Abstract
BACKGROUND AND PURPOSE:
Neuro-axonal damage is a well known sequelae of MS pathogeneses. Consequently, our aim was to test whether the ∼20% of patients with MS exhibiting a clinically benign disease course also have minimal neural dysfunction as reflected by the global concentration of their MR imaging marker NAA.
MATERIALS AND METHODS:
QNAA was obtained with nonlocalizing whole-head 1H-MR spectroscopy in 43 patients with benign RRMS (30 women, 13 men; mean age, 44.7 ± 7.3 years of age) with 21.0 ± 4.4 years (range, 15–35 years) of disease duration from the first symptom and an EDSS score of 1.9 (range, 0–3). QNAA was by divided by the brain volume (from MR imaging segmentation) to normalize it into WBNAA. All participants gave institutional review board−approved written informed consent, and the study was HIPAA compliant.
RESULTS:
The patients' lesion load was 12.2 ± 7.7 cm3. Their 8.3 ± 1.8 mmol/L WBNAA was 35% lower than that in controls (P < .001). Individual average loss rates (absolute loss compared with controls divided by disease duration) clustered around 0.22 ± 0.09 mmol/L/year (1.7%/year, assuming monotonic decline). This rate could be extrapolated from that already reported for patients with RRMS of much shorter disease duration. WBNAA did not correlate with lesion load or EDSS.
CONCLUSIONS:
Normal WBNAA is not characteristic of benign MS and is not an early predictor of its course. These patients, therefore, probably benefit from successful compensation and sparing of eloquent regions. Because they may ultimately have a rapid decline once their brain plasticity is exhausted, they may benefit from treatment options offered to more affected patients.
MS, the most common autoimmune demyelinating disorder of the CNS, is the leading cause of nontraumatic disability in young adults, affecting >2 million worldwide.1 Approximately 85% of new patients enter its RR stage characterized by cycles of relapses of varying severity and length, separated by remissions of different durations.2 RRMS is often divided into 3 disease-course patterns3: rapid, moderate, and stable (often called “benign”). The current accepted definition of the latter is full functionality reflected by an EDSS4 score of ≤3.0 at ≥15 years of disease duration.5 Although the estimated prevalence of benign MS ranges from 6% to 64% (possibly reflecting different criteria), its accepted fraction is ∼20%.6,7
The variable course of MS, its duration, high cost, and the limited availability (in some countries) of medications and their side effects make early identification and characterization of patients desirable for treatment planning and clinical study design and interpretation.8,9 Unfortunately, benign is currently only a retrospective diagnosis. Attempts to prospectively identify who will have this course have had only moderate success.7,10,11 Using imaging metrics for this purpose has so far yielded paradoxes such as higher lesion loads and magnetization transfer ratios in patients with benign MS than in more disabled patients with MS,12,13 conflicting brain atrophy rates,14–16 and global atrophy indistinguishable from patients with secondary-progressive MS of similar disease duration.17,18 Although abnormalities have been seen in lesions and normal-appearing brain matter by using diffusion tensor imaging, accurately predicting fiber tracks through complex connections and tissue disruptions remains difficult.19,20
In contrast, axonal and neuronal damage have long been implicated as a main cause of irreversible MS disability.21–24 Indeed, regional declines in the MR imaging marker, the amino acid−derivative NAA, which is almost exclusive to neurons and their processes,25 have correlated better with clinical disability than other imaging metrics.23,25–28 Because MS pathology is diffuse throughout the CNS,29 it is not surprising that the WBNAA has also shown substantial (>10%) deficits in patients with RRMS relative to matched controls,30,31 which correlate with disease duration.3
The need for reliable criteria to identify patients with MS who will remain with mild disability for the long term9,32 and the link between disability and neuronal damage22,33 provoke the hypothesis that patients with benign MS also have only (characteristic) minimal global neuronal injury, reflected by WBNAA that is similar to that in healthy controls. Here, we test this theory by comparing the WBNAA of patients meeting the “benign” criteria with values for healthy contemporaries and published values for patients with RRMS of shorter disease duration.3,30
Materials and Methods
Human Subjects
Forty-three patients with benign MS (30 women, 13 men), defined as an EDSS score ≤3.0 and a disease duration of ≥15 years at scanning time, were recruited. Their mean age was 44.7 ± 7.3 years (range, 23–54 years), their mean disease duration from first symptom was 21.0 ± 4.4 years (range, 15–35), and their mean EDSS score was 1.9 (range, 0–3). Eleven were on disease-modifying medication (6 on β-interferon and 5 on glatiramer acetate). All medicated patients had been so for at least 1 year before their examination. All had been relapse- and steroid-free for at least 3 months before their clinical examination, which included an EDSS rating. Eleven experienced short nonsevere relapses within 5 years of the scanning, while the rest have remained relapse-free since clinical diagnosis. Twenty-four age-matched controls (13 women and 11 men, 42.9 ± 12.8 years old) with no history of neurologic disease underwent the same scanning procedure as the patients. All provided institutional review board−approved written consent, and the study was HIPAA compliant.
