Abstract
Apoptosis plays a role in pemphigus IgG-dependent acantholysis; theoretically, the blockade of the caspase pathway could prevent the blistering that is caused by pemphigus autoantibodies. Using this strategy, we attempted to block the pathogenic effect of pemphigus IgG in Balb/c mice by using the caspase inhibitor Ac-DEVD-CMK. This inhibitor was administrated before the injection of pemphigus IgG into neonatal mice. The main results of the present investigation are as follows: (1) pemphigus IgG induces intraepidermal blisters in Balb/c neonatal mice; (2) keratinocytes around the blister and acantholytic cells undergo apoptosis; (3) the caspases inhibitor Ac-DEVD-CMK prevents apoptosis; (4) the inhibition of the caspase pathway prevents blister formation. In conclusion, inhibition of the caspase pathway may be a promising therapeutic tool that can help in the treatment of pemphigus flare ups.
1. Introduction
Pemphigus is an autoimmune disease characterised by the development of intraepidermal blisters. These lesions are associated with the deposition of antidesmoglein autoantibodies along desmosome structures; such depositions induce cell detachment or acantholysis. Pemphigus IgG targets include desmosomal proteins [1].
The pathogenesis of pemphigus has been better elucidated than other organ-specific autoimmune diseases. In addition to its clinical importance, such knowledge has also contributed to major advances in desmoglein biology and pathology. Historically, Beutner and Jordon first described the pemphigus antibodies [2], and a significant advance in the understanding of pemphigus pathogenesis was accomplished by Anhalt and Diaz, who reproduced the disease by injecting human pemphigus IgG into Balb/c neonatal mice. Thus, they developed the first experimental model of pemphigus [3]. Subsequently, Stanley and Amagai proposed the key concept that autoantibodies against desmogleins are both essential and sufficient for epidermal blister formation (acantholysis), with autoantibody binding impeding the normal functioning of these major adhesion proteins [4].
The family of pemphigus autoantibodies is pathogenic because the passive transfer of pemphigus IgG or the transfer of splenocytes from recombinant Dsg3-immunised Dsg3(−/−) mice into experimental animals induces blistering and acantholysis [3–5]. In a similar fashion, human maternal pemphigus autoantibodies transferred into the foetus through placental circulation induces neonatal pemphigus [6]. Pemphigus autoantibodies specifically induce blister formation, and anti-idiotype antibodies can neutralise the blistering induced by the injection of pemphigus IgG into Balb/c neonatal mice [7].
Different mechanisms of blister induction have been proposed; some of them are dependent on pemphigus autoantibodies, which trigger the dissociation of desmoglein bonds, thus leading to apoptosis [8]. The desmosome dissociation depends on a rapid and dose-dependent phosphorylation of p38 mitogen-activated protein kinase (p38MAPK) and heat shock protein 27 (HSP27). Hyperphosphorylation can be abrogated by p38MAPK inhibition, which prevents disease in pemphigus vulgaris mice [9]. However, the biphasic activation of p38MAPK suggests that apoptosis is a downstream event in pemphigus acantholysis. The earlier peak of p38MAPK activation is part of the mechanism leading to acantholysis, whereas the later peak of p38MAPK and apoptosis may not be essential for acantholysis [9].
Regarding to the possible role of apoptosis, there is an alternative explanation of acantholysis in which the cells shrink and separate without affecting the desmosomal bonds at early stages. This explanation is supported by the observation that acantholysis in early pemphigus skin lesions and keratinocyte cultures in vitro may occur in the absence of apoptosis. Additionally, secondary antibody cross-reactivity with pemphigus autoantibodies bound to the keratinocyte cell surface may result in signals which induce acantholysis [10, 11].
One experimental therapeutic approach to treat pemphigus has focused on the prevention of cell detachment by caspase inhibition [9]. This strategy is reasonable because some studies have suggested that the final phase of acantholysis in pemphigus is Fas mediated [10]. Therefore, pharmacological approaches based on modifying the apoptotic pathway have been assessed in vitro for their ability to control blistering in pemphigus [12, 13]. Here, we study the in vivo role of apoptosis in blister formation, and we propose that inhibition of the caspase pathway is a possible therapeutic approach in experimental pemphigus.
2. Methods
2.1. Patient Sera
Serum that was positive for antiepithelial antibodies taken from a patient with pemphigus vulgaris (MCA) was used for IgG purification. Clinically, the patient had active and extensive disease with oral involvement, a positive Nikolsky's sign and a skin biopsy that showed a suprabasal epidermal blister. MCA had a high titre of antiepithelial antibodies and antidesmoglein 3 antibodies, as determined using an enzyme-linked immunosorbent assay (ELISA) (described later).
The control serum was obtained from a healthy individual matched by age and sex with the pemphigus patient (MCA). This serum was used to purify normal IgG as described later.
