Summary
Kinesin-5, a widely conserved motor protein required for assembly of the bipolar spindle in vertebrates, forms homotetramers with two pairs of motor domains positioned at opposite ends of a dumbbell shaped molecule [1–3]. It has long been assumed that this configuration of motor domains is the basis of kinesin-5’s ability to drive relative sliding of microtubules [2, 4, 5]. Recently, it was suggested that in addition to the N-terminal motor domain, kinesin-5 also has a non-motor microtubule binding site in its C-terminus [6]. However, it is not known how the non-motor domain contributes to motor activity, or how a kinesin-5 tetramer utilizes a combination of four motor and four non-motor microtubule binding sites for its microtubule organizing functions. Here we show, in single molecule assays, that kinesin-5 homotetramers require the non-motor C-terminus for crosslinking and relative sliding of two microtubules. Remarkably, this domain enhances kinesin-5’s microtubule binding without substantially reducing motor activity. Our results demonstrate that tetramerization of kinesin-5’s low-processivity motor domains is not sufficient for microtubule sliding, as the motor domains alone are unlikely to maintain persistent microtubule crosslinks. Rather, kinesin-5 utilizes non-motor microtubule binding sites to tune its microtubule attachment dynamics, enabling it to efficiently align and sort microtubules during metaphase spindle assembly and function.
Keywords: kinesin-5, motor protein, microtubule organization, mitotic spindle
Results
The kinesin-5 tail domain contains an additional microtubule binding site
Full-length kinesin-5 is a homotetrameric, bipolar protein comprised of three domains (Figure 1A). The motor domain is at the N-terminus, followed by the central stalk required for dimerization of motor pairs (coil 1) and tetramerization of kinesin-5 (coils 2–4). At the C-terminus there is an additional 140 amino acid conserved tail domain whose function is unclear. To characterize the function of the non-motor tail, we generated a fluorescently labeled truncation construct of Xenopus laevis kinesin-5 (named, Kin5-Δmotor-GFP) that has the entire conserved kinesin motor domain deleted, but includes all of kinesin-5’s non-motor domains, similar to a Drosophila melonogaster kinesin-5 construct shown previously to crosslink microtubules [6, 7]. GFP was fused to the C-terminus, a site where it is unlikely to be functionally disruptive based on studies of a tagged full-length construct, Kin5-GFP [8, 9]. Comparison with Kin5-GFP by gel filtration chromatography and single particle fluorescence intensity analysis indicated that Kin5-Δmotor-GFP is homotetrameric, like the full-length construct (Figure S1A–S1C).
Figure 1. A microtubule binding site near the kinesin-5 C-terminus allows one dimensional diffusion of microtubules.
(A) Domain structures of kinesin-5 GFP-fusion constructs used in this study. (B) Schematic of the single molecule microtubule interaction assay. Rhodamine-labeled microtubules were immobilized on a glass surface and the interaction of Kin5-Δmotor-GFP was observed by single molecule TIRF microscopy. (C) Images showing Kin5-Δmotor-GFP binding to surface immobilized microtubules (top: microtubule; center: single frame; bottom: time average over 300 frames). Bar: 4µm. (D) kymograph shows Kin5-Δmotor-GFP interactions with microtubules. Bar: 4µm. (E) Schematic of the surface binding assay. (F–G) Kymographs show the movement of microtubules attached to a glass surface by Kin5-Δmotor-GFP in low salt buffer (F) and high salt buffer (G) Bars: 2 µm, 60 sec. (H) MSD calculated from motion of surface-adhered microtubules in high salt buffer (●) and low salt buffer (○). Fits represent MSD = 2Dt, error bars = sem. See also Figure S1 and Movies S1–S2.
We then examined the interaction of single molecules of Kin5-Δmotor-GFP with surface-immobilized microtubules using TIRF microscopy (Figure 1B). Previous studies have shown that full length kinesin-5 has two modes of motion on microtubules, depending on ionic strength of the assay buffer and microtubule binding state [8, 9]. On single microtubules, plus-end-directed, ATP-dependent directional motion predominates in low ionic strength buffers, while under more physiological conditions ATP-independent one-dimensional diffusive motion predominates. In physiological ionic strength, kinesin-5’s motility switches from diffusive to directional upon crosslinking a pair of microtubules. Based on these results, we mainly used two different buffer conditions throughout this study; one with low salt and one with higher salt that is closer to physiological ionic strength (see Experimental Procedures for details). In the high salt buffer we did not detect Kin5-Δmotor-GFP on microtubules (data not shown). However, upon reducing the salt concentration, we observed Kin5-Δmotor-GFP binding to bundles of microtubules (Figure 1C). Although multiple binding events could be observed on any one bundle, the association times were very short (<1 s) under our imaging conditions. We could only estimate an upper limit of 1 s for the average association time of this construct with microtubules, which is significantly lower than the previously determined value for full-length Kin5-GFP of tavg = 34 sec [8].