MR Imaging and Volumetry
All experiments were performed in a 1.5T Vision scanner using the circularly polarized transmit-receive head coil (Siemens, Erlangen, Germany). After placing the patient head-first supine into the scanner, we obtained 2 sets of images: axial proton-attenuation and T2-weighted dual turbo spin-echo (TR of 3300 ms, TE of 16 and 98 ms, 256 × 256 matrix, 250 × 250 mm2 FOV, twenty-four 5-mm thick sections) for lesion-load estimation. These were followed by sagittal 3D T1-weighted MPRAGE images for brain volumetry: TR/TE = 970/40 ms, TI = 3000 ms, 15° flip angle, 256 × 256 matrix, 210 × 210 mm2 FOV, one hundred twenty-eight 1.5-mm thick sections.
Lesions were identified on the proton-attenuation images, with the corresponding T2-weighted MR images used to increase their identification confidence. The total load was subsequently estimated by using a local thresholding segmentation method.34 Each patient's VB was then automatically segmented from their T1-weighted MPRAGE images by using the Structural Image Evaluation of Normalized Atrophy (SIENAx) package (FMRIB, University of Oxford, Oxford, United Kingdom).35
1H-MR Spectroscopy and WBNAA Quantification
MR imaging was followed by magnetic field shimming by using the manufacturer's automatic algorithm with manual adjustments to yield a consistent 15 ± 3 Hz whole-head water line width. QNAA was then obtained with nonlocalizing TE/TI/TR = 0/970/104 ms 1H-MR spectroscopy36 and scaled into an absolute amount by using phantom replacement against a 3-L sphere of 1.5 × 10−2 mol NAA in water from the SS and SR36:
VS180° and VR180° are the transmitter voltages into 50 Ω for nonselective 1-ms 180° inversion pulses on the reference and subject, respectively, reflecting their relative coil loading.
To account for the natural variations in human head sizes, we divided each patient's QNAA by their VB to yield the QNAA36:
which is a specific metric free of brain size, hence suitable for intersubject comparison. Its intra- and intersubject variability has been shown at better than ±8%.37
Although this study is cross-sectional (only 1 point was acquired per patient), the average annual projected rate of change in the i-th patient can be estimated assuming the following: 1) Before the onset of MS, their WBNAAi was similar to the 12.8 mmol/L average of healthy contemporaries in this study, ; 2) The time from first symptom is a good approximation of disease duration, though admittedly it is always an underestimation and 3) WBNAA changes are monotonic throughout the subsequent years, ΔTi. The projected decline rate is then31
Statistical Analyses
Disease duration, WBNAA, ΔWBNAA, and lesion load were summarized as means ± SDs. An exact Mann-Whitney U test was used to compare treated versus untreated patients and men versus women. ANCOVA based on ranks was used to make the same comparisons after adjusting for patient-specific covariates (eg, age and EDSS score). Specifically, each metric was converted to ranks used as the dependent variable in an ANCOVA to compare treated with untreated and male with female patients. Pearson and Spearman rank correlation coefficients were used to evaluate associations of the imaging metrics with each other and with age, EDSS score, and disease duration. Two-sided P values were declared significant when ≤.05. SAS Version 9.0 (SAS Institute, Cary, North Carolina) was used for all statistical tests, and PASS 2002 (Number Cruncher Statistical Systems, Kaysville, Utah) was used to assess their statistical power.
