Abstract
OBJECTIVE
The objective of the study was to establish categories of symptom severity based on Incontinence Symptom Index (ISI) scores and show how these categories are associated with urethral function and support.
STUDY DESIGN
Women with stress incontinence (n = 97) and asymptomatic controls (n = 98) completed the ISI. Asymptomatic women’s scores were between 0 and 6; this range was designated as absent/mild (n = 104). The median score for symptomatic women was 16; scores from 7 to 16 (n = 50) were designated as moderate, and scores of 17 or greater (n = 40) were designated as severe.
RESULTS
Urethral function differed in women with mild, moderate, and severe scores: Valsalva leak point pressure (162.3 vs 123.5 vs 101.9 cm H2O; P = .001), cough leak point pressure (202.0 vs 163.0 vs 134.3 cm H2O; P = .001), and maximum urethral closure pressure (69.1 vs 44.1 vs 35.3 cm H2O, P = .001). Loss of urethrovesical support (point Aa: −1.0 vs −0.6 vs −0.5 cm; P = .004) was found in women with moderate and severe symptoms, compared with those with mild symptoms.
CONCLUSION
Categories of symptom severity assessed by the ISI are associated with urethral function and support.
Keywords: Incontinence Symptom Index, stress urinary incontinence, symptom severity
Urinary incontinence is a common condition reported by approximately 38% of community-dwelling women.1 Its negative impact on quality of life makes it important to evaluate the severity of symptoms caused by urinary incontinence.2 The Incontinence Symptom Index (ISI) is a novel, newly validated urinary incontinence symptom questionnaire, developed with the intent of creating a clinically relevant, brief, and comprehensive index of urinary incontinence symptoms. 3 It assesses the type and frequency of symptoms, pad use, and bother caused by urinary incontinence.
Developing symptom severity groupings of scores (ie, mild, moderate, and severe) is an important component of the ISI’s development. Validated urinary incontinence questionnaires are available.4–7 Items within these instruments are associated with the presence of specific symptoms, but the overall scores have not correlated with abnormalities in urethral function and support.8–10 There remains a need for an instrument that assesses the severity of patient-reported symptoms, is readily interpreted in clinical practice, and is associated with the pathophysiology underlying urinary incontinence.
The purpose of this study was to develop clinically relevant categories of mild, moderate, and severe symptoms on the ISI and to establish their validity by associating these categories with urethral function (urethral pressure profiles and leak point pressures) and support (pelvic organ quantification findings).
Materials and Methods
This was a secondary analysis of a case-control study investigating the pathophysiology of stress urinary incontinence.11 Questionnaire, clinical, and urodynamic data were analyzed to develop clinically useful categories of urinary incontinence severity. Symptomatic women with stress-predominant urinary incontinence symptoms were recruited from university-based urogynecology and urology clinics (n = 97). These women had to report at least 2 episodes of stress incontinence on a 3-day voiding diary and to demonstrate stress incontinence during a full bladder stress test. Asymptomatic women were recruited from the community via advertisements (n = 98) and were included if they did not report any episodes of incontinence during a 3-day voiding diary and had a negative full bladder stress test.
Participants were matched for age, race, parity, and hysterectomy status. Women were excluded if they had previous surgery for urinary incontinence or pelvic organ prolapse greater than 1 cm below the hymen.
The study was approved by the University of Michigan Institutional Review Board (#2002-0636).
Pelvic examinations were performed with women in a semirecumbent position in a urodynamics chair at a 45o angle. Assessment of vaginal and uterine support was conducted using the Pelvic Organ Prolapse Quantification System (POP-Q). Urethral axis inclination measurements were made from the horizontal with a cotton-tipped swab at rest (Q-tip rest), with Valsalva (Q-tip straining), and with Kegel contraction (Q-tip Kegel). Similarly, measurements of the genital hiatus were made at rest, with Valsalva, and with Kegel contraction.
Urethral sphincter function was assessed with urethral profilometry. The bladder was filled to a volume of 300 mL. Three serial urethral pressure profile measurements were taken using an 8 Fr Gaeltec dual-tip urodynamics catheter (Medical Measurements Inc, Hackensack, NJ) with the transducer laterally oriented and averaged. Mean maximum urethral closure pressure (MUCP) was calculated by the average difference between maximum urethral pressure and resting bladder pressure. Cough leak point pressures (CLPP) and Valsalva leak point pressures (VLPP) were determined.
