Abstract
Chronic alcohol intake is associated with widespread disruptions in sleep and circadian rhythms in both human alcoholics and in experimental animals. Recent studies have demonstrated that chronic and acute ethanol treatments alter fundamental properties of the circadian pacemaker—including free-running period and responsiveness to photic and nonphotic phase-shifting stimuli—in rats and hamsters. In the present work, the authors extend these observations to the C57BL/6J mouse, an inbred strain characterized by very high levels of voluntary ethanol intake and by reliable and stable free-running circadian activity rhythms. Mice were housed individually in running-wheel cages under conditions of either voluntary or forced ethanol intake, whereas controls were maintained on plain water. Forced ethanol intake significantly attenuated photic phase delays (but not phase advances) and shortened free-running period in constant darkness, but voluntary ethanol intake failed to affect either of these parameters. Thus, high levels of chronic ethanol intake, beyond those normally achieved under voluntary drinking conditions, are required to alter fundamental circadian pacemaker properties in C57BL/6J mice. These observations may be related to the relative ethanol insensitivity displayed by this strain in several other phenotypic domains, including ethanol-induced sedation, ataxia, and withdrawal. Additional experiments will investigate chronobiological sensitivity to ethanol in a range of inbred strains showing diverse ethanol-related phenotypes.
Keywords: circadian, wheel running, ethanol, alcohol, inbred mice, C57BL/6J
Chronic alcohol intake is associated with dramatic and widespread disruptions of sleep-wake cycles and other daily biological rhythms in both human alcoholics (Brower, 2003; Kuhlwein et al., 2003; Sano et al., 1993) and experimental animals (Ehlers and Slawecki, 2000; Mukherjee and Simasko, 2009; Wasielewski and Holloway, 2001). In turn, chronobiological dysregulation may promote or sustain excessive alcohol intake and contribute to the negative health consequences associated with alcohol abuse disorders (Danel and Touitou, 2004; Rosenwasser, 2001; Spanagel et al., 2005b).
Although most studies on the chronobiological effects of alcohol have been conducted under entrained conditions, recent animal experiments have begun to explore the effects of ethanol on the phase and period of free-running circadian rhythms, parameters that directly reflect the phase and period of the underlying circadian pacemaker (Rosenwasser, 2001; Turek, 1987). Thus, chronic ethanol intake alters free-running period (Mistlberger and Nadeau, 1992; Dwyer and Rosenwasser, 1998; Rosenwasser et al., 2005a) and attenuates the phase-shifting and/or period-altering effects of brief light pulses presented during late (but not early) subjective night (Rosenwasser et al., 2005c; Seggio et al., 2007) in both rats and hamsters. Similarly, acute ethanol pretreatment also selectively attenuates the phase-shifting effects of late-night light pulses in hamsters (Ruby et al., 2009). Taken together, these findings indicate that ethanol alters the period and photic responsiveness of the circadian pacemaker.
Although the neurobiological mechanisms underlying these effects have not been fully elucidated, chronic ethanol treatment alters gene expression and neuropeptide levels within the SCN, the site of the central circadian pacemaker (Chen et al., 2004; Madeira et al., 1997). Further, GABA-A and N-methyl-D-aspartate (NMDA) receptors are well-known molecular targets for ethanol action in the central nervous system (Davis and Wu, 2001; Faingold et al., 1998) and play critical roles in regulation of the SCN pacemaker (Rosenwasser, 2003). Like ethanol, GABAergic benzodiazepines alter free-running circadian period and selectively attenuate the phase-shifting effects of late-night but not early-night light pulses (Ralph and Menaker, 1986, 1989; Subramanian and Subbaraj, 1996). Further, recent experiments have shown that direct ethanol application to the SCN can attenuate the phase-shifting effects of light and glutamate, in vivo (Ruby et al., 2009) and in vitro (Prosser et al., 2008).
The primary aim of the present experiments was to extend these observations to inbred C57BL/6J mice. This aim was motivated by a desire to establish a mouse model that could be used to investigate neurogenetic linkages between ethanol preference and circadian pacemaker phenotype. Such relationships are indicated by data showing that selective breeding for ethanol preference alters circadian phenotype in both rats (Rosenwasser et al., 2005b) and mice (Hofstetter et al., 2003), whereas mutation of the critical circadian clock gene per2 modifies ethanol preference in mice (Spanagel et al., 2005a). We chose to focus initially on the C57BL/6J strain due to its very high levels of innate ethanol preference (Belknap et al., 1993; Yoneyama et al., 2008) and its robust and highly stable circadian activity rhythms (Daan and Pittendrigh, 1976; Schwartz and Zimmerman, 1990).