A regression analysis was conducted to examine the dependence of WBNAA on disease duration and to test whether the projected rate of change in WBNAA was different between the benign cohort and that reported in moderately declining patients with RRMS.3 WBNAA was used as the dependent variable, and the model to predict WBNAA included subject group as a classification factor, disease duration as a numeric factor, and the term representing the interaction between these factors. A significant interaction would imply that the projected rate of change in WBNAA per year of disease duration is different for the 2 subject groups. The error variance was allowed to differ across subject groups to avoid the unnecessary assumption of variance homogeneity.
We used a Monte Carlo technique to simulate the longitudinal data for a single patient, assuming a consistent 1.7% annual decline rate subject to a 6% single-measurement error to estimate the necessary length of a serial study required to achieve significance.
Results
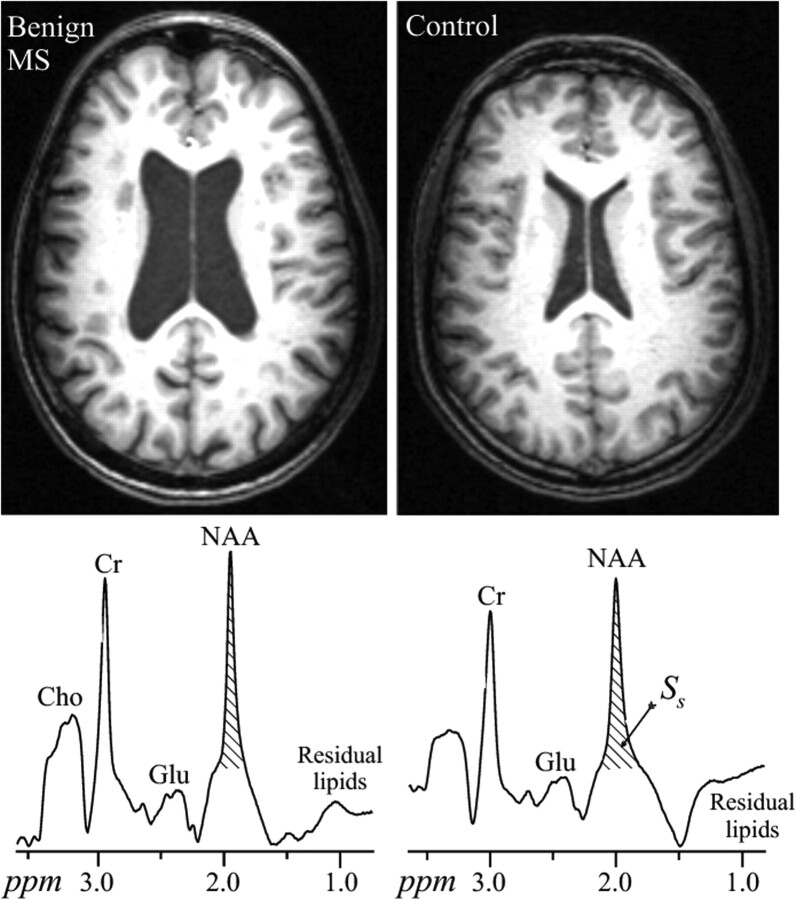
Representative axial MPRAGE images and whole-brain spectra from a patient with benign MS and a matched control are shown in Fig 1. The images illustrate typical differences in atrophy between the 2 groups as well as the T1 hypointense lesion load in the patient. The respective whole-head 1H-spectra shown demonstrate how the NAA peak area is estimated for equation 1. The mean WBNAA in the 43 patient cohort was 8.3 ± 1.8 mmol/L, highly significantly (35%, P < .001) lower than the 12.8 ± 1.2 mmol/L of the controls. Their average lesion load was 12.2 ± 7.7 cm3 or just ∼1% of the brain volume, commensurate with their disease duration regardless of course.12,38
Fig 1.
Top (left): Representative axial T1-weighted MPRAGE brain sections of a 45-year-old patient with benign MS and a 47-year-old control (right), both male. Note the atrophy in the patient compared with the control and the T1 hypointense lesion load of the patient. Bottom: The corresponding whole-head 1H-MR spectroscopy (not normalized for VB) on common intensity and frequency scales. Note the NAA peak at 2 ppm and the lipid-suppression performance. Of all the other metabolite peaks seen in the whole-head spectrum, only the NAA is implicitly localized by its biochemistry to just the brain. SS was obtained by integration for use in equation 1.