Since individual molecules of Kin5-Δmotor-GFP interact briefly with microtubules, we examined if multiple molecules of this protein could establish more persistent filament interactions (Figure 1E). For this experiment, the glass surface of an assay chamber was coated with Kin5-Δmotor-GFP and blocked to prevent non-specific attachment of microtubules. When microtubules were added, they bound to the Kin5-Δmotor-GFP on the surface, and did not release over a range of salt concentrations. Strikingly, while remaining attached to the surface each microtubule moved back and forth along its long axis (Supplemental movies S1–S2). We used kymography to track the motion of these filaments (Figures 1F and 1G) and performed mean squared displacement (MSD) analysis of filament positions (Figure 1H). The MSD of the filaments was linearly proportional to the time interval, consistent with one-dimensional diffusion on the surface. Furthermore, the diffusion constant was sensitive to ionic strength, increasing 5-fold from D = 450 +/− 40 nm2/s in low salt to D = 2600 +/− 200 nm2/s in high salt. These values are similar in magnitude to those measured for one-dimensional diffusion of microtubules along a surface coated with MCAK, member of the kinesin-13 family [10, 11]. These data suggest that a microtubule binding site outside the motor domain, most likely in the kinesin-5 tail, allows kinesin-5 to bind microtubules in a way that tolerates slippage along the filament’s axis.
The kinesin-5 tail domain is important for crosslinking microtubules
To examine how the non-motor microtubule binding site in the C-terminal tail impacts kinesin-5’s ability to crosslink and slide microtubules apart, we designed a kinesin-5 construct in which it was deleted. To identify the binding site within the tail, we aligned kinesin-5 sequences from 17 species. Strong conservation in portions of the C-terminal region was found, but no clusters of basic residues, which are common in microtubule binding domains, were identified (Figure S2). Surprisingly, the tails of many of the kinesin-5 sequences analyzed, including that of Xenopus laevis, had predicted net-negative charges, which is atypical for microtubule binding proteins [12]. Because we were unable to identify a specific sequence in the kinesin-5 tail that was likely to be responsible for microtubule binding, we deleted the entire tail region. This construct (named Kin5-Δtail-GFP), like Kin5-Δmotor-GFP, had a gel filtration elution volume and single particle fluorescence intensity distribution very similar to those of Kin5-GFP, indicating that this construct is also homotetrameric (Figure S1A–S1C).
To examine relative filament sliding we utilized an in vitro microtubule sliding assay described before [8] (Figure 2A). Rhodamine labeled, biotinylated microtubules were immobilized on glass coverslips through biotin-streptavidin linkages, and a reaction mix containing motor protein and additional non-biotinylated microtubules was added. In high salt buffer, Kin5-GFP robustly crosslinked microtubules from the solution to surface immobilized microtubules and drove their relative sliding at 45.6 +/− 1.4 nm/s (N = 20), as expected (Figure 2B, Supplemental Movie S3) [8]. In the same buffer conditions, we did not observe any microtubule crosslinking or sliding by Kin5-Δtail-GFP over a wide range of protein concentrations (data not shown). Further, we did not observe microtubule bundling in solution by Kin5-Δtail-GFP at concentrations up to 100 nM, a 20-fold excess over concentrations of Kin5-GFP that bundled microtubules (data not shown).
Figure 2. The kinesin-5 C-terminal tail domain is required for microtubule sliding at physiological ionic strength.
(A) Schematic of the microtubule sliding assay. Rhodamine-labeled microtubules from solution were crosslinked to surface-immobilized microtubules by kinesin-5, which drives relative sliding. (B–D) Kymographs show the motion of microtubules (left, red) and Kin5-GFP constructs (center, green). Bar, 3 µm. Microtubule sliding in the presence of (B) Kin5-GFP (1 nM) in high salt buffer, (C) unlabeled kinesin-5 (2 nM) and Kin5-Δtail-GFP (3 nM) in high salt buffer, and (D) Kin5-Δtail-GFP (3 nM) in low salt buffer. Dashed red lines in center kymographs indicate position of moving microtubule. Green arrows indicate Kin5-Δtail-GFP accumulated at microtubule tips. (E) Microtubule binding curves for Kin5-GFP ( ), Kin5-Δtail-GFP (
), Kin5-Δtail-GFP ( ), and Kin5-Δtail-GFP (
), and Kin5-Δtail-GFP ( ). Microtubule affinity was determined by cosedimentation of the kinesin-5 constructs with microtubules in low salt buffer with 2 mM ADP. The fraction bound was calculated from the relative fraction remaining in the pellet following cosedimentation, and binding constants were determined by fitting to a hyperbola. Error bars = sem. See also Figure S2 and Movies S3–S5.