MATERIALS AND METHODS
Experiment 1: Effects of Voluntary and Forced Ethanol Intake on Light-Induced Circadian Phase Shifting
Eight-week-old male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed individually in running-wheel cages (Coulbourn Instruments, Whitehall, PA; wheel diameter 11.5 cm) with food and fluid (either plain water or plain water and 10% [vol/vol] ethanol solution; see below) provided ad libitum. Cages were placed 2 per shelf within light- and sound-shielded enclosures equipped with exhaust fans and programmable lighting provided by incandescent lamps. Running-wheel activity was recorded and analyzed using the ClockLab interface system (Actimetrics Co., Wilmette, IL), and fluid intakes were determined at weekly intervals.
The mice were initially maintained in a light-dark (LD) 12:12 cycle, and following the establishment of stable, light-entrained rhythms, divided randomly into groups. Experiment 1 utilized 2 separate ethanol-treatment groups: a free-choice ethanol group (n = 9), which received both plain water and 10% ethanol (vol/vol) solution in 2 separate drinking bottles, and a forced ethanol group (n = 10), maintained on 10% ethanol solution as the only drinking fluid. Each of these ethanol-treated experimental groups was compared with its own water-only control group (n = 10 per group). After a 21-day baseline period to allow ethanol intakes to stabilize in the experimental groups, all animals were tested sequentially (4 tests per animal; see below) for responses to both phase-advancing and phase-delaying light pulses (15 min, 30–50 lux) using the Aschoff type II protocol (Mistlberger, 1996; Mrosovsky, 1996). In this protocol, animals are kept under an entraining LD cycle until the day of light pulse presentation, and then maintained subsequently for several days in constant darkness (DD) for assessment of the phase of the free-running rhythm. Light pulses were administered at phases expected to yield maximal phase advances (i.e., ZT 21; 9 h following the last light-to-dark transition, designated by tradition as ZT 12) and phase delays (ZT 15; 3 h after the last light-to-dark transition) in this mouse strain (Daan and Pittendrigh, 1976; Schwartz and Zimmerman, 1990). Successive phase-response tests were separated by at least 3 weeks of re-exposure to the LD cycle, ensuring stable entrainment prior to the delivery of all light pulses.
Two tests were conducted at each ZT, 1 during ongoing ethanol access and a 2nd following 24 h of ethanol deprivation. Following the 1st test conducted under ethanol deprivation, ethanol access was restored for at least 4 weeks prior to the second deprivation test. Thus, each animal was tested a total of 4 times in the same sequence: 1) ZT 15 light pulse, 2) ZT 21 light pulse, 3) ZT 15 light pulse during acute withdrawal; and 4) ZT 21 light pulse during acute withdrawal. Two potential limitations of this design should be mentioned here: First, the different test conditions followed different numbers of days of ethanol drinking experience (ranging from 30 to more than 150), and second, animals experienced a 1-week ethanol deprivation 2 weeks prior to the final phase-shift test that could have altered subsequent alcohol intake (i.e., the “alcohol deprivation effect”; Melendez et al., 2006). It is unlikely that either of these factors influenced the results presented here, however, inasmuch as the alcohol deprivation effect is generally not robust following a single 1-week deprivation episode (Melendez et al., 2006) and inasmuch as weekly ethanol intakes showed no systematic change with time after the initial 2 weeks of drinking experience (data not shown).
The magnitude and direction of circadian phase responses were determined using ClockLab's automated activity-onset detection algorithm. Prestimulus phase was estimated as the mean time of activity onset over the last 5 days of LD entrainment, and poststimulus phase was estimated by a regression line fit to activity onsets over 6 to 7 free-running circadian cycles following the test stimulus, excluding the 1st 2 activity onsets due to the possible occurrence of “transients” prior to the establishment of a steady-state free-running phase (Daan and Pittendrigh, 1976). Phase responses were then determined as the difference between these 2 phase estimates extrapolated to the 1st poststimulus activity onset.