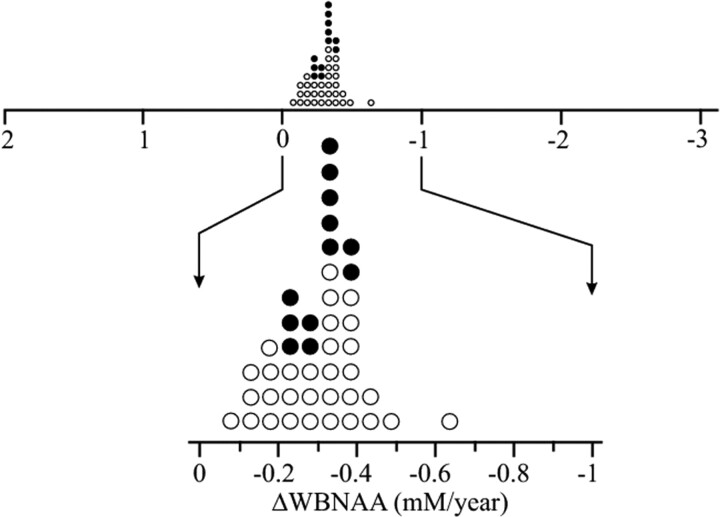
The average annual rate of WBNAA decline for each member of the cohort, ΔWBNAAi from equation 3, was −0.22 ± 0.09 mmol/L/year (∼1.7%), as shown in Fig 2 (top) for the +2…−3 mmol/L/year range reported previously for patients with RRMS of a much shorter disease duration.3,39 As the scale is narrowed (Fig 2 bottom), the uniform slow, normal WBNAA loss rate distribution becomes more apparent, regardless of medication status.
Fig 2.
Top: Dot plot of the average annual rate of WBNAA decline of the 43 individual patients with benign MS from equation 3. Closed circles are patients on disease-modifying treatment. Note the narrow distribution (0.16 mmol/L/year full width at half height) and slow (ΔWBNAA − 0.22 mmol/L/year, −1.7%) average annual decline of these patients with benign MS compared with the +2… −3 mmol/L/year range exhibited by patients with RRMS of shorter disease durations.3,39 Bottom: Zoomed detail of the average annual rate of WBNAA decline. Note that not only is the WBNAA decline slow, uniform, and normally distributed but that there is also no distinction between medicated and nonmedicated patients.
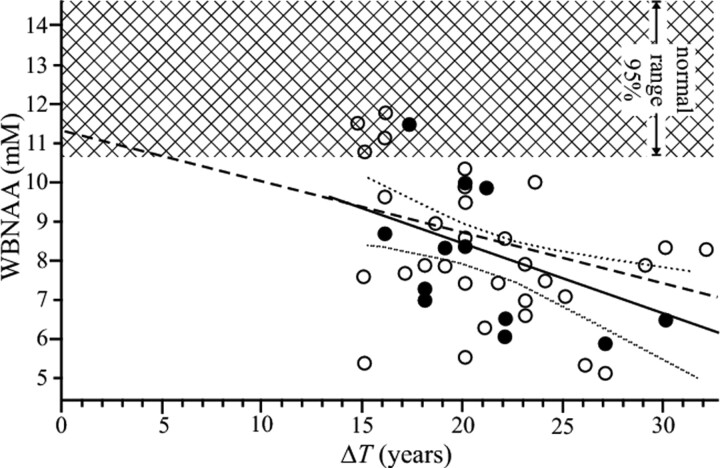
Each patient's WBNAA value was also plotted as a function of his or her disease duration (in years) in Fig 3, together with the linear regression and its 95% confidence intervals:
Fig 3.
The WBNAAi concentration of 43 patients with benign MS as a function of their ΔTi (filled circles represent patients on disease-modifying treatment). Also shown are linear predictions previously reported for patients with RRMS of shorter (3–12 years) disease durations (dashed line), the regression for the benign cohort with its 95% confidence intervals (solid and dotted lines), and the ±95% confidence intervals for the WBNAA of the 24 controls (cross-hatch). Note that the regression line for patients with RRMS of short duration falls within the 95% confidence range of the patients with benign MS and their significant loss compared with controls.
The dependence reported before for patients with RRMS of shorter disease durations3 and controls is also shown for comparison in Fig 3. The data demonstrate that our benign cohort is similar in terms of the WBNAA projected rate of change to patients with “moderate” nonbenign RRMS because the 95% confidence interval for the difference between these rates extends from −0.01 to 0.3.