2.2. Antiepithelial Antibodies
Antiepithelial autoantibodies were detected using an indirect immunofluorescence assay using cow nose as the antigen source and fluorescein isothiocyanate (FITC)-labelled goat antihuman IgG (Sigma, St. Louis, MO, USA). The serum was progressively diluted in PBS (starting at 1 : 80) and incubated for 30 minutes on 4 μm sections of cow nose. After washing three times with PBS, the slides were incubated for 30 minutes with monospecific goat antihuman IgG, IgA, or IgM. After washing, the slides were evaluated under a fluorescent microscope [14].
2.3. Determination of Antidesmoglein Antibodies Using an ELISA
The ELISA was performed using polystyrene microwell plates coated overnight at 4°C with diluted recombinant Dsg1 and Dsg3 (175 ng of Dsg/mL in TBS-Ca++, pH 7.4) (RhiGene, Inc. Woburn, USA). The active sites of the coated plates were blocked for 1 h with 1% bovine serum albumin dissolved in TBS-Ca++ with 0.05% Tween 20 at pH 7.2. After washing with TBS-Ca++ with 0.05% Tween 20, 100 μL of diluted serum (1 : 100) was applied into the coated microwell plates and incubated for 60 min at room temperature. After five washes, the plates were incubated with 100 μL of horseradish peroxidase-conjugated rabbit antihuman IgG (Fab) (Sigma). After a final wash step, the colour reaction was developed through incubation with TMB substrate solution (3,3′,5,5′-tetramethylbenzidine dihydrochloride/hydrogen peroxide; Fluka, Seelze, Germany), for 30 minutes. The reaction was stopped with 100 μL of 1 N sulphuric acid. The optical density was measured at 490 nm [15].
2.4. Isolation of Pemphigus IgG
The gamma globulin fraction was precipitated with ammonium sulphate, and the precipitates were submitted to extensive dialysis against distilled water using Slide-A-Lyzer Dialysis Cassettes (10 K MWCO Thermo Fisher Scientific Inc., Rockford, IL). IgG was purified using affinity chromatography using a HiTrap protein G HP column (polypropylene 1.6 × 2.5 cm column packed with 5 mL of recombinant protein G Sepharose). The column was equilibrated with binding buffer (20 mM sodium phosphate), and IgG was eluted in five volumes of elution buffer (0.1 M glycine-HCl, pH 2.7). The elution fractions were neutralised with Tris-HCl (pH 9.0), and the protein content was detected using a spectrophotometer at 280 nm (Beckman Coulter DU 640). Pure IgG was characterised on 10% polyacrylamide-sodium dodecylsulphate (SDS) gels [16].
2.5. Passive Transfer into Balb/c Mice
The IgG fractions from pemphigus vulgaris and normal sera purified using affinity chromatography were concentrated to 100 mg/mL and filter sterilised. A dose of 1 mg IgG per gram of body weight was intraperitoneally administered to Balb/c neonatal mice, as previously reported [3]. The animals were evaluated clinically and histologically and using immunofluorescence 24 h after injection. The experimental groups included nine animals per group, which were divided as follows: (a) a control group injected with PBS; (b) a negative control group injected with normal IgG, obtained from healthy individuals; (c) a positive control group injected with pemphigus IgG; (d) an experimental group injected intraperitoneally with Ac-DEVD-CMK (Calbiochem Cat. no. 218750) 2 h before injection with pemphigus IgG. The caspase inhibitor Ac-DEVD-CMK was dissolved in DMSO and adjusted to 20 mM in PBS to a final volume of 50 μL. Serum samples and biopsies were collected immediately after the animals were sacrificed. All 36 test animals were injected simultaneously. All experiments were conducted according to the guidelines for ethical conduct in the care and use of animals developed by the American Psychological Association (APA) (http://www.apa.org/science/anguide.html).
2.6. Tissues
Skin punch biopsies (3 mm) were performed on the experimental animals. One biopsy was processed using direct immunofluorescence and another biopsy was embedded in paraffin and processed immunohistochemical staining and haematoxylin and eosin (H&E) staining or TdT-mediated dUTP nick end labelling (TUNEL) and annexin V staining.
2.7. Direct Immunofluorescence
A 4 μm slice of skin was obtained using cryosectioning. The tissues were rinsed in 0.15 M PBS, and any possible immune depositions were detected after incubation with FITC-conjugated rabbit antihuman IgG, IgM, IgA, C1Q, C3, or C4 (Sigma). After a 30-minute incubation, the slides were rinsed, mounted in glycerol-PBS and evaluated using fluorescent microscopy (described later).