). Microtubule affinity was determined by cosedimentation of the kinesin-5 constructs with microtubules in low salt buffer with 2 mM ADP. The fraction bound was calculated from the relative fraction remaining in the pellet following cosedimentation, and binding constants were determined by fitting to a hyperbola. Error bars = sem. See also Figure S2 and Movies S3–S5.
It is possible that Kin5-Δtail-GFP cannot align microtubules on its own, but can crosslink preorganized antiparallel filaments. To examine this, we used full-length kinesin-5 that was not tagged with GFP to generate microtubule sliding pairs. Even under these conditions with relatively high concentrations of Kin5-Δtail-GFP, limited Kin5-Δtail-GFP binding was observed along the microtubules (Figure 2C, Supplemental Movie S4).
We next examined the effect of ionic strength on Kin5-Δtail-GFP binding of microtubule pairs. At low ionic strength, Kin5-Δtail-GFP clearly decorated immobilized microtubules, moved towards the plus-ends, and accumulated in bright aggregates at the microtubule tips (Fig 2D, green arrows in kymograph). Additionally, high concentrations of Kin5-Δtail-GFP were capable of microtubule bundling in solution (data not shown). Rare crosslinking and sliding events were observed (Figure 2D, Supplemental Movie S5), but the average sliding velocity was 21.9 +/– 1.3 nm/s (N = 11), consistent with the motion being driven by kinesin-5 molecules walking on one microtubule, rather than on both simultaneously. This suggests that the aggregates at the tips may be responsible for the infrequent sliding events observed.
Deletion of the C-terminal tail in Kin5-Δtail-GFP could cause microtubule crosslinking to fail because of reduced microtubule affinity. We compared the microtubule binding affinities of full length Kin5-GFP, Kin5-Δtail-GFP and Kin5-Δmotor-GFP using cosedimentation assays (Figure 2E). Full length kinesin-5 bound tightest, with a Kd of 0.19 +/– 0.05 µM. Significantly, each of the truncation constructs, Kin5-Δtail-GFP and Kin5-Δmotor-GFP, bound with more than 10-fold weaker affinity (Kd = 2.4 +/− 0.4 and 2.1 +/− 0.5 µM, respectively). This indicates that both the motor and tail domains of kinesin-5 contribute to the overall binding of full-length kinesin-5 to microtubules. Together, these results suggest that the C-terminal tail domain is required for kinesin-5’s ability to crosslink microtubules and drive their relative sliding.
The kinesin-5 tail domain promotes microtubule association and processivity
We next examined the effect of the C-terminal tail deletion on the motility of individual Kin5-Δtail-GFP molecules on single microtubules. For this we used a single molecule TIRF microscopy-based motility assay (Figure 3A). The movement of Kin5-Δtail-GFP molecules along surface immobilized microtubules was recorded in various salt conditions and compared to that of Kin5-GFP (Figures 3B–3G). The intensities of moving fluorescent spots, reflecting tetrameric motors, were fit by 2D Gaussians and single particle tracking was used to determine trajectories of the individual motors for mean squared displacement analysis (Figures 3H–3J). Average microtubule association times were determined by fitting histograms of individual run durations to single exponential functions (Figures 3K–3M).
Figure 3. The kinesin-5 tail domain increases processivity and association time with microtubules.
(A) Schematic of the single molecule motility assay. Single molecule TIRF microscopy was used to observe the motion of individual kinesin-5 molecules moving along surface-immobilized microtubules. (B–G) Kymographs showing the motion of (B–D) Kin5-GFP and (E–G) Kin5-Δtail-GFP along microtubules in PEM70 (2 mM ATP) and increasing concentrations of KCl. Motor protein concentration had to be increased to obtain sufficient numbers of binding events as ionic strength increased. [Kin5-GFP]: 60 pM in PEM70 (B), 120 pM in PEM70+40 mM KCl (C), and 240 pM in PEM70+80 mM KCl (D). [Kin5-Δtail-GFP]: 150 pM in PEM70 (E), 600 pM in PEM70+40 mM KCl (F), 1500 pM in PEM70+80 mM KCl (G). (H–J) Mean squared displacement (MSD) analysis of motility on single microtubules for Kin5-GFP in PEM70+40 mM KCl (H) and for Kin5-GFP (I) and Kin5-Δtail-GFP (J) in PEM70. Solid lines are fits to MSD = v2t2 + 2Dt+ offset. (K–M) Histograms of association times for Kin5-GFP in PEM70+40 mM KCl (K) and for Kin5-GFP (L) and Kin5-Δtail-GFP (M) in PEM70. Average association times, determined from fits to single exponentials (solid curves) are indicated. Scale bars: 3µm, error bars = sem.