No correlation was found between WBNAA and EDSS, lesion load, age, or disease duration. Additionally there was no significant difference in WBNAA when comparing sex or patients receiving treatment versus those who were not, as well as those who had a recent (within 5 years) relapse versus those who have not. There was also no correlation between disease duration and either the lesion load or the EDSS score.
The Monte Carlo simulation determined that it would require 20 scans each separated by at least 6 months to establish an individual's 1.7%/year WBNAA decline rate with statistical significance.
Discussion
Despite the importance of prospective prognostic metrics for a benign MS course, the attempts to identify these have so far proved elusive, yielding no true consensus.9 Various explanations as to why laboratory (and in particular quantitative MR imaging) metrics have fallen short have been offered and reviewed by Rovaris et al.9 While they provide interesting insights into the nature of the pathologic underpinnings of the benign phenotype, they could not identify a reliable diagnostic method that would establish this prognosis.7,10,11
The mild clinical course and accepted notion that MS targets axons and neurons23,25–28 raised the possibility that a marker of their integrity may provide such a prognosis,40 while lesion loads and atrophy rates have come up short.14–16 Additional support for this idea includes reports that approximately 20% of patients with RRMS exhibit WBNAA no different from that in healthy controls, while that in the rest was significantly lower.3,30,31 Taken together, these findings prompted our hypothesis that the WBNAA of patients with benign MS should be similar to that in healthy contemporaries, regardless of disease duration. This hypothesis also invokes a corollary that the WBNAA should, therefore, be higher in patients with benign than in nonbenign MS.
Unfortunately, the highly significant average 35% WBNAA loss by this benign cohort leads us to reject the hypothesis. Furthermore, the regression lines of WBNAA versus disease duration of our cohort similar to those extrapolated from patients of shorter (0–12 years) RRMS duration (−0.13 × years +11.4 mmol/L) causes us to also reject the corollary. Consequently, on the basis of these data, neuronal preservation is not characteristic of benign MS and WBNAA and is unlikely to be a prospective indicator for its course. Nevertheless, because none of these patients had the 1.7 mmol/L/year WBNAA loss rate reported for the “rapid” RRMS group,3 it is unlikely that any new patient with MS exhibiting such fast loss will follow a benign course.
The distribution of WBNAA decline rates of these patients with benign MS is both narrow, 0.16 mmol/L/year full width at half height, and slow, 0.22 mmol/L/year. In other words, the rate histogram is only the width of 1 year's decline, a finding distinct from previous reports in the general RRMS patient populations.3,31 This finding refutes reports that most neuroaxonal damage (NAA loss) accrues early in the disease course,30,41,42 for if it were true, longer disease duration (Ti in equation 3) would lead to lower WBNAA loss rates. Given the range of disease durations, the narrow distribution of decline rates suggests that this is probably not the case.
Although this study is cross-sectional, we are nevertheless able to estimate a WBNAA decline of 1.7%/year based on the 2 assumptions of average normal concentration at diagnosis and of a monotonic decline. On the basis of the 6% intrinsic precision of each WBNAA measurement, a longitudinal study would require subjects to be scanned at least 20 times separated by at least 6 months to establish statistical significance for such a slow rate. The 10–20 years that would be required to conduct such a longitudinal study will render it susceptible to several confounding but probably unavoidable MR imaging−equipment upgrade cycles, as well research team turnover and patient cohort attrition (ie, making it prohibitively difficult).
Because this study is not longitudinal, the reported WBNAA loss rates should be viewed as only estimates based on 1 measurement. That the WBNAA can be extrapolated from the established data for patients with moderate RRMS, however, hints at 2 possible scenarios: First, that “benign” may be an RRMS phenotype that is simply more adept in compensatory mechanisms exploiting the brain's plasticity.43 Second, although the lesion loads between this cohort (12.2 ± 7.7 cm3) and clinically dissimilar subtypes are comparable,12 patients with benign MS accumulate lesions that fortuitously miss eloquent brain regions involved in EDSS assessment.13 Their lesions have especially spared the spine, where each would have a pronounced impact without a noticeable increase in their load. Whether these 2 above scenarios work separately or in concert, their consequence is that these patients unfortunately run the risk of ultimately having a precipitous decline in the future, when their reserves are exhausted or eloquent regions are impacted. That will bring their disability in line with that of other patients with MS of similar disease duration.