2.8. Detection of Apoptotic Features
Programmed cell death was determined using two methods. First, the presence of phosphatidylserine on the outer leaflet of apoptotic membranes was detected using an Annexin-V-FLUOS Staining kit (Roche Diagnostics GmbH). Second, the presence of apoptotic DNA breaks was assessed using TUNEL staining according to the manufacturer's instructions (Roche Molecular Biochemicals, Penzberg, Germany). Positive and negative controls were included for each technique. To differentiate the true green tag of apoptotic cells from background fluorescence, tissues were counterstained with 0.5% propidium iodide, which stains nonapoptotic nuclei red. The slides were washed in PBS and evaluated using a confocal scanning microscope (LSM Axiovert 200M, Carl Zeiss, Göttingen, Germany). Combinations of fluorescein filters with excitations of 450–490 nm and rhodamine filters with emissions of 515–565 nm were used; the objectives were LCI “Plan-Neofluar,” and images were processed using a Zeiss LSM Image examiner.
2.9. Immunohistochemistry
Caspase 3, Fas and FasL were detected using immunohistochemistry in paraffin-embedded 4 μm thick sections of murine skin, which were dewaxed. Briefly, slides were permeabilised with 0.01% Triton X-100/PBS and washed three times with PBS. Endogenous peroxidase was blocked with horse serum that was heat inactivated at 56°C. After washing, tissues were incubated separately for 12 h with the following monoclonal antibodies: anticaspase 3 at a 1 : 20 dilution (35–1600, Zymed, South San Francisco, CA), anti-FasR at a 1 : 50 dilution (35–1600, Zymed), and anti-FasL at a 1 : 200 dilution (Sc-1968, Santa Cruz, CA). Dilutions were made in 10% BFS-PBS. After washing with PBS, bound antibodies were tagged with goat antimouse IgG labelled with peroxidase and diluted 1 : 100 (62–6620, Zymed). The colour reaction was induced by 2,2′diaminobenzydine in 0.06% H2O2 (Sigma, San Louis, MO), and the reaction was stopped with 2 N sulphuric acid. Finally, the slides were evaluated using a light microscope. The assays were performed in triplicate, and the results were evaluated by two observers in a blind fashion.
2.10. Caspase Activity
Caspase 3 activity was evaluated in skin extracts. Briefly, tissues were washed with cold PBS, pH 7.2, and disrupted in 200 μL of lysis buffer (1% Triton X-100, 140 mM NaCl, 1 mM EDTA, 10 mM Tris-HCI, pH 7.6, and 1 mM PMSF). Tissue extracts were homogenised and centrifuged at 14,000 rpm for 10 minutes at 4°C. The supernatants containing soluble fractions were run on 10% SDS-PAGE gels [16], which were then transferred onto nitrocellulose sheets (Hybond-C, Amersham, UK) [17]. Immunoreactive bands were identified using a 1 : 1000 dilution of a monoclonal anticaspase 3 antibody. A peroxidase-conjugated goat IgG antimouse IgG (Sigma) was used as the secondary antibody. After a 1 h incubation, immunoreactive bands were detected using chemiluminescence (ECL, RPN2106; Amersham).
Caspase 3 activity was also detected through staining of the cytoskeletal protein cytokeratin 18 (CK18), using the M30 CytoDeath antibody, which binds to a caspase-cleaved, formalin-resistant form of CK18 (Roche Applied Science, Mannheim, Germany). Paraffin-embedded tissue sections were dewaxed, and antigen retrieval was performed by heating in citric acid buffer (2 g/l citric acid, pH 6, adjusted with 1 N NaOH). Then, tissues were blocked with PBS containing 1% BSA and 0.1% Tween 20, followed by incubation with the M30 antibody for 1 h at 15 to 25°C in a humidified chamber. Tissues were then washed two times with PBS, followed by a 30-minute incubation at 37°C in a humidified chamber with a biotinylated antimouse-IgG antibody. After washing the slides with PBS, the sections were incubated with 0.5 U/mL streptavidin-POD (Roche Applied Science, Mannheim, Germany) for 30 min at 15 to 25°C in a humidified chamber, and the colour reaction was developed by incubation in a freshly prepared substrate solution of DAB substrate (Roche Applied Science, Mannheim, Germany) at 15 to 25°C until a clearly visible colour developed. The reaction was stopped using extensive rinsing in double-distilled water. Slides were counterstained with Harris haematoxylin, mounted in Kaiser's glycerol gelatin, and observed using light microscopy.
2.11. Statistical Analyses
Data were processed using a Chi-squared test with Yate's correction using the GraphPad Software, QuickCalcs; P < .005 was considered statistically significant.
3. Results
3.1. Pemphigus Serum Characterisation
The serum from a 34-year-old female patient who had pemphigus vulgaris for two years was used for IgG isolation. This serum sample was collected during a pemphigus flareup in which the patient exhibited extensive blistering affecting 70% of the skin surface in addition to the mouth and mucous epithelia. This patient tested positive for antiepithelial antibodies at a 1 : 2560 reciprocal titre for the IgG class; autoantibodies of the IgA and IgM class were negative, and she tested positive for antidesmoglein 1 at a concentration of 17.3 U/mL (normal value = 3.6) using ELISA. She also displayed antidesmoglein 3 autoantibodies at a concentration of 106 U/mL (normal value = 7).