Experiment 2: Effects of Voluntary and Forced Ethanol Intake on Free-Running Circadian Rhythms
C57BL/6J male mice were obtained from the Jackson Laboratory and individually housed in running wheel cages under prolonged constant darkness. Following a water-only baseline period of 21 days, the animals were divided randomly into 3 groups and exposed to 1 of the following drinking conditions for an additional 148 days: 1) A free-choice ethanol group was concurrently offered 10% v/v ethanol and water via separate drinking bottles (n = 12); 2) a forced ethanol group was presented 10% v/v ethanol solution as the only drinking fluid (n = 12); and 3) a control group continued to be maintained on plain water throughout the experiment (n = 11). Wheel-running activity was monitored using the ClockLab interface system, and fluid intakes were determined weekly.
Circadian rhythm parameters were determined for each of eight 3-week experimental epochs (one 3-week baseline epoch followed by seven 3-week epochs in which animals were maintained under the different drinking conditions). Free-running circadian period was determined using well-established methods implemented in the ClockLab analysis routines, including both the χ2 (nonparametric) and the Lomb-Scargle (parametric) periodogram analyses, which were averaged to yield the period estimates reported here. In addition, the peak magnitude of the Lomb-Scargle periodogram was used to estimate the robustness of free-running rhythmicity (Ruf, 1999). Finally, the total number of daily wheel turns was also determined for each animal and for each epoch of the experiment.
RESULTS
Photic Phase Shifting
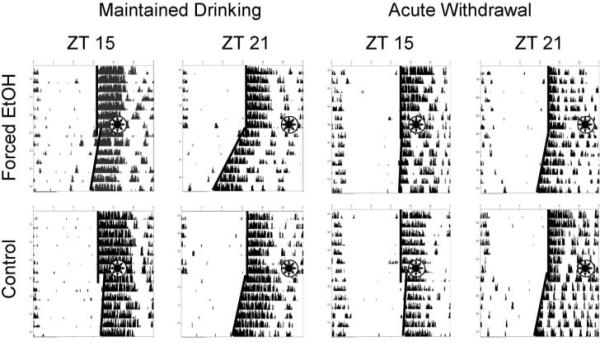
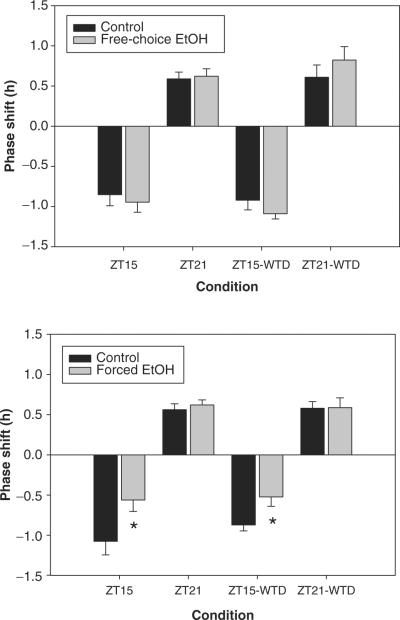
As expected from previous studies, control mice showed reliable phase delays to light pulses presented at ZT 15 and phase advances to light pulses presented at ZT 21, whereas the overall magnitude of phase delays was greater than phase advances (Figs. 1, 2). Voluntary ethanol intake had no effect on circadian phase shifting at either test phase, whether tested during maintained drinking or under acute withdrawal (Fig. 2, top). In contrast, forced ethanol intake resulted in a significant attenuation of photic phase shifting at ZT 15, but not at ZT 21, both under continued drinking (t18 = 2.33, p = 0.032) and during acute withdrawal (t18 = 2.53, p = 0.021) conditions (Fig. 1; Fig. 2, bottom). There were no significant differences between tests conducted during continued drinking and tests conducted under withdrawal at either ZT or for either group.
Figure 1.
Representative actogram segments showing light-induced phase shifts for forced ethanol and control animals, at ZT 15 and ZT 21, during either maintained drinking or at 24 h after ethanol replacement by plain water (acute withdrawal). Bold lines superimposed on each chart connect successive activity onsets prior to and following each light pulse; stars indicate the approximate times of light pulse delivery.
Figure 2.
Mean (± SEM) light-induced phase shifts (free-choice ethanol vs. controls, top; forced ethanol vs. controls, bottom). Each animal was tested a total of 4 times: at ZT 15 and ZT 21, during maintained drinking and at 24 h following ethanol withdrawal (WTD). Asterisks indicate significant attenuation of phase shifting.
