Abstract
BRCA1 functions as a tumor suppressor; recent work suggests that BRCA1 may also induce cell-cycle arrest to allow for DNA repair. We hypothesized that BRCA1 expression in prostate tumor tissue may be associated with prostate cancer progression through regulation of the cell-cycle. We used immunohistochemistry to evaluate BRCA1 protein expression in archival tumors samples from 393 prostate cancer cases in the Physicians' Health Study. The men were followed prospectively from diagnosis to development of metastases and mortality. Fifteen percent of tumors stained positive for BRCA1. BRCA1 positive tumors had substantially increased tumor proliferation index compared to negative tumors (47.0 Ki67 positive nuclei vs. 10.3, p=0.0016), and were more likely to develop lethal cancer compared to BRCA1 negative tumors (Hazard ratio=4.6; 95% Confidence interval: 2.4, 8.7). These findings strengthen the hypothesis that BRCA1 plays a role in cell-cycle control and demonstrate that BRCA1 is a marker of clinical prostate cancer prognosis.
INTRODUCTION
BRCA1 is a multifunctional tumor suppressor protein implicated in regulating the maintenance of genome integrity through the activation of DNA repair genes, heterochromatin formation, double-strand-break repair, homologous recombination events, and ubiquitination.(1–3) Recently, a more complex role for BRCA1 was proposed, whereby BRCA1 can induce arrest at different cell-cycle check-points to allow for DNA repair.(4–6)
Mutations in BRCA1 have been associated with increased risk of breast, ovarian, and more recently, prostate cancer – particularly high grade disease.(7–12) However, while mutations in BRCA1 may influence familial prostate cancer risk and progression, few studies have examined BRCA1 protein expression in prostate cancer tumor tissue, and to our knowledge, none have correlated expression with cancer progression and mortality. Recently, Schayek et al. showed that BRCA1 protein expression in prostate differentially regulates IGF-IR gene expression in an androgen-dependent manner and found significantly elevated BRCA1 levels in prostate cancer in comparison with normal prostate tissue.(13) We hypothesized that BRCA1 expression could have prognostic relevance in prostate cancer through its regulation of the cell-cycle regardless of germ-line mutations.
MATERIALS AND METHODS
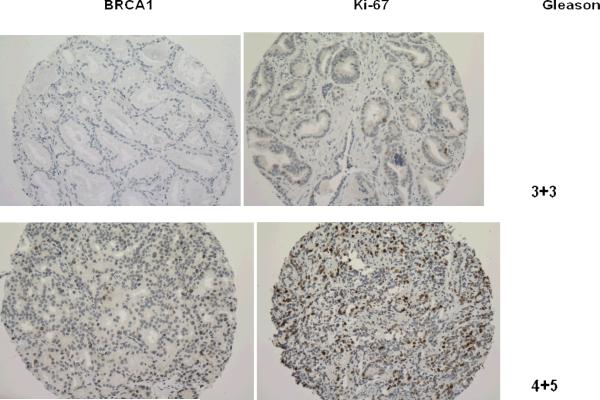
We undertook a prospective study among 392 men in the Physicians' Health Study(14, 15) (clinicaltrials.gov identifier: NCT00000500) who were diagnosed with prostate cancer from 1983 to 2004. We constructed tumor tissue microarrays from archival prostatectomy and TURP tumor tissue specimens using three 0.6 mm cores of tumor per case. Immunohistochemical staining was performed on 5 micron sections of the TMAs to assess BRCA1 expression (monoclonal MS110 antibody specific for the N-terminus of the 220 kDa BRCA1 protein, Calbiochem, diluted 1:50 after EDTA-based antigen retrieval) and cell proliferation (polyclonal anti Ki67 antibody, Vectorlab, diluted 1:2000 after citrate-based antigen retrieval). MCF7 and HCC1937 cell-lines were used respectively as positive and negative controls for BRCA1 immunostaining. Because of the small proportion of stained nuclei and the homogeneous intensity of the immunostaining, the study pathologists (MF, RF) scored tumor expression of BRCA1 manually as positive or negative; Ki67 proliferation index was scored by quantitative image analysis (Ariol SL-50, Applied Imaging) (Figure. 1). The possible heterogeneity of the immunohistochemical staining for BRCA1 was also controlled using whole sections of 14 prostate cancer cases included in the TMAs. RNA expression levels of BRCA1 were available from a subset of participants (n=116) using a gene expression profiling study that applied the DASL Illumina 6K array.(16) The study was approved by the Institutional Review Board of Partners Healthcare.
Figure 1. Immunohistochemical expression of BRCA1 according to Gleason's score.
Comparative representation of BRCA1 expression and Ki67 proliferative index in serial sections of prostate cancer tissues with different Gleason's score. The brown immunostaining is nuclear for both BRCA1 and Ki67, slides are counterstained with hematoxylin (magnification x200)
We abstracted data on age, stage and PSA levels at diagnosis from medical records, and conducted a standardized histopathological review for Gleason score.(17,18) The men were followed prospectively since diagnosis for the development of bony metastases and mortality through March 2009, without loss to follow-up.