3.2. Histology
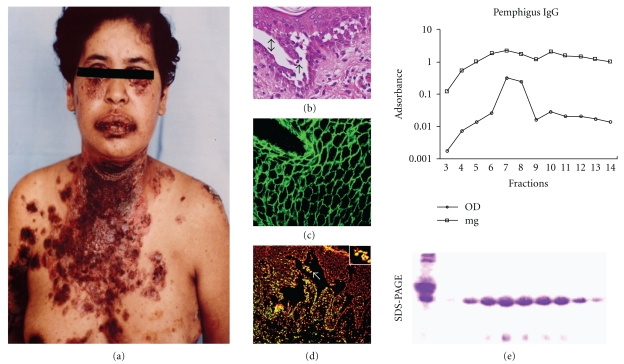
The skin biopsy stained using H&E showed a suprabasal intraepidermal blister with acantholytic cells (Figure 1). Using a direct immunofluorescence assay, extensive IgG deposition was visualised along the intercellular spaces of lesional and perilesional areas. Furthermore, we hypothesised that the pemphigus IgGs induce apoptosis because extensive apoptotic features were present in the patient's biopsy, as demonstrated using TUNEL and annexin staining techniques; no apoptotic features were observed in nonlesional skin of the patient. In contrast, apoptosis was observed in affected skin. The apoptotic cells found in the blister roof differed from apoptotic cells found following terminal skin differentiation (Figure 1).
Figure 1.
(a) Patient with pemphigus vulgaris showing extensive blistering in trunk and mouth. (b) Skin biopsy stained with H&E showing intraepidermal blister (double arrow) and acantholytic cells (arrow). (c) Patient sera showed antiepithelial antibodies using indirect immunofluorescence. (d) Intraepidermal blister showing that apoptotic cells (green) detected using TUNEL and counterstained with propidium iodide were present in the lesional area (square). (e) Isolation of pemphigus IgG using affinity chromatography. The chromatogram shows that the IgG peak was in fractions 7–9. OD: optical density. The bottom panel shows an SDS-PAGE gel of the eluted fractions.
3.3. IgG Purification
IgG purified using affinity chromatography yielded a peak in fractions 7–9, as shown using SDS-PAGE. These fractions were used to inject experimental animals. Antiepithelial IgG was positive, with a 1 : 2560 reciprocal titre exclusive of the IgG class, and antidesmoglein activity was also positive (Figure 1). Purified IgG fractions were used to induce pemphigus lesions.
3.4. Pemphigus IgG Triggers Acantholysis in Balb/c Mice
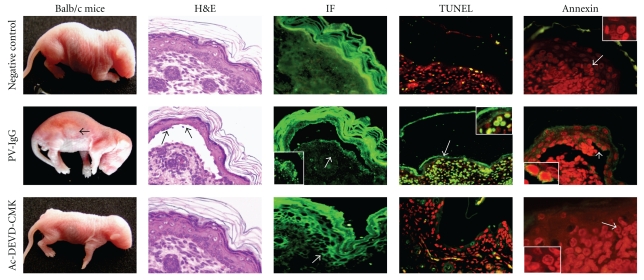
The group of animals that was injected with pemphigus IgG developed positive Nikolsky's signs, and seven of the nine mice developed blisters 24 hours after injection. Accordingly, the sera samples from these mice had positive antiepithelial antibodies, which were of human origin. Using histology, skin biopsies exhibited suprabasal intraepidermal blistering with multiple acantholytic cells, and immunofluorescence analysis showed IgG deposition along the intercellular spaces of involved and noninvolved skin. Apoptotic features were broadly distributed in lesional areas, mimicking the findings of the patient biopsy. Positive and negative controls of apoptosis were included. For the positive control, biopsies were digested with nucleases prior to staining and showed positive fluorescence along the epidermal and dermal nuclei. The negative control biopsies were processed without the TdT enzyme and thus showed no nuclear fluorescence (Figure 2).
Figure 2.
Induction of acantholysis in Balb/c mice and the prevention of blistering by caspase inhibition. The top panel shows representative figures of a negative control Balb/c mouse injected with normal IgG. This mouse did not develop blisters, show IgG deposition along intercellular spaces or initiate apoptosis. The middle panel shows a mouse injected with pemphigus vulgaris IgG that developed macroscopic abdominal and leg blisters. H&E staining shows that blisters were suprabasally located (arrow) with acantholytic cells (arrow), also PV-IgG was deposited in the roof and base (arrow) of the blister, and apoptotic cells are shown in green (arrow) as demonstrated using TUNEL and annexin staining. The bottom panel shows a mouse treated with the Ac-DEVD-CMK peptide prior to pemphigus IgG injection. The skin biopsy was taken 24 h after injection. The animal did not develop blisters despite the PV-IgG deposition along intercellular spaces. The caspase inhibitor blocked apoptosis of keratinocytes (red). PV-IgG: pemphigus vulgaris IgG. H&E: haematoxilin and eosin. IF: immunofluorescence.