Free-Running Period
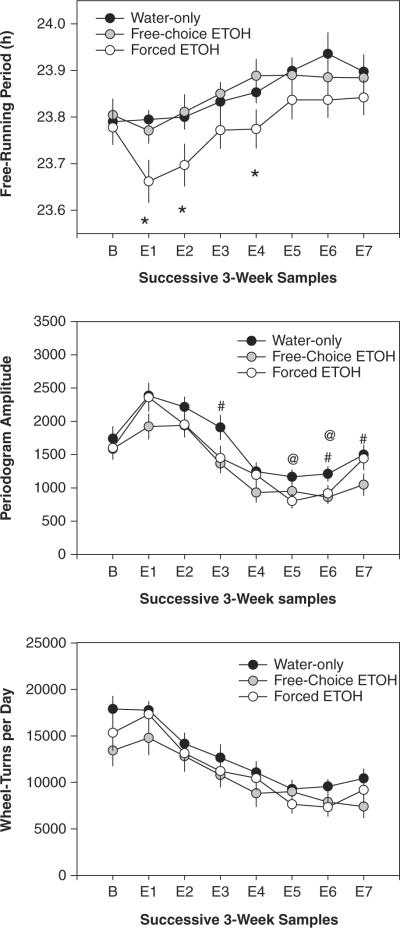
Repeated-measures analysis of variance (ANOVA) including all 8 experimental epochs detected a significant main effect of time (F7,224 = 14.38, p < 0.001), but no effect of group nor any group-by-epoch interaction, indicating that free-running periods generally lengthened over the course of long-term exposure to constant darkness in all groups (Figs. 3, 4). To examine the immediate response to the introduction of ethanol treatment, a similar analysis was conducted using only the baseline and the first 3-week ethanol treatment epoch; this analysis revealed a significant group-by-epoch interaction (F2,32 = 3.46, p = 0.044), indicating that the introduction of forced ethanol intake shortened the free-running period. Follow-up pairwise comparisons using least significant difference (LSD) tests showed no differences among groups during baseline conditions, but free-running periods in the forced ethanol group were significantly shorter than in the free-choice ethanol group or the water-only control group during the 1st, 2nd, and 4th ethanol treatment epochs (Fig. 4). In contrast, there were no significant differences between the free-choice group and the control group during any epoch. Thus, forced ethanol intake resulted in a shortening of free-running period that persisted for about 3 months of continued treatment.
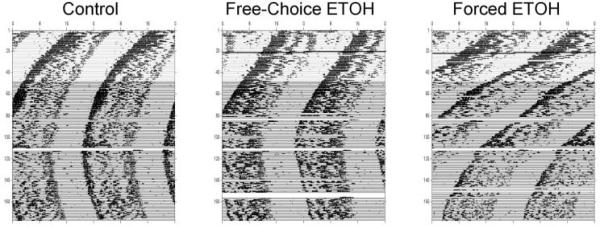
Figure 3.
Representative actograms showing free-running activity rhythms under long-term DD from 1 animal in each of the 3 groups (forced ethanol, free-choice ethanol, and water-only controls). All animals were maintained on plain water for the 1st 3 weeks of the experiment, after which ethanol was continuously available in the forced and free-choice ethanol groups (horizontal line indicates beginning of ethanol treatment on day 22).
Figure 4.
Mean (± SEM) free-running period (top), periodogram amplitude (middle), and daily activity (bottom) for all 3 groups in successive 3-week data samples. “B” indicates the initial water-only baseline, and “E1” through “E7” indicate successive 3-week samples in which ethanol was continuously available in the forced and free-choice ethanol groups. * = controls significantly different from both ethanol-treated groups; @ = controls significantly different from forced ethanol group; # = controls significantly different from free-choice ethanol group.
Periodogram Amplitude
ANOVA revealed significant main effects of experimental epoch (F7,224 = 34.39, p < 0.001) and treatment group (F2,32 = 4.08, p = 0.026) on the robustness of free-running rhythmicity, assessed by periodogram peak amplitude. The significant main effect of treatment group reflected the fact that both ethanol-treated groups showed generally lower periodogram amplitude during ethanol treatment (Fig. 4, middle). Thus, follow-up LSD tests detected significant differences between the forced ethanol group and the control group during treatment epochs 5 and 6 and between the free-choice ethanol group and the control group during epochs 3, 6 and 7, but no differences between the 2 ethanol treatment groups in any epoch.
Activity Level
ANOVA revealed a significant main effect of experimental epoch (F7,224 = 38.77, p < 0.001), reflecting the gradually decreasing activity levels displayed by both ethanol-exposed and control groups over the course of the experiment (Fig. 4, bottom). However, there was no main effect of ethanol treatment, nor any treatment-by-epoch interaction.