The paucity of serial studies of benign MS, however, renders patients' long-term outcomes relatively vague. The longest (21-year) follow-up of 47 such patients found that on the basis of EDSS alone, only 7 (15%) conditions were still considered benign, 10 patients (21%) had died with significant MS-related disability, and 8 (17%) had died of non-MS-related causes. The remaining 22 all required ambulatory assistance.11 These findings suggest that benign may be an overly optimistic misnomer due to the small number of patients who persisted in qualifying under the strict criteria and to the relatively precipitous clinical decline of the rest.11 Our results showing significant accumulating neuroaxonal injury despite apparent maintenance of clinical functionality are, therefore, consistent with that conclusion.
Admittedly the WBNAA approach trades localization for sensitivity and speed, incurring 2 limitations44: First, as mentioned above, global changes smaller than the 6%–8% sensitivity threshold are undetected. Second, regional NAA variations are averaged out and are subject to the first limitation. Such a trade-off may be acceptable, however, for diffuse pathologies. In addition, decline-rate estimates are based on 1 measurement and the assumption that at first symptom, a subject's WBNAA is no different from that of a healthy control; the model, equation 3 and Fig 3, assumes monotonic WBNAA decline throughout the long disease duration. Although these assumptions may contradict reports that (significant) NAA loss is already present and that NAA declines faster early in the MS course, cohorts in which these observations were made comprised mostly patients with early RRMS.30,41,42 Furthermore, the fact that the regression line extrapolated from patients with shorter disease duration also describes the WBNAA in subjects with benign MS indicates a decline that continues even beyond the earlier course. Finally, we did not compare the WBNAA of patients with benign MS with that in patients with nonbenign RRMS of similar disease duration due to recruitment difficulty: Most patients with MS with a long (>15 years) disease duration convert to the secondary-progressive form (50% after 10 years and >90% after 20–25 years45), rendering them either ineligible or too disabled to participate.
Conclusions
Retaining neuronal function reflected by WBNAA level similar to that in healthy contemporaries is paradoxically not a characteristic of patients with benign MS, despite the minimal disability they accumulate with very long disease durations. Instead, their NAA losses can be extrapolated for their disease duration from that of patients with RRMS of much shorter disease duration. These findings are consistent with combinations of adept compensatory mechanisms together with fortuitous sparing of eloquent brain regions. This finding corroborates WBNAA as a probe of the effect of MS on the production of this neuronal marker and may be instrumental in distinguishing “rapid” MS from the “moderate” and “benign” phenotypes due to the much faster WBNAA decline of the former. Finally, the extent of absolute NAA decline (despite a clinically benign disease course) may suggest that even these patients would probably benefit from treatment paradigms that are currently offered to the other more affected patients with RRMS.
Abbreviations
- ANCOVA
analysis of covariance
- Cho
choline
- CNS
central nervous system
- Cr
creatine
- EDSS
Expanded Disability Status Scale
- ΔTi
disease duration from first symptom
- Glu
glutamate
- 1H-MR spectroscopy
proton MR spectroscopy
- HIPAA
Health Insurance Portability and Accountability Act
- MPRAGE
magnetization-preparation rapid gradient echo
- MS
Multiple Sclerosis
- NAA
N-acetylaspartate
- QNAA
global brain NAA amount
- RR
relapsing-remitting
- RRMS
relapsing-remitting MS
- SR
reference NAA peak areas
- SS
subject NAA peak area
- VB
brain parenchyma volume
- VR
reference voltage
- VS
subject voltage
- WBNAA
whole-brain NAA concentration
Footnotes
This work was supported by National Institutes of Health grants EB01015, NS0050520, and NS39135.