Patients completed questionnaires regarding their medical and reproductive histories and completed a protocol of symptom questionnaires. Urinary incontinence symptoms and the bother associated with them were assessed by the ISI, a 10 item instrument scored on a Likert scale (range 0–4). Higher scores indicate worse symptom severity or bother. The ISI “severity” score is the sum of 8 items and ranges from 0 to 32. The ISI “bother” score is the sum of 2 items and ranges from 0 to 8. Total number of medical comorbidities was summed (including hypertension, diabetes mellitus, lung disease, heart disease, arthritis, and neurologic disease). The number of depressive symptoms was determined by responses to the Center for Epidemiologic Studies Depression Scale (CES-D).12
Statistical analysis
Using the distribution of scores within this population, clinically relevant categories were constructed for the ISI. The range of scores observed in asymptomatic women was used to develop a grouping of women with absent or “mild” symptoms. The median score of symptomatic women was used to divide the remaining women into groups with “moderate” and “severe” symptoms.
Bivariate relationships were explored between the groups with mild, moderate, and severe and ISI bother scores, demographic data, POP-Q points, Q-tip angle measurements, maximum urethral closure pressures, cough leak point pressures, and Valsalva leak point pressures with χ2 and analysis of variance (ANOVA) tests. Additional pair-wise comparisons with Student t tests were made when a significant difference was detected between the mild, moderate, and severe ISI groups with the ANOVA. An alpha of 0.05 was used for significance in all tests. All analyses were performed using STATA version 9.2. (StataCorp, College Station, TX).
Results
The distribution of ISI scores among symptomatic and asymptomatic women is presented as a histogram (Figure 1). The range of scores observed among asymptomatic controls was 0 to 6. Six individuals meeting “symptomatic” inclusion had ISI scores in this range. Thus, there were 104 individuals who had ISI scores in this range; they were considered to have absent or mild symptoms. The median ISI score of 16 for symptomatic women was used to develop groups with moderate and severe symptoms. Women with ISI scores of greater than 6 and 16 or less were considered to have symptoms of moderate severity (n = 50). Women with ISI scores of 17 or greater were considered to have symptoms that were most severe (n = 41) (Figure 2).
Figure 1.
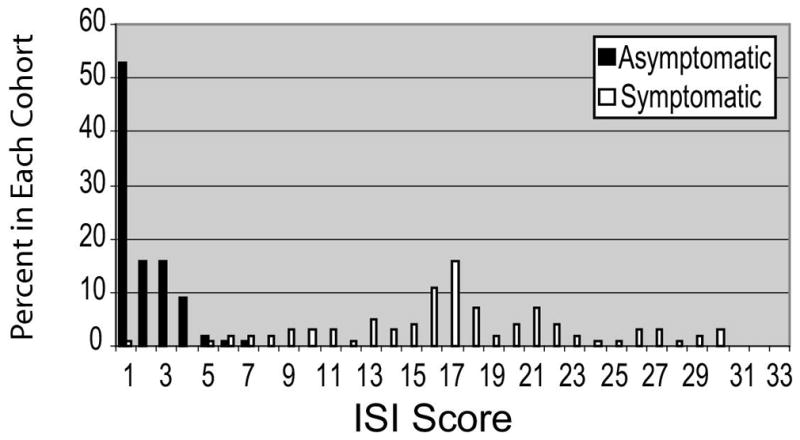
Distribution of ISI severity scores among cohorts of symptomatic and asymptomatic women
Figure 2.