Ethanol Intake
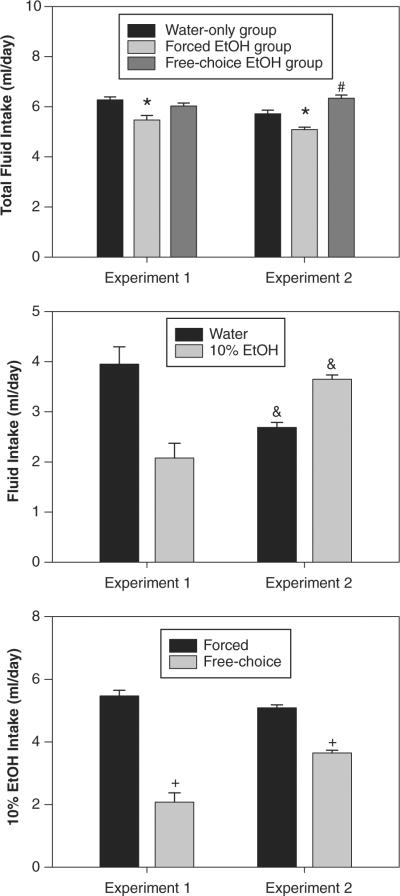
Figure 5 shows mean daily water and/or 10% ethanol intakes by volume for all groups in both experiments (for these analyses, the separate water-only control groups from the forced ethanol and free-choice ethanol comparisons in experiment 1 have been combined). Although adequate fluid intake was maintained in all groups, pairwise t tests showed that forced intake of 10% ethanol resulted in significant reductions in daily fluid intake relative to both free-choice ethanol and water-only control groups, in both experiments (Fig. 5, top). In addition, daily fluid intake was significantly higher in the free-choice ethanol group than in the water-only controls in experiment 2, but not in experiment 1 (all p < 0.05; Fig. 5, top). Comparison of water and ethanol intakes in the free-choice groups revealed that voluntary ethanol intake was higher and water intake was lower in experiment 2 than in experiment 1 (Fig. 5, middle); ethanol preference ratios (i.e., 10% ethanol intake divided by total fluid intake) averaged about 35% in experiment 1 and about 57% in experiment 2. Comparison of daily ethanol in free-choice and forced ethanol groups showed that forced ethanol intake was significantly higher than voluntary intake in both experiments (Fig. 5, bottom). Indeed, animals under forced ethanol intake conditions consumed about twice as much ethanol as did those under free-choice conditions.
Figure 5.
Mean (± SEM) fluid intakes in both experiments. (Top) Total fluid intake for forced and free-choice ethanol groups and combined water-only controls. (Middle) Water and 10% ethanol intake in free-choice ethanol groups. (Bottom) Ten percent ethanol intake in forced and free-choice ethanol groups. * = forced ethanol group significantly different from both controls and free-choice ethanol groups; # = free-choice ethanol group significantly different from controls; & = experiment 2 significantly different than experiment 1; + = free-choice ethanol group significantly different from forced ethanol group.
DISCUSSION
The present study demonstrated that chronic forced (but not free-choice) ethanol intake alters photic phase shifting and free-running circadian period in C57BL/6J mice. Such effects could reflect direct pharmacological targeting of circadian clock cells in the SCN, and indeed, recent studies have shown that ethanol application to the SCN attenuates the phase-shifting effects of light pulses in vivo (Ruby et al., 2009) and of glutamate in vitro (Prosser et al., 2008).
C57BL/6J mice were used in these studies partly because they display the highest levels of voluntary ethanol intake among all inbred strains tested to date (Belknap et al., 1993; Yoneyama et al., 2008). Nevertheless, we observed alterations in circadian pacemaker function only under conditions of forced ethanol intake, which yielded ethanol intakes about twice those seen under free-choice conditions. Inasmuch as blood ethanol concentrations were not obtained in this study, we cannot speculate regarding the blood levels necessary to produce such effects. These observations may be related to the relative ethanol insensitivity of C57BL/6J mice in other domains, including ethanol-induced sedation, ataxia, and withdrawal (Crabbe et al., 2006; Metten and Crabbe, 2005). Thus, future studies will examine the chronobiological effects of ethanol in inbred mouse strains characterized by lower preference for and greater physiological sensitivity to ethanol. Such studies will clarify the relationship between strain differences in chronobiological sensitivity to ethanol and other, better-studied ethanol-response phenotypes.
