic cardiomyopathy of the elderly. Circulation. 2002;105(4):446–451. doi: 10.1161/hc0402.102990. [DOI] [PubMed] [Google Scholar]
- Osio A, Tan L, Chen SN, Lombardi R, Nagueh SF, Shete S, et al. Myozenin 2 is a novel gene for human hypertrophic cardiomyopathy. Circ Res. 2007;100(6):766–768. doi: 10.1161/01.RES.0000263008.66799.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins MJ, Van Driest SL, Ellsworth EG, Will ML, Gersh BJ, Ommen SR, et al. Gene-specific modifying effects of pro-LVH polymorphisms involving the renin-angiotensin-aldosterone system among 389 unrelated patients with hypertrophic cardiomyopathy. Eur Heart J. 2005;26(22):2457–2462. doi: 10.1093/eurheartj/ehi438. [DOI] [PubMed] [Google Scholar]
- Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, et al. EUROGENE Heart Failure Project. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107(17):2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104(4):557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- van Dijk SJ, Dooijes D, dos Remedios C, Michels M, Lamers JM, Winegrad S, et al. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009;119(11):1473–1483. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- Van Driest SL, Vasile VC, Ommen SR, Will ML, Tajik AJ, Gersh BJ, et al. Myosin binding protein C mutations and compound heterozygosity in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44(9):1903–1910. doi: 10.1016/j.jacc.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Vasile VC, Will ML, Ommen SR, Edwards WD, Olson TM, Ackerman MJ. Identification of a metavinculin missense mutation, R975W, associated with both hypertrophic and dilated cardiomyopathy. Mol Genet Metab. 2006;87(2):169–174. doi: 10.1016/j.ymgme.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Waldmüller S, Sakthivel S, Saadi AV, Selignow C, Rakesh PG, Golubenko M, et al. Novel deletions in MYH7 and MYBPC3 identified in Indian families with familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2003;35(6):623–636. doi: 10.1016/s0022-2828(03)00050-6. [DOI] [PubMed] [Google Scholar]