We evaluated whether BRCA1 positive and negative tumor status based on immunohistochemistry differed according to Gleason score, tumor stage, PSA level, and age at diagnosis using generalized linear regression for continuous data and chi-square tests for categorical data. In addition, we assessed BRCA1 positive and negative prostate tumors for number of Ki67 positive nuclei as well as BRCA1 RNA expression levels using ANOVA. Mean Ki67 positive nuclei scores were log10 transformed prior to analysis in order to account for the uneven distribution of scores in the raw data. To assess the extent to which BRCA1 status was associated with poor progression, we used Cox proportional hazard models and examined the association between BRCA1 status and lethal prostate cancer, defined as development of distant metastases or prostate cancer-specific mortality. All statistical tests were two-sided.
This project was approved by the Partners Health Care Institutional Review Board.
RESULTS
Normal prostate tissue did not stain for BRCA1; however, 15.3% (N=60) of prostate tumor samples showed patchy nuclear positive immunostaining with a punctuate pattern (Figure 1). There was a total correspondence between the BRCA1 staining in the TMA cores and in the whole sections obtained from the selected 14 corresponding donor blocks in terms of signal intensity and percentage of positive nuclei. Cases that stained positively for BRCA1 had substantially and significantly higher Gleason score, higher PSA levels at diagnosis, and more advanced stage compared to those with tumors that did not stain for BRCA1 (Table 1). Moreover, BRCA1 positive tumors were marked by substantially increased tumor proliferation index compared to BRCA1 negative tumors (47.0 Ki67 positive nuclei vs. 10.3, p = 0.0016). Tumors staining positive for BRCA1 also demonstrated increased BRCA1 mRNA relative expression (Mean, 95% Confidence Interval : 10.5, 10.2–10.8) compared to tumors negative for BRCA1 (9.9, 9.7–10.1, p for difference=0.008).
Table 1.
Clinical characteristics of 392 men in the Physicians' Health Study according to BRCA1 status, 1983–2008
| BRCA1 Negative | BRCA1 Positive | P-value | |
|---|---|---|---|
| N | 332 | 60 | |
| Age at diagnosis (95% Cl) | 66.5 (65.7–67.2) | 67.3 (65.6–69.0) | 0.37 |
| PSA at diagnosis (95% Cl) | 10.2 (6.0–14.4) | 27.0 (15.9–38.1) | 0.0056 |
| Mean follow-up time | 11.0 (10.5–11.4) | 8.8 (7.7–9.9) | 0.0006 |
| N dead/mets (% of total) | 24 (7.2) | 16 (26.7) | <0.0001 |
| Gleason score, N (%) | 0.004* | ||
| Gleason 4–6 | 97 (29.2) | 10 (16.7) | |
| Gleason 3+4 | 116 (34.9) | 19 (31.7) | |
| Gleason 4+3 | 65 (19.6) | 10 (16.7) | |
| Gleason 8–10 | 52 (15.7) | 21 (35.0) | |
| Stage, N (%) | 0.0005* | ||
| pT2 | 207 (62.3) | 26 (43.3) | |
| pT3 | 72 (21.7) | 4 (6.7) | |
| pT4/N1 | 4 (1.2) | 4 (6.7) |
p-for-trend
During a mean follow-up of 10.6 years, 40 men died of cancer or developed bony metastases. Sixteen of the 60 men (26.7%) with BRCA1 positive tumors died of prostate cancer, compared to 24 of 332 (7.2%) men who were BRCA1 negative (Hazard ratio (HR)=4.6; 95% Confidence Interval (CI): 2.4, 8.7). This association remained statistically significant after adjusting for age at diagnosis and Gleason score (HR = 2.5; 95% CI: 1.3, 4.8). Interestingly, although BRCA1 positive tumors had substantially increased tumor proliferative index, the association of BRCA1 and lethal prostate cancer remained significant after controlling for log10-transformed Ki67 expression (HR=3.6, 95% CI: 1.6, 8.0).
DISCUSSION
This study represents the first demonstration of a direct correlation between the expression of BRCA1 and the Ki67 proliferative index in prostate cancer and further strengthens the hypothesis that BRCA1 may play a role in cell-cycle control and is a potent independent marker of clinical prognosis. Ki 67 is a well known predictor of adverse prognosis and resistance to therapy in prostate cancer. (19,20). In addition, association of increased proliferation and BRCA1 protein immunohistochemical expression was recently described in breast cancer epithelial cells from BRCA1 mutation carriers possibly as a result of EGFR pathway activation. (21) In agreement with the recent observation by Schayek et al.(13), we found that, BRCA1 was not expressed in normal prostate tissue. We hypothesize that this localization of BRCA1 only to the most aggressive tumors may reflect an inefficient attempt to upregulate DNA repair mechanisms in prostate epithelial cells with high proliferative rate and extensive genetic instability.