3.5. Pemphigus IgG Induces Caspase 3 Activation
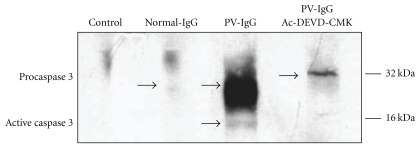
To assess whether pemphigus IgG activated caspase 3, skin extracts were characterised using SDS-PAGE, and caspase 3 was probed with an anticaspase 3 monoclonal antibody. The results showed that normal IgG did not activate caspase 3. In the control group, a protein of 32 kDa was detected, which corresponds to zymogen. In contrast, in the skin extracts of animals injected with pemphigus IgG, two bands were detected: one at 32 kDa, which corresponds to the proenzyme, and the other at 16 kDa, which corresponds to the active caspase 3 fragment (Figure 3).
Figure 3.
Mouse skin extracts were analysed using western blot and probed with a monoclonal anticaspase 3 antibody. The first line is a negative control incubated without antibody. The second line corresponds to a skin extract from a mouse injected with normal IgG. Note that a faint band appears at 32 kDa which corresponds to caspase 3 zymogen. The third line corresponds to an animal injected with pemphigus vulgaris IgG and shows a large band of pro-caspase 3 and an additional band at 16 kDa, which corresponds to active caspase 3. The skin extract from the mouse treated with Ac-DEVD-CMK and injected with pemphigus IgG exhibited only the proenzyme.
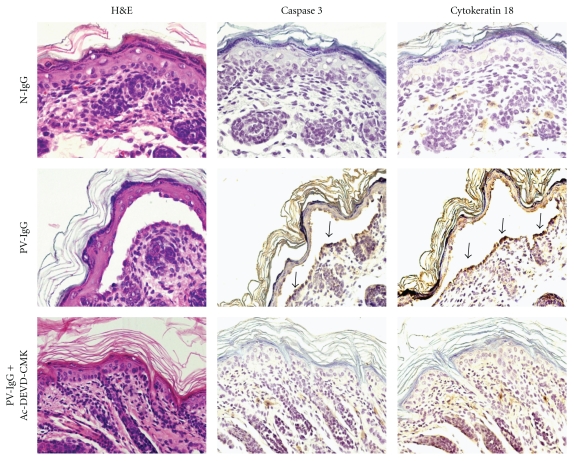
Another assay to evaluate caspase activity utilises the antibody M30, which recognises a neoepitope of cytokeratin 18 formed after caspase cleavage. This antibody does not bind the native or unmodified CK18 of normal cells. Tissues expressing CK18 apoptose upon activation of effector caspases 3, 6, 7, or 9. Animals injected with pemphigus IgG were tagged with the antibody M30, and they were found to express this surrogate marker in blister and perilesional areas but not in uninvolved skin. This tag was absent in the skin of the control group (Figure 4).
Figure 4.
Caspase 3 activity detected in mouse skin. Note the presence of caspase 3 in a blister induced by the injection of pemphigus vulgaris IgG, and the presence of cytokeratin 18 detected using the antibody M30 which recognises a neoepitope of cytokeratin 18 formed after caspase cleavage. Positive immunohistochemistry was exhibited in both cases (arrows). Note the inhibition induced by the synthetic peptide Ac-DEVD-CMK in the bottom row.
3.6. Caspases Inhibition Prevents Blistering
Our results demonstrated that PV-IgG induced blistering, and immunohistochemistry results showed a broad distribution of caspase 3 and Fas/FasL pairs in the blisters of animals treated with PV-IgG. These proteins were faintly detectable in controls. In addition, because the blisters were stained using the antibody M30, we deduce that PV-IgG induces caspase cascade activation that leads to apoptosis. Additionally, the 16 kDa active caspase 3 fragments detected in the skin of animals injected with PV-IgG demonstrated that caspase cascades were involved in blistering (Table 1).
Table 1.
Pemphigus IgG triggers acantholysis in Balb/c mice.
| Group (n = 9) | (A) Control | (B) PV-IgG | (C) Ac-DEVD-CMK+PV-IgG | P value |
|---|---|---|---|---|
| Macroscopic blisters | 0/9 | 7/9 | 0/9 | A versus B < .0037 |
| B versus C < .0037 | ||||
| Histology | Normal | Blisters, acantholysis 7/9 | No blisters | A versus B < .0037 |
| B versus C < .0037 | ||||
| Direct immunofluorescence | Negative | IgG on intercellular spaces 9/9 | IgG on intercellular spaces 9/9 | A versus B < .0002 |
| B versus C > .37 | ||||
| TUNEL | Negative | Positive 9/9 | Negative | A versus B < .0002 |
| B versus C < .0002 | ||||
| Annexin | Negative | Positive 9/9 | Negative | A versus B < .0002 |
| B versus C < .0002 | ||||
| Caspase 3 | 0/9 | 9/9 | 0/9 | A versus B < .0002 |
| B versus C < .0002 | ||||
| Fas | 9/9 faint | 9/9 strong | 9/9 faint | A versus B > .3 |
| B versus C >.32 | ||||
| FasL | 1/9 | 9/9 | 2/9 faint | A versus B < .0009 |
| B versus C < .0017 |
PV: pemphigus vulgaris, (statistically significant < 0.05), Chi-squared with Yates' correction.