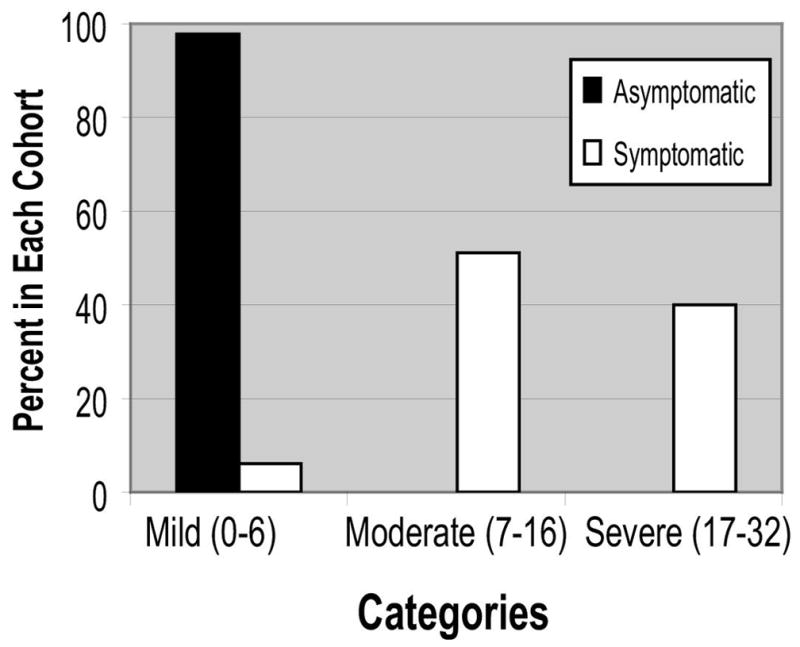
Categories developed from ISI score distribution among cohorts of symptomatic and asymptomatic women
The demographics of the mild, moderate, and severe ISI groupings are shown in Table 1. There was a difference between the groups with respect to vaginal parity, body mass index (BMI), the count of depressive symptoms by the CES-D, and the number of medical comorbidities. Pair-wise comparisons were used to determine that parity was lower among women in the severe symptom category, compared with those in the moderate group; that BMI and the count of depressive symptoms on the CES-D were greater among the women with moderate and severe symptom severity, compared with the mild group; and that the number of comorbidities was greater among the severe group compared with the mild group.
Table 1.
Demographics of women within ISI score categories
| Symptom severity ISI range (n) |
Mild 0–6 (n = 104) |
Moderate 7–16 ( n = 51) |
Severe 17–29 (n = 40) |
ANOVA P value |
|---|---|---|---|---|
| Age (y) | 47.4 ± 1.1 | 46.4 ± 1.2 | 50.3 ± 1.6 | .260 |
| BMI (kg/m2) | 27.8 ± 0.5a,b | 30.1 ± 0.9a | 31.2 ± 1.2b | .011 |
| Parity | 2.0 ± 0.1 | 1.8 ± 0.2c | 2.4 ± 0.2c | .048 |
| Weight largest infant (kg) | 3.6 ± 0.05 | 3.8 ± 0.08 | 3.6 ± 0.05 | .145 |
| Caucasian race (%) | 97.1 | 91.8 | 95.0 | .687 |
| Prior hysterectomy (%) | 9.6 | 10.0 | 15.0 | .633 |
| Currently menstruating (%) | 63.5 | 68.0 | 62.5 | .825 |
| Hormone replacement therapy use (%) | 8.7 | 10.0 | 12.5 | .784 |
| Medical comorbidities (n) | 0.7 ± 0.1b | 0.9 ± 0.1 | 1.2 ± 0.2b | .044 |
| Depressive symptoms (CES-D count) | 0.7 ± 0.1a,b | 1.9 ± 0.3a | 2.2 ± 0.4b | < .001 |
| Tobacco ever (%) | 43.3 | 48.0 | 40.0 | .739 |
| Tobacco current (%) | 15.6 | 29.2 | 31.3 | .277 |
| Lifts 30 lb more than twice a day %) | 15.4 | 14.3 | 7.8 | .389 |
| Employed (%) | 75 .0 | 64.0 | 70.0 | .364 |
Values reported as either percent (where indicated) or mean ± SE.
Pair-wise: mild vs moderate (P < .01).
Pair-wise: mild vs severe (P < .05).
Pair-wise: moderate vs severe (P < .05).
Measures of urethral function (Table 2) were associated with the symptom severity reported on the ISI. There were significant differences in MUCP between each of the ISI severity groupings. Cough and Valsalva leak point pressures were lower among the women in the severe ISI group, compared with those with either mild or moderate symptoms. Because of a small number of individuals in the mild group who had demonstrable stress incontinence during urodynamics, there were nonsignificant differences between the mild and moderate groups in cough leak point pressure (P =0.072) and Valsalva leak point pressure (P =0.117).