The present results are consistent with previous studies showing that both chronic (Seggio et al., 2007) and acute (Ruby et al., 2009) ethanol exposure attenuates photic phase shifting in Syrian hamsters. Similar attenuation of photic phase shifting has also been reported for other sedative-anxiolytic drugs (Duncan et al., 1998; Dwyer and Rosenwasser, 1998; Subramanian and Subbaraj, 1996). Nevertheless, mice and hamsters apparently differ in the circadian phase dependence of such effects. Thus, ethanol selectively attenuates phase advances to late-night light pulses in hamsters (Seggio et al., 2007; Ruby et al., 2009) and selectively attenuates phase delays to early-night light pulses in mice (present study). Although the mechanism underlying phase-dependent sensitivity to ethanol is unknown, it should be noted that hamsters generally show more robust phase advances than delays, whereas the opposite is true in mice. Thus, ethanol selectively inhibits photic phase shifting during the temporal window of maximal responsiveness in both species. In addition, direct in vitro ethanol application to the SCN inhibits both the phase-advancing and phase-delaying effects of glutamate in brain slices prepared from C57BL/6J mice (Prosser et al., 2008). This result suggests that modulatory signals originating outside the SCN are responsible for conferring phase specificity to the in vivo effects of ethanol on the photic entrainment pathway.
Despite the significant attenuation of photic phase shifting under forced ethanol conditions, we failed to observe any effect of acute ethanol withdrawal relative to maintained drinking. This test was conducted in anticipation of a possible “rebound” potentiation of photic phase shifting during acute withdrawal, a hypothesis based on the known ability of chronic ethanol treatment to up-regulate excitatory NMDA-glutamate receptors and down-regulate inhibitory GABA-A receptors, leading to central nervous system hyperexcitability that is unmasked only during ethanol withdrawal (Davis and Wu, 2001; Faingold et al., 1998). Although glutamatergic and GABAergic mechanisms are known to reciprocally modulate the circadian pacemaker's response to photic stimuli (Rosenwasser, 2003), it is not known whether adaptations to chronic ethanol occur specifically within the SCN and/or other components of the circadian timing system, or what the time course of such adaptations might be. Future experiments will examine whether other, withdrawal-sensitive inbred mouse strains exhibit ethanol withdrawal-related potentiation of photic phase shifting.
The present results are also consistent with previous studies showing that chronic ethanol intake modulates the free-running circadian period in constant darkness in hamsters (Mistlberger and Nadeau, 1992) and rats (Dwyer and Rosenwasser, 1998; Rosenwasser et al., 2005a). Importantly, effects on the free-running period occurred despite the fact that ethanol had only minor effects on the overall robustness of free-running rhythmicity, and was without significant effect on total daily activity levels, similar to our previous studies with rats (Rosenwasser et al., 2005a). Nevertheless, effects on the free-running period have been somewhat variable across studies, possibly due to species or strain differences in ethanol responsiveness. Thus, whereas Mistlberger and Nadeau (1992) originally reported period lengthening during voluntary ethanol intake in hamsters, our laboratory observed period shortening during voluntary ethanol intake in Wistar rats (Dwyer and Rosenwasser, 1998) and both lengthening and shortening of the free-running period in Long-Evans rats during forced ethanol intake (Rosenwasser et al., 2005a). Taken together, the results of these studies resemble the inconsistent effects on the free-running period seen during treatment with other anxiolytic and antidepressant drugs (Duncan et al., 1998; Rosenwasser, 1996; Subramanian and Subbaraj, 1996; Wollnik, 1992).
Animals in the free-choice ethanol groups displayed ethanol preference ratios of about 0.35 in experiment 1 and about 0.57 in experiment 2, differing both from each other and from published reports of preference ratios of 0.60 to 0.80 for this strain (Belknap et al., 1993; Yoneyama et al., 2008). These differences could be due, in part, to differences in housing conditions among the various experiments. As is common in behavioral chronobiology but uncommon in studies of ethanol preference, animals in the present study were housed individually and had continuous access to running wheels. Both running-wheel access (McMillan et al., 1995; Ozburn et al., 2008; Werme et al., 2002) and social housing (Araujo et al., 2005; Reed et al., 2001; Wolffgramm, 1990) have been shown to affect voluntary ethanol intake. Further, the relatively higher ethanol preference observed in experiment 2 may have been due, in part, to the use of prolonged exposure to constant darkness for assessment of free-running activity rhythms, inasmuch as previous research has shown that maintenance in constant darkness or exposure to short photoperiods increases ethanol preference in several rodent species (Burke and Kramer, 1974; Geller, 1971; Millard and Dole, 1983; Reiter et al., 1974; Smith et al., 1980).