In an elevated ionic strength buffer (PEM70 + 40 mM KCl), Kin5-GFP made long associations with microtubules (average association time = 29 s, Figure 3K), and the motility was mostly diffusive (v = 0.7 nm/s, D = 4.0 × 103 nm2/s, Figures 3C and 3H), consistent with previously published results [8]. In contrast, under the same conditions, Kin5-Δtail-GFP was unable to make sustained interactions with microtubules (Figure 3F), and MSD analysis was not feasible. In low salt buffer both constructs moved processively and directionally along the microtubule (Figures 3B and 3E), though the interactions of Kin5-Δtail-GFP with microtubules were generally shorter than those of full length kinesin-5 (average association times = 29 s for Kin5-GFP and 11 s for Kin5-Δtail-GFP, Figures 3L and 3M). MSD analysis showed that the average velocity of Kin5-Δtail-GFP was about 1.7-fold greater than that of Kin5-GFP (14.1 and 8.5 nm/s, respectively, Figures 3J and 3I), indicating that the tail domain can modestly attenuate kinesin-5 motility. Kin5-Δtail-GFP also exhibited a slightly higher diffusion constant than Kin5-GFP (D = 2.3 × 103 nm2/s and D = 1.4 × 103 nm2/s, respectively) suggesting that diffusive motion can be a property of the kinesin-5 motor, and that the presence of the tail can suppress diffusion in low salt conditions.
The kinesin-5 tail domain does not interfere with motility
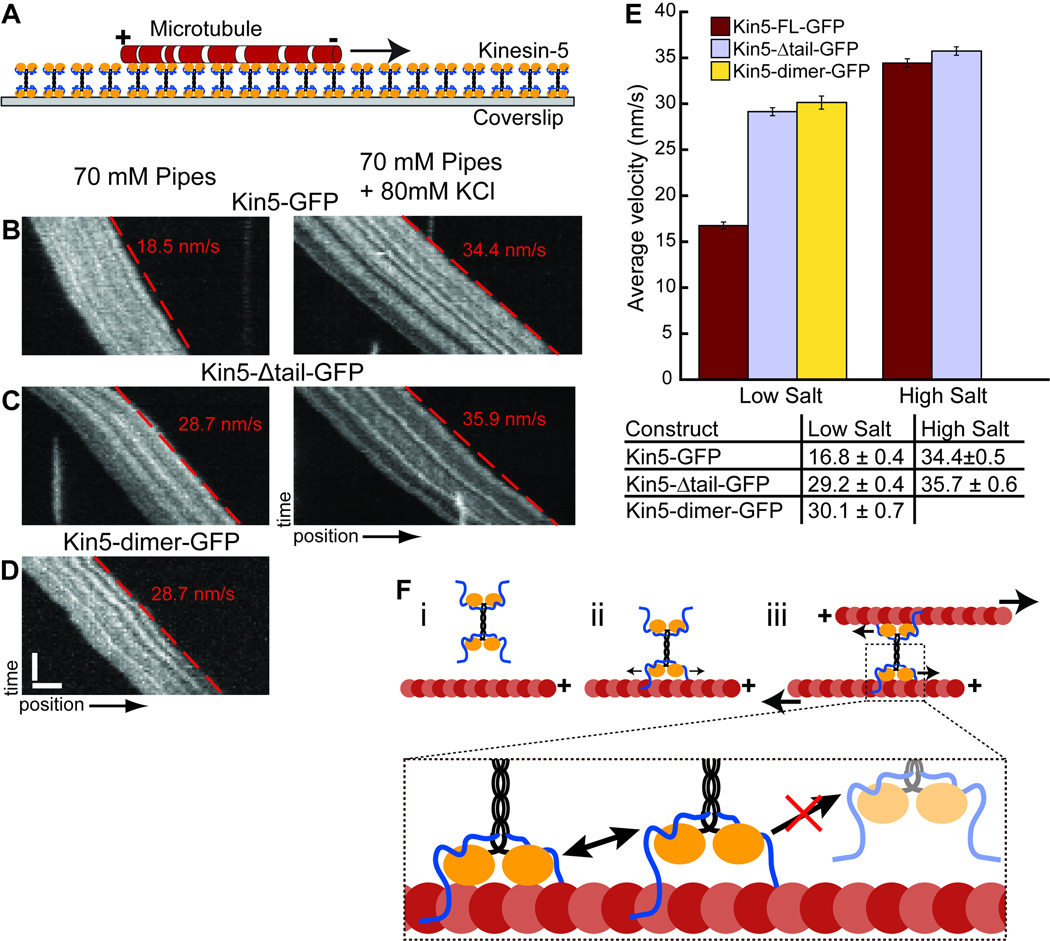
To further probe the effect of the C-terminal domain on kinesin-5 motility, we employed a microtubule surface gliding assay to examine Kin5-Δtail-GFP’s motor activity (Figure 4A). Glass coverslips were coated with different kinesin-5 constructs, and the movement of microtubules along the surface was recorded by time-lapse fluorescence microscopy. We compared the motility of Kin5-Δtail-GFP with those of Kin5-GFP and Kin5-dimer-GFP, a dimeric kinesin-5 truncation construct described earlier [8].