Cases whose prostate tumors stained positive for BRCA1 had significantly higher Gleason score, PSA at diagnosis, and tumor proliferation as well as significantly worse prognosis than those with negative BRCA1 staining. In addition, mRNA levels were also increased in the BRCA1 protein positive tumors indicating a transcriptional level control in these cases. Taken together these observations support the hypothesis that the BRCA1 gene may hold another biological function apart from its tumor suppressor activity.
Although the mechanism of cell-cycle regulation by BRCA1 still requires further exploration, we can conclude that the immunohistochemical investigation of BRCA1 protein expression represents a new tool for understanding the cell-cycle regulation in prostate cancer's development to lethal disease.
Acknowledgments
We thank the participants in the Physicians' Health Study for their long-standing participation. We are grateful to Julia Fleet, Joanne Smith, Vadim Bubes and Haiyan Zhang for their assistance with data collection and programming. This work was supported by a grant from Department of Defense (W81XWH-05-1-0562) and the Dana Farber/Harvard Cancer Center Prostate SPORE. The Physicians' Health Study is supported by grants CA34944, CA40360, and CA097193 from the National Cancer Institute and grants HL-26490 and HL-34595 from the National Heart, Lung, and Blood Institute. Dr. Mucci is a Michael Milken Scholar of the Prostate Cancer Foundation.
References
- 1.Chen J, Silver DP, Walpita D, et al. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998;2(3):317–28. doi: 10.1016/s1097-2765(00)80276-2. [DOI] [PubMed] [Google Scholar]
- 2.Scully R, Chen J, Ochs RL, et al. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90(3):425–35. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 3.Starita LM, Parvin JD. The multiple nuclear functions of BRCA1: transcription, ubiquitination and DNA repair. Curr Opin Cell Biol. 2003;15(3):345–50. doi: 10.1016/s0955-0674(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 4.Larson JS, Tonkinson JL, Lai MT. A BRCA1 mutant alters G2-M cell cycle control in human mammary epithelial cells. Cancer Res. 1997;57(16):3351–5. [PubMed] [Google Scholar]
- 5.MacLachlan TK, Somasundaram K, Sgagias M, et al. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J Biol Chem. 2000;275(4):2777–85. doi: 10.1074/jbc.275.4.2777. [DOI] [PubMed] [Google Scholar]
- 6.Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene. 2006;25(43):5854–63. doi: 10.1038/sj.onc.1209872. [DOI] [PubMed] [Google Scholar]
- 7.Agalliu I, Gern R, Leanza S, Burk RD. Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin Cancer Res. 2009;15(3):1112–20. doi: 10.1158/1078-0432.CCR-08-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas JA, Levin AM, Zuhlke KA, et al. Common variation in the BRCA1 gene and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1510–6. doi: 10.1158/1055-9965.EPI-07-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitra A, Fisher C, Foster CS, et al. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer. 2008;98(2):502–7. doi: 10.1038/sj.bjc.6604132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramus SJ, Gayther SA. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol. 2009;3(2):138–50. doi: 10.1016/j.molonc.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98(23):1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 12.Tryggvadottir L, Vidarsdottir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929–35. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 13.Schayek H, Haugk K, Sun S, True LD, Plymate SR, Werner H. Tumor suppressor BRCA1 is expressed in prostate cancer and controls insulin-like growth factor I receptor (IGFIR) gene transcription in an androgen receptor-dependent manner. Clin Cancer Res. 2009;15(5):1558–65. doi: 10.1158/1078-0432.CCR-08-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. Jama. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennekens CH, Eberlein K. A randomized trial of aspirin and beta-carotene among U.S. physicians. Prev Med. 1985;14(2):165–8. doi: 10.1016/0091-7435(85)90031-3. [DOI] [PubMed] [Google Scholar]
- 16.Setlur SR, Mertz KD, Hoshida Y, et al. Estrogen-Dependent Signaling in a Molecularly Distinct Subclass of Aggressive Prostate Cancer. J Natl Cancer Inst. 2008;100(11):815–825. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark JR, Perner S, Stampfer MJ, et al. Gleason Score and Lethal Prostate Cancer: Does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27(21):3459–64. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müntener M, Epstein JI, Hernandez DJ, et al. Prognostic significance of Gleason score discrepancies between needle biopsy and radical prostatectomy. Eur Urol. 2008;53(4):767–75. doi: 10.1016/j.eururo.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Zellweger T, Günther S, Zlobec I, et al. Tumour growth fraction measured by immunohistochemical staining of Ki67 is an independent prognostic factor in preoperative prostate biopsies with small-volume or low-grade prostate cancer. Int J Cancer. 2009;124(9):2116–23. doi: 10.1002/ijc.24174. [DOI] [PubMed] [Google Scholar]
- 20.Pollack A, DeSilvio M, Khor LY, et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22(11):2133–40. doi: 10.1200/JCO.2004.09.150. [DOI] [PubMed] [Google Scholar]
- 21.Burga LN, Tung NM, Troyan SL, et al. Altered proliferation and differentiation properties of primary mammary epithelial cells from BRCA1 mutation carriers. Cancer Res. 2009;69(4):1273–8. doi: 10.1158/0008-5472.CAN-08-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]