In summary, these results confirm and extend previous work on the chronobiological effects of chronic ethanol intake in rats and hamsters to include the C57BL/6J inbred mouse, and provide additional evidence that ethanol alters fundamental properties of the underlying circadian pacemaker. Further studies will be required to identify possible strain differences in the chronobiological effects of ethanol and ethanol withdrawal, and to determine how these effects are related genetically or physiologically to other behavioral and neurobiological effects of ethanol.
ACKNOWLEDGMENTS
Supported by a subcontract to AMR from the Integrative Neuroscience Initiative on Alcoholism (INIA-Stress), NIAAA U01 AA13641, to Oregon Health Sciences University, Kathy Grant, Principal Investigator.
REFERENCES
- Araujo NP, Camarini R, Souza-Formigoni ML, Carvalho RC, Abilio VC, Silva RH, Ricardo VP, Ribeiro Rde A, Frussa-Filho R. The importance of housing conditions on behavioral sensitization and tolerance to ethanol. Pharmacol Biochem Behav. 2005;82:40–45s. doi: 10.1016/j.pbb.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Burke LP, Kramer SZ. Effects of photoperiod, melatonin and pinealectomy on ethanol consumption in rats. Pharmacol Biochem Behav. 1974;2:459–463. doi: 10.1016/0091-3057(74)90004-5. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Ponomarev I, Prescott CA, Wahlsten D. Effects of genetic and procedural variation on measurement of alcohol sensitivity in mouse inbred strains. Behav Genet. 2006;36:536–552. doi: 10.1007/s10519-006-9067-6. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh C. A functional analysis of circadian pacemakers in nocturnal rodents II: The variability of phase response curves. J Comp Physiol A. 1976;106:253–266. [Google Scholar]
- Danel T, Touitou Y. Chronobiology of alcohol: From chronokinetics to alcohol-related alterations of the circadian system. Chronobiol Int. 2004;21:923–935. doi: 10.1081/cbi-200036886. [DOI] [PubMed] [Google Scholar]
- Davis KM, Wu JY. Role of glutamatergic and GABAergic systems in alcoholism. J Biomed Sci. 2001;8:7–19. doi: 10.1007/BF02255966. [DOI] [PubMed] [Google Scholar]
- Duncan WC, Jr, Johnson KA, Wehr TA. Decreased sensitivity to light of the photic entrainment pathway during chronic clorgyline and lithium treatments. J Biol Rhythms. 1998;13:330–346. doi: 10.1177/074873098129000165. [DOI] [PubMed] [Google Scholar]
- Dwyer SM, Rosenwasser AM. Neonatal clomipramine treatment, alcohol intake and circadian rhythms in rats. Psychopharmacology (Berl) 1998;138:176–183. doi: 10.1007/s002130050660. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Faingold CL, N'Gouemo P, Riaz A. Ethanol and neurotransmitter interactions—From molecular to integrative effects. Prog Neurobiol. 1998;55:509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Geller I. Ethanol preference in the rat as a function of photoperiod. Science. 1971;173:456–459. doi: 10.1126/science.173.3995.456. [DOI] [PubMed] [Google Scholar]
- Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in high-alcohol-preferring and low-alcohol-preferring mice. Alcohol. 2003;30:81–85. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry. 2003;54:1437–1443. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. J Neurosci. 1997;17:1302–1319. doi: 10.1523/JNEUROSCI.17-04-01302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DE, McClure GY, Hardwick WC. Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol. Drug Alcohol Depend. 1995;40:1–7. doi: 10.1016/0376-8716(95)01162-5. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Millard WJ, Dole VP. Intake of water and ethanol by C57BL mice: Effect of an altered light-dark schedule. Pharmacol Biochem Behav. 1983;18:281–284. doi: 10.1016/0091-3057(83)90377-5. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Aschoff type II method: Commentary. Chronobiol Int. 1996;13:393–394. doi: 10.3109/07420529609012663. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Nadeau J. Ethanol and circadian rhythms in the Syrian hamster: Effects on entrained phase, reentrainment rate, and period. Pharmacol Biochem Behav. 1992;43:159–165. doi: 10.1016/0091-3057(92)90652-v. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Methods of measuring phase shifts: Why I continue to use an Aschoff type II procedure despite the skepticism of referees. Chronobiol Int. 1996;13:387–392. doi: 10.3109/07420529609012662. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Simasko SM. Chronic alcohol treatment in rats alters sleep by fragmenting periods of vigilance cycling in the light period with extended wakenings. Behav Brain Res. 2009;198:113–124. doi: 10.1016/j.bbr.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Harris RA, Blednov YA. Wheel running, voluntary ethanol consumption, and hedonic substitution. Alcohol. 2008;42:417–424. doi: 10.1016/j.alcohol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. Effects of diazepam on circadian phase advances and delays. Brain Res. 1986;372:405–408. doi: 10.1016/0006-8993(86)91154-6. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. GABA regulation of circadian responses to light. I. Involvement of GABAA-benzodiazepine and GABAB receptors. J Neurosci. 1989;9:2858–2865. doi: 10.1523/JNEUROSCI.09-08-02858.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CL, Hood KE, Cortes DA, Jones BC. Genetic-environment analysis of sensitivity and acute tolerance to ethanol in mice. Pharmacol Biochem Behav. 2001;69:461–467. doi: 10.1016/s0091-3057(01)00520-2. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Blum K, Wallace JE, Merritt JH. Pineal gland: Evidence for an influence on ethanol preference in male Syrian hamsters. Comp Biochem Physiol A Comp Physiol. 1974;47:11–16. doi: 10.1016/0300-9629(74)90045-0. [DOI] [PubMed] [Google Scholar]
- Rosenwasser A. Neurobiology of the mammalian circadian system: Oscillators, pacemakers and pathways. In: Fluharty S, Grill H, editors. Progress in Psychobiology and Physiological Psychology. Vol 18. Elsevier/Academic Press; San Diego: 2003. pp. 1–38. [Google Scholar]
- Rosenwasser AM. Clonidine shortens circadian period in both constant light and constant darkness. Physiol Behav. 1996;60:373–379. [PubMed] [Google Scholar]
- Rosenwasser AM. Alcohol, antidepressants, and circadian rhythms. Human and animal models. Alcohol Res Health. 2001;25:126–135. [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav. 2005a;84:537–542. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW, Reed JD, Cotter SJ, Seggio JA. Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol. 2005b;36:69–81. doi: 10.1016/j.alcohol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiol Int. 2005c;22:227–236. doi: 10.1081/cbi-200053496. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol. 2009;296:R411–418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf T. The Lomb-Scargle periodogram in biological rhythm research: Analysis of imcomplete and unevenly spaced time series. Biol Rhythm Res. 1999;30:178–201. [Google Scholar]
- Sano H, Suzuki Y, Yazaki R, Tamefusa K, Ohara K, Yokoyama T, Miyasato K, Ohara K. Circadian variation in plasma 5-hydroxyindoleacetic acid level during and after alcohol withdrawal: Phase advances in alcoholic patients compared with normal subjects. Acta Psychiatr Scand. 1993;87:291–296. doi: 10.1111/j.1600-0447.1993.tb03374.x. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Zimmerman P. Circadian time-keeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci. 1990;10:3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Oei TP, Ng KT, Armstrong S. Rat self administration of ethanol: Enhancement by darkness and exogenous melatonin. Physiol Behav. 1980;25:449–455. doi: 10.1016/0031-9384(80)90287-5. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005a;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body's biological clock. Alcohol Clin Exp Res. 2005b;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Subramanian P, Subbaraj R. Diazepam modulates the period of locomotor rhythm in mice (Mus booduga) and attenuates light-induced phase advances. Pharmacol Biochem Behav. 1996;54:393–398. doi: 10.1016/0091-3057(95)02079-9. [DOI] [PubMed] [Google Scholar]
- Turek F. Pharmacological probes of the mammalian circadian clock: Use of the phase-response curve approach. Trends Pharmacol Sci. 1987;8:212–217. [Google Scholar]
- Wasielewski JA, Holloway FA. Alcohol's interactions with circadian rhythms. A focus on body temperature. Alcohol Res Health. 2001;25:94–100. [PMC free article] [PubMed] [Google Scholar]
- Werme M, Lindholm S, Thoren P, Franck J, Brene S. Running increases ethanol preference. Behav Brain Res. 2002;133:301–308. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Wollnik F. Effects of chronic administration and withdrawal of antidepressant agents on circadian activity rhythms in rats. Pharmacol Biochem Behav. 1992;43:549–561. doi: 10.1016/0091-3057(92)90190-q. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]