Figure 4. The kinesin-5 C-terminal tail domain reduces motility in low ionic strength.
(A) Schematic of the microtubule surface motility assay. X-rhodamine labeled microtubules bind to kinesin-5 constructs coating a glass surface. (B–D) Examples of Kymographs showing the movement of microtubules driven by Kin5-GFP (B), Kin5-Δtail-GFP (C) and Kin5-dimer-GFP (D) in high salt buffer (left) and low salt buffer (right). The microtubule velocity in each kymograph was determined from the slope of the red dashed line. (E) Average velocities for each construct in two buffer conditions. The population average for Kin5-dimer-GFP in high salt was not determined because the microtubules rapidly detached from the surface. (F) Model for the contribution of the microtubule binding site in the C-terminal tail domain to kinesin-5 motility. (i) A kinesin-5 molecule in solution prior to encountering a microtubule. (ii) The tail domain mediates the initial interaction, allowing 1-D diffusion and increases the probability of crosslinking a second microtubule. (iii) Relative filament sliding driven by kinesin-5’s two pairs of motor domains. The inset highlights how the non-motor tail domains maintain association with the filament and prevent full dissociation of kinesin-5 when the low processivity motor domains unbind. Error bars = sem.
In low salt buffer microtubule binding and gliding was observed for all three kinesin-5 constructs (Figures 4B–4D). The average gliding velocity of the two truncation constructs were similar, with v = 29.2 +/− 0.4 for Kin5-Δtail-GFP (N = 84 microtubules from 3 independent experiments) and v = 30.1 +/– 0.7 nm/s for Kin5-dimer-GFP (N = 85 microtubules from 3 independent experiments). Kin5-GFP, however, had about 1.7-fold lower average velocity (Figure 4E), with v = 16.8 +/− 0.4 nm/s (N = 84 microtubules from 3 independent experiments), consistent with the results of the single molecule assay.
Because motorless kinesin-5 showed salt dependent interactions with microtubules (Figure 1F–1H), we next examined whether the tail deletion affects the salt sensitivity of kinesin-5 motility. In high salt buffer the average gliding velocity of Kin5-Δtail-GFP increased slightly, to v = 35.7 +/− 0.6 nm/s (N = 136 microtubules from 4 independent experiments). Kin5-GFP’s velocity, however, increased by more than two-fold, to v = 34.4 +/− 0.5 nm/s for Kin5-GFP (N = 114 microtubules from 4 independent experiments), such that the velocities of the two tetrameric kinesin-5 constructs were nearly identical. In the high salt buffer, microtubules rapidly dissociated from the Kin5-dimer-GFP coated surface at all protein concentrations tested, consistent with a reduced microtubule binding affinity under these conditions.
Taken together, these results indicate the kinesin-5 non-motor tail domain enhances microtubule association but has a modest, salt-sensitive effect on motility. In low salt the presence of the tail in Kin5-GFP moderately reduces motor activity, but this impedance disappears as ionic strength is increased.