Table 2.
Urethral function analyzed by mild, moderate, and severe ISI score categories
| Symptom severity ISI range |
Mild 0–6 |
Moderate 7–16 |
Severe 17–29 |
ANOVA P value |
|---|---|---|---|---|
| Urethral function | ||||
| MUCP (mm H2O) | 69.1 ± 2.2a,b (n = 104) | 44.1 ± 2.6a,c (n = 51) | 35.3 ± 2.4b,c (n = 40) | < .001 |
| VLPP (mm H2O) | 162.3 ± 17.4b (n = 3) | 123.5 ± 6.7c (n = 37) | 101.9 ± 5.4b,c (n = 38) | .001 |
| CLPP (mm H2O) | 202.0 ± 16.0b (n = 4) | 163.0 ± 6.8c (n = 40) | 134.3 ± 6.8b,c (n = 36) | .001 |
Values reported as means ± SE.
Pair-wise: mild vs moderate (P < .001).
Pair-wise: mild vs severe (P < .001).
Pair-wise: moderate vs severe (P < .01).
Differences in urethral support (POP-Q points Aa and Ba, genital hiatus measures, and urethral axis by Q-tip test) were also observed (Table 3). There was a consistent pattern in which the group with mild symptom severity differed from the moderate and severe groups. However, the groups with moderate and severe symptom severity did not differ from one another. The moderate and severe groups did not differ with respect to urethral axis at rest by Q-tip (P = .103), urethral axis with Kegel contraction by Q-tip test (P = .123), urethral axis with straining by Q-tip (P = .636), point Aa (P = .363), point Ba (P = .502), genital hiatus at rest (P = .651), genital hiatus with Kegel contraction (P = .703), or genital hiatus with straining (P = .501).
Table 3.
Urethral support analyzed by mild, moderate, and severe ISI score categories
| Symptom severity ISI range |
Mild, 0–6 (n = 104) |
Moderate, 7–16 (n = 51) |
Severe, 17–29 (n = 40) |
ANOVA P value |
|---|---|---|---|---|
| Urethral support | ||||
| Q-tip rest (degrees) | −6.1 ± 0.1a | −2.4 ± 0.2 | 1.7 ± 0.3a | < .001 |
| Q-tip Kegel (degrees) | −20.3 ± 0.2a,b | −13.9 ± 0.3b | −8.9 ± 0.4a | .001 |
| Q-tip strain (degrees) | 24.9 ± 1.8 | 29.2 ± 2.9 | 31.3 ± 3.3 | .068 |
| POP-Q point Aa (cm) | −1.0 ± 0.1a,b | −0.6 ± 0.1b | −0.5 ± 0.1a | .004 |
| POP-Q point Ba (cm) | −0.9 ± 0.1a,b | −0.5 ± 0.1b | −0.4 ± 0.1a | .005 |
| GH rest (cm) | 2.8 ± 0.1a,b | 3.3 ± 0.1b | 3.2 ± 0.2a | .007 |
| GH Kegel (cm) | 2.4 ± 0.1a,b | 2.8 ± 0.1b | 2.8 ± 0.1a | .012 |
| GH strain (cm) | 3.4 ± 0.1a,b | 4.1 ± 0.1b | 3.9 ± 0.2a | .004 |
Values reported as means ± SE.
Pair-wise: mild vs severe (P < .01).
Pair-wise: mild vs moderate (P < .01).
Other areas of vaginal support assessed by the POP-Q did not differ. The mean (± SE) apical and posterior POP-Q points of the mild, moderate, and severe groups were the following: point C (6.3 ± 0.1 cm vs −6.3 ± 0.2 cm vs −6.2 ± 0.3 cm, P = 1.0), point D (−8.9 ± 0.1 cm vs −8.8 ± 0.1 cm vs −8.6 ± 0.3 cm, P = .8), point Ap (1.5 ± 0.1 cm vs −1.36 ± 0.1 cm vs −1.5 ± 0.1 cm, P = .8), and Bp (−1.4 ± 0.1 cm vs −1.3 ± 0.1 cm vs −1.5 ± 0.1 cm, P = .2).
Severity scores were associated with a greater impact on quality of life by the ISI bother score (r = 0.80, P < .001). The mean bother scores of the mild, moderate, and severe groups were significantly different (Figure 3).