References
- 1. Hauser SL. Multiple sclerosis and other demyelinating diseases. In: Isselbacher KJ, Wilson JD, Martin JB, et al. eds. Harrison's Principles of Internal Medicine. New York: McGraw-Hill; 1994:2287–95 [Google Scholar]
- 2. Weinshenker BG. Natural history of multiple sclerosis. Ann Neurol 1994;36:S6–11 [DOI] [PubMed] [Google Scholar]
- 3. Gonen O, Moriarty DM, Li BS, et al. Relapsing-remitting multiple sclerosis and whole-brain N-acetylaspartate measurement: evidence for different clinical cohorts initial observations. Radiology 2002;225:261–68 [DOI] [PubMed] [Google Scholar]
- 4. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52 [DOI] [PubMed] [Google Scholar]
- 5. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey—National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907–11 [DOI] [PubMed] [Google Scholar]
- 6. Ramsaransing GS, De Keyser J. Benign course in multiple sclerosis: a review. Acta Neurol Scand 2006;113:359–69 [DOI] [PubMed] [Google Scholar]
- 7. Pittock SJ, McClelland RL, Mayr WT, et al. Clinical implications of benign multiple sclerosis: a 20-year population-based follow-up study. Ann Neurol 2004;56:303–06 [DOI] [PubMed] [Google Scholar]
- 8. Confavreux C, Vukusic S. The clinical epidemiology of multiple sclerosis. Neuroimaging Clin N Am 2008;18:589–622 [DOI] [PubMed] [Google Scholar]
- 9. Rovaris M, Barkhof F, Calabrese M, et al. MRI features of benign multiple sclerosis: toward a new definition of this disease phenotype. Neurology 2009;72:1693–701 [DOI] [PubMed] [Google Scholar]
- 10. Hawkins SA, McDonnell GV. Benign multiple sclerosis? Clinical course, long term follow up, and assessment of prognostic factors. J Neurol Neurosurg Psychiatry 1999;67:148–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Costelloe L, Thompson A, Walsh C, et al. Long-term clinical relevance of criteria for designating multiple sclerosis as benign after 10 years of disease. J Neurol Neurosurg Psychiatry 2008;79:1245–48 [DOI] [PubMed] [Google Scholar]
- 12. Strasser-Fuchs S, Enzinger C, Ropele S, et al. Clinically benign multiple sclerosis despite large T2 lesion load: can we explain this paradox? Mult Scler 2008;14:205–11 [DOI] [PubMed] [Google Scholar]
- 13. Ramsaransing G, Maurits N, Zwanikken C, et al. Early prediction of a benign course of multiple sclerosis on clinical grounds: a systematic review. Mult Scler 2001;7:345–47 [DOI] [PubMed] [Google Scholar]
- 14. Traboulsee A, Dehmeshki J, Peters KR, et al. Disability in multiple sclerosis is related to normal appearing brain tissue MTR histogram abnormalities. Mult Scler 2003;9:566–73 [DOI] [PubMed] [Google Scholar]
- 15. Ceccarelli A, Rocca MA, Pagani E, et al. The topographical distribution of tissue injury in benign MS: a 3T multiparametric MRI study. Neuroimage 2008;39:1499–509 [DOI] [PubMed] [Google Scholar]
- 16. Rovaris M, Riccitelli G, Judica E, et al. Cognitive impairment and structural brain damage in benign multiple sclerosis. Neurology 2008;71:1521–26 [DOI] [PubMed] [Google Scholar]
- 17. Thompson AJ, Miller DH, MacManus DG, et al. Patterns of disease activity in multiple sclerosis. BMJ 1990;301:44–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brass SD, Narayanan S, Antel JP, et al. Axonal damage in multiple sclerosis patients with high versus low expanded disability status scale score. Can J Neurol Sci 2004;31:225–28 [DOI] [PubMed] [Google Scholar]
- 19. Audoin B, Guye M, Reuter F, et al. Structure of WM bundles constituting the working memory system in early multiple sclerosis: a quantitative DTI tractography study. Neuroimage 2007;36:1324–30 [DOI] [PubMed] [Google Scholar]
- 20. Jbabdi S, Woolrich MW, Andersson JL, et al. A Bayesian framework for global tractography. Neuroimage 2007;37:116–29 [DOI] [PubMed] [Google Scholar]
- 21. Matthews PM, De Stefano N, Narayanan S, et al. Putting magnetic resonance spectroscopy studies in context: axonal damage and disability in multiple sclerosis. Semin Neurol 1998;18:327–36 [DOI] [PubMed] [Google Scholar]
- 22. Bjartmar C, Kidd G, Mork S, et al. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol 2000;48:893–901 [PubMed] [Google Scholar]