Discussion
We have shown that tetramerization of the kinesin-5 motor domains alone is not sufficient for microtubule crosslinking. The presence of the C-terminal tail domain is also requred to crosslink and slide two microtubules apart. Interestingly, the tail domain increases kinesin-5’s microtubule association without substantially resisting motility. While we favor a simple model wherein the primary function of the tail is to mediate direct interactions with microtubules, it is difficult to rule out the alternative mechanism in which the tail domain may interact with the motor domain to allosterically regulate its microtubule affinity and motor activity. A requirement for non-motor microtubule binding sites was largely unexpected for kinesin-5, in part because it appeared that two pairs of motor microtubule binding sites would be sufficient for microtubule crosslinking. Instead, our data indicate that kinesin-5 requires a combination of eight microtubule binding sites (four non-motor and two pairs of low-processivity motor domains), and that kinesin-5 uses these motor and non-motor domains to tune its microtubule interactions so individual kinesin-5 molecules can efficiently crosslink, align and slide apart microtubule pairs.
Based on our findings we propose a model for how kinesin-5 homotetramers can walk on each filament it crosslinks (Figure 4F). When a kinesin-5 molecule in solution collides with a microtubule, the motor and nonmotor domains mediate an interaction with the filament in which it samples multiple orientations, allowing it to dwell on the filament and explore its length via 1-D diffusion. This prolonged interaction with one filament would increase the likelihood of interacting with a second. Once a second microtubule makes contact at the opposite end of the kinesin-5 tetramer, directional motility is triggered, and the motor pairs at each end of the dumbbell can move towards the microtubule plus-ends. Individual directional runs are likely to be short because kinesin-5 motors have low processivity and tend to dissociate from microtubules after walking ~67 nm [13, 14]. However, the non-motor domains would keep kinesin-5 associated with both filaments it crosslinks despite frequent stochastic dissociation of the motor domains. Together, the interactions with microtubules, which involve motor and non-motor domains, would allow kinesin-5 to efficiently slide microtubules apart without having to form large multi-protein ensembles.
Our model can help explain the two main features of kinesin-5’s distribution in the metaphase spindle. Kinesin-5 localizes to the center of the spindle, where antiparallel microtubule overlap is most prevalent. A large fraction of the kinesin-5 in this region is positionally stable despite microtubule flux towards the poles. Kinesin-5 also accumulates at the spindle poles, where microtubule minus-ends are clustered and many filaments are parallel [15]. Kinesin-5’s walking speed along microtubules is comparable to the rate of microtubule flux, but in the opposite direction. Therefore, when a kinesin-5 molecule uses its two pairs of motor domains to walk along two microtubules, it can maintain an approximately fixed position relative to the spindle, whether the two microtubules are oriented parallel or antiparallel. However, the low processivity motor domains tend to dissociate frequently from microtubules. According to our model, when this occurs the non-motor domains would allow kinesin-5 to maintain the crosslink without encumbering continued sliding driven by other molecules of kinesin-5. When one pair of a kinesin-5 tetramer’s motor domains disengages from one microtubule, the motor protein’s fixed position would still be maintained by the motility of the second pair along the other filament. When both pairs of motor domains disengage simultaneously and the crosslink is maintained solely by the non-motor domains, then kinesin-5 would be pulled along by the motion of the microtubules. Between antiparallel microtubules the motion would be balanced in both directions and the kinesin-5 molecule would have little net movement. Between parallel microtubules the motor protein would ride polewards on both, explaining the observed relative enrichment of kinesin-5 on microtubules near the spindle poles.
The eight binding site mechanism also has implications for how kinesin-5 interacts with other mechanical components of the spindle. The low processivity of the motor domains, combined with low friction microtubule interactions via the non-motor domains, could enable kinesin-5 to act as a slip-clutch, with the ability to disengage its motors but maintain microtubule crosslinks in situations where its motor activity is overwhelmed by the function of other spindle components. Thus kinesin-5 could accommodate filament motion directed by other motor enzymes, such as chromokinesins, faster transport mechanisms driven by dynein, and the formation of specialized microtubule bundles, such as the kinetochore fibers that link chromosomes to the spindle. The proposed mechanism would be distinct from those of other motor proteins that can drive relative microtubule sliding, such as kinesin-1 and the minus-end directed motor kinesin-14 [16–18], each of which has a single pair of motor domains that walks on one filament, and an additional non-motor microtubule binding site that passively binds a second filament and carries it as cargo. Kinesin-14, whose motor domains are non-processive [19], probably cannot maintain microtubule crosslinks when the motors dissociate. Therefore, kinesin-14 likely requires a large number of molecules to sustain microtubule crosslinking, such that some fraction of the molecules present between two filaments have their motor domains bound. In fact, it has been shown that a 120-fold stoichiometric excess of kinesin-14 is needed to resist the oppositely directed microtubule sliding activity of kinesin-5 [20]. A single molecule of kinesin-1, whose motors are highly processive, is likely to make sustained microtubule crosslinks, but probably would not be compliant with the functions of other cytoskeletal components as is predicted for kinesin-5.