Based on previous results, we investigated whether blocking caspases could prevent blistering. The group of animals treated with Ac-DEVD-CMK for 2 hours before injection with pemphigus IgG did not show blisters or Nikolsky's signs 24 hours after IgG administration although they had antiepithelial antibodies. The skin of these animals did not exhibit apoptotic features (Figure 2). They exhibited faint deposition of caspase 3 and Fas, but the caspases were not activated (Figure 3). No side effects of the Ac-DEVD-CMK injections were clinically detected in the mice in vivo or during necropsy.
Based on these results, we postulate that the pemphigus antibody induces disassembly of the desmosome, which in turn induces a downstream signal transduction cascade that leads to apoptosis. Control groups injected with PBS or normal IgG did not develop blisters, IgG deposition, or apoptosis (Figure 2 and Table 1).
4. Discussion
In the present work, we explored an alternative treatment approach for experimental pemphigus based on modifying caspase-related events.
The current investigation shows the following: (1) pemphigus IgG induces acantholysis in Balb/c neonatal mice, (2) pemphigus IgG-dependent acantholytic cells undergo apoptosis, and (3) caspase inhibition prevents apoptosis and acantholysis in vivo.
Previous observations have demonstrated that the blistering process in pemphigus is antibody dependent, and that blisters are caused by a mechanism that involves the binding of pemphigus IgG to the extracellular domains of desmogleins. This binding induces a downstream kinase signal transduction cascade that leads to desmoglein dissociation from the plakoglobin plate and desmosomal breakdown [18]. Simultaneously, a Fas-mediated downstream signal transduction cascade is triggered and leads to the apoptosis of acantholytic cells [19–21].
Caspases are cysteine proteases that are activated in cascades during programmed cell death. Caspase 3 is the most important caspase because it is involved in both the intrinsic and the extrinsic pathways of apoptosis. The use of caspase inhibitors to block apoptosis is based on the notion that the catalytic active site of caspases contains highly conserved motifs with characteristic sequences. This permits the interaction of complementary peptides that are designed to regulate the enzyme's activity. Caspase 3 shows a structural specificity for substrates in the S4 pocket, and this motif requires at least four amino acids to the left of the cleavage site [22, 23]. Reversible and irreversible inhibitors of caspases have been developed by coupling caspase-specific peptides to certain aldehyde, nitrite, or ketone compounds. These inhibitors can successfully block apoptosis. For instance, DEVD, which was used in the present work, is a peptide that preferentially binds caspase 3, but it can also affect other caspases such as caspase 6, 7, 8, and 10 [24].
Our results demonstrate that the acantholysis of pemphigus is linked to apoptosis; therefore, cell death is triggered by the Fas pathway. Our finding that apoptotic cells of the blister roof are different from apoptotic features observed in terminally differentiated skin of the stratum corneum strongly suggests that apoptosis in perilesional areas is caused by acantholysis and represents a pathophysiological mechanism in pemphigus [11, 25].
The present work confirms that the pan-caspase inhibitor Ac-DEVD-CMK prevents the blistering induced by human pemphigus IgG in living animals and extends the results obtained by other authors in cultured keratinocytes or cell lines [11]. These results are of potential therapeutic interest. Furthermore, the present results show macroscopic and histological evidence that Ac-DEVD-CMK prevents the development of both blisters and the initiation of apoptosis, despite these mice containing circulating human pemphigus antibodies. This suggests that apoptosis contributes to the pemphigus antibody role in desmosomal breakdown by a mechanism that needs to be further elucidated. It is possible that the cleaving effect of caspases could amplify the initial damage signal caused by pemphigus autoantibodies. However, we are certain that the unique conditions found in pemphigus that triggers apoptosis require the pemphigus autoantibodies because the injection of normal IgG did not trigger apoptosis or acantholysis. Our results were confirmed in additional experiments. The injection of neonatal Balb/c mice with PV-IgG isolated from different PV patients induced blisters and acantholysis in a similar manner to the IgG of the MCA patient, and this blistering was blocked in experimental animals by the caspase inhibitor.
To ensure that the previous results demonstrating acantholysis prevention by Ac-DEVD-CMK were real and not an epiphenomenon, we biopsied six different skin sites without clinically apparent blisters from the same animals for histological immunofluorescence and TUNEL analysis. These samples were representative of an extensive skin area of each animal. The biopsy analyses showed similar results to our previous experiments. These mice did not develop acantholysis, as demonstrated microscopically.