- 23. Arnold DL, De Stefano N, Narayanan S, et al. Axonal injury and disability in multiple sclerosis: magnetic resonance spectroscopy as a measure of dynamic pathological change in white matter. In: Comi G. ed. Magnetic Resonance Spectroscopy in Multiple Sclerosis. Milan, Italy: Springer-Verlag Italia; 2001:61–67 [Google Scholar]
- 24. Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 2007;68:S22–31, discussion S43–54 [DOI] [PubMed] [Google Scholar]
- 25. Benarroch EE. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology 2008;70:1353–57 [DOI] [PubMed] [Google Scholar]
- 26. Mainero C, De Stefano N, Iannucci G, et al. Correlates of MS disability assessed in vivo using aggregates of MR quantities. Neurology 2001;56:1331–34 [DOI] [PubMed] [Google Scholar]
- 27. Mathiesen HK, Jonsson A, Tscherning T, et al. Correlation of global N-acetyl aspartate with cognitive impairment in multiple sclerosis. Arch Neurol 2006;63:533–36 [DOI] [PubMed] [Google Scholar]
- 28. Enzinger C, Ropele S, Strasser-Fuchs S, et al. Lower levels of N-acetylaspartate in multiple sclerosis patients with the apolipoprotein E epsilon4 allele. Arch Neurol 2003;60:65–70 [DOI] [PubMed] [Google Scholar]
- 29. Miller DH, Thompson AJ, Filippi M. Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J Neurol 2003;250:1407–19 [DOI] [PubMed] [Google Scholar]
- 30. Filippi M, Bozzali M, Rovaris M, et al. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain 2003;126:433–37 [DOI] [PubMed] [Google Scholar]
- 31. Inglese M, Ge Y, Filippi M, et al. Indirect evidence for early widespread gray matter involvement in relapsing-remitting multiple sclerosis. Neuroimage 2004;21:1825–29 [DOI] [PubMed] [Google Scholar]
- 32. Sayao AL, Devonshire V, Tremlett H. Longitudinal follow-up of “benign” multiple sclerosis at 20 years. Neurology 2007;68:496–500 [DOI] [PubMed] [Google Scholar]
- 33. Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–85 [DOI] [PubMed] [Google Scholar]
- 34. Rovaris M, Filippi M, Calori G, et al. Intra-observer reproducibility in measuring new putative MR markers of demyelination and axonal loss in multiple sclerosis: a comparison with conventional T2-weighted images. J Neurol 1997;244:266–70 [DOI] [PubMed] [Google Scholar]
- 35. Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–89 [DOI] [PubMed] [Google Scholar]
- 36. Gonen O, Viswanathan AK, Catalaa I, et al. Total brain N-acetylaspartate concentration in normal, age-grouped females: quantitation with non-echo proton NMR spectroscopy. Magn Reson Med 1998;40:684–89 [DOI] [PubMed] [Google Scholar]
- 37. Benedetti B, Rigotti DJ, Liu S, et al. Reproducibility of the whole-brain N-acetylaspartate level across institutions, MR scanners, and field strengths. AJNR Am J Neuroradiol 2007;28:72–75 [PMC free article] [PubMed] [Google Scholar]
- 38. Li DK, Held U, Petkau J, et al. MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology 2006;66:1384–89 [DOI] [PubMed] [Google Scholar]
- 39. Gonen O, Oberndorfer TA, Inglese M, et al. Reproducibility of three whole-brain N-acetylaspartate decline cohorts in relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol 2007;28:267–71 [PMC free article] [PubMed] [Google Scholar]
- 40. Benedetti B, Rovaris M, Rocca M, et al. In-vivo evidence for stable neuroaxonal damage in the brain of patients with benign multiple sclerosis. Mult Scler 2009;15:789–94. Epub 2009 May 22 [DOI] [PubMed] [Google Scholar]
- 41. Chard DT, Griffin CM, McLean MA, et al. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain 2002;125:2342–52 [DOI] [PubMed] [Google Scholar]
- 42. De Stefano N, Narayanan S, Francis GS, et al. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol 2001;58:65–70 [DOI] [PubMed] [Google Scholar]
- 43. Pelletier J, Audoin B, Reuter F, et al. Plasticity in MS: from functional imaging to rehabilitation. Int MS J 2009;16:26–31 [PubMed] [Google Scholar]
- 44. Gonen O, Catalaa I, Babb JS, et al. Total brain N-acetylaspartate: a new measure of disease load in MS. Neurology 2000;54:15–19 [DOI] [PubMed] [Google Scholar]
- 45. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain 1989;112(pt 6):1419–28 [DOI] [PubMed] [Google Scholar]