Because of its essential role in cell division, kinesin-5 has been considered as a potential drug target. Like monastrol, many kinesin-5 inhibitors that have recently entered clinical trials as cancer therapeutic agents inhibit kinesin-5 by stabilizing the ADP-bound state of the motor domain, thus locking the motor in a conformation that has low affinity for microtubules [21–23]. Inhibition of full-length kinesin-5 by monastrol abolishes directional motility, but not ATP-independent 1-D diffusion on microtubules, suggesting that monastrol stabilizes kinesin-5 in a low-friction microtubule attached state [9, 24]. Significantly, monastrol treatment of cells blocks spindle pole separation, but does not eliminate localization of kinesin-5 to the monopolar spindles [25]. Our findings explain these observations because although monastrol reduces the microtubule affinity of the motor domains, microtubule association can be maintained by the binding sites in the C-terminus of the full-length homotetramer. The interaction between the kinesin-5 non-motor domain and microtubules is essential for microtubule sliding function and is a feature unique to kinesin-5. Therefore, disruption of this interaction may represent a good strategy for designing highly selective anti-cancer drugs.
Experimental Procedures
Protein constructs
Motility assays
Photobleaching and motility assays were performed on an inverted microscope (Axiovert; Carl Zeiss) as described previously [8, 9, 26], with some modifications. See supplemental information for details. Two different buffers were used for most motility experiments. The low salt buffer was PEM70 (70 mM Pipes, 1 mM EGTA, 1 mM MgCl2, pH 6.8 with KOH). The high salt buffer was PEM70 with 80 mM KCl added.
Microtubule sliding
For the microtubule relative sliding experiments, X-rhodamine labeled, biotinylated microtubules were immobilized on a glass surface using a “biotin sandwich.” See supplemental information for details.
Cosedimentation
Microtubule binding assays were performed as essentially as described [26] in PEM70 in the presence of 2 mM MgADP.
MSD analysis
Motility data were analyzed using customized software written in MATLAB (Mathworks). Moving fluorescence spots were detected as in [27] and their trajectories were recovered by single particle tracking [28] along microtubules imaged in advance. Velocity and Diffusion constant were determined from MSD analysis and fitting as in [8]. Non-linear least square fitting was applied on original MSD data using the relation MSD = v2t2+2Dt+offset. Error bars in figures indicate s.e.m. See supplemental information for details.
Highlights
Kinesin-5 contains a non-motor microtubule binding site in its C-terminal domain
The kinesin-5 C-terminal domain promotes microtubule association and processivity
The kinesin-5 C-terminal domain is required for relative sliding of microtubules
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cole DG, Saxton WM, Sheehan KB, Scholey JM. A "slow" homotetrameric kinesin-related motor protein purified from Drosophila embryos. J Biol Chem. 1994;269:22913–22916. [PMC free article] [PubMed] [Google Scholar]
- 2.Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM. A bipolar kinesin. Nature. 1996;379:270–272. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- 4.Sharp DJ, McDonald KL, Brown HM, Matthies HJ, Walczak C, Vale RD, Mitchison TJ, Scholey JM. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J Cell Biol. 1999;144:125–138. doi: 10.1083/jcb.144.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–118. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- 6.Tao L, Mogilner A, Civelekoglu-Scholey G, Wollman R, Evans J, Stahlberg H, Scholey JM. A homotetrameric kinesin-5, KLP61F, bundles microtubules and antagonizes Ncd in motility assays. Curr Biol. 2006;16:2293–2302. doi: 10.1016/j.cub.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 7.van den Wildenberg SM, Tao L, Kapitein LC, Schmidt CF, Scholey JM, Peterman EJ. The homotetrameric kinesin-5 KLP61F preferentially crosslinks microtubules into antiparallel orientations. Curr Biol. 2008;18:1860–1864. doi: 10.1016/j.cub.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapitein LC, Kwok BH, Weinger JS, Schmidt CF, Kapoor TM, Peterman EJ. Microtubule cross-linking triggers the directional motility of kinesin-5. J Cell Biol. 2008;182:421–428. doi: 10.1083/jcb.200801145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwok BH, Kapitein LC, Kim JH, Peterman EJ, Schmidt CF, Kapoor TM. Allosteric inhibition of kinesin-5 modulates its processive directional motility. Nat Chem Biol. 2006;2:480–485. doi: 10.1038/nchembio812. [DOI] [PubMed] [Google Scholar]