Another consideration is that inhibition of acantholysis and apoptosis occurred despite the expression of Fas and faint staining for FasR. These findings suggest that the pemphigus antibodies cause keratinocyte death and dissociation not only through the extrinsic, but also through the intrinsic pathway.
The Ac-DEVD-CMK peptide has previously been used in lupus experimental models to prevent apoptosis and tissue damage by antibody deposition [26, 27]. Here, we extend these previous observations to pemphigus and confirm the hypothesis that caspases inhibition can prevent blistering in pemphigus [10, 12, 13]. This experimental therapy may be a promising approach to control pemphigus flareups.
Other studies using caspase inhibitors, which were effective in blocking acantholysis, indicate that the apoptotic machinery contributes to cell dissociation. However, Waschke and Rubenstein [28–30] demonstrated that acantholysis might occur in the absence of apoptosis, which suggests that acantholysis in pemphigus can be dependent or independent of apoptosis. It has recently been demonstrated that the structural damage (acantholysis) and death (apoptosis) of keratinocytes are mediated by the same cell death enzymes; thus, the therm of apoptolysis was coined. Apoptolysis causes basal cell shrinkage and suprabasal acantholysis and may have several implications in pathogenic terms [31]. In the present study, our experimental data indicate that pemphigus IgG causes acantholysis and desmosome disassembly in association with caspase 3 activation. Such a phenomenon can be prevented by a synthetic peptide that inhibits caspase 3.
Acknowledgments
This work was supported by PROMEP-Cuerpo académico UAZ-CA-5 Autoinmunidad and partially granted by Secretaría General, UAZ. The authors thank Dr. Zelmira Lazarova from Medical College of Wisconsin for allowing them to purify IgG in her lab.
References
- 1.Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. The New England Journal of Medicine. 2006;355(17):1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- 2.Beutner EH, Jordon RE. Demonstration of skin antibodies in sera of pemphigus vulgaris patients by indirect immunofluorescent staining. Proceedings of the Society for Experimental Biology and Medicine. 1964;117:505–510. doi: 10.3181/00379727-117-29622. [DOI] [PubMed] [Google Scholar]
- 3.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. The New England Journal of Medicine. 1982;306(20):1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 4.Amagai M, Ahmed AR, Kitajima Y, et al. Are desmoglein autoantibodies essential for the immunopathogenesis of pemphigus vulgaris, or just ’witnesses of disease’? Experimental Dermatology. 2006;15(10):815–831. doi: 10.1111/j.1600-0625.2006.00499_1.x. [DOI] [PubMed] [Google Scholar]
- 5.Aoki-Ota M, Tsunoda K, Ota T, et al. A mouse model of pemphigus vulgaris by adoptive transfer of naive splenocytes from desmoglein 3 knockout mice. British Journal of Dermatology. 2004;151(2):346–354. doi: 10.1111/j.1365-2133.2004.06056.x. [DOI] [PubMed] [Google Scholar]
- 6.Avalos-Díaz E, Olague-Marchan M, López-Swiderski A, Herrera-Esparza R, Díaz LA. Transplacental passage of maternal pemphigus foliaceus autoantibodies induces neonatal pemphigus. Journal of the American Academy of Dermatology. 2000;43(6):1130–1134. doi: 10.1067/mjd.2000.110400. [DOI] [PubMed] [Google Scholar]
- 7.Alvarado-Flores E, Avalos-Díaz E, Díaz LA, Herrera-Esparza R. Anti-idiotype antibodies neutralize in vivo the blistering effect of Pemphigus foliaceus IgG. Scandinavian Journal of Immunology. 2001;53(3):254–258. doi: 10.1046/j.1365-3083.2001.00863.x. [DOI] [PubMed] [Google Scholar]
- 8.Jolly PS, Berkowitz P, Bektas M, et al. p38MAPK signaling and desmoglein-3 internalization are linked events in pemphigus acantholysis. Journal of Biological Chemistry. 2010;285(12):8936–8941. doi: 10.1074/jbc.M109.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HE, Berkowitz P, Jolly PS, Diaz LA, Chua MP, Rubenstein DS. Biphasic activation of p38MAPK suggests that apoptosis is a downstream event in pemphigus acantholysis. Journal of Biological Chemistry. 2009;284(18):12524–12532. doi: 10.1074/jbc.M808204200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arredondo J, Chernyavsky AI, Karaouni A, Grando SA. Novel mechanisms of target cell death and survival and of therapeutic action of IVIg in pemphigus. American Journal of Pathology. 2005;167(6):1531–1544. doi: 10.1016/S0002-9440(10)61239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacheco-Tovar MG, Avalos-Díaz E, Vega-Memije E, et al. The final destiny of acantholytic cells in pemphigus is Fas mediated. Journal of the European Academy of Dermatology and Venereology. 2009;23(6):697–701. doi: 10.1111/j.1468-3083.2009.03162.x. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Zhao M, Wang J, Liu Z, Diaz LA. Involvement of the apoptotic mechanism in pemphigus foliaceus autoimmune injury of the skin. Journal of Immunology. 2009;182(1):711–717. doi: 10.4049/jimmunol.182.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacheco-Tovar D, Lopez-Luna A, Bollain-y-Goytia JJ, Herrera-Esparza R, Avalos-Diaz E. Caspases pathway a therapeutic target in pemphigus. Molecular Biology of the Cell. 2008;19(supplement 19):217–218. abstract 747, (CD-ROM) [Google Scholar]