Figure 3.
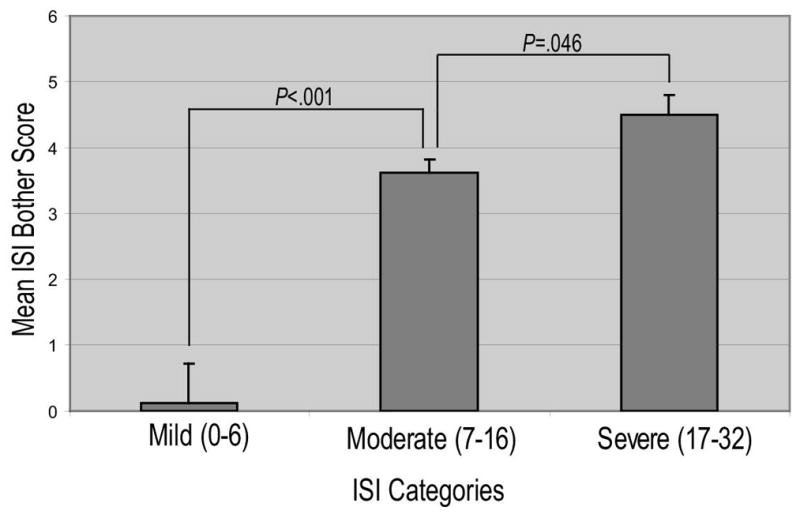
Relationship between reported mean bother score and ISI score categories
Comment
This analysis demonstrates how the ISI is associated with abnormalities of urethral function and support commonly observed during the evaluation of stress urinary incontinence. Loss of urethral support, lower intraurethral pressures, and lower leak point pressures are correlated with absent/mild, moderate, and severe categories of symptom severity. These groupings of symptom severity reflect bother or impact on quality of life and allow ISI scores to be easily interpreted.
The administration of a questionnaire is not intended to replace a thoughtful history, physical examination, and urodynamic testing. As with any clinical tool, there are individuals who do not fit the paradigm. For instance, in this analysis, a few women with stress incontinence had ISI scores that overlapped with asymptomatic continent women. This is understandable when one considers how an individual may cope with her symptoms. Women with urinary incontinence often change their activities to remain continent and their adaptations can be effective in mitigating their symptoms. Thus, even though these women may have significant anatomic and physiologic pathology, they experience fewer episodes of incontinence and use fewer pads, leading to lower symptom severity scores.
Coping with a condition such as urinary incontinence is complex, and a thorough evaluation will continue to depend on a number of different modalities including physical exam findings, voiding diaries, and urodynamics. Which modality is most likely to direct management is often based on the provider’s personal experience and cost-effectiveness.13
In our study, categories of mild, moderate, and severe ISI symptom severity scores differentiated women with progressively lower leak point pressures and lower urethral closure pressures. Other well-known urinary incontinence questionnaires have not performed as consistently when investigators have attempted to correlate symptom severity scores with urodynamics.
Lemack and Zimmern10 found a moderate correlation between a positive response on item 3 of the UDI-6 (urinary leakage with physical activity) and urodynamically demonstrable stress incontinence, but neither symptom severity scores nor any single question was associated with Valsalva leak point pressure.
Fitzgerald and Brubaker were also unable to find an association between specific questions on either the Urogenital Distress Inventory-6 or the Incontinence Impact Questionnaire and urodynamics.14 The American Urological Association Symptom Index, a validated instrument initially used to assess the severity of benign prostatic hyperplasia,7,15 has been associated with a negative impact on quality of life because of incontinence symptoms16 but has not correlated well with urodynamic findings.17–20 Thus, the fact that ISI symptom severity scores are associated with urodynamic findings is valuable and clinically useful.
The relationship of certain urodynamic findings with the ISI groupings supports the concept that urethral competence is of primary importance in the urinary continence mechanism. There were significant differences among almost all of the group comparisons of mean maximum urethral closure pressures and leak point pressures. In contrast, measures of urethral support appear to be of secondary importance because they differentiated only those who had absent/mild symptoms from those with moderate or severe symptoms.