- 10.Helenius J, Brouhard G, Kalaidzidis Y, Diez S, Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–119. doi: 10.1038/nature04736. [DOI] [PubMed] [Google Scholar]
- 11.Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, Howard J. The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell. 2003;11:445–457. doi: 10.1016/s1097-2765(03)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper JR, Wordeman L. The diffusive interaction of microtubule binding proteins. Curr Opin Cell Biol. 2009;21:68–73. doi: 10.1016/j.ceb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crevel IM, Lockhart A, Cross RA. Kinetic evidence for low chemical processivity in ncd and Eg5. J Mol Biol. 1997;273:160–170. doi: 10.1006/jmbi.1997.1319. [DOI] [PubMed] [Google Scholar]
- 14.Valentine MT, Fordyce PM, Krzysiak TC, Gilbert SP, Block SM. Individual dimers of the mitotic kinesin motor Eg5 step processively and support substantial loads in vitro. Nat Cell Biol. 2006;8:470–476. doi: 10.1038/ncb1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor TM, Mitchison TJ. Eg5 is static in bipolar spindles relative to tubulin: evidence for a static spindle matrix. J Cell Biol. 2001;154:1125–1133. doi: 10.1083/jcb.200106011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun M, Drummond DR, Cross RA, McAinsh AD. The kinesin-14 Klp2 organizes microtubules into parallel bundles by an ATPdependent sorting mechanism. Nat Cell Biol. 2009;11:724–730. doi: 10.1038/ncb1878. [DOI] [PubMed] [Google Scholar]
- 17.Fink G, Hajdo L, Skowronek KJ, Reuther C, Kasprzak AA, Diez S. The mitotic kinesin-14 Ncd drives directional microtubulemicrotubule sliding. Nat Cell Biol. 2009;11:717–723. doi: 10.1038/ncb1877. [DOI] [PubMed] [Google Scholar]
- 18.Jolly AL, Kim H, Srinivasan D, Lakonishok M, Larson AG, Gelfand VI. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc Natl Acad Sci U S A. 2010;107:12151–12156. doi: 10.1073/pnas.1004736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster KA, Gilbert SP. Kinetic studies of dimeric Ncd: evidence that Ncd is not processive. Biochemistry. 2000;39:1784–1791. doi: 10.1021/bi991500b. [DOI] [PubMed] [Google Scholar]
- 20.Hentrich C, Surrey T. Microtubule organization by the antagonistic mitotic motors kinesin-5 and kinesin-14. J Cell Biol. 2010;189:465–480. doi: 10.1083/jcb.200910125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo L, Parrish CA, Nevins N, McNulty DE, Chaudhari AM, Carson JD, Sudakin V, Shaw AN, Lehr R, Zhao H, et al. ATPcompetitive inhibitors of the mitotic kinesin KSP that function via an allosteric mechanism. Nat Chem Biol. 2007;3:722–726. doi: 10.1038/nchembio.2007.34. [DOI] [PubMed] [Google Scholar]
- 22.DeBonis S, Simorre JP, Crevel I, Lebeau L, Skoufias DA, Blangy A, Ebel C, Gans P, Cross R, Hackney DD, et al. Interaction of the mitotic inhibitor monastrol with human kinesin Eg5. Biochemistry. 2003;42:338–349. doi: 10.1021/bi026716j. [DOI] [PubMed] [Google Scholar]
- 23.Maliga Z, Kapoor TM, Mitchison TJ. Evidence that monastrol is an allosteric inhibitor of the mitotic kinesin Eg5. Chem Biol. 2002;9:989–996. doi: 10.1016/s1074-5521(02)00212-0. [DOI] [PubMed] [Google Scholar]
- 24.Crevel IM, Alonso MC, Cross RA. Monastrol stabilises an attached low-friction mode of Eg5. Curr Biol. 2004;14:R411–R412. doi: 10.1016/j.cub.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok BH, Yang JG, Kapoor TM. The rate of bipolar spindle assembly depends on the microtubule-gliding velocity of the mitotic kinesin Eg5. Curr Biol. 2004;14:1783–1788. doi: 10.1016/j.cub.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 27.Ponti A, Vallotton P, Salmon WC, Waterman-Storer CM, Danuser G. Computational analysis of F-actin turnover in cortical actin meshworks using fluorescent speckle microscopy. Biophys J. 2003;84:3336–3352. doi: 10.1016/S0006-3495(03)70058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang G, Matov A, Danuser G. Reliable Tracking of Large Scale Dense Antiparallel Particle Motion for Fluorescence Live Cell Imaging. Proc IEEE Comp Soci Conf CVPR; 2005. p. 138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.