- 14.Zagorodniuk I, Weltfriend S, Shtruminger L, et al. A comparison of anti-desmoglein antibodies and indirect immunofluorescence in the serodiagnosis of pemphigus vulgaris. International Journal of Dermatology. 2005;44(7):541–544. doi: 10.1111/j.1365-4632.2004.02541.x. [DOI] [PubMed] [Google Scholar]
- 15.Diaz LA, Arteaga LA, Hilario-Vargas J, et al. Anti-desmoglein-1 antibodies in onchocerciasis, leishmaniasis and chagas disease suggest a possible etiological link to fogo selvagem. Journal of Investigative Dermatology. 2004;123(6):1045–1051. doi: 10.1111/j.0022-202X.2004.23438.x. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. Journal of Clinical Investigation. 2005;115(11):3157–3165. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calkins CC, Setzer SV, Jennings JM, et al. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. Journal of Biological Chemistry. 2006;281(11):7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- 20.Chernyavsky AI, Arredondo J, Kitajima Y, Sato-Nagai M, Grando SA. Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. Journal of Biological Chemistry. 2007;282(18):13804–13812. doi: 10.1074/jbc.M611365200. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Brégégère F, Frušić-Zlotkin M, Feinmesser M, Michel B, Milner Y. Possible apoptotic mechanism in epidermal cell acantholysis induced by pemphigus vulgaris autoimmunoglobulins. Apoptosis. 2004;9(2):131–143. doi: 10.1023/B:APPT.0000018795.05766.1f. [DOI] [PubMed] [Google Scholar]
- 22.Ganesan R, Mittl PRE, Jelakovic S, Grütter MG. Extended substrate recognition in caspase-3 revealed by high resolution X-ray structure analysis. Journal of Molecular Biology. 2006;359(5):1378–1388. doi: 10.1016/j.jmb.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. Journal of Biological Chemistry. 1998;273(49):32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 24.Rozman-Pungerčar J, Kopitar-Jerala N, Bogyo M, et al. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death and Differentiation. 2003;10(8):881–888. doi: 10.1038/sj.cdd.4401247. [DOI] [PubMed] [Google Scholar]
- 25.Allombert-Blaise C, Tamiji S, Mortier L, et al. Terminal differentiation of human epidermal keratinocytes involves mitochondria- and caspase-dependent cell death pathway. Cell Death and Differentiation. 2003;10(7):850–852. doi: 10.1038/sj.cdd.4401245. [DOI] [PubMed] [Google Scholar]
- 26.Seery JP, Cattell V, Watt FM. Cutting edge: amelioration of kidney disease in a transgenic mouse model of lupus nephritis by administration of the caspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-(β-o-methyl)-fluoromethylketone. Journal of Immunology. 2001;167(5):2452–2455. doi: 10.4049/jimmunol.167.5.2452. [DOI] [PubMed] [Google Scholar]
- 27.Herrera-Esparza R, Villalobos R, Bollain-y-Goytia JJ, et al. Apoptosis and redistribution of the Ro autoantigen in Balb/c mouse like in subacute cutaneous lupus erythematosus. Clinical & Developmental Immunology. 2006;13(2–4):163–166. doi: 10.1080/17402520600876796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt E, Gutberlet J, Siegmund D, Berg D, Wajant H, Waschke J. Apoptosis is not required for acantholysis in pemphigus vulgaris. American Journal of Physiology. 2009;296(1):C162–C172. doi: 10.1152/ajpcell.00161.2008. [DOI] [PubMed] [Google Scholar]
- 29.Heupel WM, Engerer P, Schmidt E, Waschke J. Pemphigus vulgaris IgG cause loss of desmoglein-mediated adhesion and keratinocyte dissociation independent of epidermal growth factor receptor. American Journal of Pathology. 2009;174(2):475–485. doi: 10.2353/ajpath.2009.080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bektas M, Jolly P, Rubenstein DS. Apoptotic pathways in pemphigus. Dermatology Research and Practice. 2010;2010:8 pages. doi: 10.1155/2010/456841. Article ID 456841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grando SA, Bystryn JC, Chernyavsky AI, et al. Apoptolysis: a novel mechanism of skin blistering in pemphigus vulgaris linking the apoptotic pathways to basal cell shrinkage and suprabasal acantholysis. Experimental Dermatology. 2009;18(9):764–770. doi: 10.1111/j.1600-0625.2009.00934.x. [DOI] [PubMed] [Google Scholar]