The inconsistent ability of urethral mobility to predict the severity of symptoms has been reported in other studies as well.11,21,22 This may be due to the fact that although there is a correlation between stress incontinence and urethral hypermobility,23–26 there are many women with stress urinary incontinence who have normal urethral support.27–29 Loss of urethral support is commonly observed among women with incontinence, but measures of urethral competence appear to better account for symptom severity.
There are some limitations to consider when evaluating this study. More than 90% of these women were white; similar data in more diverse populations are needed. The definition we used for “symptomatic” women (at least 2 episodes of stress urinary incontinence in 3 days on a voiding diary and a positive full bladder stress test) is somewhat rigorous; it will be worthwhile to determine in the future whether the ISI is able to discriminate between women with an even narrower spectrum of symptom severity.
In conclusion, ISI severity scores can differentiate women with physiologic abnormalities associated with SUI and may be a valuable tool in clinical settings. In assessing the severity of symptoms, clinicians can use the ISI as a quick and effective inventory of patient perceptions, knowing that increasing severity scores are a reflection of the pathophysiology underlying urinary incontinence.
Acknowledgments
This study was supported in part by the Office for Research on Women’s Health SCOR on Sex and Gender Factors Affecting Women’s Health and the National Institute of Child Health and Human Development grant 1 P50 HD044406.
Footnotes
Presented at the 34th Annual Scientific Meeting of the Society of Gynecologic Surgeons, Savannah, GA, April 14-16, 2008.
Reprints not available from the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diokno AC, Brock BM, Brown MB, Herzog AR. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol. 1986;136:1022–5. [PubMed] [Google Scholar]
- 2.Burgio KL, Matthews KA, Engel BT. Prevalence, incidence and correlates of urinary incontinence in healthy, middle-aged women. J Urol. 1991;146:1255–9. doi: 10.1016/s0022-5347(17)38063-1. [DOI] [PubMed] [Google Scholar]
- 3.Wei JT, Dunn RL, Hoag L, Faerber G, Dorr R, McGuire EJ. The Incontinence Symptom Index (ISI): A novel and practical symptom score for the evaluation of urinary incontinence severity. in press. [Google Scholar]
- 4.Naughton MJ, Donovan J, Badia X, et al. Symptom severity and QOL scales for urinary incontinence. Gastroenterology. 2004;126(1 Suppl 1):S114–23. doi: 10.1053/j.gastro.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 5.Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) research group. Qual Life Res. 1994;3:291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 6.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women research group. Neurourol Urodyn. 1995;14:131–9. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 7.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 8.Ko DS, Fenster HN, Chambers K, Sullivan LD, Jens M, Goldenberg SL. The correlation of multichannel urodynamic pressure-flow studies and American Urological Association Symptom Index in the evaluation of benign prostatic hyperplasia. J Urol. 1995;154(2 Pt 1):396–8. doi: 10.1097/00005392-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Barry MJ, Cockett AT, Holtgrewe HL, McConnell JD, Sihelnik SA, Winfield HN. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993;150(2 Pt 1):351–8. doi: 10.1016/s0022-5347(17)35482-4. [DOI] [PubMed] [Google Scholar]
- 10.Lemack GE, Zimmern PE. Predictability of urodynamic findings based on the Urogenital Distress Inventory-6 questionnaire. Urology. 1999;54:461–6. doi: 10.1016/s0090-4295(99)00246-0. [DOI] [PubMed] [Google Scholar]
- 11.DeLancey JOL, Trowbridge ER, Miller JM, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. doi: 10.1016/j.juro.2008.01.098. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 13.Weber AM, Taylor RJ, Wei JT, Lemack G, Piedmonte MR, Walters MD. The cost-effectiveness of preoperative testing (basic office assessment vs. urodynamics) for stress urinary incontinence in women. BJU Int. 2002;89:356–63. doi: 10.1046/j.1464-4096.2001.01687.x. [DOI] [PubMed] [Google Scholar]
- 14.FitzGerald MP, Brubaker L. Urinary incontinence symptom scores and urodynamic diagnoses. Neurourol Urodyn. 2002;21:30–5. doi: 10.1002/nau.2116. [DOI] [PubMed] [Google Scholar]
- 15.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK. Correlation of the American Urological Association Symptom Index with self-administered versions of the Madsen-Iversen, Boyarsky and Maine Medical Assessment Program Symptom indexes. Measurement Committee of the American Urological Association. J Urol. 1992;148:1558–64. doi: 10.1016/s0022-5347(17)36967-7. [DOI] [PubMed] [Google Scholar]
- 16.Scarpero HM, Fiske J, Xue X, Nitti VW. American Urological Association Symptom Index for lower urinary tract symptoms in women: Correlation with degree of bother and impact on quality of life. Urology. 2003;61:1118–22. doi: 10.1016/s0090-4295(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 17.Groutz A, Blaivas JG, Fait G, Sassone AM, Chaikin DC, Gordon D. The significance of the American Urological Association Symptom Index score in the evaluation of women with bladder outlet obstruction. J Urol. 2000;163:207–11. [PubMed] [Google Scholar]
- 18.Chancellor MB, Rivas DA. American Urological Association Symptom Index for women with voiding symptoms: Lack of index specificity for benign prostate hyperplasia. J Urol. 1993;150(5 Pt 2):1706–9. doi: 10.1016/s0022-5347(17)35872-x. [DOI] [PubMed] [Google Scholar]
- 19.Nitti VW, Kim Y, Combs AJ. Correlation of the AUA symptom index with urodynamics in patients with suspected benign prostatic hyperplasia. Neurourol Urodyn. 1994;13:521–9. doi: 10.1002/nau.1930130504. [DOI] [PubMed] [Google Scholar]
- 20.Sirls LT, Kirkemo AK, Jay J. Lack of correlation of the American Urological Association Symptom 7 Index with urodynamic bladder outlet obstruction. Neurourol Urodyn. 1996;15:447–57. doi: 10.1002/(SICI)1520-6777(1996)15:5<447::AID-NAU2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Tapp K, Connolly A, Visco AG. Evaluation of Aa point and cotton-tipped swab test as predictors of urodynamic stress incontinence. Obstet Gynecol. 2005;105:115–9. doi: 10.1097/01.AOG.0000146642.68543.69. [DOI] [PubMed] [Google Scholar]
- 22.Bergman A, Koonings PP, Ballard CA. Negative Q-tip test as a risk factor for failed incontinence surgery in women. J Reprod Med. 1989;34:193–7. [PubMed] [Google Scholar]
- 23.Larrieux JR, Balgobin S. Effect of anatomic urethral length on the correlation between the Q-tip test and descent at point Aa of the POP-Q system. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:273–6. doi: 10.1007/s00192-007-0429-2. [DOI] [PubMed] [Google Scholar]
- 24.Noblett K, Lane FL, Driskill CS. Does pelvic organ prolapse quantification exam predict urethral mobility in stages 0 and I prolapse? Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:268–71. doi: 10.1007/s00192-005-1315-4. [DOI] [PubMed] [Google Scholar]
- 25.Bai SW, Kang SH, Kim SK, Kim JY, Park KH. The effect of pelvic organ prolapse on lower urinary tract function. Yonsei Med J. 2003;44:94–8. doi: 10.3349/ymj.2003.44.1.94. [DOI] [PubMed] [Google Scholar]
- 26.Cogan SL, Weber AM, Hammel JP. Is urethral mobility really being assessed by the pelvic organ prolapse quantification (POP-Q) system? Obstet Gynecol. 2002;99:473–6. doi: 10.1016/s0029-7844(01)01741-0. [DOI] [PubMed] [Google Scholar]
- 27.Latini JM, Zimmerman MB, Kreder KJ., Jr Association between Valsalva and cough leak point pressures and pelvic organ prolapse quantification in women with stress incontinence. J Urol. 2005;173:1219–22. doi: 10.1097/01.ju.0000152323.78869.93. [DOI] [PubMed] [Google Scholar]
- 28.Bradley CS, Nygaard IE. Vaginal wall descensus and pelvic floor symptoms in older women. Obstet Gynecol. 2005;106:759–66. doi: 10.1097/01.AOG.0000180183.03897.72. [DOI] [PubMed] [Google Scholar]
- 29.Zyczynski HM, Lloyd LK, Kenton K, et al. Correlation of Q-tip values and point Aa in stress-incontinent women. Obstet Gynecol. 2007;110:39–43. doi: 10.1097/01.AOG.0000267190.09976.81. [DOI] [PubMed] [Google Scholar]


